Abstract
Once seen as anomalous, facilitative interactions among plants and their importance for community structure and functioning are now widely recognized. The growing body of modelling, descriptive and experimental studies on facilitation covers a wide variety of terrestrial and aquatic systems throughout the globe. However, the lack of a general body of theory linking facilitation among different types of organisms and biomes and their responses to environmental changes prevents further advances in our knowledge regarding the evolutionary and ecological implications of facilitation in plant communities. Moreover, insights gathered from alternative lines of inquiry may substantially improve our understanding of facilitation, but these have been largely neglected thus far.
Despite over 15 years of research and debate on this topic, there is no consensus on the degree to which plant–plant interactions change predictably along environmental gradients (i.e. the stress-gradient hypothesis), and this hinders our ability to predict how plant–plant interactions may affect the response of plant communities to ongoing global environmental change. The existing controversies regarding the response of plant–plant interactions across environmental gradients can be reconciled when clearly considering and determining the species-specificity of the response, the functional or individual stress type, and the scale of interest (pairwise interactions or community-level response). Here, we introduce a theoretical framework to do this, supported by multiple lines of empirical evidence. We also discuss current gaps in our knowledge regarding how plant–plant interactions change along environmental gradients. These include the existence of thresholds in the amount of species-specific stress that a benefactor can alleviate, the linearity or non-linearity of the response of pairwise interactions across distance from the ecological optimum of the beneficiary, and the need to explore further how frequent interactions among multiple species are and how they change across different environments. We review the latest advances in these topics and provide new approaches to fill current gaps in our knowledge.
We also apply our theoretical framework to advance our knowledge on the evolutionary aspects of plant facilitation, and the relative importance of facilitation, in comparison with other ecological processes, for maintaining ecosystem structure, functioning and dynamics. We build links between these topics and related fields, such as ecological restoration, woody encroachment, invasion ecology, ecological modelling and biodiversity–ecosystem-functioning relationships. By identifying commonalities and insights from alternative lines of research, we further advance our understanding of facilitation and provide testable hypotheses regarding the role of (positive) biotic interactions in the maintenance of biodiversity and the response of ecological communities to ongoing environmental changes.
Keywords: facilitation, competition, species interactions, plant-plant interactions, stress gradient hypothesis, facilitation theory
I. INTRODUCTION
Once largely neglected, there is nowadays a broad consensus among ecologists that facilitation is a decisive mechanism for maintaining the diversity and structure of a wide range of plant communities (Callaway, 2007). Several attempts have been made to include facilitation into general ecological theory (e.g. Bruno, Stachowicz & Bertness, 2003), of which the stress-gradient hypothesis (SGH) proposed by Bertness & Callaway (1994) has been the most influential. Based on the notion that biotic interactions are dependent on environmental conditions (Gause, 1934) and the apparent prevalence of empirical studies reporting facilitative interactions under harsh environments (deserts, salt marshes and intertidal habitats), the SGH predicts that the frequency of facilitative and competitive interactions varies inversely across stress gradients. Thus, facilitation is expected to be more common in stressful conditions than in more mesic ones (Bertness & Callaway, 1994). While the SGH has significantly increased the awareness of positive plant–plant interactions worldwide, it has also been actively debated during the last decade (Lortie & Callaway, 2006; Maestre, Valladares & Reynolds, 2005, 2006; Malkinson & Tielbörger, 2010), and controversies regarding how plant–plant interactions respond to changes in environmental conditions remain largely unresolved (Holmgren & Scheffer, 2010; Soliveres et al., 2011; He, Bertness & Altieri, 2013). In spite of this controversy, assumptions of the SGH are commonly applied to current modelling approaches (Lin et al., 2012) or to studies linking facilitation with other lines of research (Bruno et al., 2003; Santoro et al., 2012). It is time to resolve these issues, particularly if we aim to refine our predictions of how plant communities respond to disturbances or environmental pressures such as those forecasted for future scenarios under ongoing global environmental change (Brooker, 2006). Moreover, the SGH is beginning to be extended to life forms other than vascular plants such as soil lichens, invertebrates or mammals (Barrio et al., 2012; Bowker, Soliveres & Maestre, 2010; Fugère et al., 2012), and a solid theory would help this research to advance further and faster.
Broadening and improving facilitation theory involves not only focusing on the behaviour of pairwise interactions along environmental gradients, but also considering the drivers and consequences of such interactions for plant communities and ecosystems. Thus, we need to understand how interactions among multiple species behave and how frequent they are in natural ecosystems, and also know their relative importance as a driver of community attributes (diversity, structure, species dynamics) and related ecosystem functions (e.g. productivity, stability, nutrient cycling). Such a shift in the focus of facilitation research from pairwise interactions to the community and ecosystem levels would also stimulate the further development of novel fields, of which the evolutionary consequences of facilitative interactions is among the most promising.
The aim of this review is to provide a unifying framework to solve current controversies regarding the behaviour of plant–plant interactions across environmental gradients. We use this framework to advance our understanding in other poorly studied topics such as: (i) how plant–plant interactions affect structure and function of plant communities and their response to ongoing global environmental change, (ii) how facilitation affects the evolution of interacting plants, and (iii) how facilitation research can be linked to other active lines of inquiry, such as plant invasions, woody encroachment, or the biodiversity–ecosystem-functioning relationship. We finally identify research gaps and propose future directions and experimental approaches that contribute to the further advancement of the theory and applications of positive plant interactions.
II. PLANT–PLANT INTERACTIONS AND THE ENVIRONMENT: CLARIFYING CONCEPTS AND RECONCILING APPARENTLY OPPOSING VIEWS
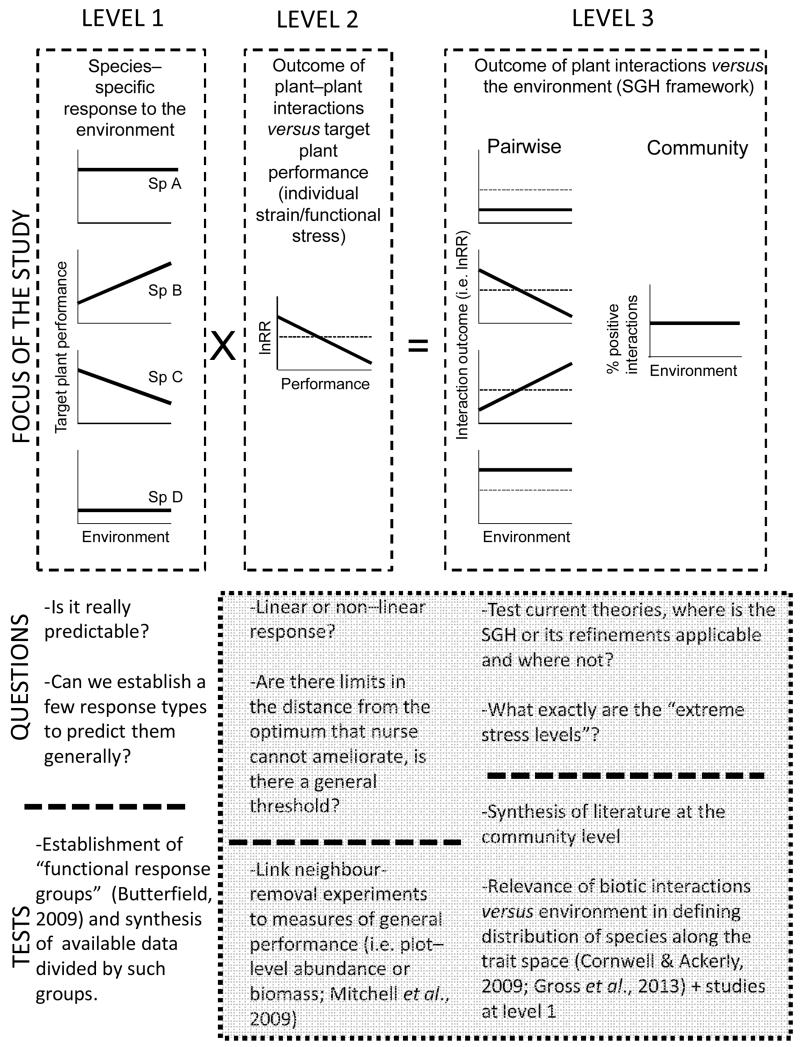
The exceptions to the original predictions of the SGH found in several empirical studies (Butterfield et al., 2010; Kitzberger, Steinaker & Veblen, 2000; López et al., 2013; Maestre & Cortina, 2004; Soliveres et al., 2011; Tielbörger & Kadmon, 2000) have fuelled a long-standing and active debate among ecologists about the nature of the response of plant–plant interactions to environmental changes. While some studies defend monotonic increases of the frequency or importance of positive interactions towards higher stress levels (He et al., 2013; Lortie & Callaway, 2006), others suggest that there is either a lack of a general response to changes in stress (Maestre et al., 2005) or that the relationship between facilitation and abiotic stress can take multiple shapes, depending on the nature and co-occurrence of the stress factors involved, or the non-linearity of the response of plant species to the environment (Holmgren & Scheffer, 2010; Maestre et al., 2009b; Malkinson & Tielbörger, 2010; Smit, Rietkerk & Wassen, 2009; Smit et al., 2007). We believe that these apparently opposing views can be largely reconciled by clarifying the scale of focus in each study (Fig. 1). First, the original predictions made by Bertness & Callaway (1994) focused on a community-level response, but these predictions have been in most cases only tested with one or a few pairwise interactions. The second important issue is how we define stress, and thus the harshness of a given environment, and whether this concept is solely applicable to particular species (as we believe) or to whole communities whose species differ widely in their ecological adaptations. These two points have crucial implications on how we understand the response of plant–plant interactions to changes in the environment, and are discussed below.
Fig. 1.
Different scales of interest typically found in the plant–plant interactions literature, relevant questions regarding their relationship with the environment and suitable tests to answer them. Species-specific (four species, Sp A–D) changes in performance across the same environmental gradient (left box; Level 1) affect the outcome of plant–plant interactions across such gradients (Level 3, pairwise). This affects the frequency of positive interactions found at the community level (Level 3, community). Plant–plant interactions become more positive with decreasing target plant performance in the open; however, for species A and D no changes in their performance are detected through the gradient, and therefore no changes in the outcome of the interactions between these latter species and their neighbours are expected. Discontinuous lines in the panels separate positive (above) and negative (below) plant–plant interaction net outcomes (represented by the lnRR [log Response Ratio] index). The grey quadrat surrounds high-priority areas for future research. SGH, stress-gradient hypothesis.
(1) On the importance of multiple plant–plant interaction drivers: insights from pairwise-level approaches
The core of previous research dealing with the response of plant–plant interactions to changes in the environment has focused on studying one or a few pairwise interactions (see Brooker et al., 2008; He et al., 2013 for reviews), and these studies have fed the existing controversy regarding the response of plant–plant interactions to environmental changes. Ecologists generally agree on the existence of multiple factors driving the outcome of pairwise plant–plant interactions. For example, the performance measure used (Goldberg et al., 1999; Maestre et al., 2005), the nature of the stress factor involved (resource or non-resource; Maestre et al., 2009b), the ontogenetic stage of the interacting species (Smit & Ruifrok, 2011; Soliveres et al., 2010; Sthultz, Gehring & Whitham, 2007), the ecological requirements of the interacting plants (Chu et al., 2008; Liancourt, Callaway & Michalet, 2005) or the evolutionary relationships of the species involved (Soliveres, Torices & Maestre, 2012c; Suzuki & Suzuki, 2012), are known to interact with the environment to define such an outcome. Added to this complexity is the fact that multiple environmental stressors, both biotic and abiotic, often co-occur in nature, and jointly shape the response of pairwise plant–plant interactions across environmental gradients (Baumeister & Callaway, 2006; Kawai & Tokeshi, 2007; le Roux & McGeoch, 2010; Smit et al., 2009; Soliveres et al., 2012a). What is the relative importance of these different (a)biotic drivers of pairwise plant–plant interactions? This is difficult to answer since most studies focus on just one or two of these multiple drivers at a time.
Recent evidence suggests that the environment is more important than the species-specific features of the species involved (He et al., 2013). It is known that the environmental conditions, both abiotic and biotic, “filter” those ecological adaptations (either functional traits or evolutionary lineages) that will thrive and those that will not (Webb et al., 2002), and thus the species-specific adaptations that will ultimately be present to interact with their neighbours. The environment can also limit the recruitment, performance or density of potential nurse plants, leading to the waning of positive interactions (Michalet et al., 2006). Moreover, variations in environmental conditions may modulate the groups of functional traits or ecological requirements that are facilitated and those that are not (Butterfield et al., 2013).
We argue, however, that species-specific factors primarily affect the outcome of pairwise interactions, with a secondary role for environmental conditions. As illustrated by Whittaker (1956) in his classic paper on changes in vegetation along aridity gradients in the Great Smoky Mountains, species forming a given community overlap to some degree in their specific requirements, but differ in their ecological optimum [see figs 2 and 3 in Whittaker (1956); discussed in detail in Chase & Leibold (2003)]. Thus, it is reasonable to think that the different pairwise interactions present in a given environment or community will differ in their outcomes depending on features of the interacting species such as ontogenetic stage, functional traits or evolutionary relationships (e.g. Greiner La Peyre et al., 2001; Miriti, 2006; Soliveres et al., 2012c; Suzuki & Suzuki, 2012). Indeed, in most studies including multiple pairwise interactions, species-specific factors of both nurse and beneficiary species shape the response of plant–plant interactions within the same environmental conditions and across environmental gradients (Chu et al., 2008; Liancourt et al., 2005; Soliveres et al., 2012c). Other evidence supports the notion that species-specific features may be more important than the environment in defining the outcome of pairwise plant–plant interactions. For example, environmental filters are relaxed due to the microclimatic amelioration provided by nurse plants (Bruno et al., 2003; Lortie et al., 2004), and this microclimatic amelioration seems to depend on the specific features of each nurse, rather than on the prevailing environmental conditions (e.g. Gómez-Aparicio, 2009).
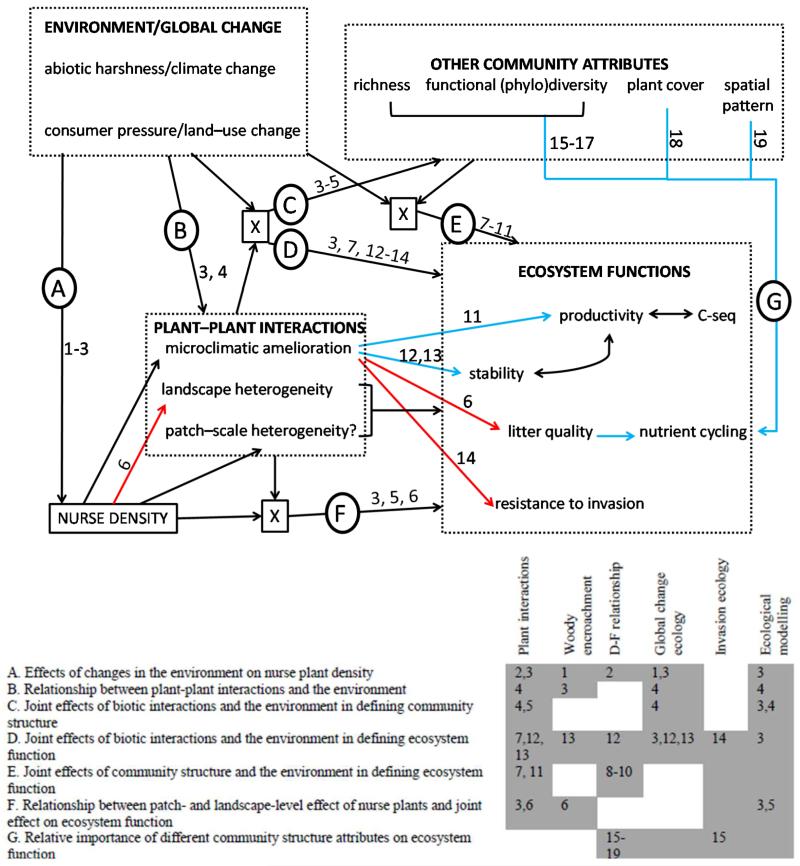
Fig. 2.
Conceptual model highlighting positive (in blue) and negative (in red) relationships between community and environmental attributes, and their effects on several ecosystem functions. Black single-headed arrows indicate likely but poorly studied relationships. X = interactions between two different components of the model (D = between plant-plant interactions and environmental conditions; F= between nurse plants’ effects at the patch level versus their density within the landscape), there is no general agreement in their outcome and cross-discipline studies are needed to solve these discrepancies. Important studies, relevant to particular parts of the model are identified with numbers and listed in the highlighted table (bottom right). 1, Van Auken (2000); 2, Michalet et al. (2006); 3, Verwijmeren et al. (2013); 4, this study; 5, Soliveres et al. (2012c); 6, Riginos et al. (2009); 7, Mulder et al. (2001); 8, Steudel et al. (2012); 9, Jucker & Coomes (2012); 10, Maestre et al. (2012); 11, Cardinale et al. (2002); 12, Butterfield (2009); 13, Wilby & Sachak (2004); 14, Reinhart (2010); 15, Zavaleta et al. (2010); 16, Forest et al. (2007); 17, Díaz et al. (2007); 18, Maestre & Escudero (2009); 19, Kefi et al. (2007). C-seq = Carbon sequestration
We now need studies aiming to assess to what extent species-specific adaptations depend on different environments and the presence of neighbours, and how this may affect the community-level importance of facilitation under such environmental conditions (Fig. 1). This will undoubtedly provide a useful link between pairwise and community-level studies, and refine our predictions on the response of plant communities to environmental changes. A combination of the approaches followed by Cornwell & Ackerly (2009; different filters of the community-level trait space) and Butterfield & Briggs (2011; relationship between plant functional traits and a regeneration niche associated with a nurse plant) appears the most promising in this regard (see also Gross et al., 2013; Hulshof et al., 2013; Schöb, Butterfield & Pugnaire, 2012). Moreover, the classification of the species pool into a few relevant groups in the functional trait space regarding the response to the presence of neighbours, and the effect of the latter on their microclimate (i.e. functional response and effect groups; Butterfield, 2009) can help to enhance the reliability and generality of current modelling approaches by introducing biotic interactions in a more refined, yet general, manner (Jeltsch et al., 2008).
(2) Can we predict changes in the outcome of pairwise interactions across environmental gradients?
Regardless of their relative importance, the multiple factors shaping pairwise plant–plant interactions and the interrelationships existing among them make it extremely difficult generally to predict the outcome of plant–plant interactions along environmental gradients. However, we can encapsulate all the species-specific and environmental drivers into a single factor: the distance to the ecological optimum of the beneficiary. In short, the further a given beneficiary species is from its ecological optimum (i.e. the lower the performance of isolated individuals), the more necessary or important will be the presence of a nurse plant (but see Mitchell, Cahill & Hik, 2009 for contradicting findings). This is referred to as “individual strain”, “functional stress” or “individual stress” in the literature (Gross et al., 2010; Lortie & Callaway, 2006; Rees, Childs & Freckleton, 2012; Soliveres et al., 2011), and we refer to it here as the “individual stress model”. There are multiple examples that support its use, including the higher facilitation found for: (i) drought-maladapted species when water stress is more important (e.g. Liancourt et al., 2005), (ii) grazing-intolerant species under increasing grazing pressure (e.g. Vandenberghe et al., 2009), or (iii) salt-sensitive species in salt marshes (e.g. Greiner La Peyre et al., 2001). Recently, He et al. (2013) showed the generality of the individual stress model with a meta-analysis of studies on pairwise interactions across the globe. They showed consistent shifts towards more positive interactions with lower target plant performance in the open (i.e. greater distance from their ecological optimum) in a variety of biomes and growth forms. The authors interpreted their results as strong support for the SGH as originally formulated. However, their results are not linked to the environmental conditions but to the performance of each particular target species. Therefore, we interpret them as strong support for the direct relationship between the outcome of pairwise interactions and the individual stress experienced by the beneficiary species (Level 2 in Fig. 1).
At this point, we should advise caution when selecting response and predictor variables for testing how plant–plant interactions change along environmental gradients. The selected response variable cannot be a by-product of the predictor (e.g. using survival away from nurse plants as a predictor and then a facilitation index built using this survival and that in the nurses as a response) as these response variables can generate spurious significant relationships and lead to misleading conclusions (Brett, 2004; Rees et al., 2012). In this regard, the ideal approach should probably follow three steps: (i) identify crucial ontogenetic phases for the population of the beneficiary species (i.e. by using matrix-based population models; Salguero-Gómez & De Kroon, 2010), (ii) perform removal experiments or planting experiments using different microsites to quantify the strength of facilitation/competition across these different ontogenetic stages (e.g. le Roux, Shaw & Chown, 2013; Soliveres et al., 2010), and (iii) relate the results to general measures of performance of the population of the beneficiary species (i.e. relative abundance or biomass at the plot scale; Miriti, Wright & Howe, 2001; Mitchell et al., 2009). A complementary future line of inquiry should focus on the linearity or non-linearity of the facilitation distance from the optimum relationship (Malkinson & Tielbörger, 2010), and on the evaluation of thresholds in the distance from the ecological optimum in which the presence of a nurse plant does not suffice to ensure recruitment of a given beneficiary (Kitzberger et al., 2000). If we know the exact shape of this relationship, and the amount of stress that a nurse can alleviate, we can make accurate predictions that can easily be incorporated into models for future species distributions and assemblages of plant communities under different global environmental change scenarios (e.g. Thuiller et al., 2013; Wisz et al., 2013).
(3) Moving from pairwise to community-level interactions: challenges ahead
By using the individual stress model, we can improve our ability to predict the outcome of particular pairwise interactions, but the concept of “stress” cannot be applied to whole communities whose members differ widely in their ecological adaptations. The problem arises when these species-specific stress levels are not linked to the overall environmental conditions. The predictability of any community-level model lacking this link vanishes because such models use these conditions (e.g. climatic data) to predict future distributions and responses of plant communities to changing environmental scenarios (Bertness & Callaway, 1994; Bruno et al., 2003; Fig. 1).
The link between species-specific stress levels and environmental gradients has led to a proposed modified SGH, which predicts a higher importance and frequency of positive plant–plant interactions at moderate levels of stress (Holmgren & Scheffer, 2010). The peaking of facilitation at moderate levels of stress is supported by other theoretical models and empirical research at the community level (Hacker & Bertness, 1999; Hacker & Gaines, 1997; Michalet et al., 2006). Other studies, however, provide support for linear and monotonic increase of the importance of facilitation at the community level (e.g. Armas, Rodríguez-Echeverría & Pugnaire, 2011; Dohn et al., 2013). Recently, a conceptual model based on empirical observations was proposed to reconcile contrasting results often found at the community level (Soliveres et al., 2011). This model states that the relationship between environmental gradients and the frequency of facilitative interactions will be predictable (either linear or unimodal) if the environmental gradient is driven by a single stressor and most species in the community perform similarly under this stress factor (discussed in Holmgren & Scheffer, 2010; Michalet et al., 2006). When multiple unrelated stress factors are present within the environmental gradient, or when there is high variability in the ecological requirements among the species pool, a relationship between the frequency of positive plant–plant interactions and environmental harshness should not be expected (López et al., 2013; Soliveres et al., 2011; Soliveres & Maestre, 2014).
To answer community-level predictions on how the frequency of positive plant–plant interactions changes across differing environmental conditions, we must necessarily use data gathered at the community level (Soliveres & Maestre, 2014). Data gathered at the level of single or a few species pairs is insufficient to test the generality of predictions from the SGH, to understand under which conditions these predictions are valid or not, and to assess the importance of factors shaping the outcome of plant–plant interactions at the community level across environmental gradients (Fig. 1). Moreover, although much has been discussed about the collapse of facilitative interactions under “extreme conditions” (Callaway, 2013; Molina-Montenegro et al., 2012; Verwijmeren et al., 2013), neither the threshold (if any) between what is considered “extreme” or “mild/moderate” environmental conditions nor the factors driving it have been defined. Does our perception of what is “extreme” vary with the community being studied and with the environmental adaptations of the occurring species? Is the co-occurrence of diverse stress factors what makes an environment “extreme”? Or is it just that we denote the conditions when facilitation collapses as “extreme”?
Another relevant issue when upscaling from the pairwise to the community scale is that plants do not interact just in pairs, but rather form multiple species assemblages (e.g. Saiz & Alados, 2011b; Verdú & Valiente-Banuet, 2011). Under this multiple-species scenario, even competitor species may be facilitated if one of them excludes a third common competitor species (Brooker et al., 2008; Levine, 1999). However, we know very little of how frequent these indirect positive interactions are, and under which environments or for which plant functional groups these interactions prevail. Indirect interactions among woody adults and seedlings, mediated by competition with grasses, have been reported in different ecosystems (Cuesta et al., 2010; Maestre, Cortina & Bautista, 2004; Saccone et al., 2010). In one of the very few attempts to study the interplay between indirect interactions and climate, Cuesta et al. (2010) showed that indirect positive effects prevailed in the wetter periods while direct facilitation was predominant during drought. Similar results were found in a more extensive study (Schöb, Armas & Pugnaire, 2013a), suggesting that indirect interactions prevail under more productive conditions.
Plants, especially in harsh environments, tend to form clumps of multiple species that interact with one another. Under such conditions, not only indirect interactions are important, but also the existence of non-hierarchical competitive networks (intransitive networks, equivalent to the rock-paper-scissors game in which no single dominant species prevails; Gilpin, 1975). These intransitive competition networks are important, yet poorly explored, mechanism for the maintenance of plant diversity (Laird & Schamp, 2006). Although intransitive networks are based on competition, and not on facilitation, the presence of nurse plants may enhance the degree of intransitivity, and therefore increase species diversity at the community level (Soliveres et al., 2011). Intransitivity is more likely to occur under more heterogeneous conditions (Allesina & Levine, 2011), such as those favoured by the presence of nurse plants. A nice example, although not discussed by the authors in their original paper, is provided by Cavieres et al. (2005). These authors found evidence of how changes in the competitive networks promoted by the cushion Azorella monantha may reduce competitive exclusion among their neighbouring plants. Although intransitive interactions have been studied mostly using mathematical models, due to difficulties in studying them empirically, new tools are becoming available to quantify changes in the degree of intransitivity in competitive networks among neighbouring species from observational data (Soliveres et al., 2011; Ulrich et al., 2014). These methodological advances open the door to assessing the frequency of intransitive loops within plant communities, and to evaluating how nurse plants, other trophic levels, or the environment affect such frequency.
The application of network theory into facilitation research has also shed substantial insights on the role of different drivers of plant–plant interactions, namely evolutionary relationships, disturbance or density-dependent effects, on the structure of the interactions among the multiple species forming plant communities (Saiz & Alados, 2011b; Verdú et al., 2009; Verdú & Valiente-Banuet, 2011). Network approaches have also been used to assess how keystone species affect interactions among the rest of the species within a community (Saiz & Alados, 2011a). We believe that network approaches may serve, among other things, to identify keystone nurse species that have disproportionate (regarding their abundance) positive effects on other species, which can help in focusing species selection in restoration efforts. Overall, these new tools and approaches offer great potential to study multiple species interactions, and hence to provide new insights into the importance of interspecific interactions on plant communities. The evidence mentioned above also indicates that the consideration of multiple-species interactions may completely change our view of how plant–plant interactions at the community level are affected by environmental conditions, and will further increase the reliability of conceptual, mathematical and empirical models aimed at predicting how these interactions will respond to ongoing global environmental change.
III. EVOLUTIONARY ASPECTS OF PLANT FACILITATION
A key theme in ecological and evolutionary studies is how, and to what degree, ecosystems – and organisms living therein– deal with the rapid human-induced climate and land-use changes (Sala et al., 2000; Vitousek et al., 1997). Classically, studies have focused on one-way adaptations of organisms to changing environments by natural selection (Jump & Peñuelas, 2005). However, species also create their own environment, and thus that of the species interacting with them (i.e. niche construction sensu Odling-Smee, Laland & Feldman, 2003). This is another, albeit largely ignored, evolutionary pathway. A recent synthesis (Castellanos & Verdú, 2012) shows that biotic factors, including the interaction with neighbouring plants, are stronger evolutionary selection forces than abiotic factors in plants. Empirical evidence also shows that species interactions largely drive the response of ecosystems to environmental changes (Cardinale, Palmer & Collins, 2002; Gilman et al., 2010). What then are the long-term consequences of facilitation for the interacting plants and their environment? Previous work has aimed at providing a theoretical framework for the evolutionary implications of facilitation (Bronstein, 2009; Laland, Odling-Smee & Feldman, 1996; Liancourt et al., 2012; Thorpe et al., 2011) and it is not our intention here to develop them further. Rather, we focus on including empirical evidence supporting or contradicting what we believe are the most important implications of facilitation on evolution, and on providing future guidelines and suggesting potential experiments to advance this topic further.
Typically, studies on the evolutionary consequences of species interactions have focused on plant–pollinator, seed–disperser, and plant–herbivore interactions (Thorpe et al., 2011). Interactions between plants have been much less considered as a mechanism for evolutionary adaptations. Thus far, this theme has received very little attention, and available studies have mostly focused on theoretical and conceptual work (Bronstein, 2009; Brooker et al., 2008; Liancourt et al., 2012; Thorpe et al., 2011; but see Aarssen & Turkington, 1985; Ehlers & Thompson, 2004; Valiente-Banuet et al., 2006). While the evolutionary implications of environmental changes have been evaluated multiple times (reviewed in Castellanos & Verdú, 2012), there is a great need for studies testing patterns of evolution in response to the presence of neighbours. So far, evidence suggests that facilitative interactions help to preserve lineages less adapted to more recent environmental (both abiotic and biotic) conditions, working as an ecological “time machine” for maladapted lineages (sensu Lortie, 2007). A crucial example for the latter is that recent (quaternary) lineages have preserved older (tertiary) drought-maladapted ones through the drier climatic conditions found during the quaternary period (Valiente-Banuet et al., 2006). Whether or not this preservation of biodiversity by benefactor species through sharp environmental changes has occurred at other times in the past, or may occur in the future, is a topic deserving further attention (Blois et al., 2013; McCluney et al., 2012).
A second major research line in the relationship between facilitation and evolution is how facilitation works as a process driving speciation. In line with the individual stress model, recent work highlights the importance of plant–plant interactions to maintain local maladapted ecotypes within a given species (Jensen & Ehlers, 2010). These results suggest that local plant communities may be much more co-evolved than assumed so far. Besides abiotic conditions, the evolutionary grazing history is also an important determinant of the outcome of plant–plant interactions. The ‘naïve’ (without grazing history) phenotype of the palatable Persicaria longiseta strongly benefits from protection against browsing, while its grazing-adapted phenotype does not (Suzuki & Suzuki, 2012). Interestingly, this study suggests that adaptations to grazing can occur within a relatively short time span (decades to centuries), which is much faster than assumed in the classical studies on evolutionary grazing history (> 10,000 years; Mack & Thompson, 1982; Milchunas, Sala & Lauenroth, 1988). This is supported by the fact that biotically selected traits hold fewer genes and therefore drive faster adaptation than abiotically driven traits (Louthan & Kay, 2011). The notion that evolutionary processes commonly take place at ecological timescales has opened up the opportunity to study feedbacks between ecology and evolution (i.e. eco-evolutionary dynamics: Carroll et al., 2007; Pelletier, Garant & Hendry, 2009; Schoener, 2011). The example of Suzuki & Suzuki (2012) is the first that places (positive) plant–plant interactions in this exciting field, and we see strong potential for novel empirical studies within this eco-evolutionary context.
There are several remaining questions regarding the role of facilitation as a speciation process. The main one is that, to generate new species, facilitative interactions do not need just to enhance the performance of maladapted ecotypes, but also to promote reproductive isolation. An obvious, yet poorly explored, mechanism is that facilitative interactions change flowering phenology of such maladapted ecotypes, thus promoting reproductive isolation from the better adapted ecotypes. Another pathway of reproductive isolation may occur when a nurse plant protects beneficiary plants against grazing, which reduces the production of flowerheads and seeds of beneficiaries outside the canopy of the nurse (Bossuyt, De Fre & Hoffmann, 2005). Studies addressing these pathways are key to assessing the role of facilitation as a driver of speciation.
To understand fully the role of facilitation in the evolution of plants, the nurse plants should also be considered (Bronstein, 2009). Two recent examples show that facilitation may come with a cost for the facilitator (Michalet et al., 2011; Schöb et al., 2013b); thus, what are the advantages of being a facilitator? Facilitation might come with benefits for the facilitator when the fitness of the population is increased by the beneficiary via promotion of seed and leaf size (Cranston et al., 2012), seed dispersal (by attractive seeds of the beneficiary), pollination (attractive flowers of the beneficiary), shared defenses, or the exact opposite (decoy; beneficiary distracts enemies of the nurse), but evidence thus far is very scarce. If we can establish how general these positive and negative outcomes for nurses are, we will undoubtedly be able to understand the implications of plant–plant interactions in the evolution of plant communities better. Only a handful of studies have considered the reciprocal effects of plant–plant interactions to date (e.g. Lortie & Reid, 2012; Michalet et al., 2011; Schöb et al., 2013b). We see a strong need for novel experimental, descriptive and modelling studies aiming to unravel the conditions under which facilitation can lead to evolutionary adaptations among the interacting plant species. Such studies would include field measurements of trait variability of nurse and beneficiary species, in each other’s presence and absence, combined with reciprocal transplant studies to identify signals for possible local adaptation to neighbouring plants (similar to Liancourt & Tielbörger, 2011; Grøndahl & Ehlers, 2008). In addition, greenhouse or common garden studies on the second- and third-generation offspring from these different populations are needed to test for the heritability of the measured altered traits. Laboratory experiments with controlled lines of both beneficiary and nurse plants in manipulated mesocosm environments would be needed to test under what conditions genetic variation due to the presence of the neighbour evolves. Modelling studies would allow populations of multiple nurse and beneficiary plants (community approach) to evolve over longer times under different scenarios (variable interaction strengths, interaction types, sets of species traits, environmental stressors and their combinations). In addition, long-term studies of species invasions are an excellent opportunity to investigate not only how invading species evolve different interactions within the novel communities (Callaway et al., 2012), but also how these invaded communities, and their members, respond to the invader in evolutionary terms (Lau, 2006; Pakeman et al., 2009).
Lastly, it has been hypothesized that systems with ubiquitous facilitative interactions may exhibit highly unstable dynamics, with some alternative states leading to local extinction (also referred to as “evolutionary suicide”; Gyllenberg & Parvinen, 2001; Kefi et al., 2008). This occurs when facilitated species, which succeed thanks to the presence of nurse species, competitively exclude the latter, preventing their own recruitment in the next generation (see Valiente-Banuet, Vite & Zavala-Hurtado, 1991, for an empirical example). This leads to either the local extinction of both nurse and facilitated species, or to sharp reductions in their population sizes (see Ferriere & Legendre, 2013, for a recent review on this topic). How this theoretical instability in communities driven by facilitation is linked to the evolutionary persistence of positive interactions observed in real world communities (e.g. Valiente-Banuet et al., 2006) is certainly a major topic to address to understand fully the evolutionary implications of plant–plant interactions. The scarce existing studies point to the spatial component as a crucial attribute to consider when investigating the conundrum between the persistence of positive plant–plant interactions and the low stability of facilitation-driven communities. For example, Kefi et al. (2008) found that short-distance seed dispersal generates patchy patterns that allow facilitation-driven populations to escape extinction, while those populations characterized by long-distance dispersal become extinct under the same conditions. Along the same lines, Filotas et al. (2010) found that facilitation-driven populations with less spatial interconnectedness were more stable than those that were highly connected. Until now, modelling has been the only available approach to address this issue. Hopefully, by investigating long-term datasets in well-studied systems showing cyclical succession (such as the Saguaro systems in Valiente-Banuet et al., 1991) will we be able to assess empirically the insights gained by modelling approaches.
IV. RELATIVE IMPORTANCE OF PLANT–PLANT INTERACTIONS FOR COMMUNITY STRUCTURE AND FUNCTIONING: IDENTIFYING GAPS IN OUR KNOWLEDGE
The importance of plant–plant interactions as a driver of plant communities has been a subject of debate since the 1980s (Goldberg & Barton, 1992; Welden & Slauson, 1986). Welden & Slauson (1986) proposed differentiation between the intensity (with versus without neighbour) and the importance (neighbour versus the remaining environmental factors affecting target plant performance) of plant–plant interactions. Although not free from caveats (Rees et al., 2012), this differentiation between intensity and importance helped to clarify contrasting results in the literature regarding plant–plant interactions (e.g. Brooker et al., 2005), and even could be exported to other active lines of inquiry in ecology, such as the relationship between disturbance and diversity, or the response of plant species to global environmental change (Kikvidze, Suzuki & Brooker, 2011). Most recent research and conceptual advances on this topic have focused on the importance of plant competition and facilitation for individuals and populations of single species (e.g. Rees et al., 2012 and references therein). However, the relative importance of plant–plant interactions versus other variables as drivers of community attributes and ecosystem functions has been largely overlooked.
It is well known that both positive and negative interactions affect the richness of plant communities (e.g. Bruno et al., 2003; Goldberg & Barton, 1992; Hacker & Gaines, 1997), and that the positive effects of nurse plants can cascade to insects (e.g. Lortie & Reid, 2012), biological soil crusts or microbial communities (Maestre et al., 2009a). However, how many species within a community depend on nurses or, in other words, what the importance is of positive plant–plant interactions for the maintenance of plant diversity, and over what spatio-temporal scales these interactions are important, is largely unknown (see discussion on this topic in Brooker et al., 2009; Ricklefs, 2008, 2009). Empirical studies (e.g. Cavieres & Badano, 2009; Soliveres et al., 2011) and theoretical work (Brooker, 2006) illustrates that facilitative interactions may influence a high proportion of the species forming a community, and that they can buffer against negative impacts from climate change. However, to what extent and under which conditions this buffering effect should be expected is very poorly understood (but see Cavieres et al., 2014; Soliveres & Maestre, 2014). As an example, Sala et al. (2000) predicted that up to 40% of actual plant communities will change in composition because of changing environmental conditions. How these predictions would change if they included positive interactions is a question that deserves to be explored.
Interactions among plants also influence other important diversity attributes, such as functional and phylogenetic diversity (Cornwell & Ackerly, 2009; Gross et al., 2013; Soliveres, Torices & Maestre, 2012b, Valiente-Banuet & Verdu, 2007; Webb et al., 2002), and the composition and spatial pattern of entire plant communities (reviewed in Brooker et al., 2008; Callaway, 2007). Separate lines of inquiry, on the other hand, have repeatedly demonstrated how these different attributes (richness, functional and phylogenetic diversity or spatial pattern) play a crucial role in the functioning of ecological communities and the services they provide (e.g. Díaz et al., 2007; Kefi et al., 2007; Maestre et al., 2012). Our aim here is to merge both bodies of knowledge to address the importance of plant–plant interactions as drivers of the structure and functioning of ecological communities. We first discuss how nurse plants can affect the structure of plant communities and then the potential implications of such effects on their functioning.
(1) Plant–plant interactions as drivers of community composition and structure: linking results at the individual and landscape spatial scales
The importance of plant–plant interactions for species richness, spatial pattern and or composition varies across community types and environments (Cavieres & Badano, 2009; Kikvidze et al., 2005; Mitchell et al., 2009; Soliveres et al., 2011). This variation seems jointly to depend on the prevailing environmental conditions and the density and functional traits of nurses (Breshears, 2006; Verwijmeren et al., 2013). Nurse plants generally ameliorate the environment beneath their canopies (i.e. positive patch-level effect), and this generates some degree of environmental heterogeneity at the landscape scale (i.e. differing water, nutrient and light conditions beneath nurse canopies than outside). This seems the main reason why nurses increase species richness and affect species composition at the landscape level (Cavieres & Badano, 2009; Tewksbury & Lloyd, 2001).
However, very few studies have related the response of these positive effects at the level of the individual plant to changes in the density of the potential nurse plant within the landscape. Literature focusing on woody encroachment (i.e. the increase in woody plant densities into former grasslands) suggests that if the density of woody plants (which also can act as nurses) increases at the landscape level, it will reach a point at which habitat heterogeneity decreases rather than increases, which could reduce the positive effect of nurses on community structure attributes (Breshears, 2006). A unique empirical example for this is found in Riginos et al. (2009), who found that the effect of Acacia trees on species richness, plant biomass and soil fertility was strongly positive, but became negative with increasing tree density at the landscape scale (but see Soliveres & Eldridge, 2013).
Finally, the positive effects of different plant functional groups, such as trees, shrubs, herbs, cushion plants or grasses, on their neighbours are widely variable because of their different morphology (Gómez-Aparicio, 2009), and these growth forms dominate under differing habitats and environments. Thus, understanding how environmental conditions, the functional traits of the nurses, and the relationship between the facilitative role of individual nurses and their density within the landscape affect ecosystem attributes is an extensive field for future research (Fig. 2).
(2) Effects of plant–plant interactions on ecosystem functioning
If studies focused on the different drivers of the importance of plant–plant interactions for community attributes such as richness, composition or spatial pattern are scarce, those focusing on ecosystem functioning are even rarer. The few available studies deal with the effect of facilitative interactions on ecosystem productivity and stability (Butterfield, 2009; Cardinale et al., 2002; Mulder, Uliassi & Doak, 2001; Wilby & Shachak, 2004), the increase in alien plant invasions due to the microclimatic amelioration provided by nurses (Bruno et al., 2003; Cavieres et al., 2005; Reinhart, 2010), or the relative importance of plant–plant interactions on nutrient cycling and soil fertility (reviewed in Callaway, 2007; see also Maestre et al., 2010). However, an increasing amount of studies are dealing with the effect of nurse plants on the functional diversity of their neighbours, or use phylogenetic diversity as a proxy to assess functional diversity. Due to the tight link between changes in functional and phylogenetic diversity, such as those promoted by nurses, and changes in ecosystem functioning (e.g. Díaz et al., 2007; Forest et al., 2007), we can use studies on the effects of facilitation on functional and phylogenetic diversity to provide a first link between facilitation among plants and ecosystem functioning.
The first step, therefore, would be to assess how plant–plant interactions respond to the environment to affect functional traits of neighbouring species (Butterfield & Callaway, 2013; Gross et al., 2013), and how these changes in functional traits relate to different ecosystem functions. It must be noted that those traits affected by the presence of a given nurse (response traits sensu Lavorel & Garnier, 2002) can differ widely from those affecting ecosystem processes (effect traits sensu Lavorel & Garnier, 2002; see also Butterfield, 2009). Thus, to link properly the effects of nurse plants on neighbours’ functional traits, and these traits to important ecosystem processes, we need to consider the existing relationship between response and effect traits. According to the framework proposed by Lavorel & Garnier (2002), those response traits related to the nutrient amelioration commonly provided by nurses are likely to be involved in nutrient cycling or productivity and, therefore, should be those considered when linking plant–plant interactions to ecosystem processes. For example, the few available studies on this topic suggest that plants growing beneath a nurse show higher leaf N contents and lower C/N ratios (Riginos et al., 2009). These response traits may also play a role as effect traits by promoting litter decomposability. Therefore, plants growing beneath nurses are likely to show higher decomposition rates than those growing in open areas, mimicking the behaviour found for cultivated versus wild plants, or high-resource versus low-resource habitats (García-Palacios et al., 2013). On the other hand, response traits related to grazing protection are likely to be less relevant to ecosystem processes (Lavorel & Garnier, 2002), and thus can be of little importance to link plant–plant interactions and ecosystem functioning.
As in the assessment of facilitation as a driver of speciation (see section III above), it is also necessary to know which facilitative interactions provide common selective forces for certain trait values, how these selective forces change with environmental conditions and how the favoured trait values affect different ecosystem functions. Again, following the individual stress model, it is unlikely that we will find common selective forces in the future, as the trait values favoured by facilitation are likely to change depending on the species-specific adaptation of each beneficiary species and the prevailing environmental conditions (Fig. 1; see also Butterfield & Callaway, 2013). Thus, generalizations about the effects of facilitation on ecosystem functioning are extremely difficult, as different functional traits are facilitated under different environments (Butterfield & Callaway, 2013). However, some commonalities arising from the current literature can help us to draw a first set of testable hypotheses to foster research in this topic. Overall, available empirical evidence suggests that facilitative interactions affect ecosystem functioning by (i) promoting higher productivity and litter quality (e.g. Cardinale et al., 2002; Schöb et al., 2012), which enhances nutrient cycling and C fixation, (ii) buffering the reduction of functional diversity expected under harsher environments (Gross et al., 2013; Schöb et al., 2012), which can promote the stability of plant productivity (Cardinale et al., 2002; Wilby & Shachak, 2004) and promote ecosystem functioning under harsher scenarios (e.g. Mulder et al., 2001), and (iii) reducing community resistance to alien plant invasions due to the microclimatic amelioration promoted by nurses (Cavieres et al., 2005; Reinhart, 2010). It is interesting to note that facilitation seems particularly important to maintain rare and subordinate species (Gross et al., 2013), which have been highlighted as crucial for the maintenance of ecosystem functions because of their low functional redundancy (Lyons et al., 2005; Mouillot et al., 2013).
The effects of facilitative interactions in the above-mentioned ecosystem-function-related variables will likely depend on the environment. In this regard, it seems clear that more functional diversity approaches (Butterfield & Callaway, 2013; Gross et al., 2013; Schöb et al., 2012, 2013a) are needed to establish general patterns on the importance and sign of the effect of plant–plant interactions on functional diversity, and how this may relate to ecosystem functioning under differing environmental conditions. Apart from the indirect effects mediated through their influence on neighbouring plants, it is obvious that nurses have their own direct effects on ecosystem functioning. Normally, they capture more resources via run-off and provide shade and milder environmental conditions, enhancing soil nutrient cycling and water capture and storage (Puigdefábregas et al., 1999; Tongway & Hindley, 2004). Thus, any study aiming to assess the role of plant–plant interactions in ecosystem functioning should consider both these direct effects and those indirectly mediated by their influence on neighbouring plants (Table 1).
Table 1.
Main topics addressed in this review, identifying the main gaps in our knowledge that should be addressed by future research, recommending approaches to address them (see text for examples) and listing theoretical improvements expected from such research. PPI, plant–plant interactions.
| Topic | Gaps in our knowledge | Approach recommended | Theoretical improvements |
|---|---|---|---|
| Pairwise interactions | Relative importance of environment and PPI as drivers of the functional traits (species-specific adaptation) present in a given community | -Quantify filtering effects on plant traits of both the environment and PPI -Relationship between plant functional traits and the outcome of PPI |
-Classification of the species pool in functional response groups (sensu
Butterfield, 2009) to improve generality and reliability of current model approaches -Identify potential benefactor species to enhance success in restoration efforts |
| Changes in the performance of species along their ecological optimum | -Population models to target crucial ontogenetic stages + experiments to quantify the effect of PPI in such stages | -Predict the response of species to environmental changes -Improve species distribution models and predict community assemblage under future climatic scenarios |
|
| Community-level interactions | Behaviour of PPI across environmental gradients | -Community-level studies across gradients driven by different uncorrelated stress factors -Quantify the importance of the type of gradient and the diversity of species-specific adaptations as drivers of the response of community-level PPI across differing environments |
-Predict the role of PPI in the response of ecological communities to global environmental change -Quantify the threshold in environmental conditions, if any, that may collapse facilitative interactions |
| Importance of interactions among multiple species | -Use observational or experimental approaches to quantify the frequency of indirect facilitative interactions and how they behave across differing environments and/or functional groups | -Improve our predictions of the importance of PPI for plant communities -Application to other research lines (e.g. persistence of shrubs in grasslands, invasion meltdown) |
|
| Evolutionary implications | Facilitation as an ecological tardis (sensu Lortie, 2007) | -Quantify the role of PPI for the maintenance of diversity across evolutionary scales | -Understand better the role of PPI on the maintenance of diversity across evolutionary scales. Improve our predictions of species loss under future environmental scenarios |
| Facilitation driving speciation | -Apply the individual stress model to intra-specific ecotypes differing in ecological adaptations + link results from different ecotypes to differences in flowering phenology that could promote reproductive isolation and drive to speciation. Quantify the heritability of altered traits/ecotypes by using second- or third-generation seeds in common garden experiments -Evaluate fitness costs for the benefactor |
-Understand better the role of PPI as an evolutionary force driving speciation–extinction -Introduction of facilitation into general evolutionary ecology theory |
|
| Facilitation and ecosystem functioning | Direct effects of PPI on ecosystem functioning | -Quantify the relative importance of PPI on community-level functional/phylogenetic diversity and ecosystem functioning -Identify density dependence in the effects of PPI on ecosystem attributes such as diversity, composition or spatial pattern |
-Unify multiple lines of research to understand better the drivers of ecosystem functioning -Quantify how PPI affect ecosystem functioning, and how their effects change across differing environments or biomes |
| Traits promoted by PPI and their effect on multiple ecosystem functions | -Identify which trait values are favoured by PPI, and link them to different ecosystem functions -Assess the role of PPI in multiple ecosystem functions simultaneously |
-Quantify direct effects on ecosystem functioning promoted by benefactors versus their indirect effects mediated by changing functional traits of their neighbours | |
| Effects on stability | -Evolutionary suicide: investigate the conundrum between the high unstability predicted for facilitation-driven communities and the ubiquituous presence of facilitative interactions in nature -Quantify the role of PPI in the maintenance of rare species, which are crucial to maintain stability in multiple functions -Link microclimatic amelioration by nurse plants with the stability in the productivity of benefactors |
-Improve our predictions on the response of communities and ecosystems to global environmental change |
Interestingly, synergistic and antagonistic relationships among different ecosystem functions affected by the presence of nurse plants can be expected, highlighting the need to study the role of facilitative interactions on multiple ecosystem functions simultaneously. For example, the higher species richness promoted by plant–plant interactions may synergistically increase the effects of changes in plant litter quality on nutrient cycling and plant productivity (Fig. 2). However, the increase in alien plant success due to microclimatic amelioration by nurses (e.g. Cavieres et al., 2005) can lead to reductions in both plant diversity and ecosystem functioning (e.g. Stachowicz & Byrnes, 2006). The effects of plant–plant interactions on ecosystem functioning are also density dependent. For example, Acacia trees enhance plant productivity when occurring at low densities, but reduce it when occurring at higher densities (Riginos et al., 2009). Furthermore, ecosystem functioning not only depends on functional diversity, but also on the spatial pattern and general cover and biomass of plants (e.g. Kefi et al., 2007; Maestre & Escudero, 2009), which are likely to be affected by plant–plant interactions (e.g. Cardinale et al., 2002; Pueyo et al., 2008). While very few studies have evaluated the relative importance of attributes such as spatial patterns and species diversity simultaneously on ecosystem functioning, even less have simultaneously studied how plant–plant interactions affect such ecosystem attributes and the ecosystem functions affected by them (but see Kikvidze et al., 2005; Maestre et al., 2010; Mitchell et al., 2009). More of these studies are needed if we are to understand the importance of plant–plant interactions as drivers of community structure and functioning. Apart from purely empirical approaches, mathematical modelling (e.g. Filotas et al., 2010) can be really helpful when addressing the complex interplay among the factors modulating the role of plant–plant interactions on ecosystem functioning.
V. CONCLUSIONS
(1) Current apparently opposing views regarding the SGH, which remain debated in the literature, can be reconciled when clearly considering and determining the species-specificity of the response, the nature of the stressor considered, and the scale of interest (pairwise interactions or community-level responses). We believe that the application of these straightforward recommendations (Fig. 1) is essential for the further development of facilitation theory and for improving our understanding and predictions of how plant communities will respond to ongoing global environmental change. These advances can also further our understanding on the evolutionary and ecological implications of plant–plant interactions for plant communities.
(2) We identified gaps in our knowledge that should be a priority for future studies. For example, we emphasized the need to address thresholds in the amount of stress that a benefactor can alleviate, and the linearity or non-linearity of the behaviour of pairwise interactions along distances from the beneficiary’s ecological optimum.
(3) We also need more community-level studies and approaches assessing interactions among multiple species to understand better the consequences of facilitative interactions for the structure of whole communities (Table 1).
(4) The SGH is rapidly expanding towards fields other than plant ecology, for which it was originally designed. It is likely that the SGH and its modifications (Fig. 1) might be applicable to other sessile organisms once the specific mechanisms that underlie their interactions have been assessed. However, for more mobile animal communities, the application of the SGH may lead to different predictions compared to plants and sessile organisms (Barrio et al., 2012), particularly at the extreme ends of environmental gradients. Development of facilitation theory for these other communities is only just beginning and there is ample room for theoretical, empirical and modelling studies.
(5) Empirical studies on the evolutionary aspects of facilitation are clearly needed. While it is now recognized that facilitation can play an important role in the maintenance of genetic diversity, very few studies have tested how positive species interactions affect the generation of new species. There is a strong need for novel experimental, descriptive and modelling studies aiming to unravel under what conditions facilitation can lead to evolutionary adaptations among interacting plant species. Such studies should also include the evolutionary aspects for the nurse species, as well as the evolutionary (grazing) history of the interacting species. We see high potential in invasion ecology as a field to test these ideas.
(6) Research on the relative importance of plant–plant interactions as drivers of community structure and functioning may benefit from approaches dealing with the interactions between environmental conditions, nurse density within landscapes and the effects of individual nurses at the scale of individual plant patches.
(7) Studies addressing the direct and indirect (mediated through their influence on the functional traits of their neighbours) effects of plant–plant interactions will help us to understand better the effects of such interactions on multiple ecosystem functions. Performing such studies across environmental gradients will help to predict the importance of biotic interactions as a driver of the response of ecological communities to ongoing global change (Fig. 2).
(8) There are multiple challenges ahead in facilitation research, and novel conceptual, empirical and modelling studies are needed to advance ecological theory with respect to facilitation.
ACKNOWLEDGEMENTS
Alison Cooper and two anonymous referees provided numerous and constructive comments that improved a previous version of this paper. Ruth Howison helped with language revisions. S.S. and F.T.M. were supported by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM).
REFERENCES
- Aarssen L, Turkington R. Biotic specialization between neighbouring genotypes in Lolium perenne and Trifolium repens from a permanent pasture. Journal of Ecology. 1985;73:605–614. [Google Scholar]
- Allesina S, Levine JM. A competitive network theory of species diversity. Proceedings of the National Academy of Sciences. 2011;108:5638–5642. doi: 10.1073/pnas.1014428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armas C, Rodríguez-Echeverría S, Pugnaire FI. A field test of the stress-gradient hypothesis along an aridity gradient. Journal of Vegetation Science. 2011;22:818–827. [Google Scholar]
- Barrio IC, Hik DS, Bueno CG, Cahill JF. Extending the stress-gradient hypothesis – is competition among animals less common in harsh environments? Oikos. 2012;122:516–523. [Google Scholar]
- Baumeister D, Callaway RM. Facilitation by Pinus flexilis during succession: A hierarchy of mechanisms benefits other plant species. Ecology. 2006;87:1816–1830. doi: 10.1890/0012-9658(2006)87[1816:fbpfds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bertness MD, Callaway R. Positive interactions in communities. Trends in Ecology & Evolution. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. Climate change and the past, present, and future of biotic interactions. Science. 2013;341:499–504. doi: 10.1126/science.1237184. [DOI] [PubMed] [Google Scholar]
- Bossuyt B, De Fre B, Hoffmann M. Abundance and flowering success patterns in a short-term grazed grassland: early evidence of facilitation. Journal of Ecology. 2005;93:1104–1114. [Google Scholar]
- Bowker MA, Soliveres S, Maestre FT. Competition increases with abiotic stress and regulates the diversity of biological soil crusts. Journal of Ecology. 2010;98:551–560. [Google Scholar]
- Breshears DD. The grassland-forest continuum: trends in ecosystem properties for woody plant mosaics? Frontiers in Ecology and the Environment. 2006;4:96–104. [Google Scholar]
- Brett MT. When is a correlation between non-independent variables “spurious”? Oikos. 2004;105:647–656. [Google Scholar]
- Bronstein JL. The evolution of facilitation and mutualism. Journal of Ecology. 2009;97:1160–1170. [Google Scholar]
- Brooker R, Kikvidze Z, Pugnaire FI, Callaway RM, Choler P, Lortie CJ, Michalet R. The importance of importance. Oikos. 2005;109:63–70. [Google Scholar]
- Brooker RW. Plant–plant interactions and environmental change. New Phytologist. 2006;171:271–284. doi: 10.1111/j.1469-8137.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Brooker RW, Callaway RM, Cavieres LA, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Valiente-Banuet A, Whitham TG. Don’t diss integration: a comment on Ricklefs’s disintegrating communities. The American Naturalist. 2009;174:919–927. doi: 10.1086/648058. [DOI] [PubMed] [Google Scholar]
- Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielborger K, Travis JMJ, Anthemlme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology. 2008;96:18–34. [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution. 2003;18:119–125. [Google Scholar]
- Butterfield B, Cavieres L, Callaway R, Cook B, Kikvidze Z, Lortie C, Michalet R, Pugnaire F, Schöb C, Xiao S. Alpine cushion plants inhibit the loss of phylogenetic diversity in severe environments. Ecology Letters. 2013;16:478–486. doi: 10.1111/ele.12070. [DOI] [PubMed] [Google Scholar]
- Butterfield BJ. Effects of facilitation on community stability and dynamics: synthesis and future directions. Journal of Ecology. 2009;97:1192–1201. [Google Scholar]
- Butterfield BJ, Betancourt JL, Turner RM, Briggs JM. Facilitation drives 65 years of vegetation change in the Sonoran Desert. Ecology. 2010;91:1132–1139. doi: 10.1890/09-0145.1. [DOI] [PubMed] [Google Scholar]
- Butterfield BJ, Briggs JM. Regeneration niche differentiates functional strategies of desert woody plant species. Oecologia. 2011;165:477–487. doi: 10.1007/s00442-010-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield BJ, Callaway RM. A functional comparative approach to facilitation and its context dependence. Functional Ecology. 2013;27:907–917. [Google Scholar]
- Callaway RM. Positive interactions and interdependence in plant communities. Springer, Dordrecht, the Netherlands: 2007. [Google Scholar]
- Callaway RM. Life at the edge, cooperation in Antarctica. Journal of Vegetation Science. 2013;24:417–418. [Google Scholar]
- Callaway RM, Schaffner U, Thelen GC, Khamraev A, Juginisov T, Maron JL. Impact of Acroptilon repens on co-occurring native plants is greater in the invader’s non-native range. Biological Invasions. 2012;14:1143–1155. [Google Scholar]
- Cardinale BJ, Palmer MA, Collins SL. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature. 2002;415:426–429. doi: 10.1038/415426a. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Castellanos MC, Verdú M. Meta-analysis of meta-analyses in plant evolutionary ecology. Evolutionary Ecology. 2012;26:1187–1196. [Google Scholar]
- Cavieres LA, Badano EI. Do facilitative interactions increase species richness at the entire community level? Journal of Ecology. 2009;97:1181–1191. [Google Scholar]
- Cavieres LA, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Schöb C, Xiao S. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecology Letters. 2014;17:193–202. doi: 10.1111/ele.12217. [DOI] [PubMed] [Google Scholar]
- Cavieres LA, Quiroz CL, Molina-Montenegro MA, Muñoz AA, Pauchard A. Nurse effect of the native cushion plant Azorella monantha on the invasive non-native Taraxacum officinale in the high-Andes of central Chile. Perspectives in Plant Ecology, Evolution and Systematics. 2005;7:217–226. [Google Scholar]
- Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. University of Chicago Press; 2003. [Google Scholar]
- Chu C-J, Maestre FT, Xiao S, Weiner J, Wang Y-S, Duan Z-H, Wang G. Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecology Letters. 2008;11:1189–1197. doi: 10.1111/j.1461-0248.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs. 2009;79:109–126. [Google Scholar]
- Cranston BH, Callaway RM, Monks A, Dickinson KJ. Gender and abiotic stress affect community-scale intensity of facilitation and its costs. Journal of Ecology. 2012 [Google Scholar]
- Cuesta B, Villar Salvador P, Puertolas J, Rey Benayas JM, Michalet R. Facilitation of Quercus ilex in Mediterranean shrubland is explained by both direct and indirect interactions mediated by herbs. Journal of Ecology. 2010;98:687–696. [Google Scholar]
- Díaz S, Lavorel S, de Bello F, Quétier F, Grigulis K, Robson TM. Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences. 2007;104:20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn J, Dembélé F, Karembé M, Moustakas A, Amévor KA, Hanan NP. Tree effects on grass growth in savannas: competition, facilitation and the stress-gradient hypothesis. Journal of Ecology. 2013;101:202–209. [Google Scholar]
- Ehlers BK, Thompson J. Do co-occurring plant species adapt to one another? The response of Bromus erectus to the presence of different Thymus vulgaris chemotypes. Oecologia. 2004;141:511–518. doi: 10.1007/s00442-004-1663-7. [DOI] [PubMed] [Google Scholar]
- Ferriere R, Legendre S. Eco-evolutionary feedbacks, adaptive dynamics and evolutionary rescue theory. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013 doi: 10.1098/rstb.2012.0081. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filotas E, Grant M, Parrott L, Rikvold PA. The effect of positive interactions on community structure in a multi-species metacommunity model along an environmental gradient. Ecological Modelling. 2010;221:885–894. [Google Scholar]
- Forest F, Grenyer R, Rouget M, Davies TJ, Cowling RM, Faith DP, Balmford A, Manning JC, Proches S, Van der Bank M, Reeves G, Hedderson TAJ, Savolainen V. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature. 2007;445:757–760. doi: 10.1038/nature05587. [DOI] [PubMed] [Google Scholar]
- Fugère V, Andino P, Espinosa R, Anthelme F, Jacobsen D, Dangles O. Testing the stress-gradient hypothesis with aquatic detritivorous invertebrates: insights for biodiversity-ecosystem functioning research. Journal of Animal Ecology. 2012;81:1259–1267. doi: 10.1111/j.1365-2656.2012.01994.x. [DOI] [PubMed] [Google Scholar]
- García-Palacios P, Milla R, Delgado-Baquerizo M, Martín-Robles N, Álvaro-Sánchez M, Wall DH. Side-effects of plant domestication: ecosystem impacts of changes in litter quality. New Phytologist. 2013;198:504–513. doi: 10.1111/nph.12127. [DOI] [PubMed] [Google Scholar]
- Gause GF. The struggle for existence. Williams and Wilkins; Baltimore: 1934. [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends in Ecology & Evolution. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Gilpin ME. Limit cycles in competition communities. American Naturalist. 1975;109:51–60. [Google Scholar]
- Goldberg DE, Barton AM. Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. American Naturalist. 1992;139:771–801. [Google Scholar]
- Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A. Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology. 1999;80:1118–1131. [Google Scholar]
- Gómez-Aparicio L. The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. Journal of Ecology. 2009;97:1202–1214. [Google Scholar]
- Greiner La Peyre M, Grace J, Hahn E, Mendelssohn I. The importance of competition in regulating plant species abundance along a salinity gradient. Ecology. 2001;82:62–69. [Google Scholar]
- Grøndahl E, Ehlers BK. Local adaptation to biotic factors: reciprocal transplants of four species associated with aromatic Thymus pulegioides and T. serpyllum. Journal of Ecology. 2008;96:981–992. [Google Scholar]
- Gross N, Börger L, Soriano-Morales SI, Bagousse-Pinguet L, Quero JL, García-Gómez M, Valencia-Gómez E, Maestre FT. Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. Journal of Ecology. 2013;101:637–649. [Google Scholar]
- Gross N, Liancourt P, Choler P, Suding K, Lavorel S. Strain and vegetation effects on local limiting resources explain the outcomes of biotic interactions. Perspectives in Plant Ecology, Evolution and Systematics. 2010;12:9–19. [Google Scholar]
- Gyllenberg M, Parvinen K. Necessary and sufficient conditions for evolutionary suicide. Bulletin of mathematical biology. 2001;63:981–993. doi: 10.1006/bulm.2001.0253. [DOI] [PubMed] [Google Scholar]
- Hacker SD, Bertness MD. Experimental evidence for factors maintaining plant species diversity in a New England salt marsh. Ecology. 1999;80:2064–2073. [Google Scholar]
- Hacker SD, Gaines SD. Some implications of direct positive interactions for community species diversity. Ecology. 1997;78:1990–2003. [Google Scholar]
- He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecology Letters. 2013;16:695–706. doi: 10.1111/ele.12080. [DOI] [PubMed] [Google Scholar]
- Holmgren M, Scheffer M. Strong facilitation in mild environments: the stress gradient hypothesis revisited. Journal of Ecology. 2010;98:1269–1275. [Google Scholar]
- Hulshof CM, Violle C, Spasojevic MJ, Mcgill B, Damschen E, Harrison S, Enquist BJ. Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. Journal of Vegetation Science. 2013;24:921–931. [Google Scholar]
- Jeltsch F, Moloney KA, Schurr FM, Köchy M, Schwager M. The state of plant population modelling in light of environmental change. Perspectives in Plant Ecology, Evolution and Systematics. 2008;9:171–189. [Google Scholar]
- Jensen C, Ehlers B. Genetic variation for sensitivity to a thyme monoterpene in associated plant species. Oecologia. 2010;162:1017–1025. doi: 10.1007/s00442-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker T, Coomes DA. Comment on “Plant species richness and ecosystem multifunctionality in global drylands”. Science. 2012;337:155. doi: 10.1126/science.1220620. [DOI] [PubMed] [Google Scholar]
- Jump AS, Peñuelas J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Kawai T, Tokeshi M. Testing the facilitation-competition paradigm under the stress-gradient hypothesis: decoupling multiple stress factors. Proceedings of the Royal Society B-Biological Sciences. 2007;274:2503–2508. doi: 10.1098/rspb.2007.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefi S, Rietkerk M, Alados CL, Pueyo Y, Papanastasis VP, Elaich A, de Ruiter PC. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature. 2007;449:213–U5. doi: 10.1038/nature06111. [DOI] [PubMed] [Google Scholar]
- Kefi S, van Baalen M, Rietkerk M, Loreau M. Evolution of local facilitation in arid ecosystems. American Naturalist. 2008;172:E1–E17. doi: 10.1086/588066. [DOI] [PubMed] [Google Scholar]
- Kikvidze Z, Pugnaire FI, Brooker RW, Choler P, Lortie CJ, Michalet R, Callaway RM. Linking patterns and processes in alpine plant communities: A global study. Ecology. 2005;86:1395–1400. [Google Scholar]
- Kikvidze Z, Suzuki M, Brooker R. Importance versus intensity of ecological effects: why context matters. Trends in Ecology & Evolution. 2011;26:383–388. doi: 10.1016/j.tree.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Kitzberger T, Steinaker DF, Veblen TT. Effects of climatic variability on facilitation of tree establishment in northern Patagonia. Ecology. 2000;81:1914–1924. [Google Scholar]
- Laird RA, Schamp BS. Competitive intransitivity promotes species coexistence. The American Naturalist. 2006;168:182–193. doi: 10.1086/506259. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee FJ, Feldman MW. The evolutionary consequences of niche construction: a theoretical investigation using two-locus theory. Journal of Evolutionary Biology. 1996;9:293–316. [Google Scholar]
- Lau JA. Evolutionary responses of native plants to novel community members. Evolution. 2006;60:56–63. [PubMed] [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- le Roux P, Mcgeoch M. Interaction intensity and importance along two stress gradients: adding shape to the stress-gradient hypothesis. Oecologia. 2010;162:733–745. doi: 10.1007/s00442-009-1484-9. [DOI] [PubMed] [Google Scholar]
- le Roux PC, Shaw JD, Chown SL. Ontogenetic shifts in plant interactions vary with environmental severity and affect population structure. New Phytologist. 2013;200:241–250. doi: 10.1111/nph.12349. [DOI] [PubMed] [Google Scholar]
- Levine JM. Indirect facilitation: evidence and predictions from a riparian community. Ecology. 1999;80:1762–1769. [Google Scholar]
- Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. [Google Scholar]
- Liancourt P, Choler P, Gross N, Thibert-Plante X, Tielbörger K. How facilitation may interfere with ecological speciation. International Journal of Ecology. 2012;2012 ID 725487. [Google Scholar]
- Liancourt P, Tielbörger K. Ecotypic differentiation determines the outcome of positive interactions in a dryland annual plant species. Perspectives in Plant Ecology, Evolution and Systematics. 2011;13:259–264. [Google Scholar]
- Lin Y, Berger U, Grimm V, Ji Q-R. Differences between symmetric and asymmetric facilitation matter: exploring the interplay between modes of positive and negative plant interactions. Journal of Ecology. 2012;100:1482–1491. [Google Scholar]
- López RP, Valdivia S, Rivera ML, Rios RS. Co-occurrence patterns along a regional aridity gradient of the subtropical andes do not support stress gradient hypotheses. PLoS ONE. 2013;8:e58518. doi: 10.1371/journal.pone.0058518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie CJ. An ecological tardis: the implications of facilitation through evolutionary time. Trends in Ecology & Evolution. 2007;22:627–630. doi: 10.1016/j.tree.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM. Rethinking plant community theory. Oikos. 2004;107:433–438. [Google Scholar]
- Lortie CJ, Callaway RM. Re-analysis of meta-analysis: support for the stress-gradient hypothesis. Journal of Ecology. 2006;94:7–16. [Google Scholar]
- Lortie CJ, Reid AM. Reciprocal gender effects of a keystone alpine plant species on other plants, pollinators, and arthropods. Botany. 2012;90:273–282. [Google Scholar]
- Louthan AM, Kay KM. Comparing the adaptive landscape across trait types: larger QTL effect size in traits under biotic selection. BMC evolutionary biology. 2011;11:60. doi: 10.1186/1471-2148-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K, Brigham C, Traut B, Schwartz M. Rare species and ecosystem functioning. Conservation Biology. 2005;19:1019–1024. [Google Scholar]
- Mack RN, Thompson JN. Evolution in steppe with few large, hooved mammals. American Naturalist. 1982;119:757–773. [Google Scholar]
- Maestre FT, Bowker MA, Escolar C, Puche MD, Soliveres S, Maltez-Mouro S, Garcãa-Palacios P, Castillo-Monroy AP, Martãnez I, Escudero AN. Do biotic interactions modulate ecosystem functioning along stress gradients? Insights from semi-arid plant and biological soil crust communities. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:2057–2070. doi: 10.1098/rstb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Bowker MA, Puche MD, Belén Hinojosa M, Martínez I, García-Palacios P, Castillo AP, Soliveres S, Luzuriaga AL, Sánchez AM. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecology Letters. 2009a;12:930–941. doi: 10.1111/j.1461-0248.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology. 2009b;97:199–205. [Google Scholar]
- Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? - A test from a semi-arid steppe. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Cortina J, Bautista S. Mechanisms underlying the interaction between Pinus halepensis and the native late-successional shrub Pistacia lentiscus in a semi-arid plantation. Ecography. 2004;27:776–786. [Google Scholar]
- Maestre FT, Escudero A. Is the patch size distribution of vegetation a suitable indicator of desertification processes? Ecology. 2009;90:1729–1735. doi: 10.1890/08-2096.1. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, García-Gómez M, Bowker MA, Soliveres S, Escolar C, García-Palacios P, Berdugo M, Valencia E, Gozalo B, Gallardo A, Aguilera L, Arredondo T, Blones J, Boeken B, Bran D, Conceição AA, Cabrera O, Chaieb M, Derak M, Eldridge DJ, Espinosa CI, Florentino A, Gaitán J, Gatica MG, Ghiloufi W, Gómez-González S, Gutiérrez JR, Hernández RM, Huang X, Huber-Sannwald E, Jankju M, Miriti M, Monerris J, Mau RL, Morici E, Naseri K, Ospina A, Polo V, Prina A, Pucheta E, Ramírez-Collantes DA, Romão R, Tighe M, Torres-Díaz C, Val J, Veiga JP, Wang D, Zaady E. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Valladares F, Reynolds JF. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. Journal of Ecology. 2005;93:748–757. [Google Scholar]
- Maestre FT, Valladares F, Reynolds JF. The stress-gradient hypothesis does not fit all relationships between plant-plant interactions and abiotic stress: further insights from arid environments. Journal of Ecology. 2006;94:17–22. [Google Scholar]
- Malkinson D, Tielbörger K. What does the stress gradient hypothesis predict? Resolving the discrepancies. Oikos. 2010;119:1546–1552. [Google Scholar]
- Mccluney KE, Belnap J, Collins SL, González AL, Hagen EM, Nathaniel Holland J, Kotler BP, Maestre FT, Smith SD, Wolf BO. Shifting species interactions in terrestrial dryland ecosystems under altered water availability and climate change. Biological Reviews. 2012;87:563–582. doi: 10.1111/j.1469-185X.2011.00209.x. [DOI] [PubMed] [Google Scholar]
- Michalet R, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, Valiente-Banuet A, Callaway RM. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecology Letters. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Michalet R, Xiao S, Touzard B, Smith DS, Cavieres LA, Callaway RM, Whitham TG. Phenotypic variation in nurse traits and community feedbacks define an alpine community. Ecology Letters. 2011;14:433–443. doi: 10.1111/j.1461-0248.2011.01605.x. [DOI] [PubMed] [Google Scholar]
- Milchunas DG, Sala OE, Lauenroth WK. A generalized model of the effects of grazing by large herbivores on grassland community structure. The American Naturalist. 1988;132:87. [Google Scholar]
- Miriti MN. Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology. 2006;94:973–979. [Google Scholar]
- Miriti MN, Wright JS, Howe HF. The effects of neighbors on the demography of a dominant desert shrub (Ambrosia dumosa) Ecological Monographs. 2001;71:491–509. [Google Scholar]
- Mitchell MG, Cahill JF, JR, Hik DS. Plant interactions are unimportant in a subarctic-alpine plant community. Ecology. 2009;90:2360–2367. doi: 10.1890/08-0924.1. [DOI] [PubMed] [Google Scholar]
- Molina-Montenegro MA, Ricote-Martínez N, Muñoz-Ramírez C, Gómez González S, Torres-Díaz C, Salgado-Luarte C, Gianoli E. Positive interactions between the lichen Usnea antarctica (Parmeliaceae) and the native flora in Maritime Antarctica. Journal of Vegetation Science. 2012 [Google Scholar]
- Mouillot D, Bellwood DR, Baraloto C, Chave J, Galzin R, Harmelin-Vivien M, Kulbicki M, Lavergne S, Lavorel S, Mouquet N. Rare species support vulnerable functions in high-diversity ecosystems. PLoS biology. 2013;11:e1001569. doi: 10.1371/journal.pbio.1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder CPH, Uliassi DD, Doak DF. Physical stress and diversity-productivity relationships: The role of positive interactions. Proceedings of the National Academy of Sciences. 2001;98:6704–6708. doi: 10.1073/pnas.111055298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche construction: the neglected process in evolution (MPB-37) Princeton University Press; 2003. [Google Scholar]
- Pakeman RJ, Pugnaire FI, Michalet R, Lortie CJ, Schiffers K, Maestre FT, Travis JMJ. Is the cask of facilitation ready for bottling? A symposium on the connectivity and future directions of positive plant interactions. Biology Letters. 2009;5:577–579. doi: 10.1098/rsbl.2009.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier F, Garant D, Hendry AP. Eco-evolutionary dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1483–1489. doi: 10.1098/rstb.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo Y, Kefi S, Alados CL, Rietkerk M. Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos. 2008;117:1522–1532. [Google Scholar]
- Puigdefábregas J, Sole A, Gutierrez L, del Barrio G, Boer M. Scales and processes of water and sediment redistribution in drylands: results from the Rambla Honda field site in Southeast Spain. Earth-Science Reviews. 1999;48:39–70. [Google Scholar]
- Rees M, Childs DZ, Freckleton RP. Assessing the role of competition and stress: a critique of importance indices and the development of a new approach. Journal of Ecology. 2012;100:577–585. [Google Scholar]
- Reinhart KO. The role of facilitative interactions in tree invasions. New Phytologist. 2010;187:559–562. doi: 10.1111/j.1469-8137.2010.03375.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Disintegration of the ecological community. The American Naturalist. 2008;172:741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. A brief response to Brooker et al.’s comment. The American Naturalist. 2009;174:928–931. doi: 10.1086/648059. [DOI] [PubMed] [Google Scholar]
- Riginos C, Grace JB, Augustine DJ, Young TP. Local versus landscape-scale effects of savanna trees on grasses. Journal of Ecology. 2009;97:1337–1345. [Google Scholar]
- Saccone P, Girel J, Brun JJ, Michalet R. Acer negundo invasion along a successional gradient: early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytologist. 2010;187:831–842. doi: 10.1111/j.1469-8137.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- Saiz H, Alados CL. Effect of Stipa tenacissima L. on the structure of plant co-occurrence networks in a semi-arid community. Ecological research. 2011a;26:595–603. [Google Scholar]
- Saiz H, Alados C. Structure and spatial self-organization of semi-arid communities through plant–plant co-occurrence networks. Ecological Complexity. 2011b;8:184–191. [Google Scholar]
- Sala OE, Stuart Chapin F, III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld MN, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. Global Biodiversity Scenarios for the Year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Salguero-Gómez R, De Kroon H. Matrix projection models meet variation in the real world. Journal of Ecology. 2010;98:250–254. [Google Scholar]
- Santoro R, Jucker T, Carboni M, Acosta AT. Patterns of plant community assembly in invaded and non-invaded communities along a natural environmental gradient. Journal of Vegetation Science. 2012;23:483–494. [Google Scholar]
- Schöb C, Armas C, Pugnaire FI. Direct and indirect interactions co-determine species composition in nurse plant systems. Oikos. 2013a;122:1371–1379. [Google Scholar]
- Schöb C, Butterfield BJ, Pugnaire FI. Foundation species influence trait-based community assembly. New Phytologist. 2012;196:824–834. doi: 10.1111/j.1469-8137.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- Schöb C, Michalet R, Cavieres LA, Pugnaire FI, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, Xiao S, Al Hayek P, Anthelme F, Cranston BH, García M-C, Le Bagousse-Pinguet Y, Reid AM, le Roux PC, Lingua E, Nyakatya MJ, Touzard B, Zhao L, Callaway RM. A global analysis of bidirectional interactions in alpine plant communities shows facilitators experiencing strong reciprocal fitness costs. New Phytologist. 2013b doi: 10.1111/nph.12641. in press. doi: 10.1111/nph.12641. [DOI] [PubMed] [Google Scholar]
- Schoener TW. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331:426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- Smit C, Rietkerk M, Wassen MJ. Inclusion of biotic stress (consumer pressure) alters predictions from the stress gradient hypothesis. Journal of Ecology. 2009;97:1215–1219. [Google Scholar]
- Smit C, Ruifrok JL. From protégé to nurse plant: establishment of thorny shrubs in grazed temperate woodlands. Journal of Vegetation Science. 2011;22:377–386. [Google Scholar]
- Smit C, Vandenberghe C, den Ouden J, Mueller-Schaerer H. Nurse plants, tree saplings and grazing pressure: changes in facilitation along a biotic environmental gradient. Oecologia. 2007;152:265–273. doi: 10.1007/s00442-006-0650-6. [DOI] [PubMed] [Google Scholar]
- Soliveres S, Desoto L, Maestre FT, Olano JM. Spatio-temporal heterogeneity in abiotic factors modulate multiple ontogenetic shifts between competition and facilitation. Perspectives in Plant Ecology, Evolution and Systematics. 2010;12:227–234. [Google Scholar]
- Soliveres S, Eldridge DJ. Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Functional Ecology. 2013;28:530–537. doi: 10.1111/1365-2435.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Eldridge DJ, Hemmings F, Maestre FT. Nurse plant effects on plant species richness in drylands: The role of grazing, rainfall and species specificity. Perspectives in Plant Ecology, Evolution and Systematics. 2012a;14:402–410. doi: 10.1016/j.ppees.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Eldridge DJ, Maestre FT, Bowker MA, Tighe M, Escudero A. Microhabitat amelioration and reduced competition among understorey plants as drivers of facilitation across environmental gradients: Towards a unifying framework. Perspectives in Plant Ecology, Evolution and Systematics. 2011;13:247–258. doi: 10.1016/j.ppees.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Maestre FT. Plant-plant interactions, environmental gradients and plant diversity: a global synthesis of community-level studies. Perspectives in Plant Ecology, Evolution and Systematics. 2014 doi: 10.1016/j.ppees.2014.04.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Torices R, Maestre FT. Environmental conditions and biotic interactions acting together promote phylogenetic randomness in semi-arid plant communities: new methods help to avoid misleading conclusions. Journal of Vegetation Science. 2012b;23:822–836. doi: 10.1111/j.1654-1103.2012.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Torices R, Maestre FT. Evolutionary relationships can be more important than abiotic conditions in predicting the outcome of plant–plant interactions. Oikos. 2012c;121:1638–1648. doi: 10.1111/j.1600-0706.2011.20309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowicz JJ, Byrnes JE. Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Marine ecology progress series. 2006;311:251–262. [Google Scholar]
- Steudel B, Hector A, Friedl T, Löfke C, Lorenz M, Wesche M, Kessler M. Biodiversity effects on ecosystem functioning change along environmental stress gradients. Ecology letters. 2012;15:1397–1405. doi: 10.1111/j.1461-0248.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Sthultz CM, Gehring CA, Whitham TG. Shifts from competition to facilitation between a foundation tree and a pioneer shrub across spatial and temporal scales in a semiarid woodland. New Phytologist. 2007;173:135–145. doi: 10.1111/j.1469-8137.2006.01915.x. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Suzuki S. Morphological adaptation of a palatable plant to long-term grazing can shift interactions with an unpalatable plant from facilitative to competitive. Plant Ecology. 2012;213:175–183. [Google Scholar]
- Tewksbury JJ, Lloyd JD. Positive interactions under nurse-plants: spatial scale, stress gradients and benefactor size. Oecologia. 2001;127:425–434. doi: 10.1007/s004420000614. [DOI] [PubMed] [Google Scholar]
- Thorpe AS, Aschehoug ET, Atwater DZ, Callaway RM. Interactions among plants and evolution. Journal of Ecology. 2011;99:729–740. [Google Scholar]
- Thuiller W, Münkemüller T, Lavergne S, Mouillot D, Mouquet N, Schiffers K, Gravel D. A road map for integrating eco-evolutionary processes into biodiversity models. Ecology Letters. 2013;16:94–105. doi: 10.1111/ele.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Tongway D, Hindley N. Landscape function analysis: a system for monitoring rangeland function. CSIRO; Canberra (Australia): 2004. [Google Scholar]
- Ulrich W, Soliveres S, Kryszewski W, Maestre FT, Gotelli NJ. Matrix models for quantifying competitive intransitivity from species abundance data. Oikos; 2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente-Banuet A, Rumebe AV, Verdu M, Callaway RM. Modern quaternary plant lineages promote diversity through facilitation of ancient tertiary lineages. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente-Banuet A, Verdu M. Facilitation can increase the phylogenetic diversity of plant communities. Ecology Letters. 2007;10:1029–1036. doi: 10.1111/j.1461-0248.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- Valiente-Banuet A, Vite F, Zavala-Hurtado JA. Interaction between the cactus Neobuxbaumia tetetzo and the nurse shrub Mimosa luisana. Journal of Vegetation Science. 1991;2:11–14. [Google Scholar]
- Van Auken OW. Shrub invasions of north american semiarid grasslands. Annual Review of Ecology and Systematics. 2000;31:197–215. [Google Scholar]
- Vandenberghe C, Smit C, Pohl M, Buttler A, Frelechoux F. Does the strength of facilitation by nurse shrubs depend on grazing resistance of tree saplings? Basic and Applied Ecology. 2009;10:427–436. [Google Scholar]
- Verdú M, Rey PJ, Alcántara JM, Siles G, Valiente-Banuet A. Phylogenetic signatures of facilitation and competition in successional communities. Journal of Ecology. 2009;97:1171–1180. [Google Scholar]
- Verdú M, Valiente-Banuet A. The relative contribution of abundance and phylogeny to the structure of plant facilitation networks. Oikos. 2011;120:1351–1356. [Google Scholar]
- Verwijmeren M, Rietkerk M, Wassen MJ, Smit C. Interspecific facilitation and critical transitions in arid ecosystems. Oikos. 2013;122:341–347. [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Webb CO, Ackerly DD, Mcpeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2002;33:475–505. [Google Scholar]
- Welden CW, Slauson WL. The intensity of competition versus its importance: an overlooked distinction and some implications. Quarterly Review of Biology. 1986;61:23–44. doi: 10.1086/414724. [DOI] [PubMed] [Google Scholar]
- Whittaker RH. Vegetation of the great smoky mountains. Ecological Monographs. 1956;26:1–80. [Google Scholar]
- Wilby A, Shachak M. Shrubs, granivores and annual plant community stability in an arid ecosystem. Oikos. 2004;106:209–216. [Google Scholar]
- Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes JA, Guisan A. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews. 2013;88:15–30. doi: 10.1111/j.1469-185X.2012.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaleta ES, Pasari JR, Hulvey KB, Tilman DG. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proceedings of the National Academy of Sciences USA. 2010;107:1443–1446. doi: 10.1073/pnas.0906829107. [DOI] [PMC free article] [PubMed] [Google Scholar]