Abstract
Plant-plant interactions are driven by environmental conditions, evolutionary relationships (ER) and the functional traits of the plants involved. However, studies addressing the relative importance of these drivers are rare, but crucial to improve our predictions of the effects of plant-plant interactions on plant communities and of how they respond to differing environmental conditions. To analyze the relative importance of –and interrelationships among– these factors as drivers of plant-plant interactions, we analyzed perennial plant co-occurrence at 106 dryland plant communities established across rainfall gradients in nine countries. We used structural equation modeling to disentangle the relationships between environmental conditions (aridity and soil fertility), functional traits extracted from the literature, and ER, and to assess their relative importance as drivers of the 929 pairwise plant-plant co-occurrence levels measured. Functional traits, specifically facilitated plants’ height and nurse growth form, were of primary importance, and modulated the effect of the environment and ER on plant-plant interactions. Environmental conditions and ER were important mainly for those interactions involving woody and graminoid nurses, respectively. The relative importance of different plant-plant interaction drivers (ER, functional traits, and the environment) varied depending on the region considered, illustrating the difficulty of predicting the outcome of plant-plant interactions at broader spatial scales. In our global-scale study on drylands, plant-plant interactions were more strongly related to functional traits of the species involved than to the environmental variables considered. Thus, moving to a trait-based facilitation/competition approach help to predict that: 1) positive plant-plant interactions are more likely to occur for taller facilitated species in drylands, and 2) plant-plant interactions within woody-dominated ecosystems might be more sensitive to changing environmental conditions than those within grasslands. By providing insights on which species are likely to better perform beneath a given neighbour, our results will also help to succeed in restoration practices involving the use of nurse plants.
Keywords: aridity, biotic interactions, competition, facilitation, phylogenetic distance, plant functional traits, semi-arid, soil fertility
Introduction
Plant-plant interactions influence the structure and composition of ecological communities, and therefore may play important roles in determining the distribution of species, secondary succession, ecosystem productivity and stability (reviewed in Brooker et al., 2008; Soliveres and Maestre, in press). Plants compete for resources, but also may improve the microclimate of their neighbours via shading, run-off capture or grazing protection (Callaway, 2007). Thus, both negative (competition) and positive (facilitation) effects of plants on their neighbours often co-occur in nature, with the net outcome of their interaction depending on species-specific and environmental factors (Maestre et al., 2009a; Soliveres et al., 2012a; He et al., 2013).
Although most research has focused on the behaviour of plant-plant interactions across environmental gradients, there is a lack of agreement on how such plant-plant interactions respond to environmental gradients (see He et al., 2013; Soliveres and Maestre, in press for last reviews in the topic). The most influential theory in this regard (the Stress Gradient Hypothesis; Bertness and Callaway, 1994) predicts a monotonic increase of the frequency of positive plant-plant interactions with environmental stress. However, unimodal relationships with a collapse of facilitative interactions under extreme stressful conditions have been also empirically observed and theoretically predicted (Hacker and Gaines, 1997; Tielbörger and Kadmon, 2000; Maestre and Cortina, 2004). A third scenario, moreover, has been recently suggested in which the relationship between plant-plant interactions and environmental conditions can be nil when variability in species’ responses to the environment within the community is high (e.g., Soliveres et al., 2011).
Part of the controversy regarding the relationship between plant-plant interactions and environmental conditions may be solved when considering the specific features of the interacting species and their relationship with the prevailing environmental conditions (Maestre et al., 2009a). Whether environmental conditions are more or less important than species-specific (functional traits or evolutionary relatedness of the interacting species) attributes, or how do these different drivers relate to each other, is poorly known (but see Schöb et al., 2012; Soliveres et al., 2012b; Butterfield and Callaway, 2013). Additionally, the different drivers of plant-plant interactions do not act in isolation, and thus they need to be studied together. Environmental conditions, functional traits and evolutionary relatedness among interacting species are strongly related to each other (Webb et al., 2002). For example, the prevailing environment determines to a certain degree the trait values or the evolutionary lineages present within a community (Weiher and Keddy, 2001; Cornwell and Ackerly, 2009) and thus those that are able to interact with each other (but see Schöb et al., 2012). Evolutionary information, conversely, is often regarded as an indirect measure of important functional traits that could be either unknown to be important or unmeasured under the assumption of evolutionary conservatism of important functional traits (Webb et al., 2002; but see Mayfield and Levine, 2010). Indeed, although current literature suggests that environmental conditions are the main driver of plant-plant interactions (e.g., He et al., 2013), the combination of environmental measurements with phylogenetic information (Soliveres et al., 2012b; Verdú et al., 2012) or functional traits (Liancourt et al., 2005, Schöb et al., 2012; Gross et al., 2013) has proven more useful to better predict the outcomes of pairwise plant-plant interactions. Thus, simultaneously studying the importance of environmental conditions, phylogeny and functional traits as drivers of plant-plant interactions will clarify how such interactions influence species distribution under changing environmental conditions. Furthermore, such a comprehensive approach would shed light on how plant-plant interactions affect the functional and phylogenetic diversity of plant communities. However, such a holistic framework has been rarely applied when studying the outcome of the variety of pairwise interactions existing within local communities, which range from strongly negative to strongly positive.
Here, we use a field study conducted in 106 drylands worldwide involving 929 pairwise plant-plant interactions (measured as their level of co-occurrence) to test the relative importance of species-specific features vs prevailing environmental conditions as drivers of plant-plant interactions. Specifically, we aimed to answer the following questions: i) what is the relative importance of environment, evolutionary relatedness and functional traits as drivers of plant-plant interactions?, ii) how do these drivers relate to, and co-determine the effects of, each other?, and iii) does the relative importance of them vary with the geographical region studied? Apart from being one of the biomes in which facilitative interactions among plants have been more extensively studied (Callaway, 2007; Brooker et al., 2008), drylands occupy a large proportion of the terrestrial surface, and provide ecosystem services that are essential for the maintenance of life on Earth (Reynolds et al., 2007). The use of facilitative interactions among plants has been invoked to restore dryland diversity in degraded environments (Cortina et al., 2011), which can help to mitigate negative impacts of climate change and desertification in these areas (Maestre et al., 2012). Therefore, solving the above-stated questions will further refine our predictions regarding how these interactions will respond to a changing environment, and will enhance the success of restoration practices involving the use of nurse plants.
Materials and methods
Study sites and sampling protocol
Field data for this study were obtained from 106 sites established across rainfall gradients within nine countries (Australia, Chile, Ecuador, Morocco, Peru, Spain, Tunisia, USA and Venezuela; see Table 1). The sites surveyed encompass the major vegetation types found in drylands: grasslands (Peru, Morocco, Spain, Tunisia and Venezuela), shrublands (Chile, Ecuador, Spain, USA) and open woodlands (Australia). Water availability is widely accepted as the main abiotic stressor in drylands and therefore was the predominant factor considered in the environmental gradients studied within each country. The different regional rainfall gradients established covered differences in annual rainfall ranging from 50 to 341 mm among sites within each region, from 4 to 30 points sampled along the different gradients, and from regions supporting grassland communities dominated by a single species to species-rich shrublands with different potential nurse species (Table 1). The fact that the plant-plant interactions studied vary widely across the different environments considered (from very positive to very negative pairwise co-occurrences [Appendix A] and from 0 to 100% facilitated species within the community [Soliveres and Maestre, under review]) clearly show that the range of environmental conditions sampled suffices to test plant-plant interactions-environment relationships.
Table 1.
Details of the different regional gradients considered.
| Country | Regional group | Ecosystem type | Range in aridity index | Number of study sites along the gradient | Number of potential nurse species | Dominant nurse type |
|---|---|---|---|---|---|---|
| Australia | AUSsh | Open woodland | 0.211-0.312 | 9 | 5 | Shrub |
| Australia | AUStr | Open woodland | 0.211-0.337 | 10 | 3 | Tree |
| Chile | Chile | Shrubland | 0.059-0.154 | 4 | 4 | Shrub |
| Ecuador | Ecuador | Shrubland | 0.431-0.739 | 16 | 9 | Shrub and Tree |
| Peru | Peru | Grassland | 0.245-0.458 | 9 | 4 | Grass and Shrub |
| United States | USA | Shrubland | 0.102-0.340 | 8 | 10 | Shrub |
| Venezuela | Venezuela | Grassland | 0.656-0.702 | 6 | 9 | Grass |
| Morocco, Spain, Tunisia | Mediterranean | Grassland | 0.125-0.416 | 30 | 1 | Grass |
| Spain | SPAspo | Grassland | 0.247-0.416 | 15 | 5 | Shrub |
| Spain | SPAnon | Shrubland | 0.241-0.431 | 22 | 4 | Shrub |
For every site, we obtained climatic variables derived from digital models using the Worldclim database (http://www.worldclim.org; Hijmans et al., 2005), from which an aridity index (precipitation/potential evapotranspiration) was derived (data from Maestre et al., 2012). To give a more readily interpretable result, we calculated Aridity as 1- aridity index, which is directly related to drought stress (higher values mean higher drought stress).
All the sites studied were sampled following the same protocol. At each site, we surveyed 30 0.5 m × 0.5 m quadrats under the canopy of the dominant plant species, which were treated as potential nurses, and the same number of quadrats in open areas far from such potential nurses (hereafter Nurse and Open microsites). These potential nurses were mainly trees or shrubs in open woodlands or shrublands, and large tussocks in grasslands (Table 1). The sampling quadrats were distributed beneath at least three (if the potential nurse was a tree), five (for shrubs) or ten (if the potential nurse was a grass) individuals at each site. Open microsites were those located > 2 m away from any adult individuals of the selected nurses, except for trees in which case open microsite were located at a distance equal to the canopy width. Composition or dominant species within these open microsites varied widely due to the range of vegetation and soil types, plant composition and productivity included in the 106 study sites. Average cover (%) and density of individuals per quadrat in the open ranged from 1.1% and 0.3 to 7.3% and 3.4, respectively. At each quadrat, we measured the number of individuals of every perennial plant species to quantify the degree of co-occurrence between each one of them and the potential nurse species. Annual plant composition substantially changes through the year, and among different years, in drylands worldwide (Whitford, 2002). Thus, we did not include annual species in our observational design to avoid confounding effects in our results or in the differences among study sites derived from sampling ‘incomplete’ communities depending on the time of the year that each site was sampled.
Co-occurrence between the potential nurse species and the rest of species forming the community is a widely used metric to assess plant-plant interactions (e.g., Badano and Cavieres, 2006; Schöb et al., 2013). Obviously, observational approaches such as that used here have some limitations, as they cannot differentiate between the outcome of plant-plant interactions per se and other drivers of plant co-occurrence such as dispersal or habitat sharing. However, co-occurrence is often assumed to be a good indicator of – and has proven to be tightly associated to- positive interactions among plants in drylands such as those studied here (Tirado and Pugnaire, 2005; Pueyo et al., 2008). Moreover, we also controlled to a certain degree for those functional traits that may drive co-occurrence among plants due to dispersal (see below), which is other main driver of co-occurrence among plants.
From the co-occurrence data, we assessed interactions at the pairwise level by performing χ2 tests using the observed number of individuals of a given species within each site beneath the potential nurse and in the open inter-spaces as the response variable. Hereafter, we refer to these χ2 tests as the plant-plant interaction metric. The metric is based on comparing the occurrence of the individuals of a given target species in the open vs. beneath the nurse in comparison with a random expectation according to the sampling effort invested for each microsite. Thus, the “expected values” were calculated as the total number of individuals of each target species observed by the relative number of quadrats within each microsite. If, say, 100 quadrats would be sampled in a given site: 50 in the open, 30 in nurse species A and 20 in nurse species B, the relative sampling effort would be 0.5, 0.3 and 0.2 for open, nurse A and nurse B microsites, respectively. Imagine a total of 100 individuals of a given target species observed in this site. Then, the expected values for this target species would be 100 × 0.5 for open, 100 × 0.3 for nurse A and 100 × 0.2 for nurse B. That is, assuming that the individuals are randomly distributed regardless of the microsite and their number only depends on the number of quadrats sampled. This random expectation (the expected values) would be compared against the observed individuals within each microsite by using the χ2 test. To aid in the interpretation of this metric, it was multiplied by −1 or 1 in non-facilitated or facilitated species (those with less or more individuals under a given nurse than in open areas) respectively. The size and the sign of our plant-plant interaction metric were mostly independent of sample size (i.e., the number of individuals recorded for each species only explained 0.8% of the variance in our plant-plant interaction metric; see Appendix A). However, to avoid confounding the effect of plant-plant interactions on either the total number of individuals or on how these individuals were distributed, we included both the number of individuals and the plant-plant interaction metric as response variables in our models (see below).
Assessment of plant functional traits, evolutionary relatedness, and the environmental gradient
We compiled from the literature available data on functional traits known to affect the co-occurrence, establishment, competitive ability and drought tolerance of plants. These were: height, growth form (grass, forb, woody), photosynthetic pathway (C3, C4/CAM) and dispersal mode (dispersed by wind, vertebrates, or other vectors). Plant height is strongly related to the ability of plants to compete for light, plant performance characteristics like growth rate, and demographic features such as longevity, time to reproduction or seed mass (Cornelissen et al., 2003; Moles et al., 2009). Growth form plays an important role in defining the outcome of interactions between individuals and their neighbors (Gómez-Aparicio, 2009; Verdú et al., 2012), and is associated with how they use the available resources, climatic factors and land use (Cornelissen et al., 2003). In our case, we sorted our plant species into three growth forms: grass, woody and forbs. Despite its simplicity, this classification follows the basic functional grouping used in current plant-plant interactions literature (e.g. Cahill et al., 2008; Gómez-Aparicio, 2009; Verdú et al., 2012). The photosynthetic pathway is a key functional attribute of any plant species, with C4 and CAM species associated with higher water-use efficiencies but lower shade tolerances (Ehrleringer et al., 1991). Finally, dispersal mode influences the degree of co-occurrence among plants, with vertebrate-dispersed species more likely to co-occur with other plant species (particularly shrubs or trees) through nucleation processes (Pueyo et al., 2008; Soliveres et al., 2012b).
In the case of the nurse species, we added to the traits described above the ability to fix nitrogen (N) and the shape of the canopy (in contact with the soil surface or not). Although important for all the species, these additional traits are crucial to define the degree of microclimatic amelioration provided by the potential nurse and to know the mechanisms through which it ameliorates environmental conditions for other species to successfully establish underneath. Plants able to fix N are more likely to improve soil fertility, and therefore to facilitate growth and survival of other species, especially in nutrient-poor soils (see Callaway, 2007 for a complete review). Canopy shape, on the other hand, was roughly divided in pyramidal canopy or canopy in full contact with the soil surface (unattached and attached to the soil, respectively). These two different canopy classes differ in their ability to capture water and nutrients coming from run-off (those in full contact with the soil capture more; Maestre et al., 2009b), to reduce incident radiation (e.g., Jankju, 2013) or to protect neighbors from grazing (e.g., Rebollo et al., 2002). Furthermore, differing nurse shapes are also known to influence seed movement and, therefore, co-occurrence among plant species (e.g., Caballero et al., 2008; Giladi et al., 2013). These features are very likely to affect how a given nurse interacts with its neighbors. Major sources of functional traits data were the BROT (Paula et al., 2009) and LEDA (Kleyer et al., 2008) databases, flora descriptions and databases available on internet (e.g. http://data.kew.org/sid/ for seeds; http://www.floravascular.com/ for Spain; http://plantnet.rbgsyd.nsw.gov.au/ for Australia; http://plants.usda.gov/java/ for USA; all accessed between January and July of 2012); and Wright et al., (2004).
Those species for which we could not acquire data on all of these traits were removed from the database. We retained 929 pairwise interactions (45.5% of the 2,040 pairwise interactions sampled), which contained information of all the traits, for further analyses. We acknowledge that other qualitative and quantitative traits, such as the specific leaf area or seed mass for beneficiaries and the leaf area index, the presence of spines or allelopathy for nurses are important drivers of plant-plant interactions (e.g., Callaway, 2007; Schöb et al., 2012, Soliveres et al., 2012a; Butterfield and Callaway, 2013; see Adler et al., 2013 and references therein for more examples where these traits are drivers of species interactions). We could gather information on the specific leaf area or seed mass for a relatively important fraction of our species, and they were included in our analyses whenever possible (“models of maximum R2” in Table 2; Appendix B). The categorical functional traits (all traits excepting plant height) were organized into a set of binomial variables, and summarized by using a non-metric multidimensional scaling ordination (NMDS, Anderson et al., 2008). This ordination technique is well suited to handle non-normal and non-continuous data (McCune and Grace, 2002) and allowed us to reduce our matrix of categorical plant trait data prior to modeling. This accomplishes the synthesis of a smaller number of vectors that correlate with sets of traits that are most informative, and summarize the separation in the trait space of the species considered. We considered the alternative of introducing multiple binomial or multi-level categorical variables to be more difficult to model and unlikely to perform any better. The NMDS ordinations were performed separately for nurse and facilitated species, and for the three different sets of analyses considered in this study (global, regional and growth form, see below). These ordinations can have solutions with either two (2D) or three (3D) dimensions or axes (McCune and Grace, 2002; Anderson et al., 2008). The 2D solution of all the ordinations conducted here showed stress values between 0.05 and 0.1. Stress values below 0.2 provide a good representation of the distance among samples (McCune and Grace, 2002; Anderson et al., 2008), and therefore we selected the 2D solution. We conducted NMDS ordinations with the PRIMER v6 statistical package for Windows (PRIMER-E Ltd., Plymouth Marine Laboratory, UK), using the Euclidean distance for the resemblance matrix and 25 re-starts to calculate stress values.
Table 2.
Summary of the standardized total effects on plant-plant interactions (sum of direct + indirect effects, standardized from 0-1) for the Structural Equation Models performed for the ten regions surveyed. Two different sets of models were performed: 1) those only including aridity and plant height as indicators of environmental conditions and plant traits, respectively (“common structure”), 2) those models including more environmental variables and traits to maximize the amount of variance explained (“model maximum R2”). PhyDis = evolutionary relatedness between interacting plants; Height = facilitated plant height; R2 = amount of variance explained in the number of individuals/plant-plant interaction metric (the latter in bold); Envir = variable(s) indicative of environmental conditions (* = only aridity, † = only fertility, ‡ = aridity + fertility), Traits = facilitated plant height and specific leaf area (height and NMDS axis in the case of Venezuela).
| Regional groups | common structure | model maximum R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Aridity | PhyDis | Height | R2 | Envir | PhyDis | Traits | R2 | |
| Chile | −0.30 | 0.07 | 0.11 | 0.15/0.41 | −0.30* | 0.07 | 0.11 | 0.15/0.41 |
| Ecuador | −0.03 | −0.07 | −0.05 | 0.02/0.11 | −0.03* | −0.07 | −0.05 | 0.02/0.11 |
| Peru | 0.29 | 0.11 | 0.24 | 0.05/0.38 | 0.29* | 0.11 | 0.24 | 0.05/0.38 |
| USA | −0.09 | 0.04 | 0.33 | 0.15/0.37 | −0.09* | 0.04 | 0.33 | 0.15/0.37 |
| Venezuela | 0.20 | 0.21 | 0.07 | 0.21/0.12 | 0.20‡ | 0.22 | 0.11 | 0.22/0.15 |
| Australia (shrubs as nurses) | 0.01 | 0.21 | −0.14 | 0.07/0.08 | −0.03* | 0.20 | 0.23 | 0.16/0.18 |
| Australia (trees as nurses) | −0.22 | −0.19 | 0.32 | 0.00/0.21 | 0.24† | −0.20 | 0.31 | 0.06/0.25 |
| Mediterranean (Spain, Morocco and Tunisia) | −0.05 | −0.19 | 0.31 | 0.03/0.11 | −0.07† | −0.19 | 0.31 | 0.02/0.20 |
| Spain (sprouting shrubs) | 0.04 | 0.07 | 0.10 | 0.02/0.12 | 0.06‡ | 0.07 | 0.27 | 0.01/0.19 |
| Spain (non-sprouting shrubs) | −0.10 | −0.02 | 0.04 | 0.06/0.07 | −0.11‡ | −0.01 | 0.17 | 0.08/0.08 |
We assembled a phylogenetic tree for these species using the Phylocom 4.1 software (Webb et al. 2008), standard phylogenetic protocols and published phylogenies (see details in Appendix C). After assembling the phylogenetic tree, we calculated the phylogenetic distance between each species pair by using the ‘cophenetic’ command of the R statistical software (R Foundation for Statistical Computing, Vienna, AT).
As a surrogate for the environmental conditions, we used the aridity level described above, with higher values of this index indicating more arid conditions. We also included soil variables widely accepted as indicators of soil fertility: organic C (%), available P (mg P · g−1 of soil) and N (the sum of ammonium, nitrate and dissolved organic N, in mg N· Kg−1 of soil; Appendix B, data extracted from Maestre et al., 2012). To reduce the amount of variables introduced in our models, soil variables (standardized by their maximum) were summarized by conducting a principal components analysis (PCA), which first axis explained 44.5% of the variance in the three variables globally (loadings for C, N and P were −0.67, −0.74 and −0.08, respectively). The second axis of this PCA ordination, more related to P, was not used since it did not increase the amount of variability explained in the model and further complicated it. In some of the regional models, and in the grass as nurse model, the addition of the first axis of this PCA (hereafter fertility) introduced multicollinearity with aridity, and therefore it was not included in the analyses of these data.
Data organization and statistical analyses
We used structural equation modeling (SEM) to evaluate the relationships between the environment (aridity and soil fertility), the traits and phylogenetic distance of the interacting species and the plant-plant interaction metric. We used SEM because it can partition causal influences among multiple variables, allowing the separation of direct and indirect effects of the different predictors (Grace, 2006). These unique properties of SEM make this technique particularly suitable to answer the questions posed in this study. In particular, we evaluated: i) the relative importance of the different predictors (plant traits, phylogenetic distance and environmental conditions), ii) the interrelationships among such predictors, and iii) the amount of the variance in the outcome of plant-plant interactions (the χ2 metric) that is directly or indirectly (mediated by a third variable) explained by each predictor. We used three separate sets of SEMs to analyze our data: a global analysis (including data from all the sites), regional analyses (using environmental gradients located in different regions of the world), and growth form analyses (using grass vs. woody species as nurses).
The first step in SEM is to establish an a priori causal model based on previous knowledge. In our case, this model included: i) a direct effect of plant traits, phylogenetic distance and environmental conditions on our proxy of plant-plant interactions, ii) a direct effect of the environment on plant functional traits and phylogenetic relationships between the interacting species caused by environmental filtering (Webb et al., 2002, Schöb et al., 2012), and iii) the influence of phylogeny on plant functional traits by evolutionary conservatism in the selected traits (Prinzing et al. 2001; see full description in Appendix C).
We applied this basic model to the different SEM analyses described in detail below. The second step in SEM is to estimate path coefficients, with their associated P-values, from the field data. We did this estimation with bootstraping because our data were positively kurtotic, and this technique is preferred to maximum likelihood estimation in these cases. The path coefficient is analogous to a partial correlation coefficient, and describes the strength and sign of the relationships among the introduced variables (Grace, 2006). It must be noted that SEM assumes linearity in the relationships between each introduced variable and all the relationships presented here fulfill this assumption. Apart from estimating single path coefficients, SEMs test the overall goodness-of-fit of the model against the dataset; for doing this we used the traditional χ2 test, the RMSEA index and the Bollen-Stine bootstrap test. It must be noted that in these indices high P-values indicate that the proposed model is a plausible causal scenario. Since all the indices rendered very similar results, we only show the χ2 tests here. Another important output of SEM analyses is the standardized total effects, which account for all the (direct and indirect) paths from a given explanatory variable to the response variable. The details of the SEMs conducted are given below. Our models also make use of composite variables (Grace and Bollen, 2008). Composites allow the effects of multiple conceptually related, but not necessarily statistically correlated, variables to be pooled into a single path to more closely match broader theoretical concepts. Error terms are fixed to zero during the construction of the composite variables, so they include all the variance of the response variable explained by the predictors introduced into a single comprehensive variable (the composite).
Global scale analysis
At this scale, we used all of our pairwise interactions (N = 929), introducing latitude and longitude of each site to account for the spatial autocorrelation present in our data (gradients were nested within spatial clusters of sites; see Maestre et al. 2012 for a related approach). At the global scale, we had a large number of categorical plant traits which would have required a complex model to include, likely with minimal information gains. The traits used in our model at the global scale were plant height (log-transformed to improve linearity in the relationships), and the first or second axis of the non-metric multidimensional scaling ordination (NMDS) conducted with our categorical functional traits for facilitated or nurse species, respectively. The remaining two NMDS axes (axis 2 for beneficiaries and axis 1 for nurses) were not considered as they were highly correlated with height, which was already introduced in the model. The first NMDS axis of the ordination conducted with the facilitated species was negatively correlated with grass growth form (Spearman’s ρ = −0.67), wind-dispersion (ρ = −0.70) and C4 photosynthetic pathway (ρ = −0.61; P < 0.001 in all cases, N = 167). The second NMDS axis of the ordination for the nurse species was positively related to dispersal by vertebrates (ρ = 0.70) and to the canopy shape attached to the soil (ρ = 0.40; P < 0.005 in all cases, N = 53). The remaining binomial traits were not well correlated with the selected NMDS axis, but were significantly correlated with nurse plant height (woody growth form ρ = 0.55; C3 photosynthetic pathway ρ = 0.35; P < 0.05 in both cases), thus their inclusion would have added little new information. We did not include the additional NMDS axes of both ordinations because they were correlated with plant height, did not improve the amount of variance explained, and further complicated the model and its interpretation. The same reason prevented us from introducing the environment × traits and environment × phylogenetic distance interactions terms in our models.
After fitting the model, we built four composite variables (Grace, 2006), each of which combine the effects of two conceptually related variables: “Spatial” (latitude + longitude), “Stress” (aridity + fertility), “Nurse” (Nurse height + NMDS axis), and “Benefit” (Facilitated height + NMDS axis)” (shown as hexagons in Fig. 1; see Appendix S3 for details). The use of these composite variables does not alter the underlying model, but collapses the effects of the variables included into a single path coefficient that aids with the interpretation of results. The latent variable, another type of synthetic variable often used to model broader theoretical constructs in SEM, was not used because latent variables seek to develop a linear vector from multiple highly correlated indicators. The ecologically meaningful concepts addressed in our models generally were not represented by highly correlated indicators (e.g., environmental conditions [climate and soils]), and thus composite variables were a better option to include these concepts in out models.
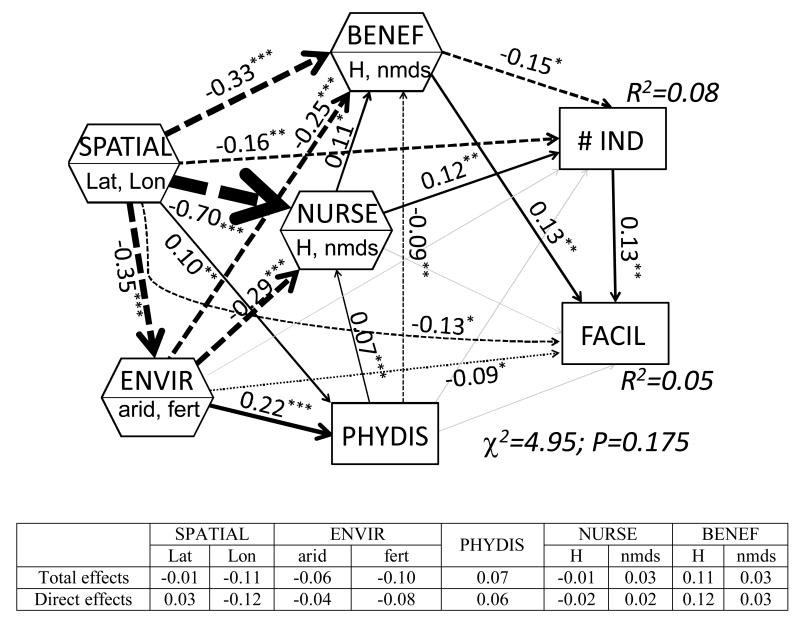
Figure 1.
Global structural equation model, depicting effects of environmental conditions (ENVIR; aridity [arid] and fertility [fert]), phylogeny (PHYDIS), and functional traits of the beneficiaries (BENEF) and the nurse (NURSE) species (see main text for details) upon our plant-plant interaction metric (FACIL) and the number of individuals of the facilitated species (# IND). H = plant height; NMDS = NMDS ordination axis performed with qualitative functional traits. Composite variables are shown with hexagons. Standardized path coefficients are shown. The width of arrows is proportional to the path coefficient, with continuous and dashed lines for positive and negative relationships, respectively. The overall goodness-of-fit test and the R2 for each variable introduced are given. P-values are: *** = P < 0.001; ** = P < 0.01; * = P < 0.05. The table shows the standardized total and direct effects on FACIL of the predictors included.
Regional analyses
In a second step, we reorganized our data into ten different “regional groups” (Table 1; see Appendix C for details). As spatial autocorrelation was no longer a problem in the within-region level, we did not include geographical predictors (latitude and longitude) in this model. To facilitate comparisons among different regions, we fitted a general and simple model structure to all of them (“common structure” in Table 2). In these models, we used aridity as our environmental measurement, phylogenetic distance between the interacting species and the height of facilitated as the only measure of plant traits. Fertility was not included in order to keep models’ structure simple and because in several regions it was highly related with aridity, which might induce multicollinearity problems. We only considered height because the NMDS ordination axis did not significantly increase the amount of variance explained nor improved the model fit in most cases, and data on specific leaf area and seed mass were not available for enough species in half of the regions. To complement these analyses, we fitted each regional model separately by introducing, from the variables available, those that explained most of the variance in our plant-plant interaction metric (“model maximum R2” in Table 2, see full description in Appendix B). Nurse functional trait data were not included into these models for three main reasons: i) nurse plants were so similar within most regions that not enough variability in their functional traits was recovered to add it as a variable in the regional models, and ii) only a simple structure such as that used could be fitted to all regions.
Nurse growth form analyses
The outcome of plant-plant interactions is known to depend on the growth form of the interacting species (Gómez-Aparicio, 2009); thus it is likely that growth form influences the effect of other drivers of plant-plant interactions (e.g., Verdú et al., 2012). While the rest of predictors assessed here are continuous variables (aridity, soil fertility, phylogenetic distance or plant height), plant growth form is not. This may obscure the influence of a widely acknowledge plant-plant interaction driver on the previous SEMs. Therefore, we evaluated changes in the relative importance of the different plant-plant interaction drivers depending on the growth form of the nurse species involved by constructing different SEMs for those pairwise interactions involving woody and grass nurses, respectively. As with the global model, we included latitude to control for spatial autocorrelation in our data (longitude could not be introduced due to multicollinearity problems when both latitude and longitude were included). SEM analyses were performed using AMOS for windows (SPSS Inc., Chicago, IL, USA).
Results
The relative importance of environment, evolutionary relatedness and functional traits as drivers of plant-plant interactions
Overall, facilitated plant traits (represented mainly by height) were more influential than environmental conditions or evolutionary relatedness in determining co-occurrence among plants. When the global data were analyzed, the height of the facilitated species (standardized total effect [STE] = 0.11), the longitude (STE = −0.12) of the study sites and soil fertility (STE = −0.10) were the best predictors of co-occurrence among plant plant species, with aridity (STE = −0.06) and the evolutionary relatedness (STE = 0.07) playing secondary roles as their effect on plant-plant interactions, once accounting for the other predictors, were weaker than those of plant height or longitude. In general, positive interactions were prevalent for taller facilitated species, more fertile soil conditions or eastern regions (Fig. 1).
Separate analyses for the ten major regions sampled (Table 2), and for the major nurse growth forms (grass vs. woody; Fig. 2), provided additional support for the importance that plant functional traits have as drivers of plant-plant interactions. Even after considering the environmental influence on plant functional traits with the standardized total effects, traits of facilitated species were more important for plant-plant interactions than the environment and the evolutionary relatedness in four of the ten regional models analyzed (Table 2, left side), and in the two models conducted for the dominant growth forms (Fig. 2). When we introduced other important functional traits that could drive species interactions in our models, such as specific leaf area or seed mass, the total amount of variance explained by the regional models increased by an additional 10% on average and plant traits were the most important driver in six of the ten models (see “models maximum R2” in Table 2). In the latter case, plant height was the most important trait (i.e., the biggest path coefficient) acting as driver of plant-plant interactions in three of the five regions analyzed (Australia trees, Spain sprouter shrubs, and Venezuela), but SLA was a better indicator of plant co-occurrence in the remaining regions (Australia shrubs and Spain non-sprouter shrubs; see full details in Appendix B).
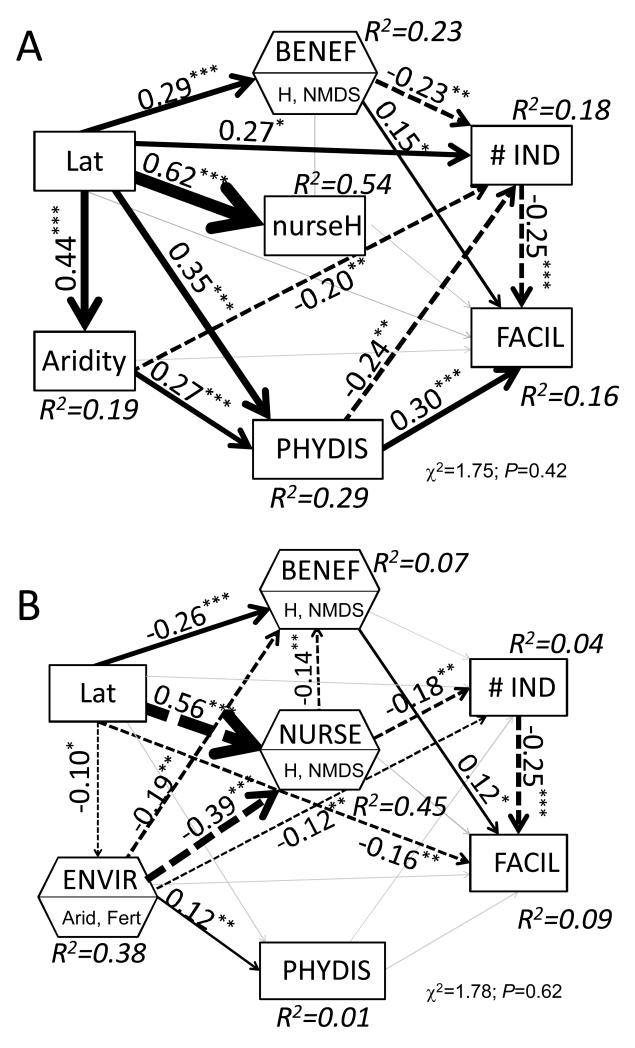
Figure 2.
Global structural equation models for grasses (A) and woody (B) plants as nurses, depicting effects of environmental conditions (aridity and fertility), phylogeny and functional traits upon our plant-plant interaction metric (FACIL) and the number of individuals of the facilitated species (# IND). Rest of legend as in Fig. 1.
Relationship among drivers of plant-plant interactions
As expected, all the different drivers of plant-plant interactions interrelated with each other (Figs. 1 and 2). Those paths from aridity to functional traits or phylogenetic distance, from phylogenetic distance to functional traits and from nurse to facilitated functional traits were significant. The stronger drivers of plant functional trait values were the spatial component (STE = −0.70 and −0.33 for nurse and facilitated traits), followed by aridity (STE = −0.29 and −0.25 for nurse and facilitated traits). The latter was the best predictor (i.e., biggest path coefficient) of pairwise phylogenetic distance (STE = 0.22). We found a relatively weak, although significant relationship between phylogenetic distance and both nurse or facilitated traits. This relationship, however, disappeared in the models separated by nurse growth-form. We found a significant relationship between nurse and facilitated traits (0.11; Fig. 1) which was much stronger for woody than for grass nurses (Fig. 2).
Conversely, the analyses conducted separately for grass and woody nurses revealed that the growth form of nurses significantly altered the importance of other plant-plant interaction drivers studied (Fig. 2). Aridity was more important in those interactions involving woody than grass nurses (STE = −0.16 vs 0.08), while the evolutionary relatedness was a much stronger driver of the effects of grass -but not woody- nurses on their neighbors (STE = 0.01 for woody nurses vs STE = 0.34 for grass nurses). The functional traits of the interacting species, however, were equally important for the interactions involving both grass and woody nurses (STE = 0.11 and 0.12, respectively).
Does the relative importance of the drivers of plant-plant interactions vary with the geographical region studied?
Even after accounting for the three major drivers of plant-plant interactions as acknowledged in current literature, we found a large effect of the geographical predictors (latitude and longitude) in both the global model and in those divided by nurse growth-form. This suggests a strong idiosyncrasy of the relative strengths of the different drivers of plant-plant interactions, as further demonstrated by the regional models (Table 2). We found a strong variability in the main drivers of plant-plant interactions (as measured by plant co-occurrence) depending on the region. For example, aridity turned out to be the major predictor of plant-plant interactions in three of the studied regions (two when considering more functional traits). Evolutionary relatedness was the most important predictor of plant-plant interactions in Venezuela, Ecuador and Australia (shrubs as nurse), and for those pairwise interactions involving grass nurses (Fig. 2). Lastly, plant height was the most important predictor in four of the studied regions, and this number increase to six when considering other important traits available in the literature (Table 2).
Discussion
We quantified the relative importance and interrelationships among environment, functional traits and evolutionary relatedness as drivers of plant-plant interactions in drylands. Our findings illustrate the wide array of mechanisms driving plant-plant interactions, and the complex relationships that exist among their major drivers. Our study also highlights the difficulty of adequately predicting the outcomes of plant-plant interactions using simple models based solely on a subset of these drivers. In spite of this complexity, we showed that readily measurable plant traits, such as plant height or growth form, can be informative predictors of plant co-occurrence in global drylands. Our results suggest that moving forward from an “abiotic/biotic stress point of view”, which has dominated the facilitation literature over the last decades, to a trait-based framework can have far reaching implications in our ability to successfully predict how these interactions, and thus whole plant communities, will respond to environmental factors (see also Butterfield and Callaway, 2013; Gross et al., 2013). The use of a trait-based framework has proven very useful to advance our knowledge in topics such as the biodiversity-function relationship (Polley et al., 2013), the response of plant communities to environmental changes (Frenette-Dussault et al., 2012), and the mechanisms underlying plant coexistence (Adler et al., 2013). Thus, moving towards a trait-based framework when studying plant-plant interactions could also help to better link these often separate, albeit tightly related, research topics (Butterfield and Briggs, 2011, Schöb et al., 2012).
The importance of plant height
The height of beneficiaries was our most informative predictor of the variance in plant species co-occurrence in drylands. In spite of the obvious limitations of not accounting for other important functional traits missing for some of our species, our results demonstrate that target plants tend to benefit more from the presence of a neighbor the taller they are. It must be noticed that the importance of facilitated plants’ height (i.e., the size of this path coefficient) prevailed for some regions even after including other important functional traits such as SLA or seed mass. These findings agree with studies from other desert systems, which identify facilitated plant height as a fundamental trait defining the outcome of plant-plant interactions (Butterfield and Briggs, 2011). The high importance of facilitated plant height as a driver of plant-plant interactions might be related to the fact that taller plants can be in disadvantage in the open (taller plants normally have bigger canopies and higher transpiration rates; Butterfield and Briggs, 2011). Thus, species holding these features might benefit from the ameliorated conditions beneath a nurse. In some cases, an alternative explanation to this high importance of facilitated height might be derived from competition by light, which seems more important than previously thought (Forseth et al., 2001; Soliveres et al., 2010). The higher growth rates that characterize taller plants may allow them to overtop their neighbors, and thus avoid the deleterious effects of shading.
Previous studies have reported an important influence of nurse height, and its interaction with the environment, as a driver of the effects of woody plants - especially trees- on the biomass of understory species (Callaway et al., 1991; Moustakas et al., 2013). However, we failed to detect such influence, since neither nurse height (Fig. 2) nor its interaction with the environment (data not shown) affected the degree of co-occurrence between plant species. A plausible explanation for these contrasting results is the different performance measures used (number of individuals vs. biomass), which are known to affect the results gathered when studying plant-plant interactions (e.g., Maestre et al., 2005). A complementary explanation is that plant-plant interactions are highly species-specific, and thus the influence of nurse height might be obscured by the mixing of different woody nurses and a variety of facilitated species in our analyses.
Interrelationship among the different drivers of plant-plant interactions
The growth form of the nurse plant strongly influenced the outcome of plant-plant interactions, and modulated the effect of both the environment and the evolutionary relatedness on such outcome. Environmental conditions and evolutionary relatedness were important mainly for interactions involving woody and graminoid nurses, respectively. The graminoid growth form is often associated with poor nurses (Gómez-Aparicio, 2009), but we found that negative interactions involving grass nurses might shift to positive ones for evolutionary distant taxa. Indeed, grass nurses can facilitate the establishment of woody seedlings beneath them, and have been recommended for the restoration of degraded drylands (Maestre et al., 2001). We speculate that the strong effect of the evolutionary relatedness on interactions involving grass, but not woody, nurses (Fig. 2) might be caused by two simple mechanisms: i) grasses are monocots and have relatively poor competitive ability against dicots (Cahill et al., 2008), and ii) the traits included in our analyses could adequately include the niche segregation between woody nurses and their neighbors, but did not adequately account for relevant differences among grass nurses and their neighbors, which were better represented by their evolutionary relatedness.
Nurse growth form also interacted with the environment to influence plant-plant interactions. Environmental conditions were more important drivers of interactions involving woody nurses than facilitated plant height or the evolutionary relatedness of the species involved (Fig. 2B). The overwhelming importance of aridity when woody nurses are involved has been illustrated previously, with works showing either an increase in the importance of facilitation in tree (nurse)-grass (facilitated) interactions with rainfall scarcity (Dohn et al., 2013) and shifts towards positive interactions with higher stress when shrubs act as nurses (Gómez-Aparicio et al., 2004; He et al., 2013). The relationship between aridity and the outcome of plant-plant interactions in drylands, however, seems to be obscured when other nurse growth forms are considered in the analyses (Maestre et al., 2005; He et al., 2013; Fig. 1). The latter supports the interrelationships between nurse growth-form and the environment found in this study.
Idiosyncratic changes in the relative importance of plant-plant interaction drivers
The highly idiosyncratic effects of different drivers of plant-plant interactions across different regions (Table 2) or nurse growth forms (Fig. 2) may partly explain the overall low variance covered by our global model (Fig. 1; see also Appendix S1). Our multi-site approach provides us with a potential explanation of these idiosyncratic results. The importance of environmental conditions increased with environmental harshness, while the importance of functional traits was augmented as the number of facilitated species involved increased (Appendix S4). We speculate that, in those environmental gradients where the stress experienced by plants is more multi-dimensional (not driven by a single dominant stress factor but by different uncorrelated ones such as low temperatures, salinity or poor soils), the degree of plant facilitation will be less related to the individual environmental gradient and neither a disadvantage of particular functional trait values nor a clear relationship between the degree of microclimatic amelioration provided by nurses and the environmental gradient will be seen (Soliveres et al., 2011, Soliveres and Maestre, 2014). The relationship between environmental conditions and the outcome of plant-plant interactions is also expected to vanish when the variability across the trait space is higher, and therefore the variability in species-specific responses to the prevailing environment present in the target community is higher; Appendix D; Soliveres et al., 2011).
Mechanisms not considered here could also be invoked to explain the results observed in the global model, that is, the relatively low explanation power of our predictors even after accounting for three of the major drivers of plant-plant interactions simultaneously. These include other well-known drivers of plant-plant interactions such as changes in grazing pressure (Smit et al., 2009; Soliveres et al., 2012a), indirect interactions among multiple species clusters (Levine, 1999; Brooker et al., 2008), the different ontogenetic stages of the individuals measured (Miriti, 2006; Soliveres et al., 2010), more complex functional classifications of the interacting plants including other important functional traits not considered here (Callaway, 2007; Appendix B), and the complex interplay between (un)measured functional traits and the multiple possible prevailing stress factors across the study sites (Butterfield and Callaway, 2013). Furthermore, two methodological issues might also account in part for the idiosyncrasies found: i) we only considered perennial plant species; however, annual species are an important fraction of the species in some of the studied regions (e.g., Chile, Australia or USA) but not in others (e.g., the Mediterranean Basin), which could generate differing results between annual- or perennial dominated communities, and ii) the link between plant co-occurrence (what we measured) and plant-plant interactions may vary with the environmental conditions or habitat-type, which could confound to a certain degree the importance of the different plant-plant interactions drivers studied. All these mechanisms could account up to some degree for the differences in the degree of co-occurrence among plant species and the idiosyncratic results found here.
Conclusion
The classification of species and communities into the functional trait space has proven to be a useful improvement in our understanding of important ecological questions, including the assemblage of ecological communities and how they scale to the ecosystem level, the response of ecosystems to global change, or the relationship between biodiversity and ecosystem functioning (Frenette-Dussault et al., 2012; Schöb et al., 2012; Gross et al., 2013; Polley et al., 2013). We provide strong empirical support for a plant-plant interaction theory based on functional traits and their relationships with other drivers of such interactions and conclude that functional traits of the interacting plants are more important than –and modulate the effects of– the environment or the evolutionary relatedness in defining pairwise interactions. Two significant testable hypotheses derive from this trait-based theory: i) plant-plant interactions at the community level may behave differently across environmental gradients depending on the nature of the gradient (i.e., the gradient is driven by one or several stress factors), and ii) the relationship between environmental gradients and plant-plant interactions at the community level may wane when a variety of functional groups are present within this community. Although we provide some results supporting these two hypotheses (i.e., Appendix D) future research should further evaluate them. Furthermore, and although we covered an important proportion and variety of dryland ecosystems, future studies focused on other dryland areas poorly studied here and including annual plant communities will undoubtedly improve our knowledge on the generality of the patterns found in our study. In addition, our findings provide insights on which species are likely to benefit from the presence of a given neighbour, which can be used for designing effective restoration strategies based on afforestation using nurse plants, and for devising methods to increase plant species diversity in order to mitigate the negative impacts of climate change and desertification in drylands.
Supplementary Material
Acknowledgements
Christoph Kueffer, Florian Jeltsch and two anonymous reviewers provided numerous comments that improved a previous version of this manuscript. This research was funded by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM). The Ciencia y Tecnología para el Desarrollo (CYTED) program funded networking activities (EPES, Acción 407AC0323). The authors deeply thank to those people who measured and allowed access to the functional trait data used in this study, especially to the creators and administrators of the LEDA and BROT databases, and to I. Wright, T. Navarro and C. Frenette-Dussault for kindly providing published and unpublished data. C. Smit provided helpful comments on a previous version of the manuscript. JRG thanks ICM PO-5, CONICYT BFP-23, and FONDECYT 1110255 for financial support.
Footnotes
Appendix A. Global model including species with >15 individuals
Appendix B. Alternative regional models
Appendix C. Methodological details
Appendix D. Relationships including standardized total effects of the regional models
References
- Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJB. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013;16:1294–1306. doi: 10.1111/ele.12157. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to software and statistical methods. PRIMER-E; Plymouth, UK: 2008. [Google Scholar]
- Badano EI, Cavieres LA. Ecosystem engineering across ecosystems: do engineer species sharing common features have generalized or idiosyncratic effects on species diversity? J. Biogeogr. 2006;33:304–313. [Google Scholar]
- Bertness MD, Callaway RM. Positive interactions in plant communities. Trends Ecol. Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres L, Kunstler G, Liancourt P, Tielbörger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R. Facilitation in plant communities: the past, the present and the future. J. Ecol. 2008;96:18–34. [Google Scholar]
- Butterfield BJ, Briggs JM. Regeneration niche differentiates functional strategies of desert woody plant species. Oecologia. 2011;165:477–487. doi: 10.1007/s00442-010-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield BJ, Callaway RM. A functional comparative approach to facilitation and its context dependence. Funct. Ecol. 2013;27:907–917. [Google Scholar]
- Caballero I, Olano JM, Loidi J, Escudero A. A model for small-scale seed bank and standing vegetation connection along time. Oikos. 2008;117:1788–1795. [Google Scholar]
- Cahill JF, Kembel SW, Lamb EG, Keddy PA. Does the phylogenetic relatedness influence the strength of competition among vascular plants? Persp. Plant Ecol., Evol. Syst. 2008;10:41–50. [Google Scholar]
- Callaway RM. Positive Interactions and Interdependence in Plant Communities. Springer; New York: 2007. [Google Scholar]
- Callaway RM, Nadkarni NM, Mahall BE. Facilitation and interference of Quercus douglasii on understory productivity in central California. Ecology. 1991;72:1484–1499. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H. Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Austr. J. Bot. 2003;51:335–38014. [Google Scholar]
- Cornwell WK, Ackerly DD. Community assembly and shifts in the distribution of trait values across an environmental gradient in coastal California. Ecol. Monogr. 2009;79:109–126. [Google Scholar]
- Cortina J, Amat B, Castillo V, Fuentes D, Maestre FT, Padilla FM, Rojo L. The restoration of vegetation cover in the Iberian southeast. J. Arid Env. 2011;75:1377–1384. [Google Scholar]
- Dohn J, Dembélé F, Karembé M, Moustakas A, Amévor KA, Hanan NP. Tree effects on grass growth in savannas: competition, facilitation and the stress-gradient hypothesis. J. Ecol. 2013;101:202–209. [Google Scholar]
- Ehrleringer JR, Sage RF, Flanagan LB, Pearcy RW. Climate change and the evolution of C4 photosynthesis. Trends Ecol. Evol. 1991;6:95–99. doi: 10.1016/0169-5347(91)90183-X. [DOI] [PubMed] [Google Scholar]
- Forseth IN, Wait DA, Casper BB. Shading by shrubs in a desert system reduces the physiological and demographic performance of an associated herbaceous perennial. J. Ecol. 2001;89:670–680. [Google Scholar]
- Frenette-Dussault C, Shipley B, Meziane D, Hingrat Y. Trait-based climate change predictions of plant community structure in arid steppes. J. Ecol. 2013;101:484–492. [Google Scholar]
- Giladi I, Segoli M, Ungar ED. Shrubs and herbaceous seed flow in a semi-arid landscape: dual functioning of shrubs as trap and barrier. J. Ecol. 2013;101:97–106. [Google Scholar]
- Gómez-Aparicio L. The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J. Ecol. 2009;97:1202–1214. [Google Scholar]
- Gómez-Aparicio L, Zamora R, Gómez JM, Hódar JA, Castro J, Baraza E. Applying plant facilitation to forest restoration in Mediterranean ecosystems: a meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 2004;14:1128–1138. [Google Scholar]
- Grace JB. Structural Equation Modeling and Natural Systems. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Gross N, Liancourt P, Choler P, Suding KN, Lavorel S. Strain and vegetation effects on limiting resources explain the outcomes of biotic interactions. Persp. Plant Ecol., Evol. Syst. 2010;12:9–19. [Google Scholar]
- Gross N, Börger L, Soriano-Morales SI, Le Bagousse-Pinguet Y, Quero JL, García-Gómez M, Valencia-Gómez E, Maestre FT. Uncovering multi-scale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. J. Ecol. 2013;101:637–649. [Google Scholar]
- Hacker SD, Gaines SD. Some implications of direct positive interactions for community species diversity. Ecology. 1997;78:1990–2003. [Google Scholar]
- He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecol. let. 2013;16:695–706. doi: 10.1111/ele.12080. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Interntl J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Holmgren M, Scheffer M, Huston MA. The interplay of facilitation and competition in plant communities. Ecology. 1997;78:1966–1975. [Google Scholar]
- Jankju M. Role of nurse shrubs in restoration of an arid rangeland: Effects of microclimate on grass establishment. J. Arid Env. 2013;89:103–109. [Google Scholar]
- Kleyer M, Bekker RM, Knevel IC, Bakker J.P, Thompson, K., Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, Klotz S, Rusch GM, Hermy M, Adriaens D, Boedeltje G, Bossuyt B, Dannemann A, Endels P, Götzenberger L, Hodgson JG, Jackel A-K, Kühn I, Kunzmann D, Ozinga WA, Römermann C, Stadler M, Schlegelmilch J, Steendam HJ, Tackenberg O, Wilmann B, Cornelissen JHC, Eriksson O, Garnier E, Peco B. The LEDA Traitbase: A database of life-history traits of Northwest European flora. J. Ecol. 2008;96:1266–1274. [Google Scholar]
- Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. [Google Scholar]
- Maestre FT, Bautista S, Cortina J, Bellot J. Potential of using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecol. Appl. 2001;11:1641–1655. [Google Scholar]
- Maestre FT, Valladares F, Reynolds JF. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol. 2005;93:748–757. [Google Scholar]
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009a;97:199–205. [Google Scholar]
- Maestre FT, Puche MD, Bowker MA, Hinojosa MB, Martínez I, García-Palacios P, Castillo AP, Soliveres S, Luzuriaga AL, Sánchez AM, Carreira JA, Gallardo A, Escudero A. Shrub encroachment can reverse desertification in Mediterranean semiarid grasslands. Ecol. Lett. 2009b;12:930–941. doi: 10.1111/j.1461-0248.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, García-Gómez M, Bowker MA, Soliveres S, Escolar C, García-Palacios P, Berdugo M, Valencia E, Gozalo B, Gallardo A, Aguilera L, Arredondo T, Blones J, Boeken B, Bran D, Conceiçaõ AA, Cabrera O, Chaieb M, Derak M, Eldridge DJ, Espinosa CI, Florentino A, Gaitán J, Gatica MG, Ghiloufi W, Gómez-González S, Gutiérrez JR, Hernández RM, Huang X, Huber-Sannwald E, Jankju M, Miriti M, Monerris J, Mau RL, Morici E, Naseri K, Ospina A, Polo V, Prina A, Pucheta E, Ramírez-Collantes DA, Romão R, Tighe M, Torres-Díaz C, Val J, Veiga JP, Wang D, Zaady E. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc. R. Soc. B. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield MM, Levine JM. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010;13:1085–1093. doi: 10.1111/j.1461-0248.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- McCune B, Grace JB. Analysis of Ecological Communities. MjM Software; Gleneden Beach, Oregon, USA: 2002. www.pcord.com. [Google Scholar]
- McIntire EJB, Fajardo A. Facilitation as a ubiquitous driver of biodiversity. New Phytol. 2013 doi: 10.1111/nph.12478. In press. doi: 10.1111/nph.12478. [DOI] [PubMed] [Google Scholar]
- Miriti M. Ontogenetic shift from facilitation to competition in a desert shrub. J. Ecol. 2006;94:973–979. [Google Scholar]
- Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, et al. Global patterns in plant height. J. Ecol. 2009;97:923–932. [Google Scholar]
- Moustakas A, Kunin WE, Cameron TC, Sankaran M. Facilitation or competition? Tree effects on grass biomass across a precipitation gradient. PLoS One. 2013;8:e57025. doi: 10.1371/journal.pone.0057025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Arianoutsou M, Kazanis D, Tavsanoglu Ç, Lloret F, Buhk C, Ojeda F, Luna B, Moreno JM, Rodrigo A, Espelta JM, Palacio S, Fernández-Santos B, Fernandes PM, Pausas JG. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:1420. [Google Scholar]
- Polley HW, Isbell FI, Wilsey BJ. Plant functional traits improve diversity-based predictions of temporal stability of grassland productivity. Oikos. 2013;122:1275–1282. [Google Scholar]
- Prinzing A, Durka W, Klotz S, Brandl R. The niche of higher plants: evidence of phylogenetic conservatism. Proc. Royal Soc. London B. 2001;268:2383–2389. doi: 10.1098/rspb.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo Y, Kéfi S, Alados CL, Rietkerk M. Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos. 2008;117:1522–1532. [Google Scholar]
- Rebollo S, Milchunas DG, Noy-Meir I, Chapman PL. The role of a spiny plant refuge in structuring grazed shortgrass steppe plant communities. Oikos. 2002;98:53–64. [Google Scholar]
- Reynolds JF, Stafford Smith DM, Lambin EF, Turner II BL, Mortimore M, Batterbury SPJ, Downing TE, Dowlatabadi H, Fernández RJ, Herrick JE, Huber-Sannvald E, Leemans R, Lynam T, Maestre FT, Ayarza M, Walker B. Global desertification: Building a science for dryland development. Science. 2007;316:847–851. doi: 10.1126/science.1131634. [DOI] [PubMed] [Google Scholar]
- Schöb C, Armas C, Guler M, Prieto I, Pugnaire FI. Variability in functional traits mediates plant interactions along stress gradients. J. Ecol. 2013;101:753–762. [Google Scholar]
- Schöb C, Butterfield BJ, Pugnaire FI. Foundation species influence trait-based community assembly. New Phytol. 2012;196:824–834. doi: 10.1111/j.1469-8137.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- Smit C, Rietkerk M, Wassen MJ. Inclusion of biotic stress (consumer pressure) alters predictions from the stress gradient hypothesis. J. Ecol. 2009;97:1215–1219. [Google Scholar]
- Soliveres S, DeSoto L, Maestre FT, Olano JM. Spatio-temporal heterogeneity in abiotic factors modulate multiple ontogenetic shifts between competition and facilitation. Persp. Plant Ecol. Evol. Syst. 2010;12:227–234. [Google Scholar]
- Soliveres S, Eldridge DJ, Maestre FT, Bowker MA, Tighe M, Escudero A. Microhabitat amelioration and reduced competition among understorey plants as drivers of facilitation across environmental gradients: towards a unifying framework. Persp. Plant Ecol., Evol. Syst. 2011;13:247–258. doi: 10.1016/j.ppees.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Eldridge DJ, Hemmings F, Maestre FT. Grazing, rainfall and species specificity interact to determine effects of woody nurse plants on plant richness in drylands. Persp. Plant Ecol., Evol. Syst. 2012a;14:402–410. doi: 10.1016/j.ppees.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Maestre FT. Plant-plant interactions, environmental gradients and plant diversity: a global synthesis of community-level studies. Persp. Plant Ecol., Evol. Syst. 2014 doi: 10.1016/j.ppees.2014.04.001. in press: http://dx.doi.org/10.1016/j.ppees.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Torices R, Maestre FT. Evolutionary relatedness can be more important than abiotic conditions in predicting the outcome of plant-plant interactions. Oikos. 2012b;121:1638–1648. doi: 10.1111/j.1600-0706.2011.20309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Tirado R, Pugnaire FI. Community structure and positive interactions in constraining environments. Oikos. 2005;111:437–444. [Google Scholar]
- Verdú M, Gómez-Aparicio L, Valiente-Banuet A. Phylogenetic relatedness as a tool in restoration ecology: A meta-analysis. Proc. R. Soc. London B. 2012;279:1761–1767. doi: 10.1098/rspb.2011.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, Ackerly DD, McPeek M, Donoghue MJ. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 2002;33:475–505. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Weiher E, Keddy P. Ecological Assembly Rules: Perspectives, Advances, Retreats. Cambridge University Press; [Google Scholar]
- Whitford WG. Ecology of desert systems. Academic Press; California, USA: 2002. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont B.B, Lee, T., Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.