Abstract
The use of robust ecological data to make evidence-based management decisions is frequently prevented by limited data quantity or quality, and local ecological knowledge (LEK) is increasingly seen as an important source of information for conservation. However, there has been little assessment of LEK's usefulness for informing prioritization and management of landscapes for threatened species, or assessing comparative species status across landscapes.
A large-scale interview survey in the Annamite Mountains (Vietnam and Lao PDR) compiled the first systematic LEK data set for saola Pseudoryx nghetinhensis, one of the world's rarest mammals, and eight other ungulates. Saola conservation is hindered by uncertainty over continued presence across much of its proposed distribution. We analysed comparative LEK-based last-sighting data across three landscapes to determine whether regional sighting histories support previous suggestions of landscape importance for saola conservation (Hue-Quang Nam: top-priority Vietnamese landscape; Pu Mat: lower priority Vietnamese landscape; Viengthong: high-priority Lao landscape) and whether they constitute an effective spatial prioritization tool for cryptic species management.
Wild pig and red muntjac may be the only Annamite ungulates with stable populations; the regional status of all other species appears to be worse. Saola have declined more severely and/or are significantly rarer than most other ungulates and have been seen by relatively few respondents. Saola were also frequently considered locally rarest or declining, and never as species that had not declined.
In contrast to other species, there are no regional differences in saola sighting histories, with continued persistence in all landscapes challenging suggestions that regional status differs greatly. Remnant populations persist in Vietnam despite heavy hunting, but even remote landscapes in Lao may be under intense pressure.
Synthesis and applications. Our local ecological knowledge data suggest that intact saola populations probably no longer exist, but individuals persist in all three landscapes, making management activities to reduce hunting pressure on ungulates in each landscape a conservation priority. Analysis of last-sighting histories can constitute an important conservation tool when robust data are otherwise unavailable, and collection of last-sighting records should be incorporated more widely into field studies and management of other highly threatened, cryptic species.
Our local ecological knowledge data suggest that intact saola populations probably no longer exist, but individuals persist in all three landscapes, making management activities to reduce hunting pressure on ungulates in each landscape a conservation priority. Analysis of last-sighting histories can constitute an important conservation tool when robust data are otherwise unavailable, and collection of last-sighting records should be incorporated more widely into field studies and management of other highly threatened, cryptic species.
Keywords: Lao, last-sighting dates, local ecological knowledge, protected area, Pseudoryx nghetinhensis, Saola, Vietnam
Introduction
The importance of evidence-based conservation, whereby management decisions for threatened species are based upon rigorous, objective research into relevant aspects of their ecology, population dynamics and threats, is increasingly recognized (Sutherland et al. 2004; Segan et al. 2010). However, species of conservation concern are frequently difficult to study, and robust data on key parameters may be unavailable. Lack of data can lead to delays in identifying or implementing necessary conservation actions, sometimes resulting in species extinctions (Groombridge et al. 2004). Wider utilization of investigative methods that can improve data availability, quality and interpretation is therefore a priority for effective protection of threatened species.
Most field research is based on ecological data collected directly by trained scientists. However, relevant information about target species is often also available from untrained local people utilizing the same environments. Local ecological knowledge (LEK), representing experiential knowledge derived from lived interactions with local environments, can provide information about the status of species and ecological resources that may be unavailable from other sources (e.g. Burbidge et al. 1988; Newing 2011). LEK is increasingly seen as an important source of data for conservation, especially for distinctive large-bodied vertebrates and/or species with socio-economic or cultural importance (Johannes, Freeman & Hamilton 2000; Jones et al. 2008; Turvey et al. 2014), and community interview surveys represent a relatively inexpensive approach for collecting comparative data across wide areas on species otherwise difficult to study.
By definition, LEK does not include records directly verified by scientists and therefore does not have the scientific validity of such data, and conservation managers must accept that there is uncertainty inherent in any interpretation of LEK. However, as with uncertainty in other areas of ecological data collection and analysis, this does not render LEK useless; instead, appropriate data collection and analytical procedures for robust interpretation of LEK are needed to minimize uncertainty. Quantitative analysis of LEK data has provided novel insights into the status and extinction drivers of many threatened species (Anadón et al. 2009; Meijaard et al. 2011; Turvey et al. 2013). In particular, community interview surveys can collect large-scale data sets of species last sightings by local observers, with analysis of sighting histories used to reconstruct extinction dynamics (Turvey et al. 2010, 2012). However, whereas LEK is now an important data source in fisheries management (Drew 2005; Zukowski, Curtis & Watts 2011), LEK data have rarely been used to inform prioritization and management of terrestrial landscapes for threatened species. There has also been little assessment of the usefulness of last-sighting data to provide novel insights into the comparative status of different species, or single species across multiple sites.
South-East Asia contains the world's highest number of threatened mammals (Schipper et al. 2008), with regional faunas experiencing ongoing range reductions and extinctions driven by human activities (Brook et al. 2014). One of the Asian mammals of highest concern is the saola Pseudoryx nghetinhensis, a distinctive forest bovid known from the northern Annamite Mountains of Vietnam and Lao People's Democratic Republic (hereafter Lao), which was only scientifically discovered in 1992 (Dung et al. 1993). The global saola population may only be in the tens or low hundreds (SWG 2009, 2013), and all available information indicates it is experiencing a severe decline caused primarily by intense hunting (Timmins et al. 2008a). The species also represents a global conservation priority on the basis of evolutionary distinctiveness (Collen et al. 2011). All Annamite large ungulates other than wild pig Sus scrofa and red muntjac Muntiacus vaginalis are also globally threatened (IUCN 2013), with densities depressed across Indochina and ‘empty forest syndrome’ increasingly widespread (Wilkie et al. 2011).
A major problem facing saola conservation is a continued lack of adequate data due to its extreme rarity, dense habitat and lack of reliably identifiable field signs (SWG 2009). Saola have never been directly observed in the wild by trained scientists, and almost all information on their distribution and ecology is derived from accounts by local villagers; however, community-based research to collect these records (Kemp et al. 1997; Diep, Long & Tuoc 2004) has not been conducted in a systematic fashion. A potential saola distribution map has been developed using locations of known records and assumptions about habitat preference (SWG 2009; Fig.1). Different landscapes with existing records have been designated high- or low-priority status on the basis of perceived likelihood of saola persisting in relatively high numbers and feasibility of effective management (SWG 2009). There is considerable uncertainty over saola distribution and status, however, and they may already be extirpated from some landscapes (SWG 2009). Standardized saola field survey and monitoring techniques have been advocated since the species’ discovery. Novel technologies for detecting saola are being explored (Schnell et al. 2012), and baseline monitoring methods for regional abundances of other Indochinese ungulates are becoming available (Gray et al. 2012; O'Kelly et al. 2012; Vongkhamheng, Johnson & Sunquist 2013). However, given the saola's wide geographical range and conservation urgency, surveys using these methods will not be comprehensive but targeted on the basis of current knowledge (SWG 2013).
Figure 1.
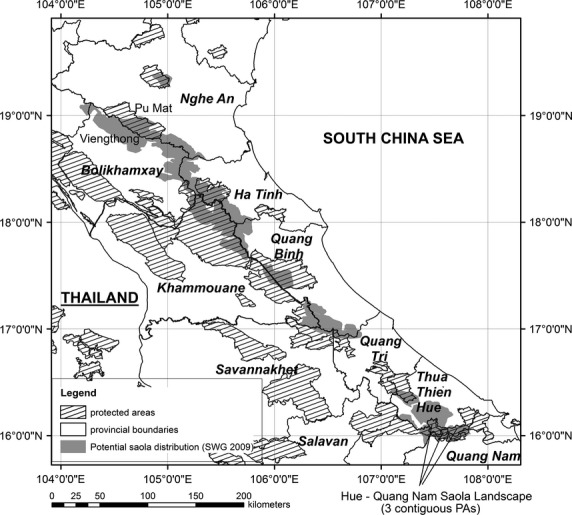
Map of potential saola distribution, showing locations of study landscapes. After SWG (2009).
It is likely that considerable LEK about saola is available in Annamite communities, because almost all saola records represent reports by local people who have encountered animals when entering forests for hunting or other resource extraction, and because hunting wild ungulates is regionally widespread and increasingly economically important (Nooren & Claridge 2001; Roberton, Trung & Momberg 2003; MacMillan & Quoc 2014). Interview data have been used to target field surveys and assess distribution, status and threat for other species in the region (Steinmetz, Chutipong & Seuaturien 2006; Newton et al. 2008; Cano & Tellería 2013). To address the crucial information gaps that continue to hinder saola conservation, we conducted a comprehensive interview survey targeting local communities in Vietnam and Lao, compiling the first systematically collected LEK data set for saola and other Annamite ungulates across three landscapes. Here, we explore the use of interview-based last-sighting time-series data for saola and other species, to determine whether LEK can provide information on species status at a wider spatial scale than the local knowledge areas of individual respondents or communities, whether it supports previous qualitative assessment of saola landscapes, and whether it can constitute an effective spatial prioritization tool for management of cryptic species.
Materials and methods
Survey Methods
Community-based fieldwork was conducted April–September 2012 in three landscapes: (i) Hue-Quang Nam (44 000 ha), comprising three contiguous protected areas (Bach Ma National Park extension, Thua Thien Hue Saola Nature Reserve, and Quang Nam Saola Nature Reserve) bordering Thua Thien Hue and Quang Nam provinces, Vietnam; (ii) Pu Mat National Park (94 000 ha), Nghe An Province, Vietnam; (iii) a c. 77 000-ha area in Viengthong District within Wildlife Conservation Society's ‘Bolikhamxay landscape’ and bordering the western boundary of Phou Sithone Endangered Species Conservation Area, Bolikhamxay Province, Lao (Fig.1). While treated here as separate landscapes, Viengthong and Pu Mat are contiguous across the Vietnam-Lao border. These different areas are occupied by different minority ethnic groups (see Appendix S1, Supporting information). Hue-Quang Nam is ranked as most important out of the four high-priority Vietnamese saola landscapes because it is considered most likely to support a viable saola population (SWG 2009); continued saola presence has recently been confirmed using camera traps (SWG 2013). Pu Mat is ranked as one of the two lower-priority Vietnamese landscapes, because it is considered likely to support only a remnant saola population at best, restricted to a reduced area of suitable habitat and possibly already extirpated (SWG 2009). Viengthong is the most poorly surveyed part of the saola's known range; Bolikhamxay Province is considered amongst the highest priorities for saola in Lao, with hopes that this region may sustain a healthier saola population than elsewhere; however, Vietnamese and Lao sites have been prioritized separately with no direct intercomparison (SWG 2009).
Villages in all landscapes rely on local forests for hunting and other non-timber forest products (Roberton, Trung & Momberg 2003; Dang Thuy Nga 2006; Viet Quang & Nam Anh 2006; MacMillan & Quoc 2014). Selection of villages in the relatively densely populated Vietnamese landscapes (Hue-Quang Nam = 46; Pu Mat = 12) aimed to achieve complete geographical coverage of spatial forest usage by community members for each landscape (Appendix S1, Fig. S1, Table S1, Supporting information). Viengthong is sparsely populated and only contains four candidate villages; due to logistic restrictions, it was only possible to survey two villages. Forest usage by these villages covers the southern part of the large forest block along the border considered likely to support an important saola population (SWG 2009) and overlapping the western part of the Phou Sithone Conservation Area (Appendix S1, Fig. S1, Table S1, Supporting information).
Interviews were conducted by trained teams of Vietnamese and Laotian students; different teams surveyed each landscape due to budgetary and/or institutional constraints. Interviewers approached community leaders for initial introductions with 4–8 key respondents considered locally to have extensive faunal knowledge and experience of forest usage; some ‘snowball sampling’ was used to find knowledgeable people, although most respondents were located by opportunistically visiting houses. In our study areas, hunting and associated faunal knowledge is considered a male domain, hence the vast majority of respondents were men; however, 15 women were also included (Hue-Quang Nam = 3; Pu Mat = 12). Community leaders and respondents were informed at the outset about the study's general aims (collecting LEK data to understand status of local mammal populations); to avoid biasing respondent selection or responses, researching saola was not given as our primary goal, and interviewers did not specifically target respondents identified as having seen saola. Interview methods followed the Zoological Society of London (ZSL)'s guidelines for ensuring appropriate ethical standards in projects involving data collection from people for research purposes, and fieldwork protocols were approved by ZSL's Ethics Committee before fieldwork began. All respondents were assured that data would be kept anonymously; interviews were only conducted following verbal consent of participants. Interviews were conducted in Vietnamese, Lao or H'mong. Before the main survey, questionnaire design/structure, interview methods and associated data collection techniques were refined in a pilot study at Pu Mat. A standard anonymous questionnaire was used which took 20–30 min to complete (Appendix S2, Supporting information). Respondents were asked for details of their most recent sightings of saola, wild pig, red muntjac, gaur Bos gaurus, serow Capricornis milneedwardsi, rhinoceros Dicerorhinus sumatrensis/Rhinoceros sondaicus, Asian elephant Elephas maximus, sambar Rusa unicolor and chevrotain Tragulus spp. Respondents were also asked about perceptions of status and trends in the local mammal fauna, including specific questions about pig and muntjac. Validation of species identification was based on elicited descriptions from respondents that focused on taxonomically useful morphological characters. No practical method was found for comparable validation of sighting dates; the use of village-specific timelines containing series of dated local events was trialled, but found to be impractical due to difficulties in cross-referencing that increased the length of interviews, and is considered unlikely to substantially aid recall or accuracy (Sudman & Bradburn 1973; see Appendices S1, S3 for further details on data validation).
Analytical Methods
All sighting records were converted to direct calendar years for analysis (Appendix S1, Supporting information); sighting data reported in analyses and figures below represent corrected data. Differences in last-sighting histories were analysed using generalized linear models (GLM) in r 3.0.2 (R Development Core Team 2013). Frequency of last-sighting dates per species per year was expressed as a proportion of total number of observations for each species sighting-history data set and regressed on year (predictor), following Turvey et al. (2012). A quasibinomial error structure was used as the data showed overdispersion. Last-sighting-history trajectories between species over time were considered significantly different if confidence intervals of regression slopes did not overlap; 83% confidence intervals were used for comparison because these give an approximate α = 0·05 test, whereas comparisons using two sets of 95% confidence intervals are too conservative (Payton, Greenstone & Schrenker 2003). Lower sighting-history slopes indicate that fewer sightings have occurred close to the present. Comparisons were investigated between different species across the overall data set, between different species within each landscape and between the same species across different landscapes.
All last-sighting data are potentially informative for understanding species declines. However, a small number of old records can produce a long ‘tail’ that greatly extends the time series used for analysis (e.g. 98·5% of our pig last-sighting records are <20 years old, but six older records push the time series under consideration back to 1972). Inclusion of a small number of such markedly older records will generate flatter overall sighting-history slopes that are harder to differentiate statistically (increasing the risk of Type II error for detecting differences between recent sighting histories) and will also substantially increase the size of confidence intervals of sighting-history slopes for commonly encountered species, as it becomes harder to use a straight line regression to approximate a sharply curving slope comprised of numerous recent sightings and a small number of older sightings. We therefore excluded the oldest 5% of last-sighting records for all species in comparative analyses of sighting-history slopes, as a standardized approach to control for this effect.
As a further test for between-landscape differences in saola sighting histories, saola reports were compared with those of a relatively common ‘reference species’ to control for spatial differences in respondent survey effort. For each respondent, time since last sighting of reference species was subtracted from date of last saola sighting. The Kruskal–Wallis test in r 3.0.2 was then used to test for an effect of landscape. Pig was used as primary reference species, but results were checked for robustness using muntjac and serow.
Optimal linear estimation (OLE), a probabilistic approach that uses the temporal distribution of independent sightings to estimate an extinction date (Solow 2005; Turvey et al. 2010), was used to investigate whether saola were likely to already be regionally extirpated from any landscape, using all available sightings per landscape. We followed Solow's (2005) implementation of the technique using the ‘sExtinct’ package in r 3.0.2. Further investigation of demographic differences and differences in respondent attitudes, awareness and experience of local faunas was conducted using analysis of variance (anova), chi-square tests, and Fisher's exact tests (including the Freeman–Halton extension for 2 × 3 contingency tables).
Results
We interviewed 450 respondents (Hue-Quang Nam = 212; Pu Mat = 193; Viengthong = 45), although not all respondents answered all questions. Following filtering of poor-quality data, 2084 dated ungulate last-sighting records are available (Hue-Quang Nam = 891; Pu Mat = 891; Viengthong = 302). Records of all target species were obtained from each landscape, except for rhinoceros from Hue-Quang Nam (Fig.2). Nearly all respondents reported sightings of muntjac (92·7%), pig (91·1%), sambar (83·1%) and serow (80·2%), whereas far fewer respondents reported sightings of elephant (38·9%), chevrotain (34·2%), saola (32·7%), gaur (7·8%) or rhinoceros (2·2%). However, substantial saola last-sighting date series were obtained from each landscape (Hue-Quang Nam = 46; Pu Mat = 61; Viengthong = 40). All rhinoceros records are >20 years old and were excluded from further analysis. Due to potential sensitivity, respondents were not asked directly whether records constituted sightings or hunting events; however, this information was voluntarily provided for most reports (Hue-Quang Nam = 99%; Pu Mat = 81%; Viengthong = 87%), with more than half representing captures of animals by the respondent (Hue-Quang Nam = 55%; Pu Mat = 60%; Viengthong = 75%).
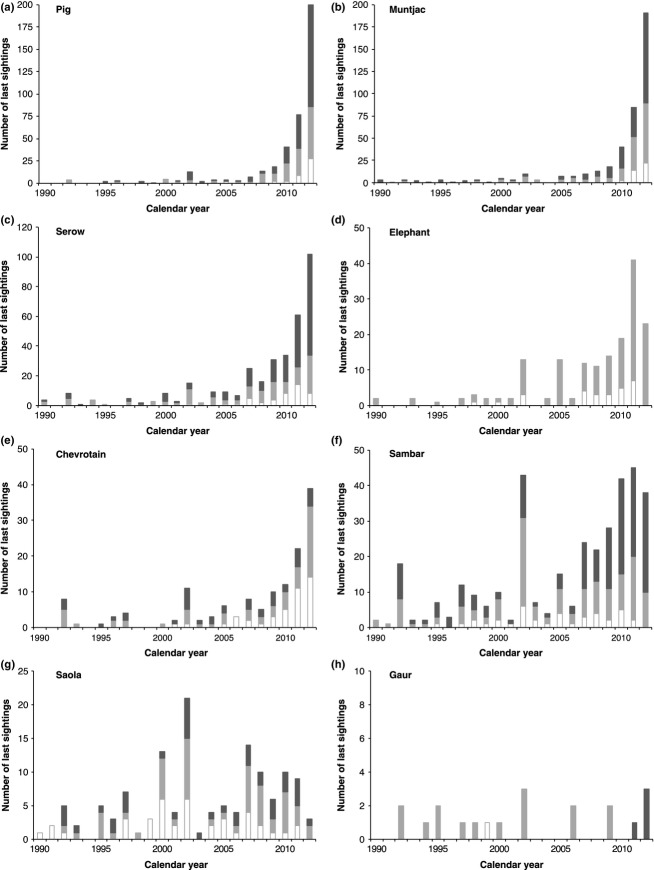
Figure 2.
Last-sighting date frequency distributions across all landscapes for eight ungulates for the period 1990–2012 (representing 94·7% of total last-sighting dates). Hue-Quang Nam = dark grey; Pu Mat = pale grey; Viengthong = white. Scale of y-axis varies between plots.
Overall, all species have significantly lower combined sighting-history slopes in comparison with pig and muntjac, and saola have a significantly lower sighting-history slope across the combined data set compared to all other species except for sambar and gaur (Fig.3, Table1). Within-landscape analyses (excluding elephant and gaur, due to only limited old records available for these taxa from some landscapes; Fig.2) show broadly similar overall patterns (Fig.4, Table2). Saola have a significantly lower sighting-history slope compared to all species other than chevrotain at Hue-Quang Nam, and all species other than sambar at Viengthong. For Pu Mat, there is no statistical difference in sighting-history slopes between chevrotain, serow, sambar or saola, with slopes for the latter three species all significantly lower than pig or muntjac.
Figure 3.
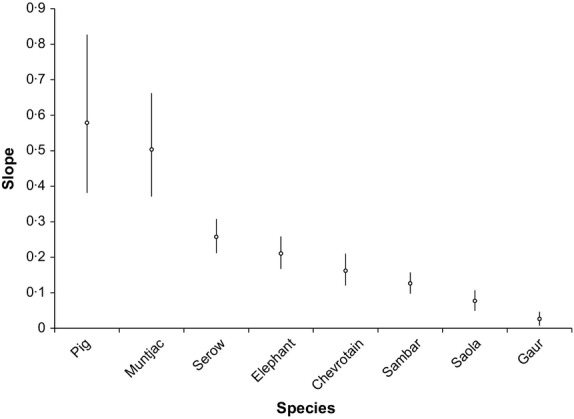
Slopes and 83% CIs of combined last-sighting-history data for all species.
Table 1.
Slopes, standard deviations, and upper and lower bounds of 83% confidence intervals for species last-sighting-history regressions
| Species | Slope | SD | Lower bound (8·5%) | Upper bound (91·5%) |
|---|---|---|---|---|
| Pig | 0·579 | 0·161 | 0·382 | 0·827 |
| Muntjac | 0·504 | 0·106 | 0·371 | 0·662 |
| Serow | 0·258 | 0·035 | 0·212 | 0·308 |
| Elephant | 0·211 | 0·033 | 0·167 | 0·258 |
| Chevrotain | 0·162 | 0·033 | 0·121 | 0·210 |
| Sambar | 0·127 | 0·022 | 0·098 | 0·157 |
| Saola | 0·077 | 0·021 | 0·049 | 0·107 |
| Gaur | 0·026 | 0·014 | 0·007 | 0·047 |
Figure 4.
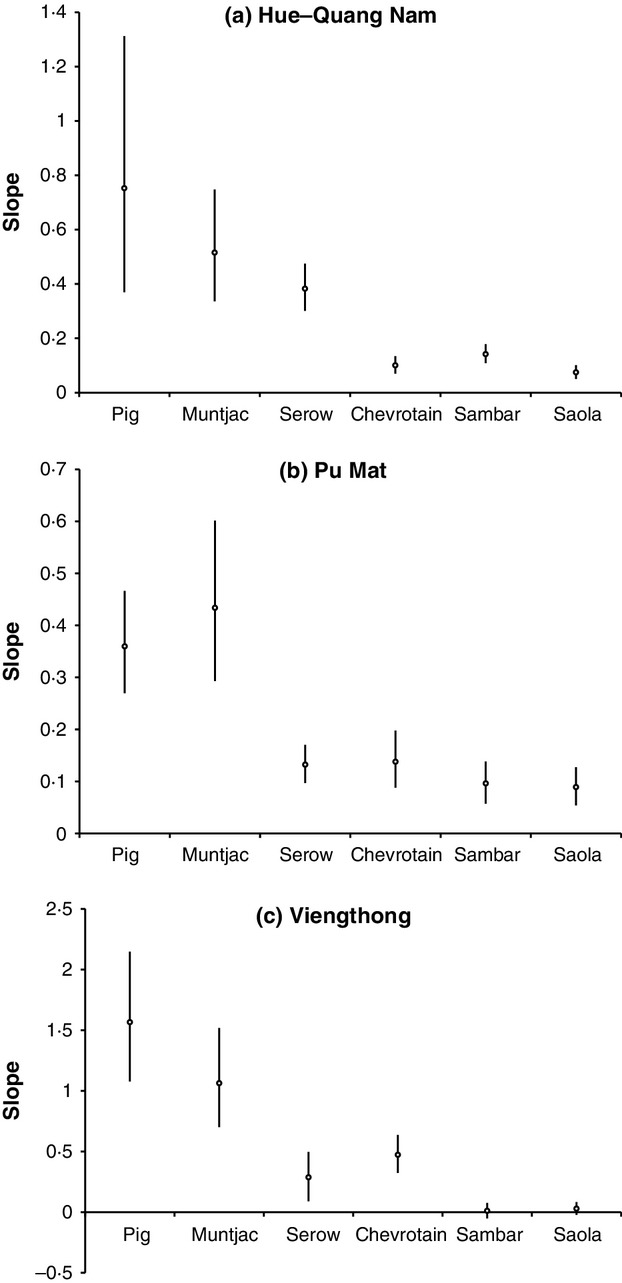
Slopes and 83% CIs of last-sighting-history data for Hue-Quang Nam, Pu Mat and Viengthong, showing data for six species (pig, muntjac, serow, chevrotain, sambar and saola) occurring at each landscape. Species shown in same order from left to right for all landscapes. Scale of y-axis varies between plots.
Table 2.
Upper and lower bounds of 83% confidence intervals for species last-sighting-history regression slopes for each landscape
| Species | Hue-Quang Nam |
Pu Mat |
Viengthong |
|||
|---|---|---|---|---|---|---|
| Lower bound (8·5%) | Upper bound (91·5%) | Lower bound (8·5%) | Upper bound (91·5%) | Lower bound (8·5%) | Upper bound (91·5%) | |
| Pig | 0·370 | 1·312 | 0·270 | 0·466 | 1·077 | 2·148 |
| Muntjac | 0·336 | 0·748 | 0·293 | 0·601 | 0·701 | 1·518 |
| Serow | 0·301 | 0·475 | 0·097 | 0·170 | 0·089 | 0·497 |
| Chevrotain | 0·070 | 0·134 | 0·088 | 0·198 | 0·322 | 0·636 |
| Sambar | 0·108 | 0·179 | 0·057 | 0·138 | −0·053 | 0·075 |
| Saola | 0·050 | 0·101 | 0·054 | 0·127 | −0·024 | 0·082 |
Analysis of saola last-sighting dates when compared with a commoner reference species also shows significant between-landscape differences (Kruskal–Wallis test, χ2 = 6·828, d.f. = 2, P = 0·033 with wild pig as reference). Post hoc pairwise comparisons (Mann–Whitney U-test) indicate that this difference arises from significantly greater intervals between sightings in Viengthong in comparison with Vietnamese sites (Wilcoxon ranked sum test, W = 1222, P = 0·012), while a pairwise comparison of Pu Mat and Hue-Quang Nam shows no significant result (sighting interval modal values: Hue-Quang Nam = 1 year, Pu Mat = 2 years, Viengthong = 12 years). These results are robust to choice of reference species.
There are significant between-landscape differences in numbers of respondents reporting saola sightings (χ2 = 76·363, d.f. = 2, P < 0·001), driven by relatively more respondents seeing saola in Viengthong compared to Vietnamese landscapes (40/45 vs. 107/405; χ2 = 69·042, d.f. = 1, P < 0·001) and more respondents seeing saola at Pu Mat than Hue-Quang Nam within Vietnam (61/193 vs. 46/212; χ2 = 4·605, d.f. = 1, P = 0·032). Respondent age is significantly correlated with landscape (Hue-Quang Nam, mean = 39·7, SD = 11·7; Pu Mat, mean = 47·7, SD = 12·5; Viengthong, mean = 44·8, SD = 9·4; F2,413 = 21·584, P < 0·001), indicating the presence of potentially confounding demographic differences. However, these demographic differences are considered unlikely to bias between-landscape patterns of recent species sighting histories, as other properties of saola sightings do not show significant between-landscape differences, whereas other species sighting histories vary between landscapes in different ways.
Optimal linear estimation conducted using each of the three separate landscape-level saola last-sighting date series produces 95% confidence intervals that overlap the survey year for each landscape (Hue-Quang Nam, CI = 2012–2014; Pu Mat, CI = 2012–2015; Viengthong, CI = 2011–2016), demonstrating no statistical support for regional disappearance of saola prior to the year that the survey was carried out anywhere across the survey area. Although most saola sightings are over a decade old (Hue-Quang Nam, mean = 13·3 years ago; Pu Mat, mean = 11·1 years ago; Viengthong, mean = 10·0 years ago), sightings were reported from all landscapes from the survey year (2012) or preceding year, and there is no significant between-landscape difference in proportion of total saola sightings per landscape over the most recent 5-year period (χ2 = 3·489, d.f. = 2, P = 0·175). Saola sighting-history slopes also do not differ significantly between landscapes, whereas other species show different between-landscape patterns: pig and muntjac have significantly steeper slopes at Viengthong compared to Pu Mat, serow have a significantly steeper slope at Hue-Quang Nam compared to Pu Mat, chevrotain have a significantly steeper slope at Viengthong compared to other landscapes, and sambar have a significantly steeper slope at Hue-Quang Nam compared to Viengthong (Fig.5, Table2).
Figure 5.
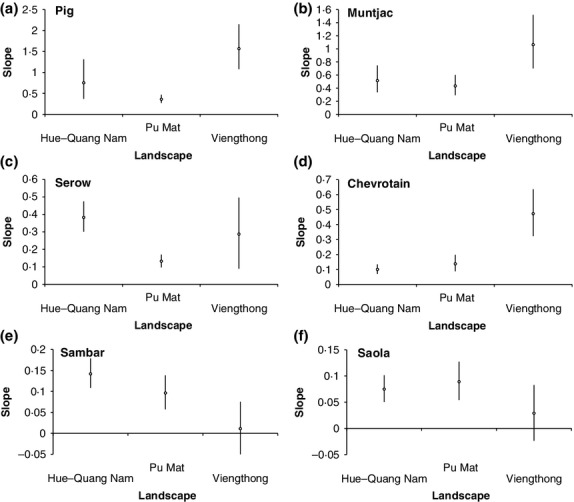
Slopes and 83% CIs of last-sighting-history data for pig, muntjac, serow, chevrotain, sambar and saola between Hue-Quang Nam, Pu Mat and Viengthong. Scale of y-axis varies between plots.
When asked which species had not declined, the only ungulates named by >1 respondent were pig (28·1%) and muntjac (12·1%); saola were never mentioned (Fig.6). However, when asked directly about these species, 10·2% of respondents specifically thought pigs had declined and 22·5% thought muntjac had declined. There were again significant between-landscape differences in perceptions of pig and muntjac decline, both in numbers of respondents specifically stating that pigs had declined (Freeman–Halton–Fisher exact test, P < 0·001) and muntjac had declined (Freeman–Halton–Fisher exact test, P < 0·001), or that pigs had not declined (χ2 = 55·872, d.f. = 2, P < 0·001) and muntjac had not declined (χ2 = 15·766, d.f. = 2, P < 0·001). Differences in numbers of respondents stating that pigs and muntjac had declined were driven both by differences between Viengthong and Vietnamese landscapes (pigs: 0/37 vs. 37/327, Fisher's exact test, P = 0·022; muntjac: 0/37 vs. 82/327, Fisher's exact test, P < 0·001) and by differences between Hue-Quang Nam and Pu Mat within the Vietnam data set for pigs (13/207 vs. 24/120; χ2 = 9·883, d.f. = 1, P = 0·002). Differences in numbers of respondents stating that pigs and muntjac had not declined were driven only by differences between Pu Mat and other landscapes (pigs: 16/155 vs. 63/126, χ2 = 52·194, d.f. = 1, P < 0·001; muntjac: 8/155 vs. 26/126, χ2 = 14·225, d.f. = 1, P < 0·001).
Figure 6.
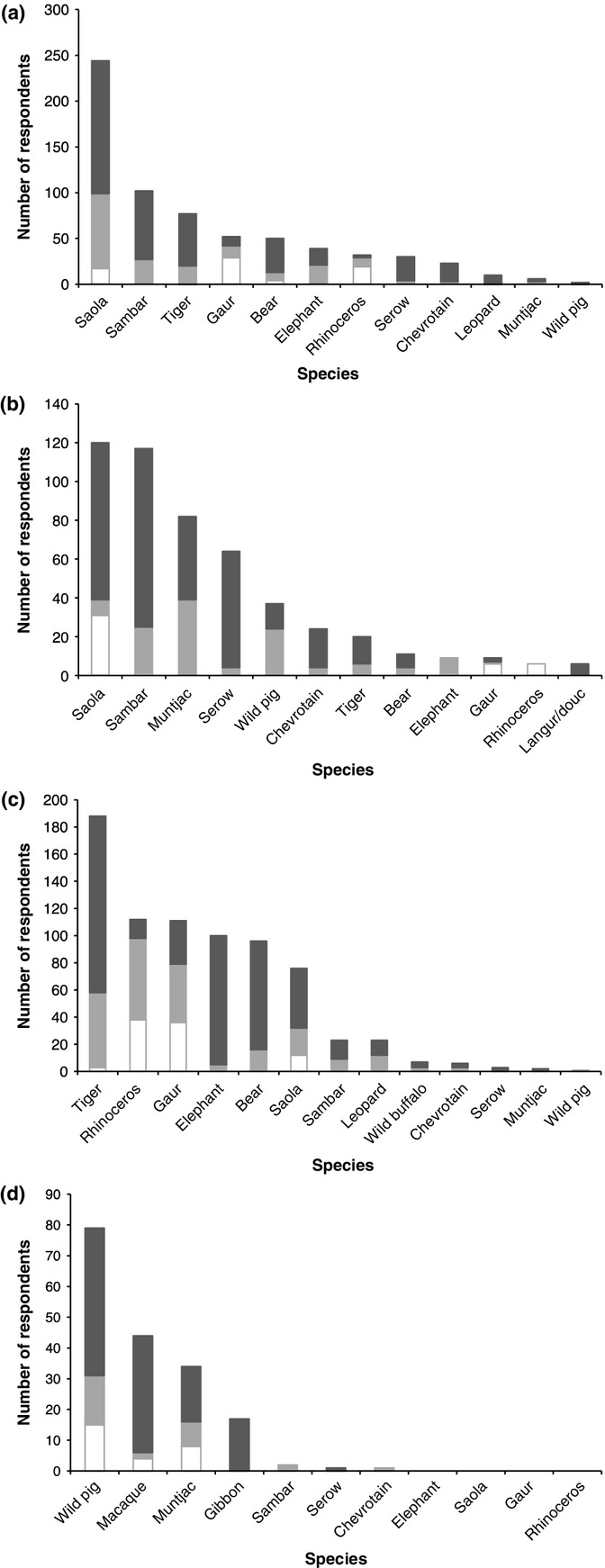
Numbers of respondents who considered that the target ungulates in our study were (a) rarest species in local landscape, (b) locally declined, (c) locally extirpated and (d) not locally declined, shown with other species also named by ≥ 5 respondents in each category. Total n = 667 (some respondents reported >1 species as locally rarest). Hue-Quang Nam = dark grey; Pu Mat = pale grey; Viengthong = white.
In Pu Mat, pigs, muntjac and sambar were the most commonly named species when respondents were asked which species had declined over the past decade; muntjac were also commonly named in Hue-Quang Nam. However, saola was the most frequently named species overall when respondents were asked which species were either locally rarest or had declined (Fig.6). There are significant between-landscape differences in numbers of respondents naming saola as one of the locally rarest species (χ2 = 12·280, d.f. = 2, P = 0·002), driven by greater numbers of respondents naming saola in Vietnamese landscapes in comparison with Viengthong (227/341 vs. 17/41; χ2 = 8·939, d.f. = 1, P = 0·003). Gaur and rhinoceros were both mentioned as being locally rarest more frequently than saola at Viengthong. There are also significant between-landscape differences in numbers of respondents who named saola as having declined (χ2 = 65·703, d.f. = 2, P < 0·001), driven by more respondents at Viengthong in comparison with Vietnamese landscapes (31/37 vs. 167/327; χ2 = 49·710, d.f. = 1, P < 0·001), and by more respondents at Hue-Quang Nam compared to Pu Mat within the Vietnam data set (81/207 vs. 8/120; χ2 = 25·478, d.f. = 1, P < 0·001). There are again significant between-landscape differences in numbers of respondents who considered saola to be locally extirpated (χ2 = 6·879, d.f. = 2, P = 0·032), driven by fewer respondents at Pu Mat compared to other landscapes (20/125 vs. 56/174; χ2 = 5·495, d.f. = 1, P = 0·019). Only 21·3% of respondents overall considered saola locally extirpated, with higher numbers of total respondents naming tiger Panthera tigris, rhinoceros, gaur, elephant and Asian black bear Ursus thibetanus (Fig.6). Both gaur and rhinoceros were consistently more often named in questions about rarity, decline or local extirpation at Viengthong in comparison with Vietnamese landscapes, where other target ungulates (notably sambar) were instead named more frequently (χ2 and Fisher's exact tests, P < 0·001 in all comparisons).
Discussion
Our results demonstrate that large-scale LEK-based sighting-history data sets can provide extensive insights into the status of threatened species for which robust data are otherwise unavailable, with important implications for informing spatial prioritization of conservation resources. Indeed, our last-sighting series represent the first quantitative comparative data for saola across key parts of its range. Although direct validation of these data is challenging in the absence of any available comparative baseline, if analysed critically, they can still constitute an invaluable resource to assist future management.
It is important to recognize that our sighting histories represent information on ungulate encounter rates over time by local forest users, rather than direct data on population status. Although accuracy of reported dating of last-sighting records ultimately has to be taken ‘at face value’ without independent means of validation, the substantial variation in sighting histories between species and landscapes, in the absence of any expected taxonomic or spatial reporting biases, strongly suggests these patterns represent genuine temporal variation in encounter rates. However, encounter rates may be affected by variation in ecological factors (abundance, population trends, detectability) and also ‘survey effort’ (including hunting effort) or other biases in sampled respondents. These factors can differ between species and landscapes, and specific sighting-history trajectories may represent complex interactions between factors through time; a lower sighting-history slope may indicate lower abundance, steeper decline, lower detectability, reduction in targeted hunting, or other cognitive and/or motivational biases. The relatively high slope for elephant probably reflects higher detectability rather than greater abundance; conversely, smaller snares that catch muntjac, pigs and chevrotain may not catch serow, sambar or saola, leading to potential downward biases in slopes for these species. No single analysis can separate these factors; we have addressed this problem by employing a range of analyses and questioning approaches (last-sighting dates; direct questions about species status). Ultimately, little information is otherwise available to inform saola conservation, and future research and management must be targeted on the basis of these data. The challenge is to interpret them as far as possible, without overinterpretation.
Pig and muntjac show the steepest sighting-history slopes, shaped by large numbers of recent sightings from all landscapes (Figs3 and 4). Direct questions reveal that these are the only ungulates that many respondents believe not to have declined, although this view is not unanimous. These may be the only ungulates with stable populations in any landscape, and other regional field and interview surveys also suggest that these species are least likely to be rare or declining (Steinmetz, Chutipong & Seuaturien 2006; Vongkhamheng, Johnson & Sunquist 2013). Conversely, combined evidence from last-sighting slopes and direct questions strongly suggests that the status of all other species is worse, again confirming previous suggestions about the regional status of these species (Duckworth & Hedges 1998; Timmins et al. 2008b; SWG 2009). Overall, relative sighting-history patterns for pig, muntjac, serow, sambar and gaur (Figs3 and 4) are identical to relative abundances for these species determined from recent Annamite field surveys, providing important support for the general ecological accuracy of our data (Vongkhamheng, Johnson & Sunquist 2013).
Our data support the contention that saola have declined more severely and/or are significantly rarer than most other Annamite ungulates. Saola have been seen by relatively few respondents and show flattened sighting-history slopes shaped by limited numbers of recent sightings at all landscapes relative to most other ungulates which have also apparently declined (Figs2–4). Saola were also frequently described as species that were rarest or declining, and never as not having declined. While relatively few respondents considered saola to be locally extirpated, the only species mentioned more frequently are those known to be extirpated or very rare (Duckworth & Hedges 1998; Brook et al. 2014). The only ungulates possibly faring worse than saola are megafaunal mammals (gaur, rhinoceros and possibly elephant).
Our data are also consistent with existing information about regional faunal histories and human impacts, providing further validation of the accuracy of the LEK data set. Pu Mat is thought to have experienced the heaviest hunting pressures (SWG 2009) and shows significantly depressed sighting-history slopes for several ungulates compared to other landscapes (Fig.5), with greatest numbers of respondents reporting pig and muntjac declines. Conversely, no respondents at Viengthong, a remote landscape considered most likely to retain healthy ungulate populations (SWG 2009), reported pig or muntjac declines. Several ungulates show steeper sighting-history slopes at Viengthong in comparison with Vietnamese landscapes (Fig.5), and more respondents here mentioned rhinoceros and gaur (Fig.6). We have no rhinoceros records more recent than 1979, but the fact that respondents in Viengthong mentioned these species suggests that, if they have vanished, this has occurred more recently than elsewhere.
Between-landscape comparisons are more challenging due to sociocultural differences between respondent populations, which can affect intensity, pattern and type of forest use. We expect average ‘survey effort’ (hunting/general forest use) to be highest in Viengthong and lowest in Pu Mat due to regional differences in respondent forest dependency (Appendix S1). However, semi-professional hunting for the wildlife trade is common in Vietnam (MacMillan & Quoc 2014), and Pu Mat in particular (Roberton, Trung & Momberg 2003), with intensive trapping conducted even in remote areas. There are no significant between-landscape differences in saola sighting histories (Fig.5); recent sightings indicate continued persistence at all landscapes, and OLE provides no evidence of extinction in any landscape. There is also no significant difference in saola last-sighting timings between Vietnamese landscapes, even if a commoner reference species is used to control for differences in respondent survey effort.
Our data on other ungulates as indicators of hunting pressure, and hence potential saola status, provide some support for suggestions that Hue-Quang Nam and Viengthong are more likely to support viable saola populations than Pu Mat (SWG 2009). However, our data strongly suggest continued saola presence at Pu Mat and provide no reason to assume saola status is worse in this landscape: significantly, more respondents reported saola sightings here compared to Hue-Quang Nam, and fewer respondents thought saola had declined or disappeared. This does not constitute strong evidence that saola status is better in Pu Mat, but challenges the suggestion that saola status differs greatly between landscapes.
Conversely, although a higher proportion of respondents at Viengthong reported sightings, a higher proportion also thought saola had declined, and there are no significant sighting-history slope differences between Viengthong and other landscapes. Time since last saola sighting when compared with a reference species tends to be longer ago in Viengthong than in Vietnamese landscapes, suggesting saola status is worse. This result could reflect differences in hunting patterns (more ‘professional hunting’ in Vietnam leading to greater likelihood of saola and reference species being seen on same hunting trip), and our data may still be consistent with a higher saola population than Vietnam. However, they are suggestive of recent decline, and the prospect of a reasonably healthy saola population seems remote.
As even continued saola presence remains uncertain across much of its proposed distribution, all three surveyed landscapes should be interpreted as important priorities for saola conservation, with population status possibly more similar between landscapes than previously thought. Our results challenge suggestions that saola are extirpated in any of these landscapes, but also that any landscape supports a population close to carrying capacity. Remnant populations persist in our Vietnamese landscapes despite heavy hunting pressures, but even remote landscapes in Lao may be under intense poaching pressure and demand from Vietnam (Nooren & Claridge 2001; McDowell, Scudder & Talbot 2013). Seven respondents from Viengthong reported seeing Vietnamese hunters in the forest within the last 3 years, and 75% here considered hunting the primary reason for wildlife declines (n = 44). Field surveys to discover intact saola populations are therefore not a conservation priority, as it is unlikely that such populations exist. However, none of these landscapes should be excluded from efforts to detect, protect or capture individual saola. Specific management activities to reduce hunting pressure (e.g. snare removal; SWG 2009) should also be maintained or increased to afford greater protection to saola and other ungulates in each landscape.
Independent corroboration of our data from other sources supports interpretation of last-sighting histories as a meaningful proxy for regional status of saola and other species. Unexpected insights into the status of different saola populations demonstrate that community interviews represent an important but underutilized source of quantitative data for evidence-based conservation, and we recommend future interview surveys to identify other critical saola landscapes, to assess potential survival of viable populations across Bolikhamxay, and locate surviving saola elsewhere in Vietnam. We also strongly recommend that collection and analysis of LEK-based species sighting histories should be incorporated more widely into field studies and conservation management of other highly threatened, cryptic species.
Acknowledgments
We thank Bill Robichaud, the IUCN Saola Working Group and ZSL's EDGE of Existence programme for support, and Andy Solow and Jennifer Crees for statistical advice. Fieldwork assistance was provided by scientific division of Pu Mat National Park Management Board, Mr Ong Dinh Bao Tri and WWF Greater Mekong Programme, Dr Pham Khac Lieu (Hue University of Science), Forest Protection Departments and Saola Nature Reserve Management Boards in Thua Thien Hue and Quang Nam provinces, Dr Nguyen Cong Thin (Vinh University) and Mr Somchay (Xiengkhoang Province People's Committee). Funding was provided by Ocean Park Conservation Foundation, Hong Kong; Darwin Initiative project 17008; and a Royal Society University Research Fellowship (UF080320).
Data accessibility
Data are available from the Figshare Digital Repository: http://figshare.com/articles/Last_Sighting_Data_Annamite_Ungulates_2012/1209569 (Wilkinson & Turvey 2014).
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Appendix S1. Ethnic groups, village selection, species identification and sighting date formats.
Table S1. Villages surveyed around the Hue-Quang Nam landscape.
Table S2. Villages surveyed around the Viengthong and Pu Mat landscapes.
Fig. S1. Surveyed villages and their approximate use zones in the Hue-Quang Nam landscape.
Fig. S2. Surveyed villages and their approximate use zones in Pu Mat National Park (Vietnam) and Viengthong District (Bolikhamxay Province, Lao PDR).
Survey questionnaires.
Key identification points for Annamite ungulates.
References
- Anadón JD, Giménez A, Ballestar R. Pérez I. Evaluation of local ecological knowledge as a method for collecting extensive data on animal abundance. Conservation Biology. 2009;23:617–625. doi: 10.1111/j.1523-1739.2008.01145.x. &. [DOI] [PubMed] [Google Scholar]
- Brook SM, Dudley N, Mahood SP, Polet G, Williams AC, Duckworth JW, Van Ngoc T. Long B. Lessons learned from the loss of a flagship: the extinction of the Javan rhinoceros Rhinoceros sondaicus annamiticus from Vietnam. Biological Conservation. 2014;174:21–29. &. [Google Scholar]
- Burbidge AA, Johnson KA, Fuller PJ. Southgate RI. Aboriginal knowledge of the mammals of the central deserts of Australia. Australian Wildlife Research. 1988;15:9–39. &. [Google Scholar]
- Cano LS. Tellería JL. Local ecological knowledge as a tool for assessing the status of threatened vertebrates: a case study in Vietnam. Oryx. 2013;47:177–183. &. [Google Scholar]
- Collen B, Turvey ST, Waterman C, Meredith HMR, Kuhn TS, Baillie JEM. Isaac NJB. Investing in evolutionary history: implementing a phylogenetic approach for mammal conservation. Philosophical Transactions of the Royal Society B. 2011;366:2611–2622. doi: 10.1098/rstb.2011.0109. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep TN, Long DT. Tuoc D. Report of survey on saola (Pseudoryx nghetinhensis. In: Johns AG, editor; Hardcastle J, Cox S, Nguyen TD, editors. “Rediscovering the Saola” Workshop Proceedings. Hanoi: WWF Indochina Programme/SFNC Project/Pu Mat National Park; 2004. & &. [Google Scholar]
- Drew JA. Use of traditional ecological knowledge in marine conservation. Conservation Biology. 2005;19:1286–1293. [Google Scholar]
- Duckworth JW. Hedges S. Tracking Tigers: A Review of the Status of Tiger, Asian Elephant, Gaur and Banteng in Viet Nam, Lao, Cambodia and Yunnan (China) with Recommendations for Future Conservation Action. Hanoi: World Wildlife Fund Indochina Programme; 1998. &. [Google Scholar]
- Dung VV, Giao PM, Chinh NN, Tuoc D, Arctander P. MacKinnon J. A new species of living bovid from Vietnam. Nature. 1993;363:443–445. &. [Google Scholar]
- Gray TNE, Phan C, Pin C. Prum S. Establishing a monitoring baseline for threatened large ungulates in eastern Cambodia. Wildlife Biology. 2012;18:406–413. &. [Google Scholar]
- Groombridge JJ, Massey JG, Bruch JC, Malcolm T, Brosius CN, Okada MM, Sparklin B, Fretz JS. VanderWerf E. An attempt to recover the po'o-uli by translocation and an appraisal of recovery strategy for bird species of extreme rarity. Biological Conservation. 2004;118:365–375. &. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. 2013. Version 2013.2. http://www.iucnredlist.org. Accessed 18 May 2014. [Google Scholar]
- Johannes RE, Freeman MMR. Hamilton RJ. Ignore fishers’ knowledge and miss the boat. Fish and Fisheries. 2000;1:257–271. &. [Google Scholar]
- Jones JPG, Andriamarovololona MM, Hockley N, Gibbons JM. Milner-Gulland EJ. Testing the use of interviews as a tool for monitoring trends in the harvesting of wild species. Journal of Applied Ecology. 2008;45:1205–1212. &. [Google Scholar]
- Kemp N, Dilger M, Burgess N. Dung CV. The saola Pseudoryx nghetinhensis in Vietnam – new information on distribution and habitat preferences, and conservation needs. Oryx. 1997;31:37–44. &. [Google Scholar]
- MacMillan DC. Quoc NA. Factors influencing the illegal harvest of wildlife by trapping and snaring among the Katu ethnic group in Vietnam. Oryx. 2014;48:304–312. &. [Google Scholar]
- McDowell D, Scudder T. Talbot LM. 2013. Vientiane Nam Theun 2 Power Company Ltd & Lao People's Democratic Republic Nam Theun 2 Multipurpose Project. Reports 21A and 21B, International Environmental and Social Panel of Experts.
- Meijaard E, Buchori D, Hadiprakarsa Y, Utami-Atmoko SS, Nurcahyo A, Tiju A, et al. Quantifying killing of orangutans and human-orangutan conflict in Kalimantan, Indonesia. PLoS One. 2011;6:e27491. doi: 10.1371/journal.pone.0027491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newing H. Conducting Research in Conservation: A Social Science Perspective. Abingdon, UK: Routledge; 2011. [Google Scholar]
- Newton P, Van Thai N, Roberton S. Bell D. Pangolins in peril: using local hunters’ knowledge to conserve elusive species in Vietnam. Endangered Species Research. 2008;6:41–53. &. [Google Scholar]
- Nga DT. Economic Value of Non-Timber Forest Products for Ka Tu People and Future Options for Song Kon Protection Forest Management Board in Quang Nam Province, Vietnam. Hanoi: WWF Greater Mekong–Vietnam Country Programme; 2006. [Google Scholar]
- Nooren H. Claridge G. Wildlife Trade in Laos: The End of the Game. Amsterdam: Netherlands Committee for IUCN; 2001. &. [Google Scholar]
- O'Kelly HJ, Evans TD, Stokes EJ, Clements TJ, Dara A, Gately M, et al. Identifying conservation successes, failures and future opportunities; assessing recovery potential of wild ungulates and tigers in eastern Cambodia. PLoS One. 2012;7(10):e40482. doi: 10.1371/journal.pone.0040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton ME, Greenstone MH. Schrenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? The Journal of Insect Science. 2003;3:34. doi: 10.1093/jis/3.1.34. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Roberton S, Trung TC. Momberg F. Hunting and Trading Wildlife: an Investigation into the Wildlife Trade in and Around the Pu Mat National Park, Nghe An Province. Nghe An, Vietnam: SFNC, Vinh; 2003. &. [Google Scholar]
- Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, Katariya V, et al. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- Schnell IB, Thomsen PF, Wilkinson N, Rasmussen M, Jensen LRD, Willerslev E, Bertelsen MF. Gilbert MTP. Screening mammal biodiversity using DNA from leeches. Current Biology. 2012;22:1980. doi: 10.1016/j.cub.2012.02.058. &. [DOI] [PubMed] [Google Scholar]
- Segan DB, Bottrill MC, Baxter PWJ. Possingham HP. Using conservation evidence to guide management. Conservation Biology. 2010;25:200–202. doi: 10.1111/j.1523-1739.2010.01582.x. &. [DOI] [PubMed] [Google Scholar]
- Solow AR. Inferring extinction from a sighting record. Mathematical Biosciences. 2005;195:47–55. doi: 10.1016/j.mbs.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Chutipong W. Seuaturien N. Collaborating to conserve large mammals in southeast Asia. Conservation Biology. 2006;20:1391–1401. doi: 10.1111/j.1523-1739.2006.00505.x. &. [DOI] [PubMed] [Google Scholar]
- Sudman S. Bradburn NM. Effects of time and memory factors on response in surveys. Journal of the American Statistical Association. 1973;68:805–815. &. [Google Scholar]
- Sutherland WJ, Pullin AS, Dolman PM. Knight TM. The need for evidence-based conservation. Trends in Ecology & Evolution. 2004;19:305–308. doi: 10.1016/j.tree.2004.03.018. &. [DOI] [PubMed] [Google Scholar]
- SWG. From Plans to Action: Proceedings of the First Meeting of the Saola Working Group. Vientiane: IUCN Lao PDR Country Programme/Saola Working Group, Asian Wild Cattle Specialist Group, IUCN Species Survival Commission; 2009. [Google Scholar]
- SWG. Conservation Through Collaboration: Proceedings of the Third Meeting of the Saola Working Group. Vientiane: Saola Working Group, Asian Wild Cattle Specialist Group, IUCN Species Survival Commission; 2013. [Google Scholar]
- Timmins RJ, Robichaud WG, Long B, Hedges S, Steinmetz R, Abramov A, Tuoc D. Mallon DP. IUCN Red List of Threatened Species. 2008a. Pseudoryx nghetinhensis. &. Version 2013.2. http://www.iucnredlist.org, Accessed 18 May 2014. [Google Scholar]
- Timmins RJ, Steinmetz R, Sagar Baral H, Samba Kumar N, Duckworth JW, Anwarul Islam M, et al. IUCN Red List of Threatened Species. 2008b. Rusa unicolor. Version 2013.2. http://www.iucnredlist.org, Accessed 18 May 2014. [Google Scholar]
- Turvey ST, Barrett LA, Hart T, Collen B, Hao Y, Zhang L, et al. Spatial and temporal extinction dynamics in a freshwater cetacean. Proceedings of the Royal Society B. 2010;277:3139–3147. doi: 10.1098/rspb.2010.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey ST, Risley CL, Barrett LA, Hao Y. Wang D. River dolphins can act as population trend indicators in degraded freshwater systems. PLoS One. 2012;7:e37902. doi: 10.1371/journal.pone.0037902. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey ST, Risley CL, Moore JE, Barrett LA, Hao Y, Zhao X, Zhou K. Wang D. Can local ecological knowledge be used to assess status and extinction drivers in a threatened freshwater cetacean? Biological Conservation. 2013;157:352–360. &. [Google Scholar]
- Turvey ST, Fernández-Secades C, Nuñez-Miño JM, Hart T, Martinez P, Brocca JL. Young RP. Is local ecological knowledge a useful conservation tool for small mammals in a Caribbean multicultural landscape? Biological Conservation. 2014;169:189–197. &. [Google Scholar]
- Viet Quang D. Nam Anh T. Commercial collection of NTFPs and households living in or near the forests. Ecological Economics. 2006;60:65–74. &. [Google Scholar]
- Vongkhamheng C, Johnson A. Sunquist ME. A baseline survey of ungulate abundance and distribution in northern Lao: implications for conservation. Oryx. 2013;47:544–552. &. [Google Scholar]
- Wilkie DS, Bennett EL, Peres CA. Cunningham AA. The empty forest revisited. Annals of the New York Academy of Sciences. 2011;1223:120–128. doi: 10.1111/j.1749-6632.2010.05908.x. &. [DOI] [PubMed] [Google Scholar]
- Wilkinson NM. Turvey ST. 2014. Figshare Digital Repository& Data from: “Interview-based sighting histories can inform regional conservation prioritisation for highly threatened cryptic species” http://dx.doi.figshare.com/articles/Last_Sighting_Data_Annamite_Ungulates_2012/1209569.
- Zukowski S, Curtis A. Watts RJ. Using fisher local ecological knowledge to improve management: the Murray crayfish in Australia. Fisheries Research. 2011;110:120–127. &. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Ethnic groups, village selection, species identification and sighting date formats.
Table S1. Villages surveyed around the Hue-Quang Nam landscape.
Table S2. Villages surveyed around the Viengthong and Pu Mat landscapes.
Fig. S1. Surveyed villages and their approximate use zones in the Hue-Quang Nam landscape.
Fig. S2. Surveyed villages and their approximate use zones in Pu Mat National Park (Vietnam) and Viengthong District (Bolikhamxay Province, Lao PDR).
Survey questionnaires.
Key identification points for Annamite ungulates.
Data Availability Statement
Data are available from the Figshare Digital Repository: http://figshare.com/articles/Last_Sighting_Data_Annamite_Ungulates_2012/1209569 (Wilkinson & Turvey 2014).