Abstract
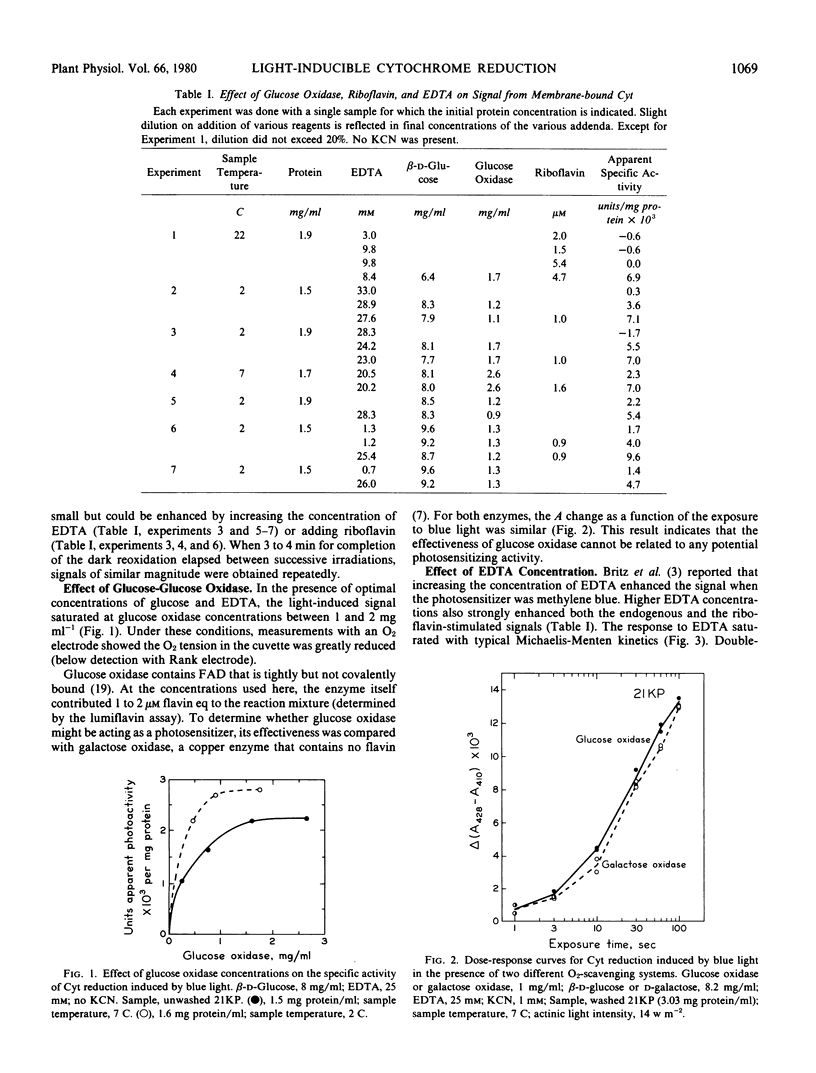
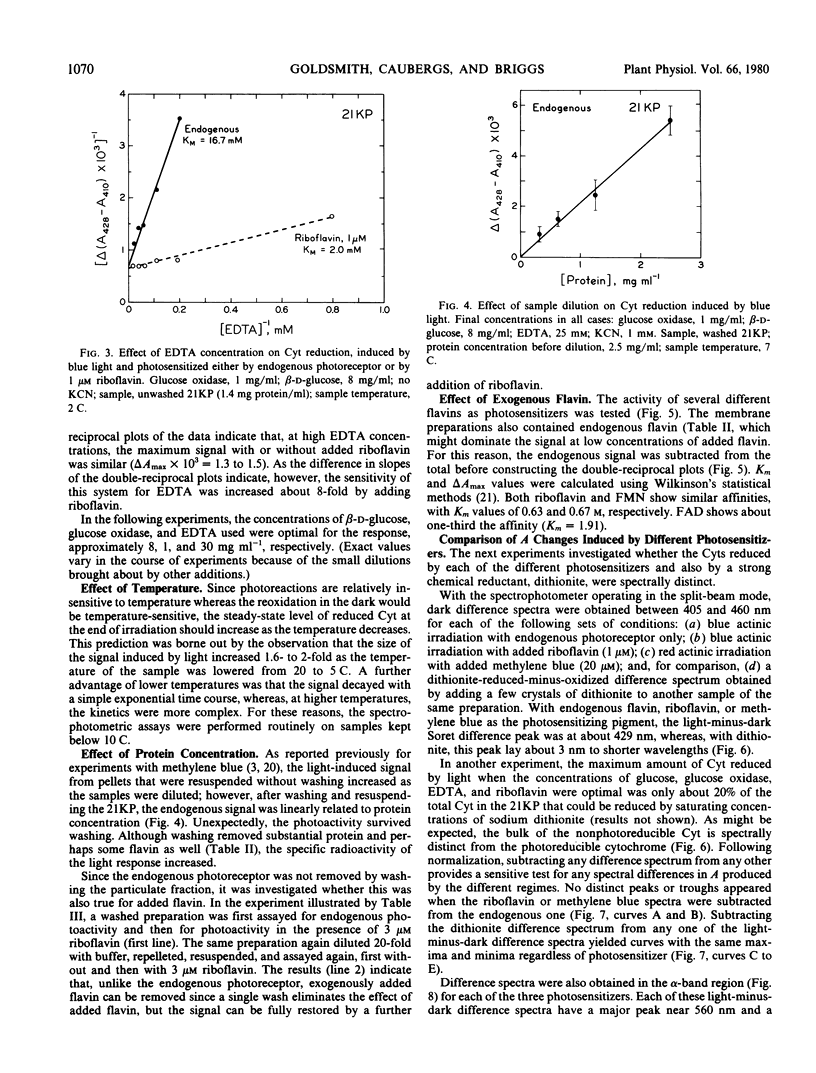
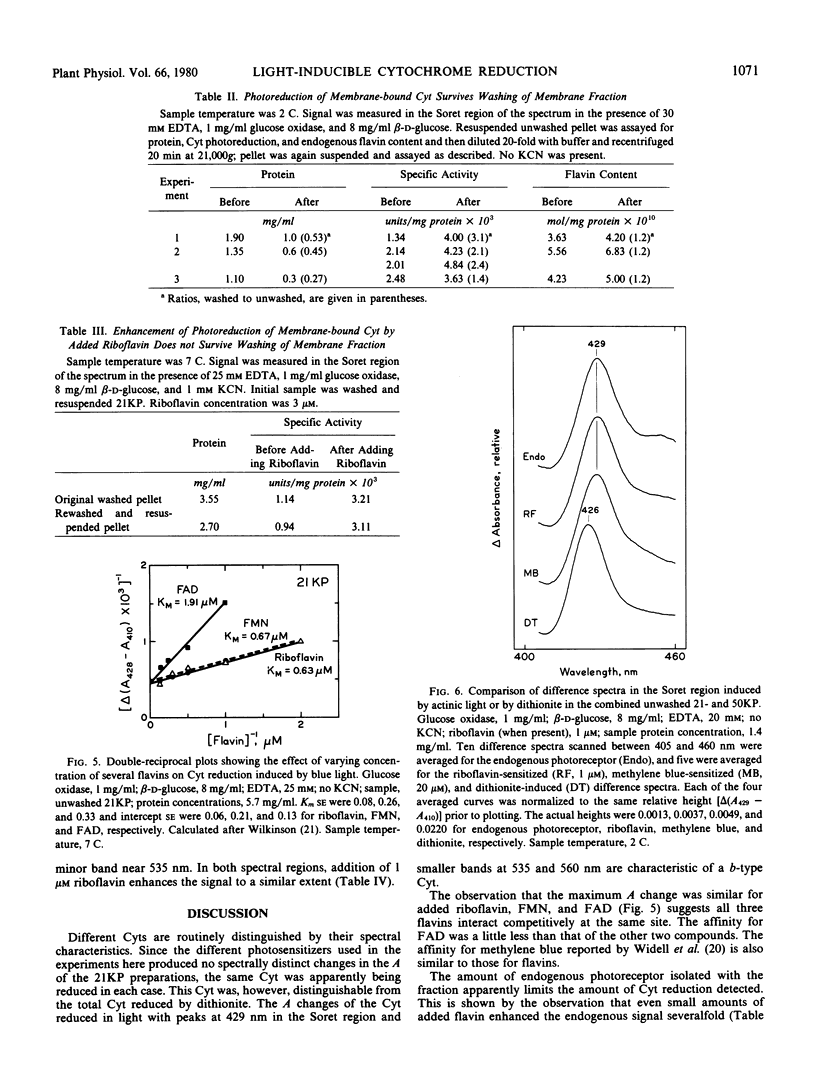
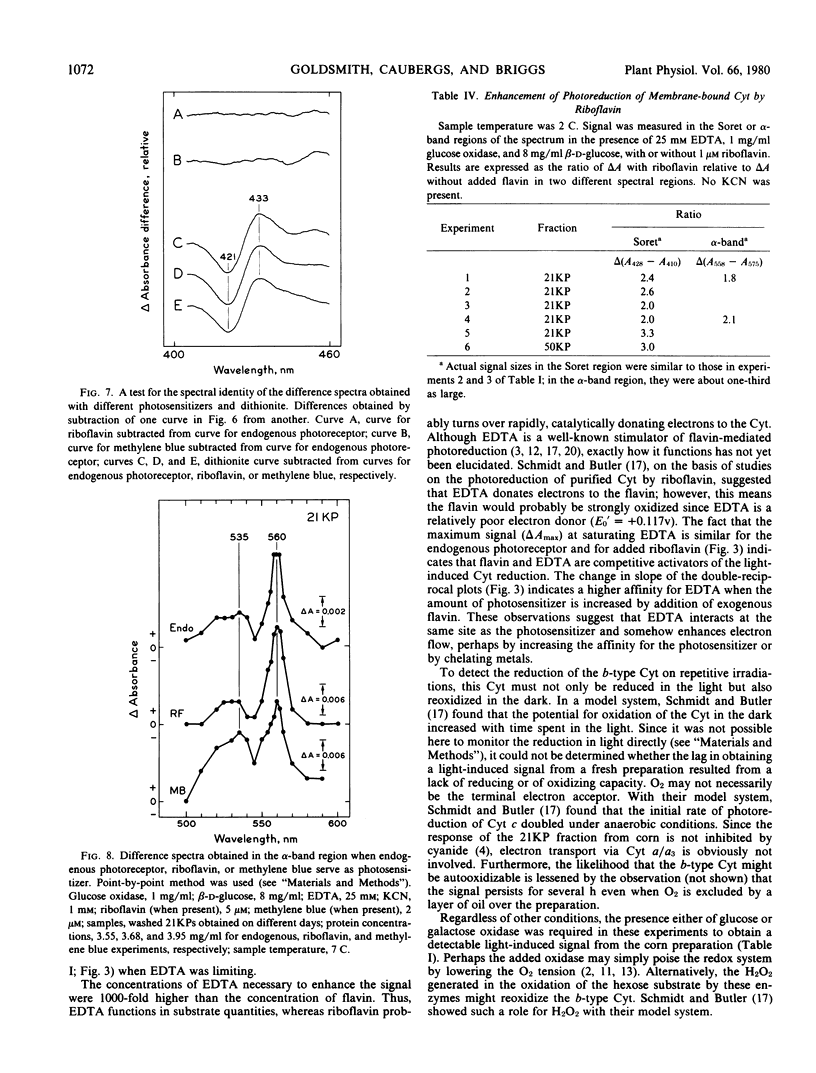
Conditions for obtaining reproducible light-induced reduction of a b-type cytochrome in membrane fractions from coleoptiles of dark-grown Zea mays L. include a glucose-glucose oxidase system that lowers O2 tension and generates H2O2, substrate amounts of ethylenediaminetetraacetic acid which, in some manner, facilitates photoreduction by both added flavin and the endogenous photoreceptor and a sample temperature below 10 C. Cytochrome reduction could be obtained by photoexcitation of either a tightly bound endogenous receptor, which is probably a flavin, or added riboflavin, flavin mononucleotide, or flavin adenine dinucleotide. The latter flavin was the least effective. The endogenous photoreceptor appears to be rather firmly bound to the membranes, suggesting that this association may also exist in vivo. When any of the above four photoreceptors or methylene blue were used to sensitize the reaction, a cytochrome with a reduced α-band near 560 nanometers and a Soret difference peak near 429 nanometers was the electron acceptor. This cytochrome could be clearly distinguished spectrally from other cytochromes that predominated in the membrane preparations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Heners P. R., Hertel R. Characterization of a Membrane Fraction Containing a b-type Cytochrome. Plant Physiol. 1977 May;59(5):941–947. doi: 10.1104/pp.59.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang-Feulner J., Rau W. Redox dyes as artificial photoreceptors in light-dependent carotenoid synthesis. Photochem Photobiol. 1975 Mar;21(3):179–183. doi: 10.1111/j.1751-1097.1975.tb06649.x. [DOI] [PubMed] [Google Scholar]
- MERKEL J. R., NICKERSON W. J. Riboflavin as a photocatalyst and hydrogen carrier in photochemical reduction. Biochim Biophys Acta. 1954 Jul;14(3):303–311. doi: 10.1016/0006-3002(54)90188-2. [DOI] [PubMed] [Google Scholar]
- Manabe K., Poff K. L. Purification and Characterization of the Photoreducible b-type Cytochrome from Dictyostelium discoideum. Plant Physiol. 1978 Jun;61(6):961–966. doi: 10.1104/pp.61.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz V., Butler W. L. Photoreceptor Pigment for Blue Light in Neurospora crassa. Plant Physiol. 1975 Feb;55(2):421–426. doi: 10.1104/pp.55.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R. The effects of light on a circadian rhythm of conidiation in neurospora. Plant Physiol. 1967 Nov;42(11):1504–1510. doi: 10.1104/pp.42.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Butler W. L. Flavin-mediated photoreactions in artificial systems: a possible model for the blue-light photoreceptor pigment in living systems. Photochem Photobiol. 1976 Jul;24(1):71–75. doi: 10.1111/j.1751-1097.1976.tb06799.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Butler W. L. Light-induced absorbance changes in cell-free extracts of Neurospora crassa. Photochem Photobiol. 1976 Jul;24(1):77–80. doi: 10.1111/j.1751-1097.1976.tb06800.x. [DOI] [PubMed] [Google Scholar]
- Swoboda B. E. The relationship between molecular conformation and the binding of flavin-adenine dinucleotide in glucose oxidase. Biochim Biophys Acta. 1969 Mar;175(2):365–379. doi: 10.1016/0005-2795(69)90014-2. [DOI] [PubMed] [Google Scholar]
- YAGI K. Chemical determination of flavins. Methods Biochem Anal. 1962;10:319–356. doi: 10.1002/9780470110270.ch10. [DOI] [PubMed] [Google Scholar]


