Abstract
Positive and negative plant-plant interactions are major processes shaping plant communities. They are affected by environmental conditions and evolutionary relationships among the interacting plants. However, the generality of these factors as drivers of pairwise plant interactions and their combined effects remain virtually unknown. We conducted an observational study to assess how environmental conditions (altitude, temperature, irradiance and rainfall), the dispersal mechanism of beneficiary species and evolutionary relationships affected the co-occurrence of pairwise interactions in 11 Stipa tenacissima steppes located along an environmental gradient in Spain. We studied 197 pairwise plant-plant interactions involving the two major nurse plants (the resprouting shrub Quercus coccifera and the tussock grass S. tenacissima) found in these communities. The relative importance of the studied factors varied with the nurse species considered. None of the factors studied were good predictors of the co-ocurrence between S. tenacissima and its neighbours. However, both the dispersal mechanism of the beneficiary species and the phylogenetic distance between interacting species were crucial factors affecting the co-occurrence between Q. coccifera and its neighbours, while climatic conditions (irradiance) played a secondary role. Values of phylogenetic distance between 207-272.8 Myr led to competition, while values outside this range or fleshy-fruitness in the beneficiary species led to positive interactions. The low importance of environmental conditions as a general driver of pairwise interactions was caused by the species-specific response to changes in either rainfall or radiation. This result suggests that factors other than climatic conditions must be included in theoretical models aimed to generally predict the outcome of plant-plant interactions. Our study helps to improve current theory on plant-plant interactions and to understand how these interactions can respond to expected modifications in species composition and climate associated to ongoing global environmental change.
Keywords: competition, facilitation, interspecific interactions, Mediterranean, phylogenetic distance, Quercus coccifera L., semi-arid, Stipa tenacissima L.
Introduction
Positive (facilitation) and negative (competition) plant-plant interactions are one of the main processes affecting the structure, diversity and dynamics of plant communities in virtually all terrestrial ecosystems (Callaway 2007, Brooker et al. 2008). These interactions depend on a number of factors, being abiotic conditions a major determinant of the interplay between facilitation and competition (Callaway 2007). The behaviour of these plant-plant interactions along abiotic gradients depend on the type and number of stressors involved (Kaway and Tokeshi 2007, Maestre et al. 2009) and the ecophysiological adaptations of the interacting species (Liancourt et al. 2005, Gross et al. 2010). However, two different general views prevail: 1) increases in abiotic stress are associated with an increase in the frequency or importance of plant-plant interactions (Bertness and Callaway 1994, Brooker et al. 2005), or 2) the relationship between these interactions and abiotic gradients is unimodal, with positive interactions prevailing at moderate levels of abiotic stress (Maestre and Cortina 2004, Michalet et al. 2006, Holmgren and Scheffer 2010).
Apart from abiotic conditions, the evolutionary relationship between the interacting species has been recently highlighted as a crucial driver of these interactions (Valiente-Banuet et al. 2006, Valiente-Banuet and Verdú 2007, Castillo et al. 2010, Verdú and Valiente-Banuet 2011). More closely related species are likely to share important ecological traits, and are therefore more likely to compete among each other (Webb et al. 2002). On the other hand, species that are more phylogenetically distant normally differ in their ecological traits and the environmental conditions they can cope with, and thus facilitative interactions among them are more likely to occur (Valiente-Banuet et al. 2006, Valiente-Banuet and Verdú 2007, Castillo et al. 2010).
The different drivers of plant-plant interactions, such as the ontogenetic stage of interacting species, abiotic factors, or herbivory, often interact among each other to define their outcome (Baumeister and Callaway 2006, Kawai and Tokeshi 2007, Smit et al. 2009, Soliveres et al. 2010, 2011). In the same way, environmental conditions and the phylogenetic distance (hereafter PD) between interacting species are likely to jointly determine the outcome of plant-plant interactions, and hence the structure and composition of plant communities. However, and to the best of our knowledge, no previous study has evaluated the combined effects and the relative roles of PD and environmental conditions as drivers of pairwise plant-plant interactions. We aimed to assess the generality of such factors, and their combined effects, as drivers of plant-plant interactions by using a large number of pairwise interactions. To do so, we evaluated the sign, intensity and importance of 197 pairwise interactions in semi-arid Stipa tenacissima L. steppes located along an environmental gradient in Spain in response to different environmental conditions and evolutionary relationships of the involved species. Specifically, we addressed the following questions: 1) What is the relative importance of environmental conditions (altitude, temperature, irradiance and rainfall), dispersal and evolutionary relationships (PD) in defining the co-occurrence of the species studied?, 2) Do these factors interact?, and 3) Does the effect of these factors depend on the nurse species? Stipa tenacissima steppes are a good model system to answer these questions, as both facilitation and competition are frequently found in this ecosystem (Maestre et al. 2001, García-Fayos and Gasque 2002, Pugnaire et al. 2011), and facilitation/competition shifts occur with changes in abiotic stress (Maestre and Cortina 2004, Soliveres et al. 2010).
Methods
Study area
We sampled 11 S. tenacissima communities along a climatic gradient spanning from the center to the south-east of Spain. Our study sites have annual precipitation and temperature values ranging from 273 mm to 488 mm and from 13°C to 17°C, respectively, which cover most of the environmental conditions where these communities are found in Spain (Maestre et al. 2007). We detected significant shifts on the direction or strength of most of the pairwise interactions occurring three times or more along the gradient studied (Table 1), which demonstrates the ecological significance of such gradient to evaluate the relationship between plant-plant interactions and environmental conditions. To minimize the experimental noise produced by environmental factors other than climate, which could affect our conclusions, all the sites shared the same general soil type (limestones), and had similar orientation and slope values. Vegetation was in all cases an open grassland dominated by S. tenacissima, with total cover values ranging from 35% to 68%. Sparse sprouting shrubs, mostly Quercus coccifera L., but also Rhamnus lycioides L. or Pistacia lentiscus L., were also present in all sites. Four environmental variables known to affect the outcome of pairwise interactions (see review in Callaway 2007) were collected for each site: irradiance, mean temperature, annual rainfall (collected from available climatic models [Ninyerola et al. 2005]), and altitude.
Table 1.
Shape of the relationship between the indicators of plant-plant interactions (Relative Interaction Index [RII] and Interaction Importance Index [Iimp]) and two different climatic factors: mean annual rainfall (R) or average daily irradiance (I). These relationships were calculated for 19 particular pairwise interactions occurring in three or more of the 11 studied plots and the mean annual rainfall or average daily irradiance in such plots. Species are organized by families. The relationship between RII/Iimp and rainfall/irradiance can be nil (0), monotonically positive or negative (+ and −, respectively) or hump-shaped (hs). The last column indicates the number of sites along the gradient in which each species was present. The indices were calculated for each nurse species (Stipa = Stipa tenacissima; Quercus = Quercus coccifera) and target species (those in the first column) using the number of individuals of the beneficiary species in each microsite as our surrogate of plant performance.
|
RII Stipa |
RII Quercus |
Iimp Stipa |
Iimp Quercus |
Sites | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| R | I | R | I | R | I | R | I | ||
| Monocots | |||||||||
| Liliaceae | |||||||||
| Asparragus horridus | hs | − | hs | − | − | 0 | + | 0 | 3 |
| Poaceae | |||||||||
| Brachypodium retusum | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Stipa offneri | hs | 0 | + | − | 0 | 0 | + | − | 5 |
| Xanthorrhoeae | |||||||||
| Asphodelus ramosus | − | 0 | 0 | − | 0 | − | 0 | − | 4 |
| Eudicots | |||||||||
| Anacardaceae | |||||||||
| Pistacia lentiscus | 0 | 0 | 0 | 0 | 0 | 0 | hs | 0 | 3 |
| Asteraceae | |||||||||
| Stahelina dubia | − | + | + | 0 | 0 | 0 | 0 | 0 | 4 |
| Cistaceae | |||||||||
| Cistus clusii | − | 0 | 0 | + | 0 | 0 | 0 | 0 | 6 |
| Fumana ericoides | − | + | − | hs | 0 | + | 0 | + | 7 |
| Fumana thymifolia | 0 | hs | 0 | 0 | 0 | + | 0 | + | 6 |
| Helianthemum cinereum | 0 | 0 | − | 0 | − | 0 | − | 0 | 6 |
| Helianthemum violaceum | hs | 0 | hs | 0 | − | + | − | + | 6 |
| Crassulaceae | |||||||||
| Sedum sediforme | 0 | hs | − | hs | 0 | hs | 0 | hs | 7 |
| Lamiaceae | |||||||||
| Rosmarinus officinalis | 0 | 0 | 0 | + | 0 | − | 0 | 0 | 8 |
| Teucrium capitatum | 0 | + | 0 | + | 0 | + | 0 | + | 7 |
| Teucrium pseudochamaepytis | 0 | − | 0 | − | 0 | 0 | 0 | 0 | 9 |
| Thymus vulgaris | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Thymus zygis | 0 | 0 | 0 | 0 | − | + | − | + | 4 |
| Polygalaceae | |||||||||
| Polygala rupestris | + | − | + | − | + | − | 0 | 0 | 4 |
| Rhamnaceae | |||||||||
| Rhamnus lycioides | 0 | 0 | 0 | 0 | 0 | 0 | − | 0 | 4 |
Evaluating species interactions
We evaluated particular pairwise interactions for the two dominant nurses in the study area (Q. coccifera shrubs and S. tenacissima tussocks, hereafter Quercus and Stipa microsites, respectively), and assessed the total number of facilitated species at each site. For doing this, we randomly selected ten S. tenacissima tussocks in each site, and sampled under their canopy using 0.5 m × 0.5 m quadrats (~30 quadrats per site). We only selected S. tenacissima individuals isolated from large shrubs, to ensure that this species acted as a nurse and not as a beneficiary. Ten paired open areas (located at least 1 m away from any Quercus or Stipa microsite, hereafter Open microsite) were also randomly selected. The same number of 0.5 m × 0.5 m quadrats sampled in each Stipa microsite was sampled in each Open microsite selected, to balance the sampling effort. Finally, the same area was also sampled under the canopies of five Q. coccifera individuals. See Appendix S1 for a complete list of the sampled species and the sign of their interactions at either Stipa or Quercus microsites. Most of the species in the study area, excepting S. tenacissima (~0.6 m2), Q. coccifera and other sprouting shrubs (>2m2), have canopy areas of less than 0.1 m2, and therefore the selected quadrat size is a proper one to sample them. The large difference between the sizes of the nurses sampled and their neighbours allowed us to assume that, regardless of their age, these larger plants were significantly affecting the microclimatic and soil conditions of their surroundings and, therefore, acting really as nurse plants (Maestre et al. 2001, Aka and Darici, 2005).
The degree of co-occurrence between neighbouring species may be influenced by other non-facilitative processes, mainly dispersal (e.g. Verdú and García-Fayos 1996, Dean et al. 1999, Gómez-Aparicio 2008). We accounted for this refuge or nucleation process by including the dispersal mechanism of the beneficiary species in our analyses. Information on the dispersal mechanism was obtained from the literature (Herrera 1995, Narbona et al. 2005, Paula et al. 2009, Blanca et al. 2009, Castroviejo et al. 2010). Since nucleation processes are mainly driven by bird or small mammal dispersal, we separated the dispersal mechanism of our beneficiary plants into two categories: “zoochory” (those seeds with fleshy fruits or known to be dispersed mainly by birds or small mammals) or ”other” (summarizing the rest of dispersal mechanisms, such as gravity or wind).
For each possible Stipa- and Quercus-beneficiary species pairs at each site, we measured the intensity and importance of plant-plant interactions, i.e. the effect that neighbours have on their target species regardless of other environmental factors (intensity), and their relative effect compared to that of other environmental factors (importance, see Brooker et al. 2005). The intensity and importance of plant-plant interactions were measured with the RII (Armas et al. 2004) and the Iimp (Seifan et al. 2010) indices, respectively. RII was calculated as (PNurse − POpen)/(PNurse + POpen), where PNurse was the number of individuals under the canopy of a nurse plant (S. tenacissima or Q. coccifera) and POpen was the number or individuals recruited in the Open microsite. Alternatively, Iimp was calculated as Iimp= Nimp/|Nimp|+|Eimp|, where Nimp and Eimp were the nurse plant and environmental contributions to the total number of individuals recruited for each species, respectively. Nimp was calculated as PNurse − POpen, and Eimp as POpen − MPOpen/Nurse, where MPOpen/Nurse is the maximum number of recruited individuals for a given species found in the entire gradient, irrespective of the microsite sampled. For these analyses we used the total number of individuals found in the ~30 0.5 m × 0.5 m quadrats per microsite sampled at each site, and calculated a unique RII and Iimp index for each species and site. We used the total number of individuals, rather than the average number of individuals by quadrat, to reduce high errors in our indices caused by the high heterogeneity usually found in the spatial distribution of semi-arid plant individuals. Finally, we calculated which species were facilitation beneficiaries (i.e. those species with more individuals recruiting under a nurse plant, but with individuals also present in open spaces, showing preference for these microsites; Butterfield 2009) in each plot. We used the number of recruited individuals as an indicator for each species performance in all our assessments because seedling recruitment is the main bottleneck in arid and semi-arid areas (e.g. Eldridge et al. 1991, Escudero et al. 1999, Maestre et al. 2001). Thus, a higher number of recruited individuals in a given microsite indicates superior environmental conditions in that microsite.
As a complementary approach, we also evaluated changes in the intensity and importance of particular pairwise interactions along the studied gradient. To do this, we selected those beneficiary species present in at least three different sites across the sampled environmental gradient (19 in total; see Table 1) and calculated Iimp and RII for the interactions between the selected species and both Stipa and Quercus as nurse plants by using the number of individuals as our proxy of plant performance. This assessment allowed us to: 1) test the ecological relevance of the environmental gradient studied (if we detect facilitation/competition shifts in these interactions we can assume that differences in environmental conditions along such gradient are wide enough to properly evaluate their effect on plant-plant interactions), 2) ensure that the different degree of co-occurrence among these species was caused mainly by the sign of the interaction among them (changes in the coocurrence with their nurse species might be mainly caused by changes in the interaction between the beneficiary and nurse species as a response to different environmental conditions), and 3) by having three or more points along the environmental gradient, we were able to test the linearity or non-linearity of the relationship between plant-plant interactions and abiotic stress, as previously recommended (Lortie 2010).
Measuring evolutionary relationships among interacting species
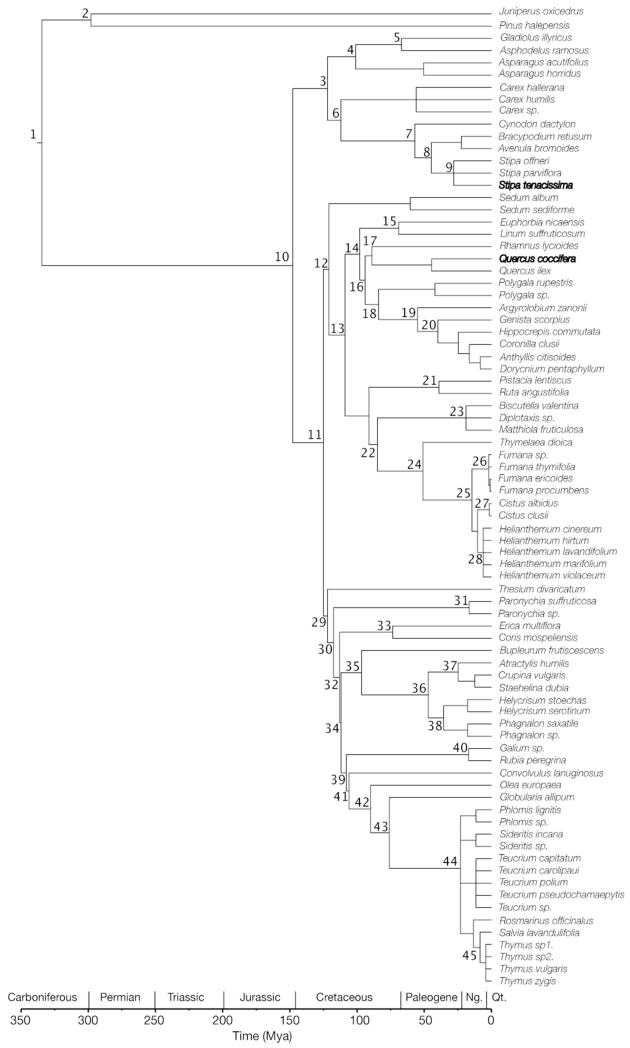
We assembled a phylogenetic tree for the 80 species included in this study using Phylomatic2 (available at: http://www.phylodiversity.net/phylomatic/phylomatic.html, Webb et al. 2008, Fig. 1). All the families in our dataset matched the family names of the angiosperm megatree used in Phylomatic (R20091110.new), which was based on the APG III phylogenetic classification of flowering plant orders and families (Angiosperm Phylogeny Group 2009). Within-family phylogenetic relationships were further resolved based on data from various published molecular phylogenies (Asteraceae [Funk et al. 2005, Susanna et al. 2006], Cistaceae [Guzmán and Vargas 2009, Guzmán et al. 2009], Fabaceae [Allan and Porter 2000, Allan et al. 2004, Wojciechowski et al. 2004], Poaceae [Bouchenak-Khelladi et al. 2004, 2010], Rubiaceae [Bremer and Erikson 2009]). After assembling the phylogenetic tree, we adjusted its branch lengths with the help of the Phylocom BLADJ algorithm, which fixes the age of internal nodes based on clade age estimates, whereas undated internal nodes in the phylogeny are spaced evenly (Webb et al. 2008). Thus, BLADJ is a simple tool that fixes the root node of a phylogeny at a specified age, as well as other nodes for which age estimates are available. It sets all other branch lengths by placing the nodes evenly between dated nodes, as well as between dated nodes and terminals (of Age 0). The Phylocom manual (Webb et al. 2008) suggests using the age estimates from Wikström et al. (2001), however, new analyses estimating divergence times for angiosperms are available since the publication of this seminal work (e.g. Anderson et al. 2005, Bell et al. 2010, Wang et al. 2010). According to Vamosi and Vamosi (2010), we used TimeTree (Hedges et al. 2006; http://www.timetree.org) to fix as many nodes in the tree as possible. TimeTree uses a tree-based (hierarchical) system to identify all published molecular time estimates bearing on the divergence of two chosen taxa (e.g. species), compute summary statistics, and present the results. We mainly used this database to fix the ages of internal nodes on our phylogenetic hypothesis, completing TimeTree results with other published sources when this database did not provide any date (see Appendix S2). The procedure described above resulted in the fixation of 45 nodes (representing almost the 70% of internal nodes of our tree, see Appendix S2). Once we assembled the phylogenetic tree, we measured the pairwise phylogenetic distance among both nurses studied (S. tenacissima and Q. coccifera) and every possible co-occurring target species in each site by using the “cophenetic” command for R statistical software (R Development Core Team 2009).
Figure 1.
Phylogenetic tree of the regional species pool. Nurse species are indicated in bold, and node labels are given in Appendix S2. Geological time scale is provided at the bottom of the figure.
Statistical analyses
To evaluate the existence of an interaction between PD and abiotic conditions, we assessed the relationships between different climatic variables (see below) and the mean PD obtained from all the 197 nurse-facilitation beneficiary species pairs in each site by using linear regressions. These species pairs were divided according to each microsite (Stipa or Quercus). The two nurses studied present large differences in ecological traits, such as root depth, productivity, litter deposition, canopy area and height (Puigdefábregas et al. 1999, Cañellas and San Miguel 2000, Filella and Peñuelas 2003). Moreover, S. tenacissima is a Monocot, while Q. coccifera is an Eudicot, which may affect importantly their competitive ability and the effect on their neighbours (Cahill et al. 2008). Hence, the effect of evolutionary relationships and climate in the interactions between both nurse-types and their neighbours were analyzed separately. We used mean annual rainfall and the average irradiance of each plot as climatic predictors. Rainfall has been widely demonstrated as a crucial driver of plant-plant interactions in semi-arid environments (e.g. Pugnaire and Luque 2001, Maestre and Cortina 2004). On the other hand, irradiance can largely affect the outcome of pairwise interactions (e.g. Maestre et al. 2003) and has also been revealed as a significant predictor of the outcome of the pairwise interactions included in this study (see results below). Thus, we evaluated the existence of interactions between average daily irradiance and PD. We also assessed the relationships between plant-plant interactions (intensity and importance) and both mean annual rainfall and average daily irradiance. These assessments were conducted using only those pairwise interactions occurring in three or more of the studied plots (19 pairwise interactions). This allowed us to evaluate of the linearity or non-linearity of the relationship between plant-plant interactions and the climatic variables considered (Lortie 2010). However, we assessed these relationships visually, as the low number of data points available (n = 3-11; Table 1) did not allow us to properly test their statistical significance. The assessment of these relationships was conducted separately for the four possible combinations resulting from the two indicators (RII or Iimp) and predictor (rainfall or irradiance) used, and the outcomes were divided into four categories: “positive” or “negative”, when the interaction indicator increased or decreased monotonically with the environmental variable used, respectively, “hump-shaped”, when the highest values of the interaction indicator occurred at moderate levels of the environmental variable, and “nill”, when the interaction indicator did not show any trend with the environmental variable used as predictor.
We analyzed the effect of PD, dispersal mechanism and environmental variables (temperature, rainfall, irradiance and altitude) on both plant-plant interaction indicators (intensity and importance) for each nurse-target species by using Regression trees (De’ath and Fabricius 2000), as implemented in the Tree package of R (R Development Core Team 2009). We used 10-fold cross-validation to fit the most parsimonious model to each dataset (De’ath and Fabricius 2000). Temperature, rainfall, irradiance, altitude, dispersal mode, and the PD between each target species and its nurse were used as predictor variables in the four regression trees performed (RII and Iimp indices for Stipa and Quercus microsites). Despite this relatively low number of predictors, we used this procedure because its flexibility and few analytical assumptions. Regression trees also allow detecting non-linear relationships, and are insensitive to the distribution of either the predictor or response variables (De’ath and Fabricius 2000). Moreover, this technique can deal with the heavily skewed nature of the PD values, a characteristic commonly found with these data (Castillo et al. 2010) and the nested nature of our experimental design (pairwise interactions nested within plots; De’ath and Fabricius 2000). Although it would be preferable to have independent climatic measurements for each of the pairwise interactions studied, this latter property of the regression trees ensure robustness against the partial lack of independence among pairwise interactions within the same plot. Since a relatively low number of predictors were significant in the regression trees (see Results below), we also performed a separate regression tree using each of the significant predictor found. These separate analyses allowed us to assess for the percentage of variance explained by each of the predictor variables, while the regression tree using all the predictors together gave us the total percentage of variance explained by all the factors included. To quantify the influence of the “possum effect” (Heard and Cox 2007), i.e. the influence of extremely unrelated or phylogenetically distant lineages, such as conifers in our case, in the results and conclusions of our analyses, we performed the regression trees with and without the three conifer-nurse pairs found. The results were identical in both cases, therefore we included all nurse-target pairs in our analyses with the confidence that our results and conclusions are not derived from the influence of a few extremely distant lineages.
Results
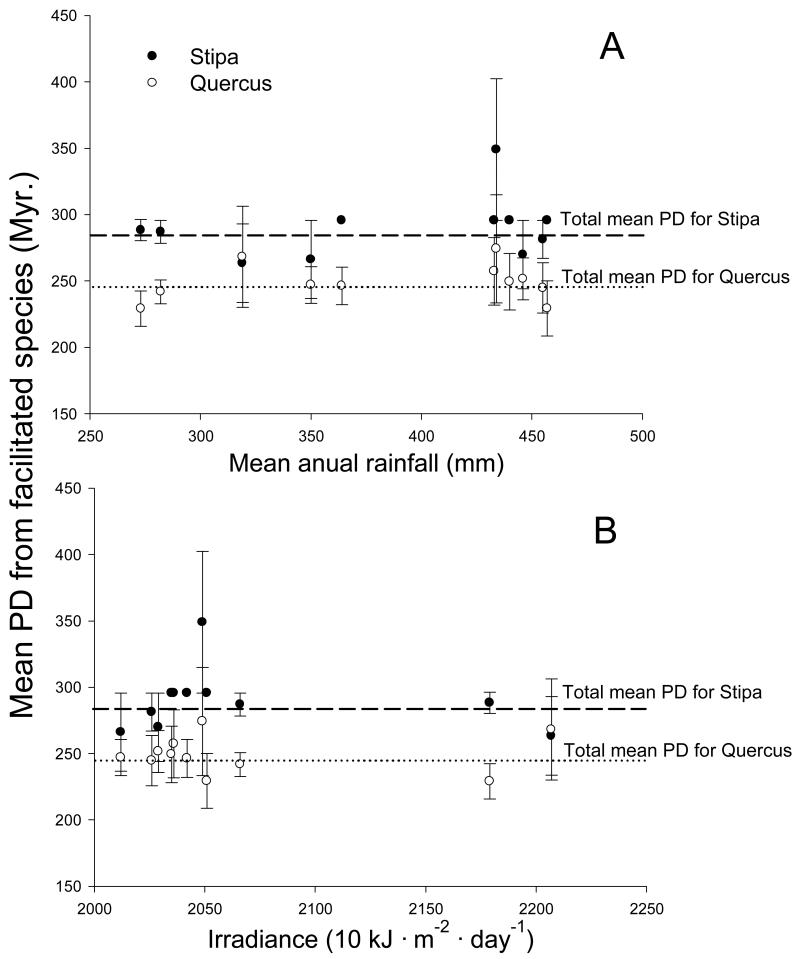
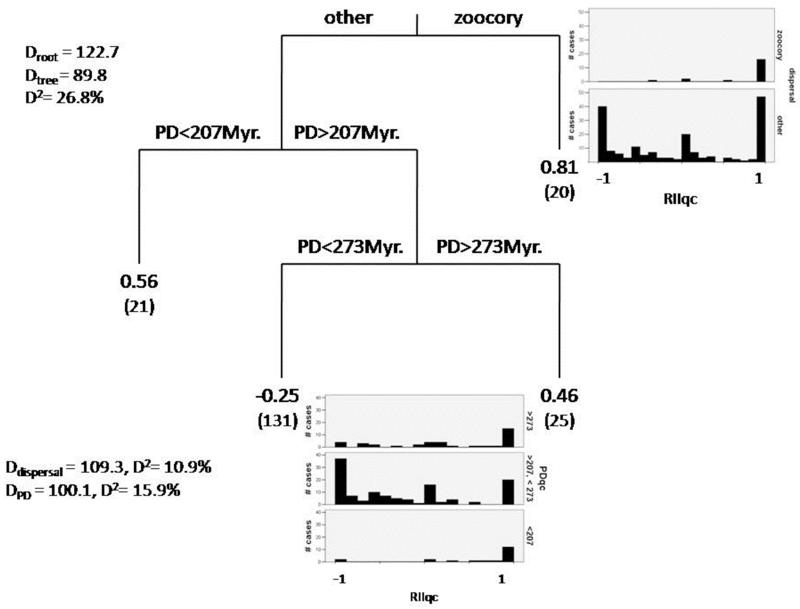
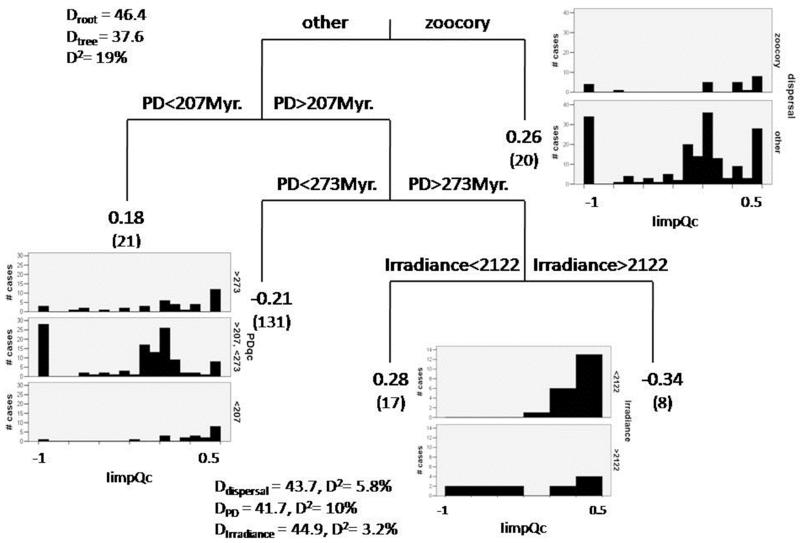
The mean PD between nurse and facilitated species remained constant across the entire environmental gradient sampled, suggesting that climatic conditions (irradiance and rainfall) and evolutionary relationships did not interact to define the outcome of pairwise interactions (Fig. 2). Neither environmental conditions (altitude, rainfall, temperature or irradiance) nor PD or dispersal mechanism were good predictors for the RII and Iimp data when S. tenacissima was the nurse (Regression trees, D2 < 5% in both cases). Conversely, regression trees predicted 27% and 19% of the variance of the RII and Iimp indices when Q. coccifera was the nurse (Figs. 3 and 4). Species dispersed by animals always rendered more positive results than those dispersed by other mechanisms with both RII and Iimp. Dispersal mechanism, therefore, explained ~10% of the variance for both indices (Figs. 3 and 4), regardless of the evolutionary relationship between the beneficiary and the nurse species (zoochory was present across the entire range of PD included). The PD was the predictor that explained most of the variance (15% of the RII and 10% of the Iimp) in both indices. When Q. coccifera was the nurse, values of this variable between 207 and 272.8 million years (Myr, i.e. taxa that started to evolve separately from Q. coccifera lineage 103.5 and 136.4 Myr. ago, respectively; see Fig. 1 and Appendix S2) rendered negative or neutral interactions, while values outside this range (i.e., PD < 207 Myr or PD > 272.8 Myr) showed mostly positive results for both RII and Iimp. Irradiance was a modulator of secondary importance for Iimp, explaining ~3% of its variance; when PD values were higher than 272.8 Myr, the interactions were positive or negative depending on the irradiance level. None of the other environmental variables were significant predictors of RII and Iimp.
Figure 2.
Relationship between the mean PD between facilitation beneficiary species and their nurses and the mean annual rainfall (A) or the average daily irradiance (B) within each study plot. No significant relationships were found (R2 < 0.1 and P > 0.3 in all the cases).
Figure 3.
Regression trees conducted with the interaction intensity index when Quercus coccifera was a nurse (RIIqc). Split values for each predictor used that became significant (dispersal and PD) are shown in each branch. Terminal nodes show the mean value of RII for each group and the number of cases in each node (between parenthesis, n = 197 cases). The general fit of the model (D2, percentage of variance explained by the model), the percentage of variance explained by each predictor, extracted from the null deviance (Deviance root), and the deviance of the final chosen tree after a 10-fold cross-validation (Deviance tree) are shown. To aid with the interpretation of these results, the histogram of frequencies of the RII values for each splitted value of the significant predictors is included in the figure. Please, notice that the histograms were constructed only with the cases remaining from the previous splits (i.e. for the histogram of RII divided by PD intervals, only species dispersed by mechanisms other than zoochory were included).
Figure 4.
Regression trees conducted with the interaction importance index when Quercus coccifera was a nurse (IimpQc). Split values for each predictor used that became significant (dispersal, PD and irradiance) are shown in each branch. Rest of legend as in Figure 3.
Sixteen of the 19 pairwise interactions occurring in three or more of the plots showed shifts in the direction, intensity or importance of their outcome along the environmental gradient studied (Table 1). However, the response of both the intensity and importance of the interaction to either rainfall or irradiance depended on the beneficiary species assayed, showing a high degree of species-specificity in the response to the environment of these pairwise interactions. To name a few examples, the interaction between Polygala rupestris and either S. tenacissima or Q. coccifera became more positive with increasing rainfall or decreasing irradiance. However, the contrary was found for Asphodelus ramosus or Fumana ericoides, whose interactions with their nurses turned more negative with increasing rainfall; or with Teucrium capitatum, which presented more positive interactions with both S. tenacissima and Q. coccifera when irradiance increased. Hump-shaped relationships between the interaction indicators and rainfall or irradiance, conversely, were common for Asparragus horridus, Helianthemum violaceum or Sedum sediforme. On the other hand, the different components of the interactions studied (intensity and importance) responded differently to both rainfall and irradiance within the same beneficiary species, with a preponderance of hump-shaped or monotonical relationships for the RII and the Iimp indices, respectively (Table 1).
Discussion
Our results revealed that PD is a better predictor of the outcome of pairwise plant-plant interactions than environmental conditions. Both the presence of fleshy fruits in the beneficiary species and the evolutionary relationship between the beneficiary and the benefactor explained the degree of co-occurrence of the large set of pairwise interactions involving Q. coccifera, but not S. tenacissima. The different predictors considered in this study included environmental conditions (temperature, irradiance, rainfall and altitude), dispersal and evolutionary relationships, all of them known to importantly affect the cooccurrence among plants (e.g. Pausas et al. 2006, Callaway 2007, Verdú and Valiente-Banuet 2011). However, we found a relatively low predictability of such factors (<30% of the variance of the interactions involving Q. coccifera, and much lower values when S. tenacissima was the nurse). This low predictability might be related to the presence of other unmeasured factors that could be affecting the outcome of the studied interactions and that were not considered in our observational design. Among them, the different ontogenetic stages of the involved plants (Miriti 2006, Valiente-Banuet and Verdú 2008, Soliveres et al. 2010), differences in herbivory pressure among sites (Callaway et al. 2000, Smit et al. 2009, Soliveres et al. 2011), or evolutionary convergent ecophysiological traits not detectable with our phylogenetic approach (Cahill et al. 2008), could account for most of the unexplained variance. The low predictability of the climatic factors was particularly surprising, as they are thought to be of paramount importance as drivers of plant-plant interactions (Callaway 2007). The visual assessment of the 19 pairwise interactions found in at least three of the studied plots allowed us to explain such low predictability; the response of pairwise interactions to different environmental conditions depends on the species involved (Greiner la Peyre 2001, Liancourt et al. 2005, Gross et al. 2010, Table 1). In other words, climatic conditions are not good general predictors for the outcome of pairwise interactions because each of these interactions will respond differently to changes in the environmental conditions.
Despite the multiplicity of factors affecting plant-plant interactions, the evolutionary relationship was an important predictor of the outcome of the interactions between Q. coccifera and its neighbours, explaining 16 and 10% of the intensity and importance of such interactions, respectively (Figs. 3 and 4). We found a double threshold in PD values that defined the sign of the interactions, with PD values between 207 and 272.8 Myr always rendering competition, and values outside these thresholds leading to facilitation. While the dominance of positive interactions among phylogenetic distant species agrees with current literature (Valiente-Banuet et al. 2006, Castillo et al. 2010), those from the lower threshold (facilitation among closely-related taxa) do not. Regardless of the exact dates of the threshold found, the idea that a threshold in the minimum PD between a nurse and its neighbour species may define the outcome of their interaction is particularly appealing. It suggests that, regardless of the time when the interacting species diverged, it is more likely to find positive interactions among distantly-related species, which likely differ in their ecological traits and therefore in the environmental conditions that they can cope with (Webb et al. 2002, Valiente-Banuet et al. 2006). Indeed, this upper threshold in PD fits surprisingly well with results from a recent study in the Mexican scrubland (Castillo et al. 2010). In that study, lower PDs always rendered negative interactions, while higher PDs could mean either positive or negative outcomes. Conversely, the lower threshold in PD found (< 207 Myr.) match with lineages that had their major radiation event during the Tertiary, as the nurse plant Q. coccifera (Bell et al. 2010). This result could be indicating that not only Quaternary, but also other Tertiary species, might maintain the regeneration niche of species evolved during the milder environmental conditions in the Tertiary (Valiente-Banuet et al. 2006). Thus, not only the evolutionary distance among interacting species, but the time and environmental conditions where these species evolved seem crucial to define the outcome of the interaction among them.
Another crucial factor determining the degree of co-occurrence among the studied species was dispersal (Pausas and Verdú 1996, Dean et al. 1999, Gómez-Aparicio 2008). This suggests that the co-occurrence of fleshy-fruited species with Q. coccifera is not only caused by a facilitatory effect of the nurse shrub per se, but rather with the role of sprouting shrubs as a refuge for animals. This refuge effect could promote an increase in the deposition of seeds by birds of animal-dispersed species and, therefore, foster the co-occurrence with the studied shrub through nucleation processes (Dean et al. 1999, Pausas et al. 2006). Unfortunately, our observational approach does not allow us to disentangle between dispersal and microclimatic amelioration as drivers of the co-occurrence of these fleshy-fruited species. Studies aimed to unravel the relative importance of both factors (dispersal processes and microclimatic amelioration) on the co-ocurrence level among species may shed some light in the relationship between plant-plant interactions and the spatial pattern in a given community (e.g. Tirado and Pugnaire 2005, Gómez-Aparicio 2008).
Previous research has repeatedly illustrated how the different drivers of plant-plant interactions jointly define their outcome, with different results obtained when considering just one or several factors affecting such interactions (Baumeister and Callaway 2006, Kawai and Tokeshi 2007, Soliveres et al. 2011). Accordingly, we expected environmental conditions and the PD between the species involved to interact and jointly determine the outcome of the studied interactions. However, not an interaction, but a hierarchy, between PD and environmental conditions can be envisaged from our results. This is demonstrated by: 1) the lack of relationship between the mean PD between facilitated species-nurse pairs and different environmental conditions (Fig. 2), and 2) the results from regression trees, which indicate that climatic conditions were only important once PD reached some threshold values (PD > 272.8 Myr; Fig. 4). The high species-specificity found in the response to environmental conditions (Table 1) and the higher importance of PD as a general predictor for the outcome of pairwise interactions cast doubt to the validity of theoretical approaches based only on including environmental factors to predict the behavior of plant-plant interactions along environmental gradients. Albeit our results certainly require further validation in other environments, they suggest that species-specific factors might be even more important than environmental conditions in defining plant-plant interactions, and therefore should be included in theoretical and conceptual models aimed to predict the outcome of plant-plant interactions (Hacker and Gaines 1997, Maestre et al. 2009, Verdú and Valiente-Banuet 2011).
Conclusions
Interactions between the different factors affecting plant-plant interactions may lead to counter-intuitive or antagonistic responses (Baumeister and Callaway 2006, Kawai and Tokeshi 2007), and thus should be studied jointly to properly infer their relative importance as drivers of such interactions (Brooker et al. 2008). Our study represents the first attempt to test the generality of environmental conditions and evolutionary relationships, and their combined effects, as drivers of plant-plant interactions by using a large number of pairwise interactions. Our results indicate that the evolutionary relationships can be more important than environmental conditions as determinants of particular pairwise interactions in semi-arid steppes, and that the relative importance of both factors depends on the species involved. They can also help to further refine current conceptual models aiming to predict how plant-plant interactions change along environmental gradients (Callaway 2007, Maestre et al. 2009), and provide new insights on the potential responses of these interactions to the predicted changes in species composition and climate associated to the ongoing global environmental change.
Supplementary Material
Appendix S1.
Table S1. Summary of the outcomes of the pairwise interactions found at each site.
Appendix S2.
Table S2. Estimated ages of branching events used for dating the phylogenetic tree. Node label indicates the node within the tree given in Figure 1.
Acknowledgements
We thank E. Pastor, B. Amat, L. Cayuela, M. D. Puche, M. Bowker, P. García-Palacios and A. Escudero for their help during different stages of this work. SS was supported by a PhD fellowship from the EXPERTAL project, funded by Fundación Biodiversidad and CINTRA S.A. This research was funded by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n° 242658 (BIOCOM) awarded to FTM.
References
- Aka H, Darici C. Carbon and nitrogen mineralization in carob soils with Kermes oak and Aleppo pine leaf litter. Eur. J. Soil Biol. 2005;41:31–38. [Google Scholar]
- Allan GJ, Porter JM. Tribal delimitation and phylogenetic relationships of Loteae and Coronilleae (Faboideae: Fabaceae) with special reference to Lotus: evidence from nuclear ribosomal ITS sequences. Am. J. Bot. 2000;87:1871–1881. [PubMed] [Google Scholar]
- Allan GJ, et al. Molecular phylogenetic evidence for the geographic origin and classification of Canary Islands Lotus (Fabaceae: Loteae) Mol. Phylog. Evol. 2004;32:123–138. doi: 10.1016/j.ympev.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Anderson CL, et al. Dating phylogenetically basal eudicots using rbcL sequences and multiple fossil reference points. Am. J. Bot. 2005;92:1737–1748. doi: 10.3732/ajb.92.10.1737. [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J Linn. Soc. 2009;161:105–121. [Google Scholar]
- Armas C, et al. Measuring plant interactions: A new comparative index. Ecology. 2004;85:2682–2686. [Google Scholar]
- Baumeister D, Callaway RM. Facilitation by Pinus flexilis during succession: A hierarchy of mechanisms benefits other plant species. Ecology. 2006;87:1816–1830. doi: 10.1890/0012-9658(2006)87[1816:fbpfds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bell CD, et al. The age and diversification of the angiosperms re-visited. Am. J. Bot. 2010;97:1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- Bertness MD, Callaway RM. Positive interactions in communities. Trends Ecol. Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Blanca G, et al., editors. Flora Vascular de Andalucía Oriental. 4 s. Consejería de Medio Ambiente, Junta de Andalucía; Sevilla: 2009. [Google Scholar]
- Bouchenak-Khelladi Y, et al. Large multi-gene phylogenetic trees of the grasses (Poaceae): Progress towards complete tribal and generic level sampling. Mol. Phylog. Evol. 2008;47:488–505. doi: 10.1016/j.ympev.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Bouchenak-Khelladi Y, et al. Biogeography of the grasses (Poaceae): A phylogenetic approach to reveal evolutionary history in geographical space and geological time. Biol. J. Linn. Soc. 2010;162:543–557. [Google Scholar]
- Bremer B, Eriksson O. Time tree of Rubiaceae: Phylogeny and dating the family, subfamilies and tribes. Int. J. Plant Sci. 2009;170:766–793. [Google Scholar]
- Brooker RW, et al. The importance of importance. Oikos. 2005;109:63–70. [Google Scholar]
- Brooker RW, et al. Facilitation in plant communities: the past, the present, and the future. J. Ecol. 2008;96:18–34. [Google Scholar]
- Butterfield BJ. Effects of facilitation on community stability and dynamics: synthesis and future directions. J. Ecol. 2009;97:1192–1201. [Google Scholar]
- Cahill JF, et al. Does the phylogenetic relatedness influence the strength of competition among vascular plants? –Persp. Plant Ecol. Evol. Syst. 2008;10:41–50. [Google Scholar]
- Callaway RM. Positive Interactions and Interdependence in Plant Communities. Springer; 2007. [Google Scholar]
- Callaway RM, et al. Facilitation by unpalatable weeds may conserve plant diversity in overgrazed meadows in the Caucasus Mountains. Oikos. 2000;89:275–282. [Google Scholar]
- Cañellas I, San Miguel A. Biomass of root and shoot systems of Quercus coccifera shrublands in Eastern Spain. Ann. For. Sci. 2000;57:803–810. [Google Scholar]
- Castillo JP, et al. Neighborhood phylodiversity affects plant performance. Ecology. 2010;91:3656–3663. doi: 10.1890/10-0720.1. [DOI] [PubMed] [Google Scholar]
- Castroviejo S, et al. Flora Ibérica. 2010 http://www.floraiberica.org/
- Dean WR, et al. Large trees, fertile islands, and birds in arid savanna. J. Arid Env. 1999;41:61–78. [Google Scholar]
- De’ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- Eldridge DJ, et al. Soil-surface characteristics, microtopography and proximity to mature shrubs: effects on survival of several cohorts of Atriplex vesicaria seedlings. J. Ecol. 1991;79:357–364. [Google Scholar]
- Escudero A, et al. Factors controlling the establishment of Helianthemum squamatum, an endemic gypsophile of semi-arid Spain. J. Ecol. 1999;87:290–302. [Google Scholar]
- Filella EI, Peñuelas J. Partitioning of water and nitrogen in co-occurring Mediterranean woody shrub species of different evolutionary history. Oecologia. 2003;137:51–61. doi: 10.1007/s00442-003-1333-1. [DOI] [PubMed] [Google Scholar]
- Funk VA, et al. Everywhere but Antarctica: Using a supertree to understand the diversity and distribution of the Compositae. Biol. Skrifter. 2005;55:343–374. [Google Scholar]
- Garcia-Fayos P, Gasque M. Consequences of a severe drought on spatial patterns of woody plants in a two-phase mosaic steppe of Stipa tenacissima L. J. Arid Env. 2002;52:199–208. [Google Scholar]
- Gómez-Aparicio L. Spatial patterns of recruitment in a Mediterranean tree (Acer opalus subsp. granatense): linking the fate of seeds, seedlings, and saplings in heterogeneous landscapes at different scales. J. Ecol. 2008;96:1128–1140. [Google Scholar]
- Greiner La Peyre MK, et al. The importance of competition in regulating species abundance along a salinity gradient. Ecology. 2001;82:62–69. [Google Scholar]
- Gross N, et al. Strain and vegetation effects on local limiting resources explain the outcomes of biotic interactions. Persp. Plant Ecol. Evol. Syst. 2010;12:9–19. [Google Scholar]
- Guzmán B, Vargas P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Organ. Div. Evol. 2009;9:83–99. [Google Scholar]
- Guzmán B, et al. Adaptive radiation in Mediterranean Cistus (Cistaceae) Plos ONE. 2009;4:e6362. doi: 10.1371/journal.pone.0006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker SD, Gaines SD. Some implications of direct positive interactions for community species diversity. Ecology. 1997;78:1990–2003. [Google Scholar]
- Heard SB, Cox GH. The Shapes of Phylogenetic Trees of Clades, Faunas, and Local Assemblages: Exploring Spatial Pattern in Differential Diversification. Am. Nat. 2007;169:E107–E118. doi: 10.1086/512690. [DOI] [PubMed] [Google Scholar]
- Hedges SB, et al. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Dispersal systems in the Mediterranean: ecological, evolutionary and historical determinants. Ann. Rev. Ecol. Syst. 1995;26:705–727. [Google Scholar]
- Holmgren M, Scheffer M. Strong facilitation in mild environments: the stress gradient hypothesis revisited. J. Ecol. 2010;98:1269–1275. [Google Scholar]
- Kawai T, Tokeshi M. Testing the facilitation-competition paradigm under the stress-gradient hypothesis: decoupling multiple stress factors. Proc. R. Soc. Lond. B. 2007;274:2503–2508. doi: 10.1098/rspb.2007.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liancourt P, et al. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. [Google Scholar]
- Lortie CJ. Synthetic analysis of the Stress-Gradient Hypothesis. In: Pugnaire FI, editor. Positive plant interactions and community dynamics. Fundación BBVA & CRC Press, Taylor & Francis Group; 2010. pp. 125–149. [Google Scholar]
- Maestre FT, et al. Potential for using facilitation by grasses to establish shrubs on a semi-arid degraded steppe. Ecol. Appl. 2001;11:1641–1655. [Google Scholar]
- Maestre FT, et al. Positive, negative and net effects in grass-shrub interactions in Mediterranean semiarid grasslands. Ecology. 2003;84:3186–3197. [Google Scholar]
- Maestre FT, et al. Ecología del esparto (Stipa tenacissima L.) y los espartales de la Península Ibérica. Ecosistemas. 2007;16:117–136. [Google Scholar]
- Maestre FT, et al. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009;97:199–205. [Google Scholar]
- Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc. R. Soc. Lond. B. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Cortina J. Remnant shrubs in Mediterranean semi-arid steppes: effects of shrub size, abiotic factors and species identity on understorey richness and occurrence. Acta Oecol. 2005;27:161–169. [Google Scholar]
- Michalet R, et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Miriti MN. Ontogenetic shift from facilitation to competition in a desert shrub. J. Ecol. 2006;94:973–979. [Google Scholar]
- Narbona E, et al. Explosive seed dispersal in two perennial Mediterranean Euphorbia species (Euphorbiaceae) Am. J. Bot. 2005;92:510–516. doi: 10.3732/ajb.92.3.510. [DOI] [PubMed] [Google Scholar]
- Navarro FB, et al. Stipa tenacissima as a nurse plant of the endemic species Haplophyllum bastetanum near Granada, SE Spain. Appl. Veg. Sci. 2008;11:63–72. [Google Scholar]
- Ninyerola M, et al. Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. Universidad Autónoma de Barcelona; Bellaterra, Spain: 2005. [Google Scholar]
- Paula S, et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:1420. [Google Scholar]
- Puigdefábregas J, et al. Scales and processes of water and sediment redistribution in drylands: results from the Rambla Honda field site in Southeast Spain. Earth Sci. Rev. 1999;48:39–70. [Google Scholar]
- Pugnaire FI, et al. Facilitation and succession under the canopy of the leguminous shrub, Retama sphaerocarpa, in a semi-arid environment in south-east Spain. Oikos. 1996;76:455–464. [Google Scholar]
- Pugnaire FI, et al. Positive plant interactions in the Iberian Southeast: mechanisms, environmental gradients, and ecosystem function. J. Arid Env. In press. [Google Scholar]
- Pugnaire FI, Luque M. Changes in plant interactions along a gradient of environmental stress. Oikos. 2001;93:42–49. [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- Seifan M, et al. Facilitating an importance index. J. Ecol. 2010;98:356–361. [Google Scholar]
- Smit C, et al. Inclusion of biotic stress (consumer pressure) alters predictions from the stress gradient hypothesis. J. Ecol. 2009;97:1215–1219. [Google Scholar]
- Soliveres S, et al. Spatio-temporal heterogeneity in abiotic factors modulate multiple ontogenetic shifts between competition and facilitation. Persp. Plant Ecol. Evol. Syst. 2010;12:227–234. [Google Scholar]
- Soliveres S, et al. Temporal dynamics of herbivory and water availability interactively modulate the outcome of a grass-shrub interaction in a semi-arid ecosystem. Oikos. 2011;120:710–719. [Google Scholar]
- Susanna A, et al. The Cardueae (Compositae) revisited: insights from ITS, trnL-trnF, and matK nuclear and chloroplast DNA analysis. Ann. Miss. Bot. Garden. 2006;93:150–171. [Google Scholar]
- Tirado R, Pugnaire FI. Community structure and positive interactions in constraining environments. Oikos. 2005;111:437–444. [Google Scholar]
- Valiente-Banuet A, Verdú M. Facilitation can increase the phylogenetic diversity of plant communities. Ecol. Lett. 2007;10:1029–1036. doi: 10.1111/j.1461-0248.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- Valiente-Banuet A, et al. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc. Natl. Acad. Sci. USA. 2006;103:16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi JC, Vamosi SM. Key innovations within a geographical context in flowering plants: towards resolving Darwin’s abominable mystery. Ecol. Lett. 2010;13:1270–1279. doi: 10.1111/j.1461-0248.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- Verdú M, García-Fayos P. Nucleation processes in a Mediterranean bird-dispersed plant. Func. Ecol. 1996;10:275–280. [Google Scholar]
- Verdú M, Valiente-Banuet A. The relative contribution of abundance and phylogeny to the structure of plant facilitation networks. Oikos. 2011;120:1351–1356. [Google Scholar]
- Wang H, et al. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proc. Natl. Acad. Sci. USA. 2010;106:3853–3858. doi: 10.1073/pnas.0813376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, et al. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 2002;33:475–505. [Google Scholar]
- Webb CO, et al. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Wikström N, et al. Evolution of the angiosperms: calibrating the family tree. Proc. R. Soc. Lond. B. 2001;268:2211–2219. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski MF, et al. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004;91:1846–1862. doi: 10.3732/ajb.91.11.1846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Table S1. Summary of the outcomes of the pairwise interactions found at each site.
Appendix S2.
Table S2. Estimated ages of branching events used for dating the phylogenetic tree. Node label indicates the node within the tree given in Figure 1.