In the original activation of T lymphocytes, distinct cytokine genes and genes for other functionally relevant proteins are imprinted for reexpression upon secondary activation by antigen [1, 2]. To measure this imprinting independently of a particular antigen, T cells can be stimulated with PMA and ionomycin, mimicking TCR stimulation [3]. PMA directly activates protein kinase C and hence leads to the nuclear translocation of NF-κB via phosphorylation of its antagonist IκB [4–6]. Ionomycin induces increased cytoplasmic Ca2+ concentrations that activate calcineurin [7, 8]. Calcineurin dephosphorylates NFAT and thus enables its translocation to the nucleus [9–11]. NFAT, NF-κB, and AP-1, which are also activated by PMA/ionomycin, induce the transcription of imprinted genes [1, 12, 13]. The nuclear translocation of NFAT occurs in an all-or-none fashion, due to the multiple dephosphorylation events required, a process mediated by the Ca2+-dependent phosphatase calcineurin [11]. Thus, when a population of T cells is restimulated, calcineurin activity determines the frequency of cytokine-producing cells in that population, rather than the amount of cytokine expressed per cell [14, 15].
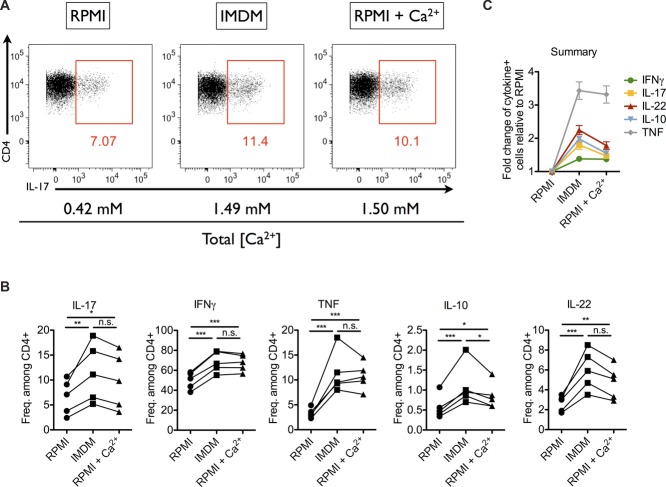
When addressing the functional potential of Th cells in murine T cell transfer-induced colitis [16], we compared the frequencies of cytokine-producing mucosal Th cells, isolated ex vivo and restimulated with PMA and ionomycin in RPMI (Roswell Park Memorial Institute 1640) or in IMDM (Iscove's modified Dulbecco's medium). In IMDM, the frequencies of Th cells expressing IFN-γ, IL-17, IL-10, IL-22, or TNF were consistently higher than in RPMI. The difference in frequencies ranged from 1.4-fold for IFN-γ to more than threefold for TNF (Fig.1 and Supporting Information Fig. 1). This effect was also observed for Th cells from spleen and mesenteric LNs of colitic mice (data not shown).
Figure 1.
Frequencies of cytokine-expressing Th cells upon restimulation with PMA/ionomycin in RPMI, IMDM, and Ca2+-supplemented RPMI. Small intestine lamina propria (SI LP) cells of colitic mice were stimulated with PMA/ionomycin in RPMI, IMDM, or Ca2+-supplemented RPMI. (A) Intracellular staining for IL-17 in SI LP Th cells. (B) Frequencies of IL-17, IFN-γ, TNF, IL-10, and IL-22-expressing cells among SI LP Th cells stimulated in RPMI, IMDM, and Ca2+-supplemented RPMI, as determined by flow cytometry. Lines represent cells from individual mice. (C) Fold change in frequencies of cytokine-expressing cells, stimulated in IMDM or Ca2+-supplemented RPMI compared to RPMI, as determined by flow cytometry. Data are shown as mean ± SEM, n = 5 mice/group and are from one experiment representative of four independent experiments with similar results. (B) *p < 0.05, **p < 0.01, and ***p < 0.001 by one-way ANOVA for repeated measurements and Tukey's post hoc test.
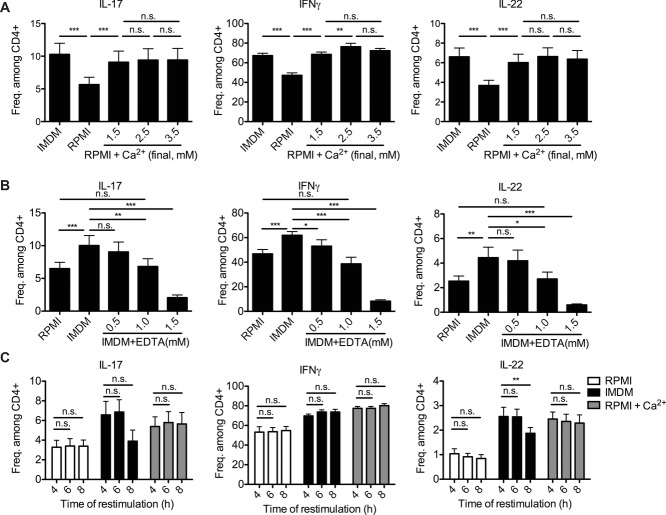
One major difference in the formulations of RPMI versus IMDM of potential relevance for PMA/ionomycin stimulation is the concentration of Ca2+. While RPMI contains 0.42 mM Ca2+, IMDM contains 1.49 mM. Indeed, supplementation of RPMI with Ca2+ to a total concentration of 1.5 mM was sufficient to trigger frequencies of cytokine-producing cells in RPMI comparable to IMDM (Fig.1). Increasing the Ca2+ concentration of RPMI even further, from 1.5 to 2.5 and 3.5 mM, had no significant effect on the frequencies of IL-17- or IL-22-expressing Th cells (Fig.2A). For IFN-γ, we observed a minor increase upon restimulation with 2.5 mM of Ca2+ compared with that at 1.5 mM (Fig.2A, middle). Taken together, a Ca2+ concentration of the medium of at least 1.5 mM reveals the maximal frequencies of cytokine-expressing Th cells upon PMA/ionomycin stimulation.
Figure 2.
Quantitative and kinetic analysis of cytokine expression of Th cells restimulated with PMA/ionomycin in different Ca2+ concentrations. (A, B) SI LP cells of colitic mice were stimulated with PMA/ionomycin in IMDM, RPMI, or (A) Ca2+-supplemented RPMI or (B) IMDM supplemented with EDTA. (A) The frequencies of Th cells expressing IL-17, IFN-γ, and IL-22 were measured by flow cytometry. Data are shown as mean + SEM of 9 mice/group, pooled from two independent experiments. (B) The frequencies of Th cells expressing IL-17, IFN-γ, and IL-22 were measured by flow cytometry. Data are shown as mean + SEM of 9 mice/group, pooled from two independent experiments. (C) LP Th cells of colitic mice were stimulated in RPMI, IMDM, or Ca2+-supplemented RPMI for 4, 6, or 8 h. The frequencies of Th cells expressing IL-17, IFN-γ, and IL-22 were measured by flow cytometry. Data are shown as mean + SEM of 4 mice/group, from one experiment. (A–C) *p < 0.05, **p < 0.01, and ***p < 0.001 by one-way ANOVA for repeated measurements and Tukey's post hoc test.
Conversely, lowering the concentration of available Ca2+ of IMDM by the Ca/Mg-chelating reagent EDTA, resulted in a dose-dependent reduction in the frequencies of cytokine-expressing cells (Fig.2B). Since EDTA binds Ca2+ in an equimolar fashion and with 100× higher affinity than Mg2+ [17], 1 mM of EDTA reduced the concentration of available Ca2+ from 1.49 mM to approximately 0.5 mM, comparable to the Ca2+ concentration of RPMI ([Ca2+] = 0.42 mM). Indeed, addition of 1.0 mM EDTA to IMDM reduced the frequencies of cytokine-producing cells to those obtained in RPMI, as shown for IL-17, IFN-γ, and IL-22 in Figure2B.
Maximizing the PMA/ionomycin stimulation by Ca2+ supplementation of RPMI had minimal impact on the viability of the activated Th cells, nor did it change the kinetics of cytokine expression. The viability of the Th cells 4 h after onset of PMA/ionomycin treatment was 78.6 ± 1.8 in RPMI, 76.7 ± 1.8 in IMDM, and 77.3 ± 2.1 in CaCl2-supplemented RPMI (data not shown). The frequencies of cytokine-expressing Th cells differed between media, but were comparable at 4, 6, and 8 h after onset of each stimulation, as shown for IL-17-, IFN-γ-, and IL-22-expressing Th cells in Figure2C. In particular, the frequencies of cytokine-producing Th cells in RPMI did not increase at 6 or 8 h to frequencies in IMDM or in CaCl2-supplemented RPMI (Fig.2C).
We show here that conventional RPMI, routinely used for the activation of Th lymphocytes with PMA and ionomycin, contains too little Ca2+ for maximal ionomycin stimulation. A significant underestimation of antigen-experienced Th cells imprinted for expression of distinct cytokines is the consequence, as demonstrated here for Th cells isolated ex vivo from inflamed tissue or secondary lymphoid organs of mice with colitis. Increasing the Ca2+ concentration of RPMI from the regular 0.42 to ≥1.5 mM is sufficient to correct this effect and obtain a more accurate estimation of the functional potential of polyclonal Th cell populations.
Methods
Mice
Specific pathogen free C57BL/6J and C57BL/6 Rag1−/− mice were obtained from Charles River (Sulzfeld, Germany). All animal experiments were performed in accordance with institutional, state, and federal guidelines.
T cell transfer colitis
Colitis was induced as described before [16]. Briefly, CD4+ T cells from spleen and LNs of C57BL/6J donors were purified by MACS using mouse CD4 microbeads (L3T4, Miltenyi Biotec). Viable CD4+CD45RBhiCD25− cells were isolated by FACS. A total of 4 × 105 cells were injected i.v. into each of the C57BL/6 Rag1−/− recipients. Mice were sacrificed 2–3 weeks after transfer, when signs of diarrhea and weight loss became apparent.
Isolation of lamina propria (LP) mononuclear cells
LP mononuclear cells were isolated from colon and small intestine (SI) as described before [18]. In brief, fat was removed from colon and SI. They were then opened longitudinally and washed with PBS. The epithelial layer was stripped off by two rounds of incubation in calcium/magnesium-free HBSS with 5 mM EDTA and 10 mM HEPES for 20 min at 37°C with 100 RPM shaking. To obtain a single cell suspension of the LP, colons and SIs were minced into small pieces and incubated three times for 20 min with 0.5 mg/mL Collagenase D (Roche), 0.5 mg/mL DNase I (Sigma), and 0.05 U/mL Dispase (BD) in calcium/magnesium-containing HBSS with 10 mM HEPES at 37°C with 100 RPM shaking. For the SI, LP mononuclear cells were separated from debris by centrifugation over a Percoll gradient (GE Healthcare).
Restimulation and flow cytometry
Ca2+-supplemented RPMI was obtained by adding 1.08 mM of CaCl2 to RPMI ([Ca2+final] = 1.5 mM). IMDM ([Ca2+] = 1.49 mM), RPMI (Life Technologies, [Ca2+] = 0.42 mM),, and Ca2+-supplemented RPMI ([Ca2+] = 1.50 mM) were supplemented with 10% FCS and contained 25 mM HEPES. For intracellular cytokine staining, 1–2 × 106 cells were restimulated with 10 ng/mL PMA (Sigma) and 1 μg/mL ionomycin (Santa Cruz) in the respective media at 5 × 106–1 × 107 cells/mL for a total of 4 h. After 1 h, brefeldin A (BioLegend) was added to a final concentration of 5 μg/mL. Following washing with PBS, cells were stained with a fixable live/dead staining (pacific orange succinimidyl ester, Life Technologies) for 20 min on ice. After 20 min fixation using the BD Cytofix/Cytoperm buffer, cells were washed with and stained in 0.5% w/v Saponin (Sigma) for 20 min on ice. A total of 1–2 × 105 cells were measured with a FACSCanto II (BD).
Antibodies
| Epitope | Color | Clone | Manufacturer |
|---|---|---|---|
| CD3 | Allophycocyanin-eFluor 780 | 145–2C11 | eBioscience |
| CD4 | Pe-Cy7 | RM4–5 | eBioscience |
| IFN-γ | PerCP-Cy5.5 | XMG1.2 | eBioscience |
| IL-17 | FITC | TC11–18H10 | BioLegend |
| IL-10 | Pe | JES5–16e3 | eBioscience |
| IL-22 | Allophycocyanin | IL22JOP | eBioscience |
| TNF | AlexaFluor405 | MP6-XT22 | Custom |
Data presentation and statistics
Individual data points refer to Th cells isolated from individual colitic mice and are presented as mean + SEM unless stated otherwise. Multiple comparisons were tested for significant differences by one-way ANOVA for repeated measurements followed by Tukey's post hoc test with *p < 0.05, **p < 0.01, and ***p < 0.001.
Acknowledgments
We are grateful to P. Saikali for fruitful discussions. We would like to thank C. Winsauer, A. Okhrimenko, M. Bardua, C. Haftmann, K. Lehmann, P. Maschmeyer, R. Riedel, F. Siracusa, M. Weber, and K. Westendorf for the provision of samples and/or experimental support. We are grateful to J. Kirsch and T. Kaiser, operators of the flow cytometry core facility (FCCF). This work was supported by the German Research Council (DFG SFB633, SFB 650 and GRK1121) and the European Research Council advanced grant ERC-2010-AdG_20100317 Grant 268987. J.Z. designed and performed the research, analyzed the data, and wrote the paper. A.R. designed and supervised the research and wrote the paper. H.-D. C. designed the research, analyzed the data, and wrote the paper.
Glossary
- IMDM
Iscove's modified Dulbecco's medium
- LP
lamina propria
- RPMI
Roswell Park Memorial Institute 1640
- SI
small intestine
Conflict of interest
The authors declare no financial or commercial conflict of interest.
The detailed Materials and methods for Technical comments are available online in the Supporting information
References
- 1.Löhning M, et al. Adv Immunol. 2002;80:115–181. doi: 10.1016/s0065-2776(02)80014-1. [DOI] [PubMed] [Google Scholar]
- 2.Rebhahn JA, et al. Eur. J. Immunol. 2014;44:2216–2229. doi: 10.1002/eji.201444645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truneh A, et al. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- 4.Castagna M, et al. J. Biol. Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 5.Sen R, Baltimore D. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Baltimore D. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 7.Lyall RM, et al. Biochem. Soc. Trans. 1980;8:720–721. doi: 10.1042/bst0080720a. [DOI] [PubMed] [Google Scholar]
- 8.Yang SD, et al. Biochem. Biophys. Res. Commun. 1982;106:1419–1425. doi: 10.1016/0006-291x(82)91272-4. [DOI] [PubMed] [Google Scholar]
- 9.Jain J, et al. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 10.Shaw KT, et al. Proc. Natl. Acad. Sci. USA. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamura H, et al. Mol. Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 12.Su B, et al. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 13.Rao A, et al. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 14.Koeck J, et al. J. Biol. Chem. 2014;289:26752–26761. doi: 10.1074/jbc.M114.587865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podtschaske M, et al. PLoS One. 2007;2:e935. doi: 10.1371/journal.pone.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powrie F, et al. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 17.Skoog DA, West DM, Holler FJ. Fundamentals of analytical chemistry. New York: Saunders College Publishing; 1988. p. 418. [Google Scholar]
- 18.Sanos SL, Diefenbach A. Methods Mol. Biol. 2010;612:505–517. doi: 10.1007/978-1-60761-362-6_32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.