Abstract
Cognitive impairment is emerging as an important therapeutic target in patients with psychiatric illnesses, including major depressive disorder (MDD). The objective of this general overview is to briefly review the evidence for cognitive impairment in MDD and to summarize a representative sample of cognitive assessment tools currently available to assess cognitive function in depressed patients. Study results in MDD patients with cognitive dysfunction are somewhat inconsistent, likely due to the heterogeneity of the disorder as well as the use of diverse assessment tools. Measuring cognitive changes in this population is challenging. Cognitive symptoms are typically less severe than in patients with schizophrenia and bipolar disorder, requiring greater sensitivity than afforded by existing tools. Preliminary evidence suggests antidepressant treatments may improve cognitive functioning as a direct result of ameliorating depressive symptoms; however, any procognitive effects have not been elucidated. To evaluate antidepressant efficacy in MDD patients with cognitive dysfunction, a standardized cognitive battery for use in clinical trials is essential.
Keywords: cognitive assessment, cognitive dysfunction, major depressive disorder, antidepressant
INTRODUCTION
Cognitive dysfunction is a core component of major psychiatric disorders, such as schizophrenia, bipolar disorder (BD), and major depressive disorder (MDD).[1–4] Although it was initially thought that cognitive impairments in MDD were state-related, there is increasing evidence suggesting that cognitive abnormalities persist beyond depressive episodes.[5] Longitudinal studies have shown that poor concentration, poor memory, and difficulty making decisions are common symptoms in patients with MDD and often persist in individuals who meet the clinical criteria for remission.[5–10] Based on clinical ratings and patient self-reports, approximately 90% of 2,541 patients enrolled in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study had difficulty with concentration and decision making.[11] Moreover, 22% of remitters reported residual cognitive difficulties.[10] In an earlier study, residual problems with apathy, inattentiveness, forgetfulness, word-finding difficulty, and mental slowing were reported in >30% of responders.[12]
In addition to the subjective reports of cognitive difficulties in patients with MDD, numerous studies have used objective neurocognitive testing to delineate the deficits in patients with MDD and to identify clinical correlates that may be predictive of cognitive outcomes. The number of publications addressing cognition in MDD has risen sharply in the last decade (Fig.1), reflecting the growing interest in cognition as a therapeutic target and emphasizing the need for standardized, validated assessment tools for use in patients with MDD. An exhaustive review of these results is beyond the scope of this publication. However, the following examples serve to illustrate limitations that are important to critically evaluating the literature in this area. Most studies designed to investigate specific cognitive deficits in MDD have been small, cross-sectional studies that used different patient populations and widely varying neurocognitive batteries (Table 1). As a result, not all studies measured impairment in the same cognitive domains, and the sensitivity of the assessment tools also varied. There is a lack of consensus in the field regarding the optimal instrument or battery to assess cognitive functioning in MDD. Thus, the aim of the present work is to briefly describe the most robust neurocognitive deficits reported in MDD to date, as well as to give an overview of the most commonly used scales and tasks to measure cognitive deficits in this population.
Figure 1.
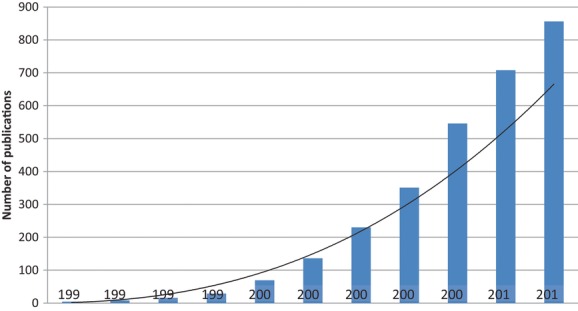
Cumulative number of publications indexed in PubMed between January 1, 1991, and January 1, 2013, using the search terms “cognitive function” or “cognition” and “major depressive disorder” [not stroke, not bipolar, not bias, not CBT (Cognitive-Behavioral Therapy), not electroconvulsive] and “adult” [MeSH].
Table 1.
Comparison of neurocognitive assessments in patients with schizophrenia, BD, and MDDa
| Schizophrenia/BD | MDD (meta-analyses) | |||
|---|---|---|---|---|
| Neurocognitive domain | MATRICS consensus cognitive battery[32] | Remission from MDD[6] | First MDD episode[13] | Executive function in nonpsychotic MDD[51] |
| Reasoning and problem solving | Neuropsychological Assessment battery (NAB): Mazes | Phonological fluency; semantic fluency; Stroop test; Wisconsin Card Sorting Test | Attentional switching Trail-Making Test B; verbal fluency letter and semantic fluency Cognitive flexibility Wisconsin Card Sorting Test (WCST); Modified Card Sorting Test (MCST); CANTAB Intradimensional/extradimensional shift (ID/ED) | Phonological fluency; semantic fluency; Wisconsin Card Sorting Test; Stroop Interference Test; Trail-Making Test B |
| Processing speed | Trail-Making Test A; brief assessment of cognition in schizophrenia (symbol coding subtest); category fluency | Trail Making A and B; symbol digit Modalities test (SDMT); Familiar faces; Boston Naming Test | Trail-Making Test A; digit symbol-coding; SDMT (written version) | |
| Attention/vigilance | Continuous performance test: identical pairs | Digit span forward; spatial span forward | ||
| Working memory | Wechsler Memory Scale; spatial span; letter-number span | Letter–number sequencing (LNS) | Digit span backward; spatial span backward | |
| Verbal learning and memory | Hopkins Verbal Learning Test (HVLT) | Rey Auditory Verbal Learning Test (RAVLT); Category Cued Recall | Logical Memory 1 and 2; RAVLT total and delayed recall; California Verbal Learning Test-second edition (CVLT-II) total recall, short-delayed free recall and long-delayed free recall; HVLT total and delayed recall; Buschke Selective Reminding Test (SRT) | |
| Visual learning and memory | Brief Visuospatial Memory Test | Rey–Osterrieth Complex Figure Test (RCFT) | Visual reproduction 1 and 2; Rey Complex Figure Test (RCFT) 30-min delayed recall; Wechsler Memory Scale (WMS); Visual Memory Index | |
| Social cognition | Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) | |||
Boldface tests are used more than once.
BD, bipolar disorder; MATRICS, Measurement and Treatment Research to Improve Cognition in Schizophrenia; MDD, major depressive disorder.
COGNITIVE IMPAIRMENT IN MDDM
Decades of research suggest that neurocognitive deficits are a major component of MDD, yet there is large variation both in the patient samples included in studies as well as the neurocognitive batteries used to assess cognitive impairment. Nevertheless, several recent meta-analyses suggest that there are several cognitive domains that do appear to be consistently affected in MDD. Although a comprehensive review of neurocognitive deficits in MDD is beyond the scope of the present work, we summarize findings from recent meta-analyses to provide an estimate of the most consistently affected domains.
Cognitive impairment appears to be present early in the course of the illness. One meta-analysis of data from 13 studies (15 samples) compared adults experiencing their first major depressive episode (N = 644; mean age, 39.4 ± 10.2 years) and healthy controls (N = 570) found that patients performed significantly worse than controls on a range of tests.[13] There were small to medium effect sizes in psychomotor speed, attention, visual learning and memory, executive functioning, attentional switching, verbal fluency, and cognitive flexibility.[13] Conversely, there were no differences between patients and controls on working memory and verbal memory. Some demographic factors, such as age and level of education, appeared to explain some of the heterogeneity in cognition; namely, it was found that patients were on average older than controls, which may have contributed to worse performance on tasks of psychomotor speed, visual learning, and executive functions. Higher levels of education in patients may have mitigated deficits in learning and memory, as well as attentional switching. Inpatient status, which may reflect symptom severity, was associated with slower psychomotor speed and poorer working memory, verbal learning and memory, and visual learning and memory. Subjects treated with antidepressant medications had better cognitive flexibility, but poorer memory and verbal learning than did subjects who were not taking antidepressants.
A recent meta-analysis, including patients with more chronic MDD, suggests that patients demonstrate significant impairment in response inhibition, cognitive flexibility, and semantic verbal fluency, with large effect sizes (Cohen's d = 1.18, 1.11, and 0.92, respectively).[14] Strategic planning and organization were moderately impaired (d = 0.44). Although only three of the 15 studies evaluated the effect of antidepressant medication treatment on cognition, results suggest that executive function improved with antidepressant treatment.
The importance of subsyndromal symptoms in MDD is highlighted in a recent study of 88 patients with remitted MDD (Hasselbalch et al., 2012).[6] Compared with healthy controls, patients with remitted MDD demonstrated residual impairment on measures of processing speed and cognitive flexibility. Within the same sample, the cumulative duration of depressive episodes was correlated with the degree of global cognitive impairment, even when controlling for current depressive symptoms.[15] This suggests that as the disorder progresses cognition becomes more impaired. Moreover, the presence of psychotic features during a period of previous depression was associated with worse cognitive functioning in the remitted state.[15] In a systematic review by Hasselbalch et al. ([5]), including 11 studies for a total of 500 remitted unipolar depression patients and 417 healthy controls, it was found that not only the severity of depressive symptoms, but also the melancholic subtype negatively affect cognition. Authors also showed that early onset was mostly associated with deficit on episodic memory, whereas executive functions and processing speed were more affected in late-onset patients.[5] It was also reported that a higher number of depressive episodes is associated with more pronounced cognitive deficits.[8, 16] No correlations were found between residual cognitive symptoms and treatment with antidepressants, antipsychotic agents, anticonvulsive mood stabilizers, or numbers of electroconvulsive shock treatments.
In a very recent meta-analysis of cognition in euthymic MDD including 27 studies (895 euthymic MDD patients and 993 healthy controls), Bora et al. ([17]) showed that euthymic MDD patients have somewhat lower global cognition compared to healthy subjects (d = .47).[17] It was also found that MDD patients underperformed compared to healthy subjects in tasks measuring attention, executive functions, working memory, and verbal learning with moderate effects sizes (d ranged from .39 to .59). Differences were noted between early-onset (between 18 and 50 years, depending on the study) and late-onset patients (after 50–65 years), with early-onset patients showing smaller effect sizes compared to controls in most of the cognitive domains and late-onset patients presenting with larger effect sizes on global cognition and in particular on verbal memory.
Overall, results from these studies support the notion that cognitive function is impaired in MDD, including early in the course of the illness and during remission. Moreover, certain aspects of cognitive dysfunction, such as psychomotor speed and memory, appear to be more strongly related to mood state. Others, such as attention and executive functioning more broadly, seem to be less affected by mood state variables and appear to be more trait-like in their presentation.[18]
SOCIAL COGNITION IN MDD
In addition to the traditional neurocognitive domains described above, pronounced deficits in cognitive domains closely related to social functioning have been observed in MDD. These deficits have mostly been studied within the context of facial emotion recognition and perception,[19–21] attributional biases,[22] and, more broadly, within the larger context of Theory of Mind (ToM). Although a thorough review of these deficits is beyond the scope of this work, the evidence to date suggests that social cognition is impaired in MDD, and that this impairment is present early in the course of the illness.[23] A recent meta-analysis demonstrated that patients with MDD are impaired in perceiving facial emotional expressions;[24] in fact, there were no differences in the degree of impairment in this domain between patients with MDD and patients with BD. Similarly, attributional biases have long been known to be a trait-like feature of MDD, with patients tending to attribute negative events to personal and internal factors rather than to external factors.[22, 25, 26] There is some evidence that these attributional biases persist even in the remitted state.[27]
Studies that have assessed the broad concept of ToM in MDD have done so using a variety of measures assessing a range of skills, such as the ability to detect a social faux pas, the capacity to understand subtle social cues, and the ability to appreciate humor. Recent studies suggest that patients with MDD are indeed impaired in these areas[23, 28–30] and that these impairments remain even after controlling for nonsocial cognitive impairment.[23]
COGNITION AS A THERAPEUTIC TARGET IN MDD
Interest in cognitive dysfunction as a therapeutic target has highlighted some limitations in available methodologies used to understand their impact on everyday function, which is a critical factor of complete recovery. Research into cognitive disabilities in patients with schizophrenia and, to a lesser extent, BD is considerably more advanced than in individuals with MDD. A decade ago, the National Institute of Mental Health and the US Food and Drug Administration, along with academic institutions and the pharmaceutical industry, established the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative.[31] The result was the MATRICS Consensus Cognitive Battery (MCCB).[31, 32] Although the MCCB was originally developed to be used in clinical trials targeting cognition in schizophrenia, recent studies have demonstrated its suitability to effectively capture neurocognitive deficits in BD patients as well, regardless of their clinical state at the time of testing.[33, 34] There is a critical need to expand these efforts to the cognitive dysfunction associated with patients with MDD, yet very little work has been done in this area. Just as in other disorders, clinically significant improvement requires an amelioration of neurocognitive impairment. Furthermore, as neurocognition reliably distinguishes patients with MDD from controls, it is a logical endpoint, in addition to mood symptom status, for clinical trials. One hurdle that must be overcome in implementing this approach is the lack of neurocognitive measures traditionally employed in clinical trials.
ASSESSMENT TOOLS
The Hamilton Depression Rating Scale (HAM-D)[35] and the Montgomery-Åsberg Depression Rating Scale (MADRS)[36] are clinician-administered assessments of depressive symptoms that are the most frequently used depression rating scales in clinical trials. However, neither of these scales evaluates cognition in any depth and both rely on a clinician's subjective opinion based upon a patient's report. On the HAM-D, a single item assesses psychomotor functioning, whereas a single item on the 10-item MADRS assesses concentration.[36] The 16-item Quick Inventory of Depressive Symptomatology (QIDS-16) assesses both of these items, but is also based on a subjective rating.[37]
The patient-reported Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ) assesses multiple cognitive symptoms in patients with MDD and is simple enough to be suitable for use in routine clinical practice as well.[38] However, it is important to note that a patient's subjective experience of cognitive dysfunction is not always related to performance on objective neuropsychological measures. A recent study compared outcomes using the clinician-administered Screen for Cognitive Impairment in Psychiatry (SCIP) with results from the CPFQ in patients with MDD.[39] There was no significant correlation between the severity of impairment measured with the SCIP and patient reports of cognitive symptoms. Although the SCIP is not validated in MDD, similar observations were made in patients (with BD) who complained of cognitive impairment. Subjective cognitive complaints in patients with BD have consistently been shown to be poorly correlated with objective performance on cognitive tests.[39–41] When patients with BD are directly compared with patients with MDD, similar rates of cognitive complaints are noted; however, more severe objective cognitive impairment is generally seen in patients with BD.[42] In this study, subjective cognitive impairment was significantly associated with the severity of depressive symptoms, but not with the categorical diagnosis of MDD or BD. This is perhaps not surprising, given the documented negative cognitive bias associated with depression that may cause patients to overestimate the severity of their impairments.
OBJECTIVE ASSESSMENT TOOLS: LESSONS LEARNED FROM MATRICS
Cognitive deficits are qualitatively similar in schizophrenia and bipolar and unipolar depression, with evidence of impaired underlying attention, verbal learning, and executive functioning across these diagnoses.[31] However, the severity of impairment differs between these disorders, with the most severe deficits seen in patients with schizophrenia, followed by BD, and the least severe deficits noted in individuals with MDD.
Although there are hundreds of standardized neurocognitive tests available,[43] it is important to consider the adoption of a more uniform battery when taking into account the similarities and differences seen among multiple neuropsychiatric disorders. Moreover, some cognitive tests may be optimized for use when considering treatment effects on neurocognitive functioning. The MCCB was developed with this in mind, with a specific focus on clinical trials for cognition in schizophrenia. It comprises 10 tests to evaluate seven cognitive domains: processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning/problem solving, and social cognition (Table 1).[32, 44] In validation studies in patients with schizophrenia, the MCCB demonstrated excellent test–retest reliability, small practice effects, and strong correlations with functional capacity. Better performance on tests of processing speed, visual learning, and attention/vigilance were found to be correlated with competitive employment, suggesting that these attributes were strongly correlated with functional capacity.[45]
As the MCCB is among the most frequently used and most comprehensive neurocognitive assessment battery in clinical trials of psychiatric disorders, it may be helpful to use this battery as a reference in evaluating other tests used to measure cognition in MDD (Table 1). An assessment of MCCB suitability for use in BD clinical trials showed that this battery was generally adequate in this less severely impaired population.[34] Patients with BD were significantly impaired relative to healthy controls on all domains except reasoning/problem solving and social cognition. In this study (N = 80), results were similar for individuals who were euthymic and those who were mildly symptomatic, suggesting that subclinical mood symptoms did not appreciably affect performance. This study also highlighted the possibility that not all of the MCCB subtests are sensitive enough to assay the more subtle deficits common in nonpsychotic psychiatric subjects. For example, the effect size of the deficit noted on the Hopkins Verbal Learning Test was smaller than prior results found when using the more difficult California Verbal Learning Test.[34] Thus, when considering optimal assessment measures in affective disorders, including MDD, it is critical to determine which tasks will capture the expected variance in performance.
Although the MCCB is the most frequently used neurocognitive battery in clinical trials in schizophrenia, other cognitive batteries more specific to affective disorders have been developed. One such example is the Brief Assessment of Cognition in Affective Disorders (BAC-A), an extension of the Brief Assessment of Cognition in Schizophrenia.[46] The BAC-A comprises eight subtests that assess the domains of motor speed, attention, verbal memory, working memory, verbal fluency, reasoning and problem solving, emotion inhibition, and affective interference; these last two domains are not included in the BACS as they are thought to be particularly relevant to affective disorders. This battery is potentially well-poised to effectively assay cognitive functioning in MDD and is currently being used in several ongoing clinical trials in this disorder. One potential problem, however, is the omission of tests that assess social cognition. As this domain is known to be altered in MDD, a battery that includes this domain would be ideal.
In order to assess the sensitivity of specific measures to detect cognitive deficits in patients with MDD, Lim et al.[47] recently conducted a meta-analysis of data from 22 studies that compared cognitive function in a total of 955 patients with MDD and 7,664 healthy controls (Table 2). Effect sizes were significant for all tests except the Finger Tapping Test, Trail-Making Test B, delayed verbal memory, and both immediate and delayed visual memory, suggesting that the majority of tests could discriminate between individuals with MDD and healthy controls. However, heterogeneity among studies using the same tests was statistically significant for all tests except the Finger Tapping Test and the Wisconsin Card Sorting Test, and neither age nor medication status could account for the substantial heterogeneity observed.
Table 2.
Meta-analysis of studies comparing cognition in patients with MDD and healthy controls[47]
| Cognitive domains | Tests (no. of studies analyzed) | Heterogeneity and effect size (MDD vs. healthy control) |
|---|---|---|
| Attention | Backward Digit Span Test (n = 10) | χ2 = 19.66; df = 9 (P = .02); I2 = 54% |
| Z = 4.29 (P < .0001) | ||
| Continuous Performance Test (n = 2) | χ2 = 2.94; df = 1 (P = .09); I2 = 66% | |
| Z = 2.49 (P = .01) | ||
| Processing speed | Trail-Making Test A (n = 9) | χ2 = 26.65; df = 8 (P = .0008); I2 = 70% |
| Z = 3.07 (P = .002) | ||
| Digit Symbol Test (n = 9) | χ2 = 39.59; df = 8 (P < .00001); I2 = 80% | |
| Z = 2.75 (P = .006) | ||
| Finger Tapping Task (n = 2) | χ2 = 0.16; df = 1 (P = .69); I2 = 0% | |
| Z = 1.76 (P = .08) | ||
| Executive function | Stroop Test (n = 6) | χ2 = 14.57; df = 5 (P = .01); I2 = 66% |
| Z = 4.96 (P < .00001) | ||
| Trail-Making Test B (n = 10) | χ2 = 885.88; df = 9 (P < .00001); I2 = 99% | |
| Z = 1.23 (P = .22) | ||
| Wisconsin Card Sorting Test (n = 8) | χ2 = 4.86; df = 7 (P = .68); I2 = 0% | |
| Z = 5.04 (P < .00001) | ||
| Verbal Fluency using FAS form (n = 12) | χ2 = 28.36; df = 11 (P = .003); I2 = 61% | |
| Z = 4.56 (P < .00001) | ||
| Memory | Verbal memory (immediate recall, n = 6; delayed recall, n = 5) | Immediate recall |
| χ2 = 26.17; df = 5 (P < .0001); I2 = 81% | ||
| Z = 2.70 (P = .007) | ||
| Rey Auditory Verbal Learning Test (RAVLT); Luria Verbal Learning Test; Wechsler Memory Scale-Revised (WMS-R); California Verbal Learning Test-II (CVLT-II) | Delayed recall χ2 = 53.88; df = 4 (P < .00001); I2 = 93% Z = 1.05 (P = .29) | |
| Visual learning and memory; Wechsler Memory Scale-Revised (WMS-R; n = 4) | Immediate visual memory | |
| χ2 = 17.74; df = 3 (P = .0005); I2 = 83% | ||
| Z = 0.69 (P = .49) | ||
| Delayed visual memory: χ2 = 27.55; df = 3 (P < .00001); I2 = 89% | ||
| Z = 0.39 (P = .70) |
MDD, major depressive disorder.
TREATMENT EFFECTS
At this time, there is no evidence to suggest that a formal assessment of cognitive dysfunction will facilitate diagnosis or inform choice of antidepressant medication in patients with MDD, nor are there any agents approved for the treatment of cognition in MDD. Two ongoing, large-scale studies of treatment optimization in MDD (the international Study to Predict Treatment Outcomes in Depression [iSPOT-D] and Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care [EMBARC]) include baseline neurocognitive functioning as predictors of treatment response in addition to follow-up assessments; to date, however, the evidence linking baseline neurocognitive functioning to treatment response is inconsistent. There is evidence to suggest that antidepressants may improve cognition in patients with MDD, although this improvement is likely to require several months of treatment[18] and the effects of different antidepressant treatments and how they compare is not known. The importance of focusing on this domain in an effort to enhance quality of life and promote a more complete recovery is evidenced by ongoing clinical trials in patients with MDD that have cognitive measures as their primary endpoints. In preliminary studies, both the selective serotonin reuptake inhibitor escitalopram and the serotonin–norepinephrine reuptake inhibitor duloxetine were found to improve baseline scores for episodic and working memory as well as processing speed and executive function.[48, 49] Patients receiving duloxetine showed a significantly larger improvement than those in the escitalopram group; however, practice effects cannot be ruled out, as there was no placebo arm.
Nevertheless, neurocognitive improvement following antidepressant treatment is likely closely related to the improvement in depressive symptoms facilitated by the medication, and no specific procognitive effects of antidepressant agents have been described that are independent of broader symptom improvement. As such, it is not currently possible to disentangle the effects of antidepressant treatment and symptom improvement on neurocognition, leaving open the possibility of pseudospecificity. It is likely that neurocognition improves similarly with amelioration of depressive symptoms after psychosocial treatments, such as cognitive behavioral therapy, although this has not been well-studied. There is preliminary evidence that cognitive-emotional training directly targets problematic biases in MDD and is able to improve them, with concomitant improvements in depressive symptomatology.[50] These intriguing preliminary results suggest that nonpharmacological treatments may be able to target problematic cognitive biases as well as, if not better than, medication. In addition, the neurocognitive benefits of neuromodulatory interventions have not been well-studied. Further work is needed to separate the effects, if possible, of neurocognitive improvement, mood state, treatment type, and treatment response. Moreover, there is evidence that some aspects of neurocognitive impairment remain even in remission;[17] future work is needed to target this lingering dysfunction, particularly as it may increase risk for relapse.
FUTURE DIRECTIONS
Converging data suggest that cognitive impairment is a concern in patients with MDD. Although this area of research is still emerging, it is important to consider these deficits as possible treatment targets, as highlighted in ongoing work in patients with schizophrenia and BD.
Developing a standardized cognitive battery for use in MDD clinical trials is the obvious next step in assessing and treating patients with this disorder. Although this can be informed by prior work, such as the MATRICS initiative, the appropriateness of cognitive outcome measures in a disorder-specific manner should be considered. The evidence in MDD suggests that, for this disorder, tests that assess facial affect processing—in addition to the more traditional domains of psychomotor speed, fluency, verbal learning, and verbal memory—may promise the detection of cognitive changes that co-occur with treatment response. These cognitive tests can not only be informative in treatment trials, but can also be integrated with improved imaging techniques to develop a clearer understanding of the cellular and neurological pathologies underlying cognitive dysfunction in MDD and across diagnoses.
Acknowledgments
Assistance with writing and manuscript preparation was provided by Ann C. Sherwood, Ph.D. (medical writer), of The Medicine Group and funded by the Takeda Pharmaceutical Company, Ltd. and H. Lundbeck A/S.
Conflict of interest
Dr. Katherine E. Burdick has participated on an advisory board for Dainippon Sumitomo Pharma (DP) and has participated in a Continuing Medical Education event funded by Takeda and Lundbeck. Drs. Manuela Russo and Katie Mahon report no conflicts of interest.
REFERENCES
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38(6):771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 3.Bora E, Yucel M, Pantelis C, Berk M. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatr Scand. 2011;123(3):165–174. doi: 10.1111/j.1600-0447.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- 4.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 5.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 2011;134(1–3):20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology. 2012;26(5):642–651. doi: 10.1037/a0029301. [DOI] [PubMed] [Google Scholar]
- 7.Majer M, Ising M, Kunzel H, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. 2004;34(8):1453–1463. doi: 10.1017/s0033291704002697. [DOI] [PubMed] [Google Scholar]
- 8.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89(1–3):125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Reischies FM, Neu P. Comorbidity of mild cognitive disorder and depression—a neuropsychological analysis. Eur Arch Psychiatry Clin Neurosci. 2000;250(4):186–193. doi: 10.1007/s004060070023. [DOI] [PubMed] [Google Scholar]
- 10.Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynes BN, Rush AJ, Trivedi MH, et al. Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med. 2007;5(2):126–134. doi: 10.1370/afm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fava M, Graves LM, Benazzi F, et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry. 2006;67(11):1754–1759. doi: 10.4088/jcp.v67n1113. [DOI] [PubMed] [Google Scholar]
- 13.Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140(2):113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5–10. doi: 10.1089/cap.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. The cumulative load of depressive illness is associated with cognitive function in the remitted state of unipolar depressive disorder. Eur Psychiatry. 2013;28(6):349–355. doi: 10.1016/j.eurpsy.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. 1998;28(5):1027–1038. doi: 10.1017/s0033291798006862. [DOI] [PubMed] [Google Scholar]
- 17.Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 18.Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust N Z J Psychiatry. 2009;43(12):1105–1117. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- 19.Asthana HS, Mandal MK, Khurana H, Haque-Nizamie S. Visuospatial and affect recognition deficit in depression. J Affect Disord. 1998;48(1):57–62. doi: 10.1016/s0165-0327(97)00140-7. [DOI] [PubMed] [Google Scholar]
- 20.Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27(3):320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- 21.Rubinow DR, Post RM. Impaired recognition of affect in facial expression in depressed patients. Biol Psychiatry. 1992;31(9):947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- 22.Alloy LB, Abramson LY, Metalsky GI, Hartlage S. The hopelessness theory of depression: attributional aspects. Br J Clin Psychol. 1988;27(Pt 1):5–21. doi: 10.1111/j.2044-8260.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 23.Ladegaard N, Larsen ER, Videbech P, Lysaker PH. Higher-order social cognition in first-episode major depression. Psychiatry Res. 2014;216(1):37–43. doi: 10.1016/j.psychres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188(3):303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Alloy L, Lipman A, Abramson L. Attributional style as a vulnerability factor for depression: validation by past history of mood disorders. Cogn Ther Res. 1992;16(4):391–407. [Google Scholar]
- 26.Sweeney PD, Anderson K, Bailey S. Attributional style in depression: a meta-analytic review. J Pers Soc Psychol. 1986;50(5):974–991. doi: 10.1037//0022-3514.50.5.974. [DOI] [PubMed] [Google Scholar]
- 27.Ball HA, McGuffin P, Farmer AE. Attributional style and depression. Br J Psychiatry. 2008;192(4):275–278. doi: 10.1192/bjp.bp.107.038711. [DOI] [PubMed] [Google Scholar]
- 28.Uekermann J, Channon S, Lehmkamper C, Abdel-Hamid M, Vollmoeller W, Daum I. Executive function, mentalizing and humor in major depression. J Int Neuropsychol Soc. 2008;14(1):55–62. doi: 10.1017/S1355617708080016. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, LaBar KS, Smoski M, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziobek I, Fleck S, Kalbe E, et al. Introducing MASC: a movie for the assessment of social cognition. J Autism Dev Disord. 2006;36(5):623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- 31.Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 32.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 33.Yatham LN, Torres IJ, Malhi GS, et al. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC) Bipolar Disord. 2010;12(4):351–363. doi: 10.1111/j.1399-5618.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 34.Burdick KE, Goldberg TE, Cornblatt BA, et al. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36(8):1587–1592. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Rush AJ, Bernstein IH, Trivedi MH, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59(6):493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fava M, Losifescu DV, Pedrelli P, Baer L. Reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire. Psychother Psychosom. 2009;78(2):91–97. doi: 10.1159/000201934. [DOI] [PubMed] [Google Scholar]
- 39.Svendsen AM, Kessing LV, Munkholm K, Vinberg M, Miskowiak KW. Is there an association between subjective and objective measures of cognitive function in patients with affective disorders. Nord J Psychiatry. 2012;66(4):248–253. doi: 10.3109/08039488.2011.626870. [DOI] [PubMed] [Google Scholar]
- 40.Burdick KE, Endick CJ, Goldberg JF. Assessing cognitive deficits in bipolar disorder: are self-reports valid? Psychiatry Res. 2005;136(1):43–50. doi: 10.1016/j.psychres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Aran A, Vieta E, Colom F, et al. Do cognitive complaints in euthymic bipolar patients reflect objective cognitive impairment? Psychother Psychosom. 2005;74(5):295–302. doi: 10.1159/000086320. [DOI] [PubMed] [Google Scholar]
- 42.Miskowiak K, Vinberg M, Christensen EM, Kessing LV. Is there a difference in subjective experience of cognitive function in patients with unipolar disorder versus bipolar disorder? Nord J Psychiatry. 2012;66(6):389–395. doi: 10.3109/08039488.2012.658862. [DOI] [PubMed] [Google Scholar]
- 43.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 44.Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1–3):124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Lim J, Oh IK, Han C, et al. Sensitivity of cognitive tests in four cognitive domains in discriminating MDD patients from healthy controls: a meta-analysis. Int Psychogeriatr. 2013;24(9):1543–1557. doi: 10.1017/S1041610213000689. [DOI] [PubMed] [Google Scholar]
- 48.Herrera-Guzman I, Gudayol-Ferre E, Herrera-Guzman D, Guardia-Olmos J, Hinojosa-Calvo E, Herrera-Abarca JE. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J Psychiatr Res. 2009;43(9):855–863. doi: 10.1016/j.jpsychires.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Herrera-Guzman I, Herrera-Abarca JE, Gudayol-Ferre E, et al. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Res. 2010;177(3):323–329. doi: 10.1016/j.psychres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Iacoviello BM, Wu G, Alvarez E, et al. Cognitive-emotional training as an intervention for major depressive disorder. Depress Anxiety. 2014;31(8):699–706. doi: 10.1002/da.22266. [DOI] [PubMed] [Google Scholar]
- 51.Wagner S, Doering B, Helmreich I, Lieb K, Tadic A. A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr Scand. 2012;125(4):281–292. doi: 10.1111/j.1600-0447.2011.01762.x. [DOI] [PubMed] [Google Scholar]


