Abstract
We previously detected enterovirus D68 (EV-D68) in children with severe acute respiratory infections in the Philippines in 2008–2009. Since then, the detection frequency of EV-D68 has increased in different parts of the world, and EV-D68 is now recognized as a reemerging pathogen. However, the epidemiological profile and clinical significance of EV-D68 is yet to be defined, and the virological characteristics of EV-D68 are not fully understood. Recent studies have revealed that EV-D68 is detected among patients with acute respiratory infections of differing severities ranging from mild upper respiratory tract infections to severe pneumonia including fatal cases in pediatric and adult patients. In some study sites, the EV-D68 detection rate was higher among patients with lower respiratory tract infections than among those with upper respiratory tract infections, suggesting that EV-D68 infections are more likely to be associated with severe respiratory illnesses. EV-D68 strains circulating in recent years have been divided into three distinct genetic lineages with different antigenicity. However, the association between genetic differences and disease severity, as well as the occurrence of large-scale outbreaks, remains elusive. Previous studies have revealed that EV-D68 is acid sensitive and has an optimal growth temperature of 33 °C. EV-D68 binds to α2,6-linked sialic acids; hence, it is assumed that it has an affinity for the upper respiratory track where these glycans are present. However, the lack of suitable animal model constrains comprehensive understanding of the pathogenesis of EV-D68. © 2014 The Authors. Reviews in Medical Virology published by John Wiley & Sons Ltd.
Introduction
Human enterovirus D68 (EV-D68) is a member of species enterovirus D (EV-D), which belongs to the genus Enterovirus and the family of Picornaviridae. Only five EV-D serotypes have been identified thus far: enterovirus D70 (which is associated with acute hemorrhagic conjunctivitis) [1], enterovirus D94 (which is a causative agent of acute flaccid paralysis) [2], enteroviruses D111 and D120 (which were identified in non-human primates) [3], and EV-D68.
Enterovirus D68 was first isolated from four hospitalized pediatric patients with lower respiratory tract infections in California, USA, in 1962 [4]. Oropharyngeal specimens collected from the four patients were inoculated onto primary rhesus monkey kidney cells, leading to the isolation of the following four strains, Fermon, Franklin, Robinson, and Rhyne, with typical enterovirus-like cytopathic effects [4]. The four isolates were tested for serological identification by using neutralization (NT) tests with immune sera specific for a panel of viruses. The test revealed that the isolates were antigenically distinct from other any known enteroviruses [4]. Therefore, the four isolates were proposed to be members of a new serotype of the Picornaviridae family. The Fermon strain was selected as a representative strain of this new serotype, because the four strains had identical antigenic properties [4].
After the initial identification of EV-D68 in 1962, detection of this virus was rarely reported until the early 2000s. However, we detected EV-D68 in children hospitalized with severe acute respiratory infections in the Philippines in 2008–2009, which was followed by a number of similar reports from different parts of the world [5]. Most EV-D68 viruses have been detected in patients with acute respiratory infections. A considerable number of these cases were severe, and some were fatal [6–8]. However, the mechanisms underlying the recent global increase in EV-D68 detections are still not fully understood. Moreover, there is limited information about the virological characteristics of EV-D68, despite the increasing epidemiological and clinical significance of this virus.
In this review, we summarize current knowledge about EV-D68, by reviewing published articles and analyzing the EV-D68 sequence data deposited in GenBank.
Genome Structure
Enterovirus is a non-enveloped virus containing a single-stranded RNA genome with positive polarity [9]. The viral RNA encodes four structural proteins, namely, VP1, VP2, VP3, and VP4. Structural proteins VP1, VP2, and VP3 comprise the outer surface of the virion, whereas VP4 resides inside the protein shell of the virion [9].
The VP1 gene is generally considered the most variable region of the enterovirus genome. Therefore, it has been used for classifying viruses into different serotypes and genotypes [9]. This is why the VP1 sequences of enteroviruses, including those of EV-D68, have been extensively studied. A number of EV-D68 VP1 sequences from different parts of the world including Asia [6,10–15], Africa [7], Europe [8,16–21], Oceania [22], and the USA [7,23], have been deposited in GenBank. The EV-D68 strains detected in recent years are classified into three genetic groups based on the phylogenetic tree generated for VP1 nucleotide sequences (Figure1) [8,11]. These genetic groups are designated as lineages 1, 2, and 3 in this review, although there are variations in the names given to the three genetic groups in other reports: clades A–C [7,18], clusters 1–3 [13], and lineage 1, which contains sub-lineages 1 and 2, and lineage 2 [21]. Mutations have accumulated in two specific regions of the VP1 sequences: the BC and DE loops [8]. Despite the variety of geographical sources for EV-D68, the strains detected in recent years have similar VP1 sequences as long as they belong to the same genetic lineage [24]. All strains classified as lineage 3 share a unique sequence characteristic, nucleotide deletions at positions 2806–2808 in the VP1 region compared with the Fermon strain, which results in an amino acid deletion at this position (Figure2b) [8,10]. This unique genetic signature suggests a common origin for this genetic lineage. It is known that VP1 of enteroviruses has β-barrel structures containing eight β-strands, B, C, D, E, F, G, H, and I, and that these strands are connected by seven loop structures: the BC, CD, DE, EF, FG, GH, and HI loops [9]. The loop structures, including the BC and DE loops, are generally located on the viral surface and are associated with antigenic epitopes [25–27]. Therefore, it has been suggested that unique sequence variations in the BC and DE loops might cause altered antigenicity in these viruses [8,13]. Subsequently, we confirmed that there are antigenic differences between the recently detected EV-D68 strains and the Fermon strain, as well as among the lineages of recently detected strains [24].
Figure 1.
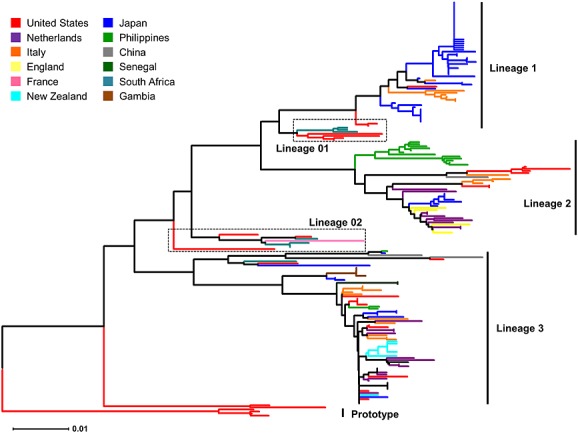
Phylogenetic relationships among enterovirus D68 strains detected in different countries. The phylogenetic tree for the VP1 region was generated using the neighbor-joining method, as implemented in mega software (http://www.megasoftware.net/). The partial VP1 sequences at nucleotide positions 2446–3033 corresponding to the Fermon strain (GenBank accession number: AY426531) were obtained from GenBank
Figure 2.
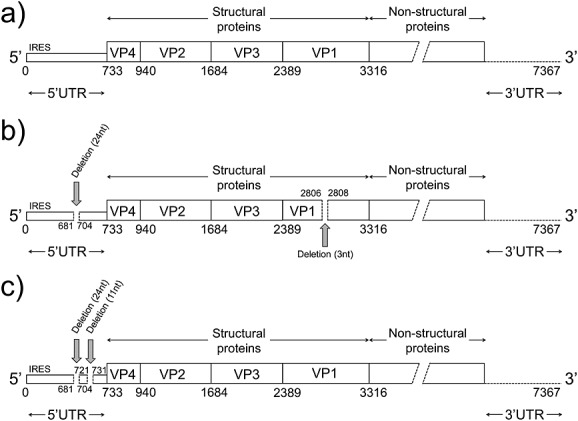
Genome structure of enterovirus D68 (EV-D68). The genome structures of the Fermon strain and lineage 2 (a), lineage 3 (b), and lineage 1 (c) of EV-D68 are depicted. Each genome region is indicated with a bar, and the nucleotide positions of these regions are annotated with numbers below the bars
Selection analyses conducted on the VP1 amino acid sequences in several studies have revealed that most of the positively selected codons are found in the BC and DE loop regions [13,24]. These findings suggest that antigenic epitopes might be located in the BC and DE loop regions. However, more detailed studies are required to determine the antigenic epitopes of the virus.
Recent studies using the Bayesian Markov chain Monte Carlo approach have estimated that genetic diversity in the VP1 region increased after the late 1990s, which may have resulted in the emergence of the three lineages [8]. The emergence of genetically variant EV-D68 strains might be associated with the increasing trend of EV-D68 detections in recent years. However, considering that most of the VP1 sequences available in GenBank are from strains detected in recent years, the rapid increase in VP1 sequence variation might simply be a result of sampling greater numbers of viruses from different geographical locations. Careful observations are required to draw any conclusions from these data.
Compared with the VP1 region, VP2, VP3, and VP4 have been less intensively studied, and only a limited number of sequences have been deposited in GenBank. We previously reported an analysis of VP2 and VP3 amino acid sequences from EV-D68 [24]. Unique amino acid mutations in the VP2 and VP3 regions that can differentiate recently detected strains from the Fermon strain, as well as recent strains of one genetic lineage from another, have been identified; however, such mutations were found less frequently than those in the VP1 region [24].
The 5′UTR is generally the most conserved genomic region in enteroviruses [9] and is commonly used as a target region for RT-PCR and sequencing for screening purposes [5]. The majority of the EV-D68 detections reported in recent years were based on positive results from RT-PCR and sequencing targeting the 5′UTR of EV-D68. The 5′UTR sequences of EV-D68 are known to be similar to those of human rhinoviruses (HRV) [28]; therefore, some of the recent EV-D68 strains were detected by PCR and sequencing using primers designed against the 5′UTR of HRV [6,19]. As the most conserved region, it was reported that the 5′UTR sequences were similar among the strains detected during the same outbreaks [6,19]. However, some genetic variations have been reported at the 3′ end of the 5′UTR, the spacer region between the internal ribosome entry sites and the open reading frame of the VP4 region [7,12]. Some strains were reported to have a 24-nucleotide deletion at positions 681–704 of the Fermon strain (Figure2b), and some of them had an additional 11-nucleotide deletion at positions 721–731 (Figure2c) [7,12]. Strains of lineage 1 were likely to have deletions at both positions 681–704 and 721–731. In contrast, lineage 3 strains had deletions only at positions 624–704, whereas those of lineage 2 had no deletions in the 5′UTR. The role of the spacer region in the pathogenesis of EV-D68 is still unresolved. Therefore, the significance of these deletions remains elusive.
Biological Features
Acid liability
In the report of the initial identification of EV-D68 in 1962, Schieble et al. demonstrated that the Fermon strain was acid stable [4]. In contrast, Blomqvist et al. reported in 2002 that two sublines of the Fermon strain (VR-561 and VR-1076) were acid sensitive, which was confirmed by similar experiments [29]. The report by Oberste et al. in 2004 concurred with that of Bloqvist et al. concerning the acid sensitivity of the Fermon strain and additionally showed that five other clinical isolates including MN89 (Minnesota, 1989), MN98 (Minnesota, 1989), MD02-1 (Maryland, 2002), TX01 (Texas, 2001), and TX02 (Texas, 2002) were also acid sensitive [28]. The variation observed in the preceding results might be caused by the use of different methods, including cell lines or strains used in each experiment. Oberste et al. also demonstrated that EV-D68 proliferated more efficiently at 33 °C than at 37 °C [28]. The acid sensitivity profile and optimal growth temperature indicate that EV-D68 strains share similar biological characteristics with HRV. These shared similarities might underlie the mechanisms for their respiratory tract tropism.
Receptor specificity
It was previously reported that EV-D68 strongly agglutinates guinea pig erythrocytes [4]. We additionally confirmed that EV-D68 agglutinates those from turkey, but not chicken or sheep (Imamura et al., unpublished data). However, the mechanisms controlling the agglutination activity of EV-D68 have not been documented. In 1991, neuraminidase treatment of HeLa cell monolayers was shown to result in an approximately 90% reduction of EV-D68 attachment and replication, suggesting that sialic acids (SAs) were a possible receptor for EV-D68 [30]. We investigated further the SA binding specificities of EV-D68 using glycan array analysis as well as enzymatically modified erythrocytes and found that EV-D68 had a stronger affinity with α2,6-linked SAs (NeuAcα2, 6Gal) than α2,3-linked SAs (NeuAcα2, 3Gal) [24]. It is known that α2,6-linked SAs are dominantly expressed in the upper respiratory tract whereas α2,3-linked SAs are expressed in the lower respiratory tract of human airways [31]. These results suggest that EV-D68 may have an affinity for the upper respiratory tract, compared with the lower respiratory tract. However, several epidemiological studies have shown that the EV-D68 detection rate was significantly higher among patients with lower respiratory tract infections than among those with upper respiratory tract infections [10,13]. Hence, mechanisms other than the distribution of potential receptors might be responsible for severe respiratory infections. It was previously reported that EV-D68 is associated with asthma exacerbations in children [32]. Therefore, enhanced immune responses in the lower respiratory tract might cause severe respiratory illnesses observed in EV-D68 infections.
It is also worth mentioning that EV-D68 has affinity for glycans with N-glycolyl neuraminic acid termini, which are commonly found in non-human species [24,33,34]. Thus, it might be possible that animals other than human are susceptible to EV-D68 infection. However, to date, EV-D68 has only been detected in humans.
Enterovirus 70 (EV70), which is another EV-D species member, is known to utilize α2,3-linked SAs for attachment and infection in cell culture [35]. The amino acid sequences of the capsid regions (VP4, VP2, VP3, and VP1) of EV70 share >76% similarity with those of EV-D68 [28]. Despite their sequence similarity, the pathogenesis of EV-D68 and EV70 in human appears to vary. The receptor specificities might underlie the mechanism controlling such clearly distinct tissue tropisms for these two enterovirus serotypes.
Antigenicity
We have shown by hemagglutination inhibition (HI) and NT tests that the Fermon strain has only limited cross-reactivity against the antisera generated for the EV-D68 strains detected in recent years [24]. This has led to the suggestion that the Fermon strain differs significantly from the EV-D68 strains that have been circulating in recent years. This result is in line with a previous study that demonstrated that the NT titer of sera collected from pregnant Finnish women against the Fermon strain decreased as the collection year progressed [36]. We found that antigenic differences were also present among the EV-D68 strains circulating in recent years: the HI and NT titer differed significantly among the strains from three different genetic lineages. Emergence of antigenically variant EV-D68 strains may be one of the reasons underlying the worldwide increase in detection of this virus in recent years.
Pathogenesis in animal models
Schieble et al. examined the pathogenesis of EV-D68 in suckling mice by inoculating them with four strains of EV-D68 (Fermon, Franklin, Robinson, and Rhyne) via the intracerebral and intraperitoneal routes [4]. The mice were observed for 2 weeks post-inoculation, after which the muscle and brain tissue subsequently harvested were passaged to new mice. No evidence of pathogenicity was observed for the Fermon, Franklin, or Robinson strain during passages of up to three times. However, mice inoculated with the Rhyne strain, which had been passaged twice in mice, developed limb tremor and weakness. Notably, mice developed paralysis and died after inoculation with the Rhyne strain that had been passaged three times [4]. This demonstrates the neuro-virulence of EV-D68 in mice, which is consistent with a case report describing the detection of EV-D68 in CSF collected from a fatal case of meningomyeloencephalitis [37]. However, a study by Schieble et al. did not document any pathogenic effects of EV-D68 in the respiratory systems of suckling mice. Therefore, despite the etiological importance of EV-D68 among patients with acute respiratory tract infections, animal models for EV-D68 respiratory infections have still not been established.
Epidemiology
After its initial identification in 1962, detection of EV-D68 was reported only rarely until the early 2000s [38]. In fact, enterovirus surveillance conducted in the USA between 1970 and 2005 identified only 26 EV-D68 cases [38]. Epidemiological data on EV-D68 have also been limited. The largest case series was seven EV-D68-positive cases among military recruits in the USA between 2004 and 2005 [39]. However, increased detection of EV-D68 has been reported from different parts of the world [5], including Asia [6,10–15], Africa [7], Europe [8,16–21], Oceania [22], and the USA [7,23] since the late 2000s (Table 1). Initially, it was pointed out that the increasing trend of EV-D68 detections in recent years might be due to sampling bias or the introduction of highly sensitive detection methods such as PCR. However, retrospective testing using stored respiratory samples from Yamagata, Japan, and the Netherlands revealed that EV-D68 detection had actually increased in recent years [8,11]. In those studies, EV-D68 had been detected with no more than 10 cases per year until 2009, however, a significantly increased number of positive cases were reported in 2010: 40 in Yamagata and 24 in the Netherlands.
Table 1.
Incidence of worldwide EV-D68 infections in recent years
| Location [references] | Period of EV-D68 detection | Study period | Study population | Number of EV-D68-positive cases (overall detection rate, %) | Number of fatal cases | Age of EV-D68-positive cases | Diagnosis |
|---|---|---|---|---|---|---|---|
| The Netherlands [8] | 1996, 1997, 1998, 1999, 2000, 2001, 2003, 2006, 2007, 2008, 2009, 2010 | 1994–2010 | ILI surveillance, children cohorts for wheezing illness, cystic fibrosis, and amniotic fluid study | 70 (70/12,743, 0.55%) | 0 | 0.8 months–80s | ILI, ARI |
| South Africa [7] | May 2000–May 2001 | ND | Hospitalized children | 8 | 0 | 5 months–1 year 11 months | Respiratory illness (hospitalized) |
| Japan (Yamagata) [11] | Sep–Oct 2005, Sep 2006, Aug–Oct 2007, Sep 2009, Aug–Oct 2010 | 2005–2010 | Pediatric outpatients with ARI | 55 (55/6307, 0.87%) | 0 | 5 months–15 years (mean: 5.2 years) | URTI, LRTI, others (e.g., asthma) |
| China (Beijing) [14] | Aug–Oct 2006, Aug 2008 | Aug 2006–Apr 2010 | Adult patients admitted to outpatient clinics and hospitals | 13 (13/6942, 0.18%) | 0 | 18–67 years (median: 34 years) | Mild ARTI |
| The Gambia [7] | Jun 2008 | ND | Hospitalized children | 5 | 0 | <2 years | Respiratory illness (hospitalized) |
| Philippines (Leyte) [6,10] | Oct 2008–Feb 2009, Jun–Aug 2011 | May 2008–Dec 2011 | Hospitalized pneumonia patients, outpatients with ILI | 33 (33/6056, 0.54%) | 4 | 1 month–76 years (median: 1 year 8 months) | Pneumonia, ILI |
| Italy (Pavia) [16,17] | Oct 2008–Apr 2009 | Oct 2008–Sep 2009 | Pediatric and adult patients hospitalized with ARI | 12 (12/1500, 0.80%) | 0 | 1 month–57 years | URTI, LRTI |
| USA (New Hampshire) [37] | Autumn 2008 | CR | CR | 1 | 1 | 5 years | Meningomyeloencephalitis |
| USA (Arizona) [7] | Oct 2009 | ND | ND | 1 | 0 | 1 year 11 months | Fever, cough, and rhinitis |
| The Gambia [7] | Jun 2008 | ND | Hospitalized children | 5 | 0 | <2 years | Respiratory illness (hospitalized) |
| Thailand [13] | Jun–Sep 2009, Feb–Oct 2010, Jun–Sep 2011 | Feb 2006–Nov 2011 | Children with ARTI | 25 (25/1810, 1.38%) | 0 | 7 months–15 years (mean: 7.6 years) | Pneumonia, ILI |
| China (Chongqing, Beijing, Tianjin) [15] | Jun 2009–Jun 2012 | Sep 2010–Aug 2011 | Hospitalized children and adult outpatients with ARTI | 9 (9/2150, 0.42%) | 0 | 7 children, 2 adults | ARTI (e.g., pneumonia, URTI, and asthma) |
| USA (New York) [7] | Aug–Oct 2009 | ND | Outpatients with respiratory illnesses | 16 | 0 | 14–47 years | Respiratory illness (outpatients) |
| France (Champagne-Ardenne) [19] | Sep–Nov 2009 | Sep 2009–Jun 2010 | Pediatric patients admitted to a hospital with acute airway diseases | 10 (10/651, 1.53%) | 0 | 6 months–10 years (median: 3.8 years) | Asthma, bronchiolitis |
| USA (Arizona) [7] | Oct 2009 | ND | ND | 1 | 0 | 1 year 11 months | Fever, cough, and rhinitis |
| England (London) [21] | Nov–Dec 2009, Sep–Dec 2010 | Nov 2009–Dec 2010 | Patients with respiratory symptoms attending primary care and hospitals | 17 | 1 | 7 weeks–45 years (mean: 12.2 years, median: 5.8 years) | Respiratory symptoms |
| Italy (Pavia) [18] | Jan 2010–Dec 2012 | 2010–2012 | Patients who stayed or visited a hospital with respiratory tract infections | 9 (9/3736, 0.24%) | 0 | 7 pediatrics (4 months–6 years, median 15 months), 2 adults | URTI, LRTI |
| Senegal [7] | Feb–Mar 2010 | ND | Hospitalized children | 3 | 0 | 2, 23, and 32 years | Respiratory illness (hospitalized) |
| New Zealand [22] | Mar–Aug 2010 | ND | ND | 15 | 0 | 1 months–48 years (median: 11 years) | Bronchiolitis, asthma, cough, coryza, wheeze, strider, pertussis, sepsis, heart failure, burns |
| Japan (Osaka) [12] | Jul–Sep 2010 | Oct 2009–Oct 2010 | Children with RTI | 15 (15/448, 3.35%) | 0 | 3 months–5 years (mean: 2 years 10 months) | Pneumonia, bronchopneumonia, bronchitis, LRTI, asthma, pharyngitis, febrile convulsion |
| Japan (Yamaguchi) [32] | Jul–Sep 2010 | Jul–Sep 2010 | Hospitalized children with history of asthma | 26 (26/35, 74.3%) | 0 | Mean: 4 years | Asthma attack |
| Netherlands [20] | Aug–Nov 2010 | 2009–Jan 2011 | Children hospitalized with respiratory infections | 24 (24/252, 9.52%) | 0 | 1 months–72 years (median: 14 years) | Respiratory infections (hospitalized) |
Summary information for EV-D68 incidence. The data include the study sites, year of detection, case numbers, age distribution, and diagnosis. The numbers of reported cases of EV-D68-positive respiratory illnesses in the USA in 2014 have not been included in this table because of the limited information at present.
ILI, influenza-like illness; RTI, respiratory tract infections; URTI, upper respiratory tract infections; LRTI, lower respiratory infections; ARTI, acute respiratory tract infections; ARI, acute respiratory infections; ND, unknown; CR, case report.
It is not fully understood whether EV-D68 has cyclic patterns of increased detections. The retrospective studies in Yamagata and the Netherlands suggested that EV-D68 was circulating every year, although the number of cases was significantly larger in 2010 for unknown reasons. In the Philippines, we identified EV-D68 three times: first in 2008–2009 [6], second in 2011 [10], and third in 2013–2014 (Furuse and Chaimongkol et al., unpublished data). It is possible that EV-D68 outbreaks occur in cyclic patterns at 2-year intervals in the Philippines, suggesting that the frequency of outbreaks may vary between countries.
Increased detection of EV-D68 has recently been reported in the USA [40]. From mid-August to 10 October 2014, a total of 691 cases have been reported in 46 states. Most of the cases occurred among children with severe respiratory illnesses, and some cases were fatal [40]. Detection of EV-D68 in the USA was previously reported in New York and Arizona in 2009 [7]. It is still unclear whether the present outbreak is associated with any changes that may have occurred in EV-D68 viruses that have been circulating in this country.
Molecular Epidemiology
The EV-D68 strains circulating in recent years are classified into three genetic groups as mentioned earlier [8,11]. For the VP1 region, strains belonging to lineages 1 and 2 are likely to form clusters containing viruses from the same geographical origins on the phylogenetic tree. Strains from Yamagata, Japan (blue lines), form a cluster in lineage 1, those from the Philippines form a cluster in lineage 2 (green lines), and those from Italy form a cluster in lineages 1 and 2 (orange lines; Figure1). In contrast, strains belonging to lineage 3 are less likely to show geographical clustering patterns (Figure1). These data suggest that transmission at the global level might be more common for lineage 3 than for lineages 1 and 2.
The EV-D68 strains identified in each geographical area are likely to consist of strains of multiple genetic lineages. In Yamagata, Japan, strains belonging to all three lineages were detected during a period of increased detection in 2010 (Figure3). In Italy, England, Thailand, and the Philippines, co-circulation of lineages 2 and 3 strains was reported (Figure3). In contrast, strains of only one lineage were detected each year in some of the study sites, including New Zealand (lineage 3 only); New York, USA (lineage 3); China (lineage 3); and Osaka, Japan (lineage 1; Figure3). Enterovirus 71 (EV71), which also belongs to the genus Enterovirus, is known to have three genogroups and several genotypes [41,42]. None of the specific genogroups or genotypes has been reported to be associated with an increased risk of severe illness. However, it is likely that genotype replacement is associated with the occurrence of large outbreaks [43]. Nevertheless, such a replacement of EV-D68 lineages has not been observed in any of the previous studies in which multiple EV-D68 lineages were detected. In Yamagata, Japan, the majority of the EV-D68 strains detected during the increased detections in 2010 belonged to lineage 1, which was the same genetic lineage as the strains detected in the same area before 2010 [11]. The mechanisms responsible for the increase in detections of EV-D68 might differ from those of viruses of the same genus, such as EV71.
Figure 3.
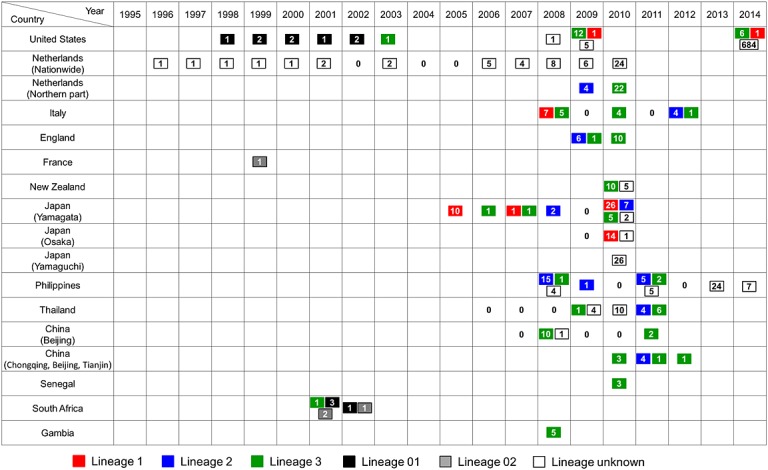
Circulation of different genetic lineages of enterovirus D68 (EV-D68) strains in recent years. The number of EV-D68 cases detected in a year at each study site is indicated in the box. Each year for which EV-D68 testing was conducted and no EV-D68-positive cases were found is shown as “0,” without a box in the column. Each year when no EV-D68 testing was reported is left blank
Clinical Significance
Detection of EV-D68 has been exclusively reported in studies where respiratory samples from patients with acute respiratory infections or asthma were tested, indicating that EV-D68 might only be associated with respiratory illnesses (Table 1). The importance of including healthy controls in viral etiological studies has been recently recognized, as some viral pathogens have also been detected in healthy individuals [44]. In the sentinel general practice (GP) network surveillance for influenza-like illness (ILI) conducted in the Netherlands, EV-D68 was detected in 57 out of 9979 patients (0.57%) with respiratory symptoms, whereas only one out of 567 healthy individuals (0.17%) was positive for EV-D68 [8]. The EV-D68 detection rate was higher among patients than healthy controls, suggesting that EV-D68 is more likely to be associated with respiratory diseases in humans.
Several study groups have compared EV-D68 detection rates in patients with different disease severities. In the epidemiological study for acute respiratory infections in the Philippines, the EV-D68 detection rate was significantly higher among patients hospitalized with pneumonia (9/1187, 0.76% of pediatric cases, and 2/456, 0.44% of adult cases) than among outpatients diagnosed as ILI (1/3597, 0.028%; p < 0.0001) [10]. Similarly, in a population-based study among children with acute respiratory tract illnesses in Thailand, the EV-D68 detection rate was higher in the hospitalized cases (1.5%, 9/597) than it was in the outpatients (1.3%, 16/1213), but the data lacked statistical significance [13]. In the Netherlands, respiratory samples were collected in the sentinel GP network surveillance for ILI, and EV-D68-positive acute respiratory infections were more commonly accompanied with severe respiratory symptoms, such as dyspnea (11/57, 36.67%, vs 22/833, 10.95%; p = 0.0002) and bronchitis (13/57, 23.21%, vs 46/833, 5.67%; p < 0.0001), than were EV-D68-negative cases [8]. These studies indicate that EV-D68 infections are more likely to be associated with severe respiratory illnesses. Clinical manifestations of the pediatric patients were reported in the recent outbreaks in the USA, including the clusters in Kansas City and Chicago [45,46]. Many of these patients had clinical characteristics indicative of severe illness, including difficulty of breathing, hypoxia, admission to an intensive care unit, and the requirement for mechanical ventilation. Such reports from the USA are consistent with previous studies indicating that EV-D68 infections are associated with severe respiratory illnesses. It is noteworthy that, of 30 pediatric patients whose respiratory samples were positive for EV-D68 in the USA, 21 (70%) had a previous history of asthma or wheezing [46]. An association between EV-D68 infections and asthma was also reported in Japan [32]. It might be possible that children with a history of asthma or wheezing illnesses are more likely to develop severe EV-D68 infections.
So far, a total of six fatal cases involving EV-D68 infections have been reported: three pediatric patients and one adult patient with pneumonia in the Philippines [6,10], one adult patient with severe respiratory symptoms in London, UK [21], and one pediatric patient admitted to the emergency department with meningomyeloencephalitis in New York, USA [37]. These reports indicate that EV-D68 infection can result in a fatal outcome. It should be mentioned that the two adult patients who died in the Philippines and London had comorbidities of hepatic cirrhosis and HIV infection, respectively [10,21]. These reports suggest that EV-D68 is also capable of causing fatal illness in adults with underlying diseases.
Susceptibility to EV-D68 infection per age group has not been fully defined, because most detected cases of EV-D68 have been reported from studies targeting pediatric patients. Only a few studies compared the EV-D68 detection rates among different age groups. In the ILI study conducted in the Netherlands, EV-D68 cases were most commonly identified in 50- to 59-year-olds, and the EV-D68-positive rate was lowest in children <10 years of age [8]. We previously reported that the EV-D68 detection rate was higher among pediatric pneumonia patients compared with adult pneumonia patients in the Philippines, but the data lacked statistical significance [10]. Because the disease severity of the study subjects differed, it is difficult to compare these two results. Further studies are needed to define the prevalence of EV-D68 infections in different age groups.
In several studies, co-detection of other viral pathogens in respiratory samples was reported for some of the EV-D68-positive cases [7,13]. In a study conducted in Thailand, 9 out of 13 EV-D68-positive cases had co-infections: one had RSV group A (RSV-A), three had influenza B virus, and five had influenza A(H1N1)pdm [13]. In another study, two patients in South Africa were co-infected with RSV-A, and one patient in the Gambia was co-infected with human parainfluenza virus type 1 [7]. In both studies, a possible association between disease severity and co-infections was not discussed, presumably because of the limited number of cases in which more than one virus was detected.
The etiological association between EV-D68 and other diseases also remains elusive. To the best of our knowledge, only two studies have identified EV-D68 in non-respiratory samples: one was a case report of a pediatric fatality from meningomyeloencephalitis (with radiological evidence of pneumonia) who was positive for EV-D68 in CSF [37], and the other was where EV-D68 was detected in serum collected from pneumonia patients who were positive for EV-D68 in respiratory samples [47]. Any association between the presence of EV-D68 in serum and disease severity has not been defined. However, that EV-D68 was detected in the CSF of a patient raises the possibility that EV-D68 might be capable of causing systemic spread through viremia. EV-D68 infections can result in very severe outcomes once the virus is spread to the CNS from the respiratory tract. It was recently reported that EV-D68 was detected in nasopharyngeal specimens collected from pediatric patients with polio-like illnesses in the USA: two patients in California in 2012–2013 and four in Colorado in 2014 [48,49]. Preceding respiratory illnesses were reported in those patients, by which systemic spread of EV-D68 from the respiratory tract was suggested as the underlying mechanism for those cases, as was also suggested for the fatal case of EV-D68-positive meningomyeloencephalitis in New York [37]. It was previously shown that one of the four EV-D68 strains detected in 1962 (the Rhyne strain) replicated in the brains and muscles of suckling mice and caused paralysis in those mice [4]. It might be possible that EV-D68 has an affinity for the CNS, as do most other enteroviruses [9].
Conclusions
The number of reported cases of EV-D68 greatly increased in the late 2000s, and the etiological significance of EV-D68 in acute respiratory infection has been strongly suggested. The virological properties of EV-D68 have also been studied, revealing that it is acid sensitive, binds to α2,6 SAs, and has a unique antigenicity corresponding to its genetic lineages. However, further studies are required to fully define the pathogenesis and clinical significance of EV-D68 in humans.
Future Perspectives
The clinical and epidemiological significance of EV-D68 infections has been suggested after the worldwide increase in EV-D68 detections in recent years. However, there are still a number of unsolved questions, including those related to the virological characteristics and pathogenesis of EV-D68. For example, it is still not fully understood why EV-D68 infections occasionally develop into severe lower respiratory tract infections, whereas EV-D68 recognizes α2,6 SAs, which are more common in the upper respiratory tract. It is known that patients infected with influenza A(H1N1)pdm 2009, which recognizes α2,6 SAs in the upper respiratory tract, occasionally develop severe lower respiratory tract illnesses such as acute respiratory distress syndrome [50]. Enhanced immune response induced in the airway could be the possible mechanisms also for severe EV-D68 infections. However, details of the immune responses induced by infection with EV-D68 have not been documented in vivo or in vitro. The functions of the non-structural proteins of EV-D68 have also been poorly documented. Indeed, only two previous studies have analyzed the role of the 3C protease of EV-D68 in antiviral development [51,52]. A more comprehensive understanding of the pathogenesis of EV-D68 is currently difficult to obtain because of the lack of suitable animal models for EV-D68 respiratory infections. Therefore, further studies are required to fully define the pathogenesis of EV-D68.
Acknowledgments
This work was supported by a grant-in-aid from the Japan Initiative for Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology.
Conflict of Interest
The authors have no competing interest.
References
- 1.Chatterjee S, Quarcoopome CO, Apenteng A. Unusual type of epidemic conjunctivitis in Ghana. British Journal of Ophthalmology. 1970;54:628–630. doi: 10.1136/bjo.54.9.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smura TP, Junttila N, Blomqvist S, et al. Enterovirus 94, a proposed new serotype in human enterovirus species D. Journal of General Virology. 2007;88:849–858. doi: 10.1099/vir.0.82510-0. [DOI] [PubMed] [Google Scholar]
- 3. Picornaviridae.com http://www.picornaviridae.com/enterovirus/ev-d/ev-d.htm [10 October 2014]
- 4.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. American Journal of Epidemiology. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR. Morbidity and Mortality Weekly Report. 2011;60:1301–1304. [PubMed] [Google Scholar]
- 6.Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerging Infectious Diseases. 2011;17:1430–1435. doi: 10.3201/eid1708.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. Journal of General Virology. 2012;93:1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in the Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Knipe DM, Howley PM. Fields Virology. Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 10.Imamura T, Suzuki A, Lupisan S, et al. Molecular evolution of enterovirus 68 detected in the Philippines. PLoS One. 2013;8:e74221. doi: 10.1371/journal.pone.0074221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda T, Mizuta K, Abiko C, et al. Acute respiratory infections due to enterovirus 68 in Yamagata, Japan between 2005 and 2010. Microbiology and Immunology. 2012;56:139–143. doi: 10.1111/j.1348-0421.2012.00411.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerging Infectious Diseases. 2011;17:1494–1497. doi: 10.3201/eid1708.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linsuwanon P, Puenpa J, Suwannakarn K, et al. Molecular epidemiology and evolution of human enterovirus serotype 68 in Thailand, 2006–2011. PLoS One. 2012;7:e35190. doi: 10.1371/journal.pone.0035190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang Z, Gonzalez R, Wang Z, et al. Coxsackievirus A21, enterovirus 68, and acute respiratory tract infection, China. Emerging Infectious Diseases. 2012;18:821–824. doi: 10.3201/eid1805.111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu QB, Wo Y, Wang HY, et al. Enterovirus 68 is one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. Journal of Medical Microbiology. 2013;63:408–414. doi: 10.1099/jmm.0.068247-0. [DOI] [PubMed] [Google Scholar]
- 16.Piralla A, Baldanti F, Gerna G. Phylogenetic patterns of human respiratory picornavirus species, including the newly identified group C rhinoviruses, during a 1-year surveillance of a hospitalized patient population in Italy. Journal of Clinical Microbiology. 2011;49:373–376. doi: 10.1128/JCM.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piralla A, Lilleri D, Sarasini A, et al. Human rhinovirus and human respiratory enterovirus (EV-D68 and EV104) infections in hospitalized patients in Italy, 2008–2009. Diagnostic Microbiology and Infectious Disease. 2012;73:162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. Journal of Medical Virology. 2013 doi: 10.1002/jmv.23821. Doi: 10.1002/jmv.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renois F, Bouin A, Andreoletti L. Enterovirus 68 in pediatric patients hospitalized for acute airway diseases. Journal of Clinical Microbiology. 2013;51:640–643. doi: 10.1128/JCM.02640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. Journal of Clinical Virology. 2011;52:103–106. doi: 10.1016/j.jcv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One. 2012;7:e36005. doi: 10.1371/journal.pone.0036005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd AK, Hall RJ, Wang J, et al. Detection and whole genome sequence analysis of an enterovirus 68 cluster. Virology Journal. 2013;10:103. doi: 10.1186/1743-422X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wylie KM, Wylie TN, Orvedahl A, et al. Genome sequence of enterovirus D68 from St. Louis, Missouri, USA. Emerging Infectious Diseases 2015 [31 October 2014]. DOI: 10.3201/eid2101.141605. [DOI] [PMC free article] [PubMed]
- 24.Imamura T, Okamoto M, Nakakita SI, et al. Antigenic and receptor binding properties of Enterovirus 68. Journal of Virology. 2014;88:2374–2384. doi: 10.1128/JVI.03070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. Journal of Virology. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norder H, Bjerregaard L, Magnius L, Lina B, Aymard M, Chomel JJ. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. Journal of General Virology. 2003;84:827–836. doi: 10.1099/vir.0.18647-0. [DOI] [PubMed] [Google Scholar]
- 27.McPhee F, Zell R, Reimann BY, Hofschneider PH, Kandolf R. Characterization of the N-terminal part of the neutralizing antigenic site I of coxsackievirus B4 by mutation analysis of antigen chimeras. Virus Research. 1994;34:139–151. doi: 10.1016/0168-1702(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 28.Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. Journal of General Virology. 2004;85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 29.Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. Journal of Clinical Microbiology. 2002;40:4218–4223. doi: 10.1128/JCM.40.11.4218-4223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology. 1991;180:814–817. doi: 10.1016/0042-6822(91)90098-v. [DOI] [PubMed] [Google Scholar]
- 31.Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Research. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa S, Hirano R, Okamoto-Nakagawa R, Ichiyama T, Shirabe K. Enterovirus 68 infection in children with asthma attacks: virus-induced asthma in Japanese children. Allergy. 2011;66:1618–1620. doi: 10.1111/j.1398-9995.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 33.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y, Ito T, Suzuki T, et al. Sialic acid species as a determinant of the host range of influenza A viruses. Journal of Virology. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander DA, Dimock K. Sialic acid functions in enterovirus 70 binding and infection. Journal of Virology. 2002;76:11265–11272. doi: 10.1128/JVI.76.22.11265-11272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-D68 in vitro: implications for pathogenesis. Journal of Medical Virology. 2010;82:1940–1949. doi: 10.1002/jmv.21894. [DOI] [PubMed] [Google Scholar]
- 37.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Archives of Pathology & Laboratory Medicine. 2011;135:793–796. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- 38.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. MMWR Surveillance Summary. 2006;55:1–20. Enterovirus surveillance—United States, 1970–2005. [PubMed] [Google Scholar]
- 39.Wang Z, Malanoski AP, Lin B, et al. Broad spectrum respiratory pathogen analysis of throat swabs from military recruits reveals interference between rhinoviruses and adenoviruses. Microbial Ecology. 2010;59:623–634. doi: 10.1007/s00248-010-9636-3. [DOI] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention http://www.cdc.gov/non-polio-enterovirus/outbreaks/EV-D68-outbreaks.html [10 October 2014]
- 41.Brown BA, Oberste MS, Alexander JP, Jr, Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. Journal of Virology. 1999;73:9969–75. doi: 10.1128/jvi.73.12.9969-9975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan YF, Sam IC, AbuBakar S. Phylogenetic designation of enterovirus 71 genotypes and subgenotypes using complete genome sequences. Infection, Genetics and Evolution. 2010;10:404–12. doi: 10.1016/j.meegid.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Huang SW, Hsu YW, Smith DJ, et al. Reemergence of enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. Journal of Clinical Microbiology. 2009;47:3653–3662. doi: 10.1128/JCM.00630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. New England Journal of Medicine. 2013;368:633–43. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephenson J. CDC tracking enterovirus D-68 outbreak causing severe respiratory illness in children in the Midwest. JAMA. 2014;312(13):1290. doi: 10.1001/jama.2014.13256. [DOI] [PubMed] [Google Scholar]
- 46.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR. Morbidity and Mortality Weekly Report. 2014;63(36):798–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza and Other Respiratory Viruses. 2014;8:21–24. doi: 10.1111/irv.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neurology http://www.neurology.org/content/82/10_Supplement/P3.335 [10 October 2014]
- 49. Center for Infectious Disease Research and Policy (CIDRAP), University of Minnesota http://www.cidrap.umn.edu/news-perspective/2014/09/cdc-notes-more-ev-d68-sees-link-earlier-polio-illness [10 October 2014]
- 50.Topfer L, Menk M, Weber-Carstens S, et al. Influenza A (H1N1) vs non-H1N1 ARDS: analysis of clinical course. Journal of Critical Care. 2014;29(3):340–6. doi: 10.1016/j.jcrc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Tan J, George S, Kusov Y, et al. 3C protease of enterovirus 68: structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. Journal of Virology. 2013;87:4339–4351. doi: 10.1128/JVI.01123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang Z, Li L, Lei X, et al. Enterovirus 68 3C protease cleaves TRIF to attenuate antiviral responses mediated by toll-like receptor 3. Journal of Virology. 2014 doi: 10.1128/JVI.03138-13. Doi: 10.1128/JVI.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


