Abstract
Background & Aims
The organic solute transporters alpha and beta (OSTα-OSTβ) form a heterodimeric transporter located at the basolateral membrane of intestinal epithelial cells and hepatocytes. Liver injury caused by ischaemia-reperfusion, cancer, inflammation or cholestasis can induce a state of hypoxia in hepatocytes. Here, we studied the effect of hypoxia on the expression of OSTα-OSTβ.
Methods
OSTα-OSTβ expression was measured in Huh7 cells and primary human hepatocytes (PHH) exposed to chenodeoxycholic acid (CDCA), hypoxia or both. OSTα-OSTβ promoter activity was analysed in luciferase reporter gene assays. Binding of hypoxia-inducible factor-1 alpha (HIF-1α) to the OSTα-OSTβ gene promoters was studied in electrophoretic mobility shift assays (EMSA).
Results
Expression of OSTα and OSTβ increased in PHH under conditions of hypoxia. Exposure of Huh7 cells or PHH to CDCA (50 μM) enhanced the effect of hypoxia on OSTα mRNA levels. In luciferase assays and EMSA, the inducing effect of low oxygen could be assigned to HIF-1α, which binds to hypoxia responsive elements (HRE) in the OSTα and OSTβ gene promoters. Site-directed mutagenesis of either the predicted HRE or the bile acid responsive FXR binding site abolished inducibility of the OSTα promoter, indicating that both elements need to be intact for induction by hypoxia and CDCA. In a rat model of chronic renal failure, the known increase in hepatic OSTα expression was associated with an increase in HIF-1α protein levels.
Conclusion
OSTα-OSTβ expression is induced by hypoxia. FXR and HIF-1α bind in close proximity to the OSTα gene promoter and produce synergistic effects on OSTα expression.
Keywords: bile acid transport, chronic renal failure, gene regulation, ligand, nephrectomy, nuclear receptor, organic anion transport, rat liver
Bile acids undergo extensive enterohepatic cycling. After being synthesized in the liver, bile acids are excreted into bile and the intestine, subsequently re-absorbed into the portal venous blood and finally transported back to the liver. The enterohepatic circulation of bile acids is maintained by bile acid uptake transporters and excretion pumps, located at the basolateral and apical membranes of intestinal, hepatic and renal cells, which are regulated by changes in the intracellular concentration of bile acids (1).
Any reduction in the regular availability of oxygen in human tissues leads to hypoxia. Various conditions can cause hypoxia or induce hypoxia modulators, such as ischaemia, ischaemic reperfusion injury, cancer and inflammation. In addition, cholestasis induces hypoxia in the liver (2–4) and low oxygen levels regulate bile salt transporters. However the exact mechanism by which hypoxia modulates the expression of these transport proteins has not been elucidated. Furthermore, no inducing effects on the expression of transport proteins have so far been shown in the literature. An important part of the cellular response to the lack of oxygen is mediated by hypoxia-inducible factor 1 (HIF-1). HIF-1 consists of a hypoxia regulated subunit α (HIF-1α) and a constitutively expressed subunit β (HIF-1β), also known as the aryl hydrocarbon receptor nuclear translocator protein (ARNT). Under normoxic conditions HIF-1α is hydroxylated and consequently ubiquitinated, so that the half-life of HIF-1α is heavily reduced by proteasomal degradation. Under low oxygen conditions, the oxygen dependent hydroxylase activity is reduced and HIF-1α is less prone to degradation (5,6). Accumulated HIF-1α dimerises with ARNT, translocates to the nucleus and binds to the hypoxia responsive element (HRE) in the promoter region of a given target gene. With very little variation, the core HRE is composed of the sequence ACGTG (7).
The heterodimeric organic solute transporter α/β (OSTα/β), encoded by the genes SLC51A and SLC51B, respectively, is a bile acid transporter expressed in the liver, intestine and kidney. In hepatocytes the OST heterodimeric transporters are located in the basolateral membrane, where they are assumed to mediate basolateral efflux of organic anions including bile acids, thereby potentially protecting hepatocytes from intracellular accumulation. In enterocytes OSTs are located in the basolateral membrane, where they are responsible for the efflux of bile acids into the portal venous blood (8,9).
Cholic acid and chenodeoxycholic acid (CDCA) are the two primary bile acids in humans (10). The nuclear farnesoid X receptor (FXR), also known as the bile acid receptor (BAR), has been identified as an important mediator of CDCA-dependent regulation of gene expression (11,12). CDCA is an agonistic ligand of FXR and leads to the regulation of a whole battery of genes involved in bile acid metabolism and transport in both the liver and intestine. We previously showed that CDCA transactivates the OSTα and OSTβ genes via an FXR dependent pathway. (9).
In this study, we investigated in vitro to what extent hypoxic conditions affect the expression of OSTα/β in the liver using hepatocyte-derived cell lines as well as a chronic renal failure (CRF) rat model. We characterize the transcriptional pathway that mediates hypoxic effects on OSTα/β gene expression. By exposing cells to elevated bile acid concentrations and hypoxic conditions in parallel, we also simulate the combined effects of hypoxia and cholestasis on the transcriptional regulation of OSTα/β.
Material and methods
Chemicals
Chenodeoxycholic acid (CDCA) and dimethylsulphoxide (DMSO) were purchased from Sigma Aldrich and Invitrogen respectively. Maintenance and transfection media as well as supplements for cell culture were obtained from Invitrogen (Basel, Switzerland) (OptiMEM, Penicillin/Streptomycin), PAA Laboratories (Les Mureaux, France) [fetal bovine serum (FBS), RPMI 1640 medium] and Lonza (Walkersville, MD, USA) (Hepatocyte maintenance medium with ultraglutamine). Short interfering RNAs (siRNAs) and the TransIT-TKO transfection reagent used in knock-down experiments were purchased from Dharmacon (Lafayette, CO, USA). The Fugene HD reagent used in transfection experiments for luciferase gene reporter assays was obtained from Roche Diagnostics (Basel, Switzerland). Antibodies used in Western blot and EMSA analysis were obtained from Abcam (Cambridge, UK) (β-actin antibody Ab8227), Santa-Cruz Biotechnology Inc. (Dallas, TX, USA) (anti-HIF-1α antibody sc13515, antimouse Ostα antibody sc10078), Merck Millipore (Darmstadt, Germany) (anti-HIF-1α antibody ABE279) and Pierce/Thermo Fisher Scientific (Wohlen, Switzerland) (horseradish peroxidase-conjugated goat antimouse antibody #32460). MultiScribe Reverse Transcriptase and TaqMan Gene Expression Assays used for real-time PCR-based expression analysis were purchased from Applied Biosystems (Rotkreuz, Switzerland). Acrylamide 40%/Bisacrylamide 2% used for SDS-Page gel preparation was purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Cell culture
Huh7 cells (American Type Culture Collection; Molsheim, France) were cultured in RPMI 1640 medium supplemented with 10% FBS plus 100 U/ml penicillin and 100 μg/streptomycin. The isolation and culture of PHH has been described in detail (13). Upon arrival, primary human hepatocytes (PHH) were kept for 5 h in hepatocyte maintenance medium with ultraglutamine before any further treatment. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 at either regular atmospheric or 0.2% oxygen partial pressure (Ruskin Invivo 400 system; Ruskin Technology Ltd, Bridgend, UK).
RNA isolation and real-time PCR
Huh7 cells were seeded in twelve-well plates and, when 80% confluent, subjected to normoxia or hypoxia as well as to treatment with 50 μM CDCA or empty vehicle (DMSO). Total RNA was isolated using TRIzol reagent. Two micrograms of each RNA sample were reverse transcribed by random priming (Reverse Transcription System). Two microlitres of cDNA were used in each real-time PCR reaction. Real-time PCR was performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems) using the TaqMan Gene Expression Assays Hs00380895_m1, Hs00418306_m1 and Hs99999070_m1 for human OSTα, OSTβ and Vascular endothelial growth factor A (VEGFa, used as an hypoxia marker) respectively.
Constitutively expressed β-actin mRNA was measured as an internal standard for sample normalization (4310881E). Relative mRNA levels were calculated using the comparative threshold cycle (ΔCT) method. Each PCR was performed in triplicate and all experiments were repeated three times.
Transfection with siRNA
Huh7 cells were transfected in twelve-well plates at 80% confluence. Per transfection, 5 μl of TransIT-TKO transfection reagent were mixed with 120 μl of serum-free OptiMEM followed by the addition of either the SMARTpool siRNA targeting HIF-1α or FXR or siControl non-targeting siRNA no. 1 to a final concentration of 25 nM.
After an incubation at room temperature for 10 min, the siRNA mixtures were added to 600 μl RPMI 1640 medium supplemented with 10% FBS in each well. Six hours after transfection, cells were subjected to CDCA/hypoxia treatment for 18 h. Cellular RNAs were extracted using TRIzol reagent and subjected to real-time PCR as described above.
In silico analysis of the OST gene promoter region
The first 5000 bp upstream of the transcription start site within the OSTα and OSTβ 5′ flanking regions were scanned for putative HRE. The analyses were based on the reference sequences NT_029928 and NW_925884 (NCBI database) respectively. The Genomatix Matinspector program (http://www.genomatix.de) was used to perform the scan.
Reporter gene constructs and expression vectors
Promoter fragments of the human OSTα and OSTβ genes were PCR amplified using primers described previously (9), generating amplicons of partial sequences corresponding to the reference sequences listed in the NCBI database (NT_029928 and NW_925884). The PCR products were cloned into the luciferase (Luc) vector pGL3basic to generate the OSTα (−1475/+161) and OSTβ (−4748/+29) reporter constructs. Point mutations were introduced into putative transcription factor binding sites using the QuikChange site-directed mutagenesis kit; the oligonucleotides are listed in Table 1. The sequences of all constructs were confirmed by DNA sequencing (Microsynth, Balgach, Switzerland). The mammalian expression plasmids pCMX-FXR and pCMX-RXR were kindly provided by Dr D. Mangelsdorf (University of Texas Southwestern Medical Center).
Table 1.
Sequences of oligonucleotides used for EMSAs and site-directed mutagenesis. The 5′ overhangs are marked in blue, the core hypoxia responsive elements are underlined and the introduced mutations are marked in red. The reverse complementary oligonucleotides are not shown
 |
Electromobility shift assay (EMSA)
Oligonucleotides used in EMSA analysis (Table 1) were designed to have a 5′-AGCT overhang at both ends when annealed, allowing radioactive labelling by fill-in reactions. Twenty-five nanograms of annealed oligonucleotides were labelled in a 10 μl reaction mix containing 25 units MultiScribe™ Reverse Transcriptase and corresponding reaction buffer, as well as 0.25 mM dGTP/dCTP/dTTP plus 20 μCi [α-32P]dATP. Unincorporated nucleotides were removed using Microspin G-25 columns. HIF-1α protein was synthesized using the TNT rabbit reticulocyte lysate-coupled in vitro transcription/translation system. One microlitre of synthesized protein was added to each reaction. Approximately 50 000 cpm (0.5–1.5 ng) of the radioactive probe was added per reaction. Protein-DNA complexes were formed in binding buffer [10 mM Tris HCl (pH 8.0), 40 mM KCl, 0.05% (vol/vol) Igepal CA-630, 6% (vol/vol) glycerol, 1 mM DTT and 50 ng/ml poly(dI-dC)-poly(dI-dC)] in a total volume of 20 μl for 10 min at 30°C. In super-shift experiments, 1 μg of HIF-1α sc13515 antibody was added to the reaction 30 min before adding the radioactively labelled oligonucleotide and incubated at 4°C. In competition experiments, a 10-, 50- or 250-fold molar excess of the competing oligonucleotides was added immediately before adding the radioactively labelled probe. Samples were loaded onto pre-electrophoresed 5% (acrylamide/bis 30:1) native acrylamide gels and run at 200 V in 0.5 × 44 mM Tris-borate, 1 mM EDTA (TBE) for 1.5 h. Gels were fixed in 10% (vol/vol) acetic acid for 10 min, rinsed with water, dried onto Whatman DE81 paper under vacuum, and exposed to Kodak BioMax MR-1 (Milian AG, Nesselnbach/Niederwil, Switzerland) films at −80°C.
Transient transfections and reporter assays
Huh7 cells were seeded into 48-well plates at a density of 25 000 cells/well and transfected with 400 ng of the indicated Luciferase reporter constructs or the empty vector pGL3 basic and 100 ng of phRG-TK reporter plasmid to normalize for transfection efficiency. The Fugene HD reagent was used at a ratio of 3 μl per 1 μg DNA. Six hours after transfection, cells were treated with either 50 μM CDCA or vehicle (DMSO) and subjected to either normoxic or hypoxic conditions (0.2% oxygen) for 18 h. Cells were lysed and luciferase activities determined using the Luciferase Assay System and a GloMax Multi luminometer (Promega, Madison, WI, USA). Transfection experiments were performed three times. Gene reporter activities obtained for the empty pGL3basic under each treatment condition and for the wild-type construct in the normoxic control, respectively, were set to one, and fold activities were calculated relative to these activity levels.
Preparation of protein extracts
For the preparation of whole cell protein extracts, cells grown in six-well plates were washed with ice-cold phosphate buffered saline (PBS) and lysed by a 5-min incubation in 150 μl of ice-cold lysis buffer supplemented with complete protease inhibitors [50 mM Tris _HCl (pH 8.0), 150 mM NaCl, 1% (vol/vol) Igepal CA-630, 0.5% (wt/vol) Na-deoxycholate, 1 mM EDTA, 0.1% (wt/vol) SDS and 10% (vol/vol) glycerol]. For the preparation of proteins from CRF to sham-operated rat livers, the hepatic tissue was homogenized in RIPA buffer at 4°C (Polytron, Kinematica, Lucerne, Switzerland) and centrifuged. In both cases, protein concentrations were determined with the bicinchoninic acid (BCA) Protein assay (Thermo scientific). Samples were stored at −20°C until further use.
Immunoblot analysis
Thirty micrograms of Huh7, 15 μg of PHH or 20 μg of liver tissue derived whole protein extract were used for Western blot analysis. The samples were separated on 12% SDS-polyacrylamide gels and electroblotted onto Amersham hybond ECL nitrocellulose membranes. Membranes were blocked overnight (cell protein extracts) or for 1 h (liver derived protein extracts) in 5% (wt/vol) non-fat milk in PBS-Tween 20 [0.1% (vol/vol) Tween 20 in PBS; PBS-T] blocking solution. The membranes were probed with antibodies against HIF-1α (ABE279 diluted 1:1000 in blocking solution), an antibody raised in rabbit against an OSTα peptide (obtained from the laboratory of N. Ballatori, diluted 1:500 in blocking solution), or an antibody against mOstα cross-reactive with hOSTα (sc10078 diluted 1:800 in blocking solution). All membranes were incubated overnight at 4°C. The next day, the membranes were washed three times with blocking solution and incubated with secondary horseradish peroxidase-conjugated goat antimouse antibody at a concentration of 10 ng/ml for 1 h. Blots were washed three times with blocking solution, twice with PBS-T, and analysed using the SuperSignal West Femto Maximum Sensitivity Substrate and the Fusion FX luminescence detector system (Vilber Lourmat, Marne-La-Vallée, France). To ensure equal loading of the protein samples, blots were stripped with Restore Plus Western Blot Stripping Solution, reblocked, and reprobed for constitutively expressed β-actin antigen. β-actin probing and detection were performed as described above except that the β-actin antibody was used at a concentration of 200 ng/ml and horseradish peroxidase-conjugated goat antirabbit antibody was used as secondary antibody at a concentration of 10 ng/ml. The relative intensities of the β-actin bands were used to normalize the results for OSTα expression. The experiments were repeated with at least three independent batches of PHH obtained from different donors and Huh7 cells at different passages.
Animal models
Liver tissue for immunoblot analysis was obtained from male Sprague-Dawley rats that had undergone either 5/6 nephrectomy (chronic renal failure group, CRF) or sham surgery (Sham), as described previously (14). Rats were killed 8 weeks after surgery. The livers were removed immediately, snap frozen in liquid nitrogen and stored at −80°C for subsequent homogenization.
Statistical analysis
Quantitative data obtained from luciferase gene reporter assays and real-time TaqMan PCR are shown as mean values ±1 SD. Average results were statistically evaluated using the one sample t-test or the unpaired t-test respectively. A P-value of <0.05 was considered to be significant. Statistical analysis was performed using graphpad prism 5.
Results
OSTα-OSTβ mRNA levels are increased by exposing cells to CDCA or hypoxia
The relative mRNA expression of OSTα/β was determined in Huh7 cells and PHH using TaqMan real-time PCR. As shown in Figure1, mRNA levels of OSTα and OSTβ were significantly increased in Huh7 cells upon treatment with the FXR agonist CDCA. OSTα mRNA levels were also increased in Huh7 cells exposed to 0.2% oxygen pressure (hypoxia) for 18 h. Interestingly, a greater than additive effect on OSTα mRNA levels in Huh7 cells and PHH could be observed when cells were exposed simultaneously to 50 μM CDCA and 0.2% oxygen for 18 h (Fig.1a and b). OSTβ mRNA was induced in both Huh7 cells and PHH by CDCA (Fig.1c), but only in PHH by hypoxia (Fig.1d). In PHH, a small but non-significant additive effect on OSTβ mRNA expression was observed under a combined regimen of 50 μM CDCA and 0.2% oxygen for 18 h (Fig.1d).
Fig 1.
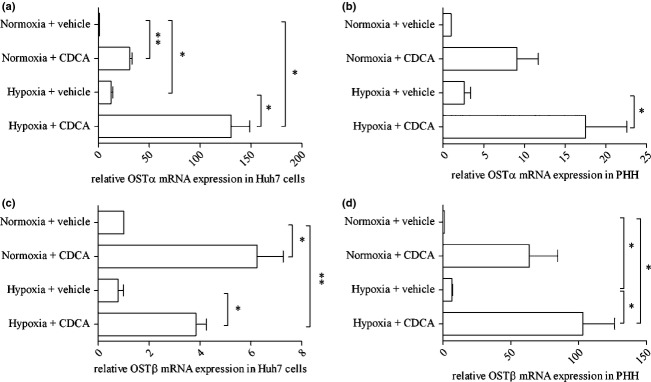
OSTα-OSTβ mRNA levels are increased after 18 h of treatment with CDCA or hypoxia. After 18 h of treatment with 50 μM CDCA in DMSO and exposure to either normoxia or hypoxia, cells were harvested and mRNA levels were determined by TaqMan real-time PCR. Figures a–d show average results of at least three independent experiments, *P < 0.05, **P < 0.01. (a) Relative OSTα mRNA determined in Huh7 cells. All treatment combinations show significant upregulation of OSTα mRNA. (b) Relative OSTα mRNA determined in PHH. A significant upregulation of OSTα mRNA is seen under combined treatment conditions. (c) and (d) Significant upregulation of OSTβ mRNA is seen only with CDCA in both Huh7 cells (c) and PHH (d). In PHH, a significant increase in OSTβ mRNA was induced by hypoxia (d).
HIF-1α binds to hypoxia response elements in the OSTα and OSTβ promoters
A search for putative HRE within the 5′-flanking region of OSTα revealed only one element at position −1345 upstream of the predicted transcription start (HRE1). Interestingly, the predicted HRE appears to be located in close proximity to a functionally relevant FXR binding site within the OSTα promoter (9). Only five base pairs separate these two binding elements. In the case of OSTβ, three different putative HRE were identified, at −202 (HRE1), −3961 (HRE2) and −4103 HRE3) base pairs upstream of the predicted transcription start.
The in vitro binding of HIF-1α to the newly identified, putative HREs within the OSTα and OSTβ promoters was assessed by electrophoretic mobility shift assay (EMSA). Oligonucleotides carrying a functional VEGFa HRE (15) were used as a positive control in all experiments. To confirm specific binding of HIF-1α, super-shift experiments were performed using a HIF-1α specific antibody. As demonstrated in Figure2a, the predicted OSTα HRE was able to bind HIF-1α. In the case of OSTβ the binding intensity varied among the three sites. The binding to the putative HRE at position −4103 upstream of the transcription start (short HRE3) appeared to be the strongest. Therefore, this binding site was selected to further elucidate its functional relevance. The capacity of the wild-type or mutated annealed oligonucleotides to compete with VEGFa HRE for HIF-1α binding was shown by competition experiments (Fig.2b). Three different molar excesses of each of the different annealed oligonucleotides were applied to otherwise identical binding reactions of the reference VEGFa oligonucleotide. When the core elements of the HRE in OSTα and the HRE3 in OSTβ were mutated, their capacity to compete for binding to HIF-1α was strongly impaired when compared to the wild-type.
Fig 2.
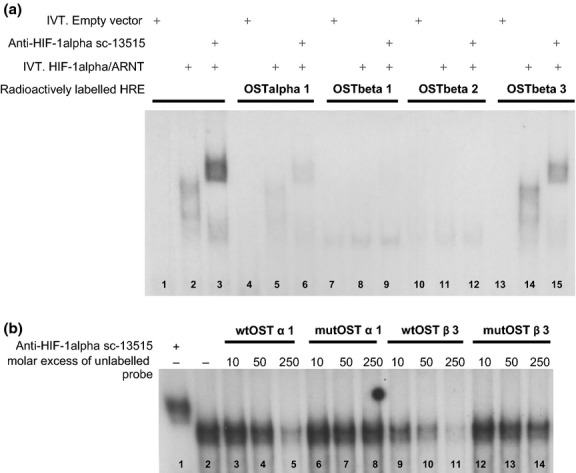
HIF-1α binds to putative hypoxia responsive elements in OSTα and OSTβ promoters in vitro. EMSA was performed to determine the binding of HIF-1α to predicted putative HREs within the OSTα and OSTβ promoters. (a) Radiolabeled annealed oligonucleotides containing four different putative HREs were incubated with either a mix of in vitro translated (IVT) HIF-1α and ARNT or a negative control (in vitro translated empty vector backbone) and run on a native polyacrylamide gel. Super-shift reactions were performed by addition of anti-HIF-1α antibody to the binding reactions. (a) Positive shift and super-shift reactions were observed for the positive control using labelled oligonucleotides containing the VEGFa HRE (lanes 2 and 3), for the HRE within the OSTα promoter (lanes 5 and 6) as well as for HRE3 in the OSTβ promoter (lanes 14 and 15). (b) Shows a competition EMSA. Three different molar excesses of unlabelled wild-type (wt) or mutated (mut) HRE-containing oligonucleotides were added to a mix of in vitro translated HIF-1α and ARNT together with radiolabeled VEGFa HRE oligonucleotides. In lane 1, the supershift was induced with the anti-HIF-1α antibody. In lane 2, no competing unlabelled oligonucleotide was added. Wild-type HRE oligonucleotides were able to compete off the binding of HIF-1α and ARNT in a dose dependent manner. Oligonucleotides containing the HREs in mutated form were not able to compete successfully (lanes 6–8 and 12–14).
The inducing effect of hypoxia and CDCA on OSTα expression is dependent on the transcription factors HIF-1α and FXR
The increase in OSTα mRNA levels induced by hypoxia or CDCA was significantly reduced when Huh7 cells were transfected with siRNAs targeting HIF-1α (Fig.3a). A comparable effect was seen when transfecting cells with siRNA targeting FXR, inducing a reduction in the response to CDCA treatment and suggesting a functionally important role for HIF-1α and FXR in OSTα regulation (Fig.3a). In contrast, an siRNA-dependent knock-down of HIF-1α did not show any significant effect on the expression of OSTβ mRNA when the cells were subjected to hypoxia (Fig.3b). The increase in OSTβ mRNA levels upon CDCA treatment was reduced when siRNA against FXR was transfected, confirming the known functional role for FXR in regulating OSTβ gene transcription (Fig.3b).
Fig 3.
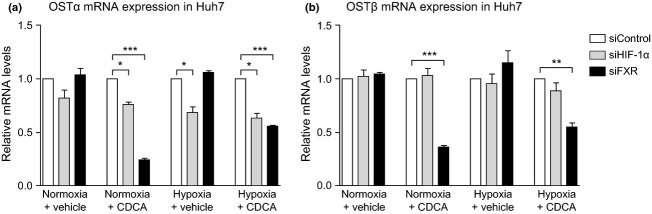
The upregulation of OSTα mRNA by hypoxia and CDCA is dependent on HIF-1α and FXR. The figure shows the relative OST mRNA levels in Huh7 after transfection with siRNA targeting HIF-1α or FXR. The results of each treatment condition (CDCA, hypoxia, or both) are shown relative to cells transfected with a random scrambled siRNA (siControl). The figure shows average results of at least three independent experiments, with *P < 0.05, **P < 0.01 and ***P < 0.001. (a) Knock-down of HIF-1α significantly reduces the induction of OSTα mRNA in cells treated with CDCA or hypoxia, either alone or in combination. As expected, a knock-down of FXR abrogates the inducing effect of CDCA on OSTα mRNA expression. (b) While knock-down of FXR significantly reduces the inducing effect of CDCA on OSTβ mRNA expression, HIF-1α knock-down does not influence the induction of OSTβ mRNA in cells treated with CDCA under either normoxic or hypoxic conditions.
The hypoxia-induced upregulation of OSTα expression is mediated by the newly identified HRE
Luciferase gene reporter constructs carrying subcloned OSTα and OSTβ promoter fragments were used in dual luciferase assays. When the wild-type OSTα promoter construct was transfected into Huh7 cells, a significant increase in luciferase activity was observed treating cells with both 50 μM CDCA and 0.2% oxygen. When the treatment conditions were applied separately, only a slight, non-significant increase in luciferase activity was observed (Fig.4a). In contrast, no inducing effects of the different treatment conditions on luciferase activity were observed when transfecting the OSTβ promoter construct (Fig.4b).
Fig 4.
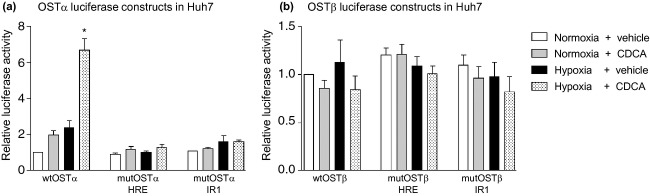
Hypoxia induces luciferase activity in promoter constructs of OSTα, an effect that is dependent on the newly identified hypoxia responsive element. Dual luciferase assays with OSTα and OSTβ promoter constructs transfected into Huh7 cells are represented as relative luciferase activity, normalized to the pcDNA3.1 empty vector backbone. Plasmids containing either the wild-type (wt) promoter or promoters with HRE or IR1 in mutated form (mut) were transfected into cells that were treated for 18 h with 50 μM CDCA or DMSO and exposed to either normoxia or hypoxia. (a) A significant increase (*P < 0.05) in promoter activity of wt OSTα was seen in cells treated with hypoxia and CDCA together. A non-significant trend was observed for single treatments. The inducing effects were markedly reduced when either the HRE or the IR1 were mutated. (b) Luciferase activity of the OSTβ promoter construct was not significantly changed by any of the treatment conditions applied. Mutation of either the HRE or IR1 site did not significantly affect the basal activity.
The increased luciferase activity induced by a combined treatment with CDCA and hypoxia was lost when the core element of the putative HRE within the OSTα promoter construct was artificially mutated from ACGTG to ACCTC. A similar observation was made when the functionally relevant FXR binding site described previously (9) was mutated (Fig.4a). The mutation of the HRE or FXR binding site in the OSTβ promoter construct did not reduce the basal luciferase activity (Fig.4b).
Hypoxia treatment increases OSTα protein levels in Huh7 cells and PHH
To determine the regulation of OSTα at the protein level, Western blot analysis was performed. The anti-OSTα antibodies sc100078 as well as a non-commercial antibody generated in the laboratory of Ballatori and coworkers (data not shown) were used to detect the target protein. Exposure to 0.2% oxygen for 18 h clearly induced the expression of OSTα three-fold at the protein level in Huh7 cells (Fig.5a) and two-fold in PHH (Fig.5b).
Fig 5.
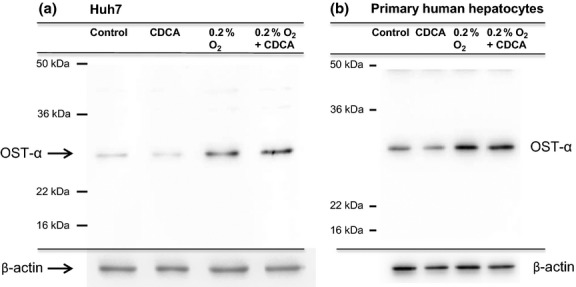
Hypoxia treatment for 18 h increases OSTα protein levels in Huh7 cells and PHH. Cells were treated with 50 μM CDCA or DMSO alone and were either exposed to hypoxia or kept under normoxic conditions for 18 h. Total protein from whole Huh7 cell extracts (30 μg) (a) or 15 μg from whole cell extracts isolated from PHH (b) were run on an SDS-PAGE gel and immunoblotted. An anti-OSTα antibody was used to probe the membrane, followed by stripping and probing with β-actin antibody. Exposure of the cells to hypoxia-induced protein expression in both Huh7 (three-fold) and PHH (two-fold).
Rats suffering from chronic renal failure show concordantly upregulated HIF-1α and OSTα protein in liver tissue
We previously showed a marked increase in OSTα expression in liver tissue of rats that had undergone 5/6 nephrectomy to simulate CRF conditions (14). Because the mechanism of hepatic OSTα induction in the CRF model was not resolved, we hypothesized that an increase in HIF-1α expression could be involved. We therefore analysed the expression of OSTα and HIF-1α protein in CRF rats compared to sham-operated controls. As shown in Figure6, rats suffering from CRF showed markedly increased OSTα and HIF-1α protein levels as compared to controls.
Fig 6.
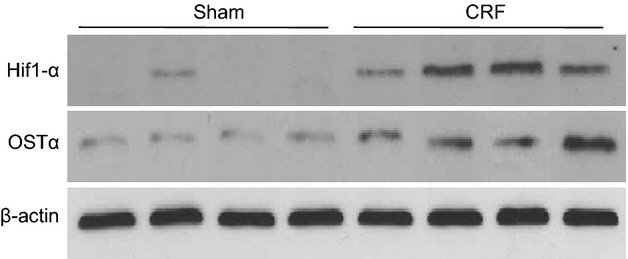
The hepatic expression of HIF-1α and OSTα is significantly induced in rats suffering from chronic renal failure. Four sham-operated rats and four CRF rats that had undergone 5/6 nephrectomy were compared with regard to the expression level of HIF-1α and OSTα in hepatic tissue 8 weeks after the procedure. Western blot analysis shows a significant increase in both HIF-1α and OSTα under CRF conditions compared to sham-operated controls. Actin was probed for assay normalization.
Discussion
Tissue hypoxia in the liver can be caused by several forms of liver injury (2–4). In cholestasis the toxic effect of bile acids within hepatocytes is prevented by adaptive responses such as induction of bile acid efflux transporters. This study shows that both hypoxia and elevated bile acid levels have the potential to modulate the expression of bile acid transporters via transcription factor mediated pathways, as demonstrated here for the heterodimeric transport protein OSTα-OSTβ. To determine whether hypoxic conditions affect expression at the transcriptional level, relative mRNA levels were measured in both the hepatoma-derived cell-line Huh7 and PHH treated with hypoxia or CDCA, separately or in combination. The inducing effect of CDCA on OSTα-OSTβ expression was reported previously (9). Here, we show that hypoxia alone is able to increase OSTα and OSTβ mRNA. In Huh7 cells, the combination of CDCA treatment and hypoxia appeared to induce a greater than additive elevation of OSTα mRNA levels, suggesting a possible synergistic effect. In all experiments in which relative OSTα and OSTβ mRNA levels were measured, the induction of VEGFa mRNA was confirmed as a positive control for the effect of hypoxia on gene expression. CDCA alone had no effect on relative VEGFa mRNA levels (data not shown). The inducing effect of hypoxia on OSTα was also evident at the protein level in both Huh7 cells and PHH after 18 h. This was confirmed in the same samples using a second OSTα antibody provided by the laboratory of N. Ballatori (data not shown). Activation of OSTβ gene transcription by CDCA was also observed, as described previously (9,16). A significant inducing effect of hypoxia on OSTβ was found in PHH but not Huh7 cells (Fig.1c and d).
The binding of HIF-1α to the HRE in the OST promoter regions was analysed in vitro by EMSA as shown in Figure2. HIF-1α binds to the HRE identified in the OSTα promoter and preferentially to one of the three HRE detected in the OSTβ promoter (Fig.2a). When the core of the HRE was mutated HIF-1α binding was significantly reduced in both OSTα and OSTβ, as shown by the competition EMSA in Figure2b. Using siRNA to knock-down HIF-1α and FXR, the inducing effects of CDCA and hypoxia on OSTα regulation could be disrupted (Fig.3a). In contrast to OSTα, no relevant effect of anti-HIF-1α siRNA on OSTβ expression was observed, suggesting that the overall magnitude of OSTβ regulation by HIF-1α may be less than for OSTα.
Dual luciferase assays using wild-type and mutated OSTα promoter constructs confirmed the importance of the predicted HRE for the regulation of OSTα gene expression by hypoxia (Fig.4a). Interestingly, the induction of the OSTα promoter by CDCA was reduced when an OSTα promoter construct carrying the mutated HRE site was employed. This observation could be explained by the close proximity of the HRE sequence and the FXR binding site. Mutation of the HRE could affect the functionality of the FXR binding site. In the case of the OSTβ promoter, no relevant effect of CDCA and hypoxia on luciferase reporter activity was seen (Fig.4b), again suggesting that HIF-1α may not activate OSTβ gene transcription to the degree that OSTα gene transcription is induced. The absence of an effect of CDCA on the promoter activity of OSTα and OSTβ in dual luciferase assays (Fig.4) does not contradict previous results (9), as the present set-up relied on shorter incubation times and on the activity of endogenously expressed transcription factors only.
Dimerization of OSTα with OSTβ is thought to be essential for transport function (17–19). Despite the functional synergism, several reports suggest that OSTα and OSTβ appear to be regulated differently. This was concluded from the divergent effects that transcription factors exert on the two promoters, or from the fact that certain transcriptional effects appear to regulate only one of the subunits. (20–23). It has been shown that OSTβ is important for both the trafficking of OSTα to the plasma membrane as well as for its function (24). However, the lack of co-immunoprecipitation of the mature, glycosylated form of OSTα upon immunoprecipitation of OSTβ suggests that the primary interaction may occur early in the biosynthetic pathway and may be transient (16). This would support the mutual independence of the subunits in substrate transport and gives rise to the theory that different dimerization partners exist for the OST subunits. Another indication of the possibility of alternative binding partners or conformations of OSTα is the existence of different splicing variants that have been deposited in the available databases. The exact role of these splice variants has not been investigated.
OSTα-OSTβ is considered to be an efflux system for bile acids across the basolateral membrane of hepatocytes that allows extrusion of bile acids into sinusoidal blood. This could protect hepatocytes from the intracellular accumulation of potentially toxic bile acids, e.g. during cholestatic liver injury or under conditions of hypoxia. Hypoxia caused by arterial liver ischaemia has previously been shown to decrease expression of the Na+-dependent bile acid uptake transporter Ntcp and the bile salt export pump Bsep, which could lead to cholestasis (25). Induction of OSTα in this setting could represent a rescue mechanism for reducing the intracellular bile acid load when the canalicular efflux pump shows reduced expression.
Chronic renal failure is associated with hypoxia in the kidney. In this study, we show that the previously observed increase in OSTα expression in the liver tissue of rats suffering from CRF (14) could be attributable to the increased expression of HIF-1α (Fig.6). It is assumed that chronic hypoxia occurring during CRF is a multifactorial event caused by damage of renal arterioles, a distortion of peritubular capillaries and imminent interstitial fibrosis. Hypoxia itself is able to induce strong profibrogenic effects leading, among other pathways, via induction of HIF-1α dependent signalling to further destruction of peritubular capillaries, fibrotic remodelling of tubular cells and thus, to the further progression of kidney failure (26–28). Induction of HIF-1α in the liver suggests that hypoxic stimuli occurring during CRF modulate HIF-1α dependent signalling pathways in other tissues as well.
In conclusion, the expression of OSTα and OSTβ is significantly induced by bile acids and hypoxia. While the CDCA-dependent induction of OSTα-OSTβ expression is known to be mediated by FXR, the hypoxia effect is mediated by the transcription factor HIF-1α through newly detected, functionally relevant HIF-1α responsive elements within the OSTα and OSTβ gene promoters. In the case of the OSTα promoter, FXR and HIF-1α bind in close proximity and putatively interact to produce synergistic effects on OSTα expression.
Acknowledgments
We thank Christian Hiller for excellent technical assistance. We are grateful to Margot Crucet for assistance with the handling of the hypoxia chamber. We thank Dr N. Ballatori and coworkers for providing an antibody against human OSTα.
Financial support: This work was supported by Swiss National Science Foundation (SNF) grant numbers 320030_144193/1 (to GAKU) and PDFMP3_127259/1 (ProDoc program), the Swiss National Center for Competence in Research NCCR-Kidney.ch, and the International Fellowship Program (grant no. 246539) on Integrative Kidney Physiology and Pathophysiology (IKPP).
Conflicts of interest: The authors do not have any disclosures to report.
Glossary
- CDCA
chenodeoxycholic acid
- CRF
chronic renal failure
- EMSA
electrophoretic mobility shift assay
- HRE
hypoxia responsive element
- OSTα/β
organic solute transporter α/β
- PCR
polymerase chain reaction
- PHH
primary human hepatocytes
- VEGFa
vascular endothelial growth factor A
References
- 1.Pellicoro A, Faber KN. Review article: the function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment Pharmacol Ther. 2007;26:149–60. doi: 10.1111/j.1365-2036.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- 2.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–7. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622–33. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toki F, Takahashi A, Suzuki M, et al. Development of an experimental model of cholestasis induced by hypoxic/ischemic damage to the bile duct and liver tissues in infantile rats. J Gastroenterol. 2011;46:639–47. doi: 10.1007/s00535-010-0330-5. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–61. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 6.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 7.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 8.Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. In: Fromm MF, Kim RB, editors. Drug Transporters. Berlin, Heidelberg: Springer; 2011. pp. 169–203. Handbook of Experimental Pharmacology 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landrier J-F, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–85. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Chiang JY. Bile acid signaling in liver metabolism and diseases. J Lipids. 2012;2012:754067. doi: 10.1155/2012/754067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y-D, Chen W-D, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–95. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 13.Lee SML, Schelcher C, Demmel M, Hauner M, Thasler WE. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. J Vis Exp. 2013:e50615. doi: 10.3791/50615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gai Z, Chu L, Hiller C, et al. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am J Physiol Renal Physiol. 2014;306:F130–7. doi: 10.1152/ajprenal.00114.2013. [DOI] [PubMed] [Google Scholar]
- 15.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soroka C, Xu S, Mennone A, Lam P, Boyer J. N-Glycosylation of the alpha subunit does not influence trafficking or functional activity of the human organic solute transporter alpha/beta. BMC Cell Biol. 2008;9:57. doi: 10.1186/1471-2121-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–8. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ostα and Ostβ, that constitute the organic solute and steroid transporter. Biochem J. 2007;407:363–72. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seward D, Koh A, Boyer J, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278:27473–82. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- 20.Khan AA, Chow ECY, Porte RJ, Pang KS, Groothuis GMM. Expression and regulation of the bile acid transporter, OSTα-OSTβ in rat and human intestine and liver. Biopharm Drug Dispos. 2009;30:241–58. doi: 10.1002/bdd.663. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Wong C. Oleanolic acid is a selective farnesoid X receptor modulator. Phytother Res. 2010;24:369–73. doi: 10.1002/ptr.2948. [DOI] [PubMed] [Google Scholar]
- 22.Noble CL, Abbas AR, Lees CW, et al. Characterization of intestinal gene expression profiles in Crohn's disease by genome-wide microarray analysis. Inflamm Bowel Dis. 2010;16:1717–28. doi: 10.1002/ibd.21263. [DOI] [PubMed] [Google Scholar]
- 23.Schaap FG, Van Der Gaag NA, Gouma DJ, Jansen PLM. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–35. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 24.Christian WV, Li N, Hinkle PM, Ballatori N. β-Subunit of the Ostα-Ostβ organic solute transporter is required not only for heterodimerization and trafficking but also for function. J Biol Chem. 2012;287:21233–43. doi: 10.1074/jbc.M112.352245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouassier L, Beaussier M, Schiffer E, et al. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G25–35. doi: 10.1152/ajpgi.00175.2006. [DOI] [PubMed] [Google Scholar]
- 26.Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–80. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- 27.Kairaitis LK, Wang Y, Gassmann M, Tay YC, Harris DC. HIF-1alpha expression follows microvascular loss in advanced murine adriamycin nephrosis. Am J Physiol Renal Physiol. 2005;288:F198–206. doi: 10.1152/ajprenal.00244.2003. [DOI] [PubMed] [Google Scholar]
- 28.Choi YJ, Chakraborty S, Nguyen V, et al. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491–7. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]


