Abstract
Aim
The global spread of woody plants into grasslands is predicted to increase over the coming century. While there is general agreement regarding the anthropogenic causes of this phenomenon, its ecological consequences are less certain. We analyzed how woody vegetation of differing cover affects plant diversity (richness and evenness) and multiple ecosystem functions (multifunctionality) in global drylands, and how this changes with aridity.
Location
224 dryland sites from all continents except Antarctica widely differing in their environmental conditions (from arid to dry-subhumid sites) and woody covers (from 0 to 100%).
Methods
Using a standardized field survey, we measured the cover, richness and evenness of perennial vegetation. At each site, we measured 14 ecosystem functions related to soil fertility and the build-up of nutrient pools. These functions are critical for maintaining ecosystem function in drylands.
Results
Species richness and ecosystem multifunctionality were strongly influenced by woody vegetation, with both variables peaking at relative woody covers (RWC) of 41-60%. This relationship shifted with aridity. We observed linear positive effects of RWC in dry-subhumid sites. These positive trends shifted to hump-shaped RWC-diversity and multifunctionality relationships under semiarid environments. Finally, hump-shaped (richness, evenness) or linear negative (multifunctionality) effects of RWC were found under the most arid conditions.
Main conclusions
Plant diversity and multifunctionality peaked at intermediate levels of woody cover, although this relationship became increasingly positive under wetter environments. This comprehensive study accounts for multiple ecosystem attributes across a range of woody covers and environmental conditions. Our results help us to reconcile contrasting views of woody encroachment found in current literature and can be used to improve predictions of the likely effects of encroachment on biodiversity and ecosystem services.
Keywords: aridity, species evenness, species richness, semiarid, thicketization, shrub encroachment, soil
INTRODUCTION
Overgrazing or alterations in rainfall patterns and atmospheric CO2 concentrations due to human activity are among the major causes of widespread increases in the cover and abundance of woody vegetation into former grasslands worldwide (Schlesinger et al., 1990; Van Auken, 2000; Sankaran et al., 2008). This phenomenon, hereafter referred to as woody encroachment, will likely increase in intensity and extent under predicted future environmental scenarios (Kulmatinski & Beard, 2012; Blois et al., 2013). Woody encroachment is known to affect structural and functional attributes of ecosystems, including plant spatial patterns (Schlesinger et al., 1990), plant composition and diversity (Ratajczak et al., 2012; Quero et al., 2013; Soliveres & Eldridge, 2013) and nutrient cycling (Throop & Archer, 2007; Knapp et al., 2008; Eldridge et al., 2013). Alteration to nutrient cycles results in substantial changes in the provision of key ecosystem services such as forage production (Riginos et al., 2009), carbon storage (Knapp et al., 2008; Jackson et al., 2000a) and the regulation of climate (Blois et al., 2013). Woody encroachment is particularly prominent across arid, semi-arid and drysubhumid ecosystems (drylands; Van Auken, 2000; Sankaran et al., 2008; Maestre et al., 2009), where it has been traditionally linked to land degradation and desertification (Schlesinger et al., 1990; MEA, 2005). In these areas, many billions of dollars have been spent worldwide to reduce the cover of woody plants to enhance the pastoral value for livestock using a range of chemical and mechanical techniques (e.g., Teague, 1994; Daryanto & Eldridge, 2010).
While the links between woody encroachment and desertification have dominated scientific and management efforts for years, there are multiple lines of evidence for positive or neutral effects of encroachment on ecosystem structure and functioning (Maestre et al., 2009; Barger et al., 2011; Quero et al., 2013). Indeed, a recent review found little evidence to support the contention that woody encroachment has an overall negative impact on dryland ecosystems (Eldridge et al., 2011). Current research suggests that the magnitude, and particularly the sign, of woody encroachment effects on ecosystems is highly variable, and may depend on both environmental factors and the degree of shrub dominance (Breshears, 2006; Soliveres & Eldridge, 2013). Environmental conditions are particularly important to understand the impacts of woody encroachment. For example, aridity alters the effects of encroachment on C sequestration or herbaceous productivity (Jackson et al., 2000a; Knapp et al., 2008; Blaser et al., 2013) and the increase in grazing pressure influences the effects of encroachment on plant diversity and several ecosystem functions (Eldridge et al., 2013). Additionally, despite the lack of strong empirical support, it has been suggested that the effects of woody encroachment vary with the degree of encroachment (i.e., the density or cover of woody species; Breshears, 2006). Positive effects are expected under intermediate levels of encroachment for attributes such as diversity (Blaum et al., 2007; Sirami et al., 2009), while the capture of resources, such as C or water, may linearly increase towards higher woody densities (Breshears, 2006; Riginos et al., 2009; Eldridge et al., 2013). These major drivers of the effect of woody encroachment (the degree of encroachment and environmental conditions) are likely to simultaneously and interactively influence the structure and function of natural ecosystems. However, they are generally considered in isolation. Most studies of encroachment have tended to focus on either a single site or similar environmental conditions (reviewed in Archer et al., 2001; House et al., 2003; Breshears, 2006), or consider paired plots (encroached vs unencroached) without accounting for true gradients in woody encroachment. This narrow focus hinders our capacity to develop general models of woody plant effects on dryland ecosystems across a broad range of environments. Furthermore, most previous research on the ecological effects of woody encroachment has evaluated a limited set of ecosystem attributes at any one time (see Eldridge et al., 2011 for a review), despite the fact that ecosystems are valued primarily for the multiple functions and services they provide, and woody encroachment can affect multiple structural and functional variables simultaneously (MEA, 2005; Eldridge et al., 2011; Quero et al., 2013).
Using a space-for-time substitution, we address the role of different degrees of woody abundance (which might help to explain the ecological consequences of woody encroachment; Breshears, 2006) on multiple ecosystem structural and functional variables across a wide variety of communities and environmental conditions. We used data from 224 dryland sites from all continents except Antarctica, to analyze the effect of differing degrees of woody dominance on ecosystem structure and multiple ecosystem functions, and to explore how environmental conditions alter such effects. We measured the relative cover of woody species together with plant diversity (species richness and evenness) and 14 ecosystem functions related to soil fertility and the buildup of nutrient pools, which were analysed both separately and simultaneously by using an index of multifunctionality (Maestre et al., 2012). Our results show how both aridity and woody dominance interact to affect plant diversity and ecosystem multifunctionality in global drylands. As drylands are particularly sensitive to climate change and woody encroachment (Van Auken, 2000; Kulmatinsky & Beard, 2012), our results provide new insights into the potential consequences of widespread woody plant increase on dryland biodiversity and ecosystem functioning.
MATERIALS AND METHODS
Study sites and environmental data
We sampled 224 dryland sites located in 16 countries from all continents except Antarctica (Argentina, Australia, Brazil, Chile, China, Ecuador, Iran, Israel, Kenya, Mexico, Morocco, Peru, Spain, Tunisia, USA and Venezuela). These sites covered a wide range of woody cover (from 0 to 100% relative woody cover), environmental conditions (66-1219 mm average annual rainfall, −1.8 °C-27.8°C average annual temperature, 69-4668 m.a.s.l., 3-83% total plant cover, more than 25 different soil types according to the FAO [UN Food and Agricultural and Organization] classification), and vegetation (grasslands, shrublands and savannas) and land management (from protected areas to rangelands) types (see Maestre et al., 2012 for more details).
In all of these sites, we established four 30 m-long transects in which the total plant and woody coverage were measured using a line-intercept method. From these data, we calculated the relative woody cover (RWC) as the percentage of the total plant cover that was occupied by woody species. We used relative woody cover (woody cover * 100/total plant cover) instead of woody cover per se to account for the marked differences in total cover among the sampled sites, which could potentially influence any conclusions on the effects of total plant cover on ecosystem functioning in drylands (Greene et al., 1994; Maestre & Escudero, 2009). Within each site, we also registered the total number of perennial plant species and their cover within 80 1.5 × 1.5 m sampling quadrats (see Maestre et al., 2012). These data were used to calculate species richness (total number of plant species found within each site) and species evenness (Hurlbert’s PIE [probability of inter-specific encounter]; Hurlbert, 1971).
We acknowledge that woody encroachment is pre-eminently a temporally dynamic process, and that repeated measurements through time or historical records would be necessary to properly assess it. However, space-for-time substitutions have proven useful to assess temporal dynamics (reviewed in Hammond & Kolasa, 2014), and are particularly suitable when such continuous measurements are simply not possible due to the scale and extent of a study such as ours. Moreover, studying ecosystems across a whole shrubland-grassland continuum (i.e., differing in woody relative cover), such as that used here, can provide important insights on the ecological implications of woody encroachment (Breshears, 2006).
For every site, we obtained climatic variables derived from digital models using the Worldclim database (http://www.worldclim.org; Hijmans et al., 2005), from which an aridity index (mean annual precipitation/potential evapotranspiration) was derived. To facilitate the interpretation of our results, we used 1- aridity index in our analyses (higher values of aridity mean lower water availability; see Delgado-Baquerizo et al., 2013).
Measuring ecosystem functions
Dryland ecosystems are naturally heterogeneous (e.g., Schlesinger et al., 1990). Therefore, an appropriate way of analysing soil properties is by using a stratified sampling design in which the heterogeneity in soil properties is taken into account. Thus, we used a stratified sampling design taking into account the dominant microsites within each site. We sampled the top 7.5 cm of the soil from up to three different microsites per site; these microsites always included a location from areas of bare soil devoid of vascular plants, as well as microsites dominated by perennial vegetation (e.g. under trees, shrubs, or tussock grasses, depending on the dominant growth forms within each site). Five samples were collected for each microsite, yielding between 10 and 15 samples per site, which were sieved (< 2 mm fraction) and air-dried at room temperature before physicochemical analyses. In each soil sample, we measured, in the same laboratory to avoid experimental noise, 14 variables: nitrate (NO3−) and ammonium (NH4+) availability, organic C, total N, available inorganic P, aminoacids, proteins, pentoses, hexoses, aromatic compounds, phenols, potential N transformation rate and the activity of two extracellular enzymes (β-glucosidase and phosphatase). These variables, which were evaluated as detailed in Maestre et al., (2012), provide a good proxy of key ecosystem functions linked to soil fertility, the ability of soils to capture and retain water, nutrient cycling, biological productivity, and build-up of nutrient pools (see Maestre et al., 2012; Delgado-Baquerizo et al., 2013 for full rationale). Data from individual replicates were averaged to obtain site-level estimates by using the mean values observed in bare ground and vegetated areas, weighted by their respective cover at each site. With these site-level estimates, we calculated a multifunctionality index representing the ability of each site to maintain high levels of multiple ecosystem functions simultaneously (Zavaleta et al., 2010). The multifunctionality index (M) was calculated as the average of the Z-scores of each of the 14 variables measured (Maestre et al., 2012). The M index is statistically robust (Maestre et al., 2012), is being increasingly used (e.g., Pendleton et al., 2014; Wagg et al., 2014) and is related to other widely used multifunctionality metrics (Byrnes et al. 2014).
Multifunctionality measurements may obscure specific responses for the different functions. Thus, we analysed the effect of both aridity and woody cover on each of the 14 functions measured separately. All of the functional variables measured were either positively correlated or uncorrelated. The only exception was the relationship between organic C and available P, which was slightly negative (r = −0.15, P < 0.05, n = 224). The relationships between aridity and woody cover and these variables were also fairly consistent (see Results below). Overall, this suggests that there were no trade-offs among the different functions measured and, therefore, we will not discuss them separately here.
Statistical analyses
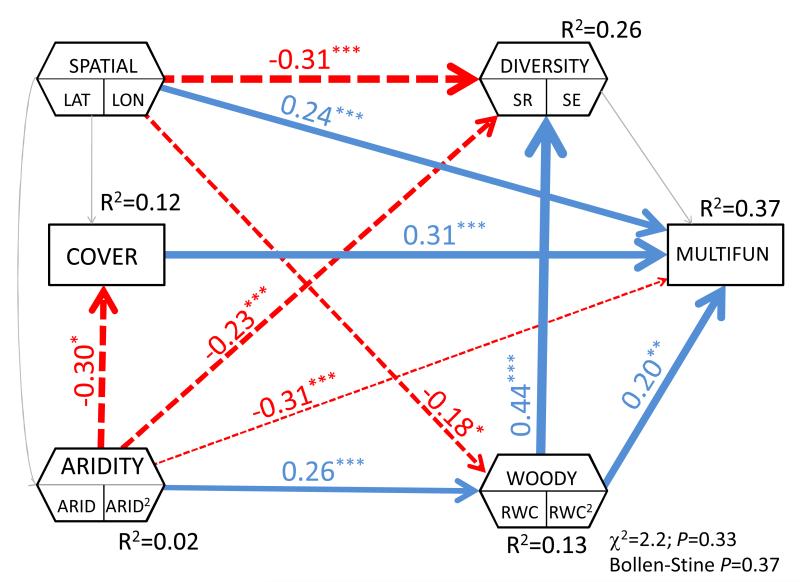
The statistical analyses included two sequential steps. First, we assessed the relationship among the several geographical, environmental, plant and soil variables evaluated by using Structural Equation Modelling (SEM; Grace, 2006) with the whole dataset. This modelling approach is a synthesis of path analysis, factor analysis, and maximum likelihood techniques that has been widely used as a tool for causal inference and for partitioning causal influences among multiple variables. The first step in SEM is to establish an a priori causal model establishing the expected relationships among the variables introduced based on previous knowledge. In our case, we established an a priori model assuming that both geographical variables (latitude and longitude) and aridity affect to some degree the rest of the variables introduced in the model (plant species richness, plant species evenness, total plant cover, relative woody cover and ecosystem multifunctionality; e.g., Lomolino et al., 2006; Sankaran et al., 2008). Total plant cover was expected to affect ecosystem multifunctionality (Maestre & Escudero, 2009) and to correlate with both species richness and evenness due to shared causal influences external to the model. Relative woody cover, in turn, was expected to affect plant richness and evenness, together with ecosystem multifunctionality (Eldridge et al., 2013). There is ample evidence that both species richness and evenness affect ecosystem multifunctionality (e.g., Isbell et al., 2009; Maestre et al., 2012 and references therein); thus, we also considered this relationship in our a priori model.
The second step in SEM is to estimate path coefficients, with their associated P-values, from the field data. We estimated coefficients with bootstrapping because some of our data were positively kurtotic or non-normal, and this technique is preferred to maximum likelihood estimation in these cases. The path coefficient is analogous to a partial correlation coefficient, and describes the strength and sign of the relationships among the introduced variables (Grace, 2006). Apart from estimating single path coefficients, SEM tests the overall goodness-of-fit of the model against the dataset. To do this, we used the traditional χ2 test, the RMSEA index and the Bollen-Stine bootstrap test. All of these indices (Fig. 1) show that our model fits, and that our proposed model structure is a plausible network of causal relationships among our variables. We acknowledge that other a priori model structures would be possible, and that inferring causality from observational studies is always a challenge. However, the goodness-of-fit metrics of our model (Fig. 1), and the ample evidence demonstrating causality in the effect of both diversity (richness and evenness) and woody cover on multiple ecosystem functions such as those measured here (e.g., Isbell et al., 2009; Eldridge et al., 2011), clearly support the direction of the paths chosen.
Figure 1.
Structural equation modeling depicting effects of aridity, geographical, and plant variables upon ecosystem multifunctionality. Composite variables are shown with hexagons. The width of arrow is proportional to the path coefficient, with blue (positive), red (negative) and grey (non-significant) lines indicating the sign of the relationships. The overall goodness-of-fit test and the R2 for each variable introduced are included. P-values of patch coefficients are as follows: *** = P < 0.001; ** = P < 0.01; * = P < 0.05. ARID = aridity level, RWC = relative woody cover, LAT = latitude, LON = longitude, WOODY = woody dominance, represented by relative woody cover (RWC), COVER = total plant cover, SR = species richness, SE = species evenness, and MULTIFUN = multifunctionality.
Nevertheless, we also considered an alternative a priori model structure including the effects of multifunctionality on species richness, evenness, total plant cover and relative woody cover. Although less likely, according to current literature, this a priori model structure was also a plausible causal scenario (χ2 = 0.54; df = 1; P = 0.46; Fig. S1). However, the path from multifunctionality to RWC was not significant (standardized path coefficient [SPC] = 0.11; P = 0.12), and neither were those paths linking multifunctionality with richness (SPC = 0.12; P = 0.09) or evenness (SPC = 0.12; P = 0.10). While the causal links from diversity and woody cover to multifunctionality have previously been widely demonstrated, the sign of the effect of woody encroachment on both diversity and multifunctionality -and its interaction with the environment- are poorly understood. We believe that the structure of our proposed model can provide important insights into this topic and do not consider the alternative model structure further.
Inspection of our data indicated that both aridity and relative woody cover had a hump-shaped relationship with ecosystem multifunctionality, which could be well-modeled with a second order polynomial regression. To model these non-linear relationships, we included a composite variable in our model including both x and x2, x being the raw predictor (aridity or relative woody cover). Composite variables were also used to model the cumulative effects of multiple geographical predictors (latitude and longitude) and the cumulative effect of the two elements of plant diversity measured (richness and evenness). The use of these composite variables does not alter the underlying model, but collapses the effects of the variables included (two in our case) into a single path coefficient, aiding interpretation of model results (Grace, 2006).
We also explored the interactions between aridity and woody dominance as drivers of diversity and multifunctionality as they are very likely to interact (Jackson et al., 2000a; Knapp et al., 2008). For doing this, we divided our study sites into drysubhumid (aridity level = 0.55-0.2; N = 27), semiarid (aridity level = 0.54-0.79 N = 151) and arid (aridity level = 0.80-1.00 N = 46) environments. We fit separate regressions (linear or quadratic, depending on the shape of the relationship) for each variable and aridity level to assess for the effect of relative woody cover on species richness, evenness and ecosystem multifunctionality. As relative woody cover deviated from normality, we re-analyzed this data by using classes of relative woody cover to verify the results. These results yielded qualitatively similar results as those presented in the main text (Appendix S2). Species richness and evenness were square root- and arcsine- transformed, respectively, before statistical analyses to normalize them. SEM analyses were performed using AMOS for windows (SPSS Inc., Chicago, IL, USA), the rest of the analyses were conducted with SPSS 13.0 for Windows (Chicago, IL, USA).
RESULTS
Our structural equation model explained 37% of the variance in multifunctionality (Fig. 1); its relationships with both aridity (based on FAO’s aridity level) and relative woody cover (proportion of total plant cover occupied by woody species) were positively hump-shaped, with multifunctionality peaking under semiarid conditions and with relative woody cover values between 41 and 60%. The effects of both relative woody cover and aridity were relatively homogeneous when considering the 14 measured soil variables separately, with most peaking at 50-60% relative woody cover and 0.5-0.6 aridity level values (Fig. 2). Our structural model also showed that plant diversity (richness and evenness) decreased with aridity or latitude (Fig. 1). However, relative woody cover was its strongest predictor and, the relationships between species richness or evenness and relative woody cover were similarly hump-shaped, with both variables peaking at relative woody covers of 61-80% (Fig. 1).
Figure 2.
Representation of the relationship between relative woody cover (%) and aridity (from 0 to 1) with the 14 soil variables measured. Green, red and blue triangles indicate linear positive, linear negative and hump-shaped relationships. Triangle length shows those values of the predictor at which the response variable peak in hump-shaped relationships (the three main values are shown as concentric circles to facilitate the interpretation of the diagram).
Woody encroachment is the increase in woody abundance into former grasslands. An alternative way of assessing the role of woody encroachment, therefore, would be to test how absolute woody cover (irrespective of total plant cover within each site) affected diversity and multifunctionality considering solely those sites in our database located in grasslands, excluding shrublands and other vegetation types (Fig. S3). Using absolute instead of relative woody cover yielded similar results, with substantial positive effects of woody cover on multifunctionality and species evenness up to values of 40%, the maximum value recorded in grasslands within our global survey. Species richness, in turn, peaked at woody cover levels of ~25%.
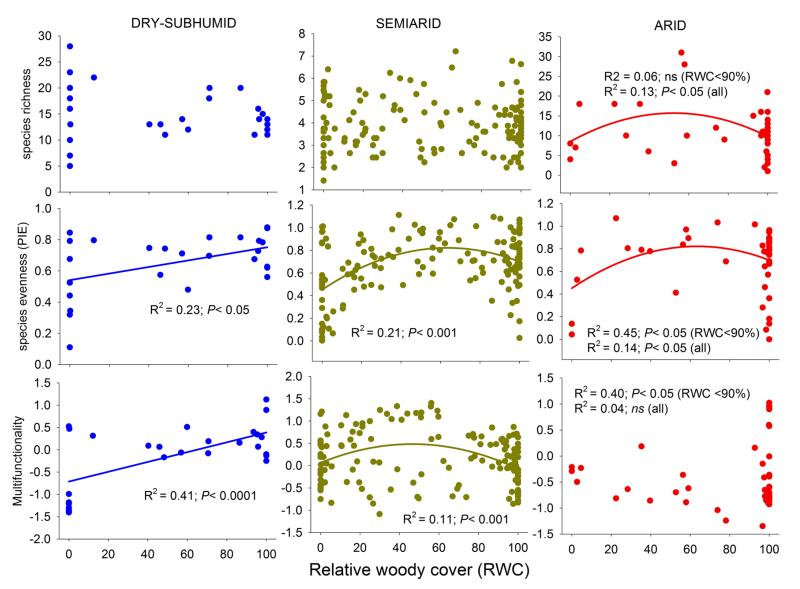
Aridity altered the effect of relative woody cover on species richness, evenness and multifunctionality. The positive linear trend in evenness and multifunctionality with increasing relative woody cover found in dry-subhumid sites changed to a unimodal relationship under semiarid conditions (Fig. 3). Under the most arid conditions, the unimodal relationships were retained for evenness and richness, but shifted to a linear negative relationship for multifunctionality. The negative relationship between relative woody cover and multifunctionality or species evenness in arid environments was obscured by the high variability found at sites with very high relative woody cover (>90%). When these high cover sites were excluded, trends were more clearly defined for evenness (R2 = 0.45, P < 0.05) and multifunctionality (R2 = 0.40, P < 0.05), but not for species richness (Fig. 3).
Figure 3.
Relationships between relative woody cover and species richness, evenness or ecosystem multifunctionality across differing aridity levels. Significant relationships and their statistics are shown within each panel. For arid environments, regression results with (all) and without (RWC<90%) the sites with RWC > 90% are shown. N = 27, 151 and 46 for dry-subhumid, semiarid and arid environments, respectively.
DISCUSSION
Examining trends in ecosystem structure and functioning across gradients of varying woody dominance helps us to understand the ecological consequences of woody encroachment, and guides the management and policy of key environmental areas such as desertification, deforestation, grazing, fire management and climate change (Breshears, 2006 and references therein). Despite the considerable interest in woody encroachment from land managers, scientists and government agencies, very few studies had attempted to assess how both aridity and woody dominance simultaneously affect plant diversity and ecosystem functioning at the global scale (Jackson et al., 2000a) and none has considered how the degree of woody dominance influence such effects in global drylands.
Hump-shaped relative woody cover-diversity and relative woody cover-multifunctionality relationships
The results of our global study are remarkably consistent with the few existing regional studies that indicate hump-shaped responses of diversity across gradients of woody cover (Blaum et al., 2007; Sirami et al., 2009; but see Báez & Collins, 2008). They provide clear evidence that these hump-shaped relationships are also applicable at the global scale, and extend these relationships to include critical ecosystem functions related to soil fertility, productivity and the ability to capture and retain resources.
The ecological mechanisms behind the hump-shaped responses of diversity across gradients of woody cover have been discussed previously, and relate mainly to heterogeneity in environmental conditions and resources between canopy and inter-canopy microsites, and to the connectivity among vegetated patches (Huxman et al., 2005; Breshears, 2006; Riginos et al., 2009). Woody canopies reduce incident radiation and increase levels of water infiltrability and soil fertility compared with inter-canopy microsites (Breshears, 2006; Throop & Archer, 2007; Maestre et al., 2009). These markedly different environmental conditions increase the amount of available niche space (e.g., Moro et al., 1997; Dean et al., 1999), and may increase overall species richness at the site-level (Maestre et al., 2009; Soliveres et al., 2012). However, once a given threshold in woody density or cover is reached, higher woody densities create a more homogeneous environment, with shading extending to the open interspaces (Martens et al., 2000) and similar water availabilities in woody and open microsites due to the overlap of woody plant roots (Breshears, 2006). Once a threshold in woody dominance has been reached, shade-intolerant species or those that cannot compete with woody plants for soil moisture disappear. Put simply, our results indicate that, at low densities of woody plants, increases in woody cover and density increase habitat heterogeneity and therefore niche space, leading to greater diversity of both plants and animals and increased heterogeneity of resources. At higher densities, however, increasing densities have an homogenizing influence. Our study is unique in that the comprehensive global database used provides strong evidence that this cover threshold lies between 41 and 60% relative woody cover (Fig. 3), or close to 25% absolute woody cover (Fig. S3).
Interestingly, the hump-shaped relationship found between plant richness and woody cover has also been observed in bird (Sirami et al., 2009) and mammal (Blaum et al., 2007) assemblages. Like plants, animals can also benefit from the increase in heterogeneity, and therefore niche space within the landscape at average levels of woody encroachment (e.g., more insect species to feed from, increase in fleshy-fruited plants or more available nesting spaces; e.g., Dean et al., 1999). However, at higher woody densities those species requiring open areas for habitat will disappear, promoting the same hump-shaped response observed for plants. Interestingly, observations of woody thresholds based on empirical data for bird (~40%; Sirami et al., 2009) and mammal richness (~20%; Blaum et al., 2007) are similar to our values for plants (Figs. 3 and S3).
Our results for ecosystem multifunctionality tracked those found with plant diversity, but are likely driven by different mechanisms. Although soil C is hypothesized to increase linearly with increasing woody cover (Reich et al., 2001; Breshears, 2006), we failed to detect such a pattern when evaluating multiple ecosystem functions at the high end of our relative woody cover gradient. Several potential mechanisms might explain this observation: 1) consistent with the Jornada desertification model (Schlesinger et al., 1990), dominance of woody plants over herbaceous plants at high levels of relative woody cover might be expected to enhance wind erosion and topsoil removal (Huxman et al., 2005), compromising the ability of the soil to sequester resources, and 2) the higher water demand of woody dominated areas (relative woody cover > 80%) creates intense plant-plant competition for moisture, compromising their ability to capture and recycle nutrients (Breshears, 2006; Knapp et al., 2008). These mechanisms might explain, in part, why some studies show increases (Maestre et al., 2009; Barger et al., 2011; Eldridge et al., 2011) while others demonstrate decreases (Schlesinger et al., 1990; Archer et al., 2001) in soil fertility with increasing woody cover. These contrasting results might be explained, at least in part, by the different woody densities in which each study was performed (e.g., relative woody cover = 26% in Maestre et al., 2009 vs 60% in Archer et al., 2001) and, hence, highlight the necessity of considering differing levels of woody dominance to properly assess the role of woody encroachment in global nutrient cycles.
Interactions between woody dominance and aridity
Surprisingly few studies have addressed how water availability influences the effects of woody plants on ecosystem structure and function. These studies stem from very divergent degrees of woody encroachment and show contradicting results (Jackson et al., 2000a; Knapp et al., 2008). Our global survey showed that, within drylands, increasing dominance of woody plants increases plant diversity and ecosystem multifunctionality under the wettest situations, and linearly decreases them under the driest situations. Intermediate semiarid situations, instead, exhibited a hump-shaped relationship between woody dominance and plant diversity and ecosystem multifunctionality. Our results contrast with those previously found under a similar range of rainfall conditions (Jackson et al., 2000a), which might be explained by 1) differing degrees of woody cover between theirs and our database (discussed extensively above), or 2) the influence of soil depth sampled (Dean et al., 2012). Jackson et al., (2000a) took samples at deeper soil depths, and soil nutrients are more widely distributed across the soil profile, and therefore might be showing lower values in the surface when woody plants become more dominant (Jackson et al., 2000b). Thus, the negative effect of woody encroachment we found under arid conditions could be derived from the lower values of C in shallow soils in situations where woody plants are more dominant. An alternative explanation is the extreme inter-site variability in the relative woody cover-ecosystem functioning relationships found in arid areas. For example, both positive and neutral responses of soil C across gradients of woody encroachment have been previously found in sites within the same area under arid conditions (Eldridge et al., 2013), and both strongly positive (Wheeler et al., 2007) and negative (Schlesinger et al., 1990; Archer et al., 2001) responses of soil C have been reported in arid areas of south-western United States. Indeed, the high variability in our own results resulted in non-significant trends (Fig. 3). The high variability among arid sites might be due to differences in management across study sites (i.e., grazing pressure). For example, overgrazing might lead to the loss of plant diversity at the time that prevents herbaceous cover from growing sufficiently to reduce erosion risk (Schlesinger et al., 1990; Greene et al., 1994). Grazing effects, which are particularly relevant in the most arid sites (Illius & O’Connor, 1999), can dampen the positive effects of woody encroachment on both diversity and multifunctionality under arid conditions (Eldridge et al., 2013; Soliveres & Eldridge, 2013). High inter-site variability might also result from a more variable response of woody-dominated systems to prolonged droughts, compared with grasslands (Breshears, 2006; Knapp et al., 2008). This could explain the dampening of the positive effect of woody plants on ecosystem functioning in arid areas, which experience a greater range of extreme climatic events (Holmgren et al., 2006).
The more positive effect of woody encroachment on ecosystem multifunctionality in wetter environments is consistent with findings from North American grasslands where the positive effect of woody encroachment on aboveground ANPP increases with increasing precipitation (Knapp et al., 2008). Because more water is stored within deeper soil layers in more mesic environments (Scholes & Archer, 1997; Kulmatinski & Beard, 2012), the higher water demand of woody plants does not stifle nutrient capture and storage (Knapp et al., 2008), and may increase productivity at the site level when woody plants are more abundant (Hughes et al., 2006). Overall, our results show that the increase in aridity predicted under future climatic scenarios may compromise the generally positive role of woody dominance in dryland structure and functioning (Eldridge et al., 2011). This reduction in the positive effects of woody dominance on ecosystem structure and function seems to be particularly relevant in those areas shifting from semiarid to arid climates, which are already more sensitive to increasing aridity due to important changes in nutrient stoichometry (Delgado-Baquerizo et al., 2013).
In summary, our study provides clear evidence that increasing woody plant dominance positively influences plant diversity and ecosystem multifunctionality in drylands, with both diversity and multifunctionality peaking at 41-60% of relative woody cover. The interactions between aridity and relative woody cover suggest that the increase in aridity predicted for drylands with climate change (Feng & Fu, 2013) will hinder the positive effect of woody encroachment, particularly in those areas shifting from semiarid to arid climates. By investigating the interrelationships among climate and woody dominance using a global survey, our results not only provide a major advancement to forecast the ecological consequences of woody encroachment, but also can help to refine management and policy actions in drylands worldwide.
Supplementary Material
BIOSKETCH.
Santiago Soliveres has a range of interests in dryland community ecology ranging from plant-plant interactions, diversity-ecosystem functioning relationships, restoration of roadsides and mine sites, the effect of grazing or climate on plant diversity, or the ecology of biological soil crusts. However, his latter research has mainly focused on woody encroachment, its ecological consequences and how the widely extended woody encroachment phenomenon interact with differing environmental conditions to simultaneously drive drylands’diversity and functioning.
ACKNOWLEDGEMENTS
We specially thank Donaldo Bran, Omar Cabrera, Alex Cea, Mohamed Chaieb, Abel A. Conceição, Mchich Derak, Carlos I. Espinosa, Adriana Florentino, Juan Gaitán, Wahida Ghiloufi, Susana Gómez-González, Julio R. Gutiérrez, Elizabeth Guzmán, Rosa M. Hernández, Elisabeth Huber-Sannwald, Victoria Ochoa, Beatriz Gozalo, Enrique Valencia, Ana Prado-Comesaña, Miguel Berdugo, Miguel García-Gómez, Mohammad Jankju, Rebecca L. Mau, Maria Miriti, Jorge Monerris, Vicente Polo, Aníbal Prina, Eduardo Pucheta, Roberto Romão, Duilio Torres, Cristian Torres-Díaz, James Val, Deli Wang and Eli Zaady for their contribution to the database used. This research was supported by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM).
Footnotes
Supporting Information
Supplementary information can be found in the online version of this article
REFERENCES
- Archer S, Boutton TW, Hibbard KA. Trees in grasslands: biogeochemical consequences of woody plant expansion. In: Schulze ED, et al., editors. Global Biogeochemical Cycles in the Climate System. Academic Press; 2001. pp. 115–130. [Google Scholar]
- Báez S, Collins SL. Shrub invasion decreases diversity and alters community stability in northern chihuahuan desert plant communities. PLoS ONE. 2008;3:e2332. doi: 10.1371/journal.pone.0002332. doi:10.1371/journal.pone.0002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger NN, Archer SR, Campbell JL, Huang C, Morton JA, Knapp AK. Woody plant proliferation in North American drylands: a synthesis of impacts on ecosystem carbon balance. Journal of Geophysical Research. 2011;116:G00K07. doi:10.1029/2010JG001506. [Google Scholar]
- Blaser WJ, Sitters J, Hart SP, Edwards PJ, Olde Venterink H. Facilitative or competitive effects of woody plants on understorey vegetation depend on N-fixation, canopy shape and rainfall. Journal of Ecology. 2013;101:1598–1603. [Google Scholar]
- Blaum N, Rossmanith E, Popp A, Jeltsch F. Shrub encroachment affects mammalian carnivore abundance and species richness in semiarid rangelands. Acta Oecologica. 2007;31:86–92. [Google Scholar]
- Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. Climate change and the past, present, and future of biotic interactions. Science. 2013;341:499–504. doi: 10.1126/science.1237184. [DOI] [PubMed] [Google Scholar]
- Breshears DD. The grassland-forest continuum: trends in ecosystem properties for woody plant mosaics? Frontiers in Ecology and the Environment. 2006;4:96–104. [Google Scholar]
- Byrnes JEK, Gamfeldt L, Isbell F, Lefcheck JS, Griffin JN, Hector A, Cardinale BJ, Hooper DU, Dee LE, Duffy JE. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods in Ecology and Evolution. 2014 doi: 10.1111/2041–210X.12143. [Google Scholar]
- Daryanto S, Eldridge DJ. Plant and soil surface responses to a combination of shrub removal and grazing in a shrub-encroached woodland. Journal of Environmental Management. 2010;91:2639–2648. doi: 10.1016/j.jenvman.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Dean C, Stephen H, Roxburgh SH, Harper RJ, Eldridge DJ, Watson IW, Wardell-Johnson GW. Accounting for space and time in timbered rangelands. Ecological Engineering. 2012;38:51–64. [Google Scholar]
- Dean WRJ, Milton SJ, Jeltsch F. Large trees, fertile islands, and birds in arid savanna. Journal of Arid Environments. 1999;4:61–78. [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL, et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature. 2013;502:672–676. doi: 10.1038/nature12670. [DOI] [PubMed] [Google Scholar]
- Eldridge DJ, Soliveres S, Bowker MA, Val J. Grazing dampens the positive effects of shrub encroachment on ecosystem functions in a semi-arid woodland. Journal of Applied Ecology. 2013;50:1028–1038. [Google Scholar]
- Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG. Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecology Letters. 2011;14:709–722. doi: 10.1111/j.1461-0248.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Fu Q. Expansion of global drylands under a warming climate. Atmospheric Chemistry and Physics. 2013;13:10081–10094. [Google Scholar]
- Grace JB. Structural Equation Modeling and Natural Systems. Cambridge University Press; 2006. [Google Scholar]
- Greene RSB, Kinnell PIA, Wood JT. Role of plant cover and stock trampling on runoff and soil-erosion from semi-arid wooded rangelands. Australian Journal of Soil Research. 1994;32:953–973. [Google Scholar]
- Hammond MP, Kolasa J. Spatial variation as a tool for inferring temporal variation and diagnosing types of mechanisms in ecosystems. PLoS ONE. 2014;9:e89245. doi: 10.1371/journal.pone.0089245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Holmgren M, Stapp P, Dickman CR, Gracia C, Graham S, Gutiérrez JR, et al. Extreme climatic events shape arid and semiarid ecosystems. Frontiers in Ecology and the Environment. 2006;4:87–95. [Google Scholar]
- House JI, Archer SR, Breshears DD, Scholes RJ. Conundrums in mixed woody–herbaceous plant systems. Journal of Biogeography. 2003;30:1763–1777. [Google Scholar]
- Hughes RF, Archer SR, Asner GP, Wessman CA, McMurtry C, Nelson J, et al. Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Global Change Biology. 2006;12:1733–1747. [Google Scholar]
- Hurlbert SH. The nonconcept of species diversity: a critique and alternative parameters. Ecology. 1971;52:577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- Huxman TE, Wilcox BP, Breshears DD, Scott RL, Snyder KL, Small EE, et al. Ecohydrological implications of woody plant encroachment. Ecology. 2005;86:308–319. [Google Scholar]
- Illius AW, O’Connor TG. On the relevance of nonequilibrium concepts to arid and semiarid grazing systems. Ecological Applications. 1999;9:798–813. [Google Scholar]
- Isbell FI, Polley HW, Wilsey BJ. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecology Letters. 2009;12:443–451. doi: 10.1111/j.1461-0248.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Banner J.L, Jobbágy, E.G., Pockman WT, Wall DH. Ecosystem carbon loss with woody plant invasion of grasslands. Nature. 2002;418:623–626. doi: 10.1038/nature00910. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Schenk HJ, Jobbágy EG, Canadell J, Colello GD, Dickinson RD, et al. Belowground consequences of vegetation change and their treatment in models. Ecological Applications. 2000b;10:470–483. [Google Scholar]
- Knapp A, Briggs JM, Collins SL, Archer SR, Bret-Hart MS, Ewers BE, et al. Shrub encroachment in North American grasslands: shifts in growth form dominance rapidly alter control of ecosystem carbon inputs. Global Change Biology. 2008;14:615–623. [Google Scholar]
- Kulmatinski A, Beard KH. Woody plant encroachment facilitated by increased precipitation intensity. Nature Climate Change. 2013;3:833–837. [Google Scholar]
- Lomolino MV, Riddle BR, Brown JH. Biogeography. 3rd Sinauer; 2006. [Google Scholar]
- Maestre FT, Escudero A. Is the patch size distribution of vegetation a suitable indicator of desertification processes? Ecology. 2009;90:1729–1735. doi: 10.1890/08-2096.1. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Bowker MA, Puche MD, Hinojosa BM, Martínez I, García-Palacios P, et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecology Letters. 2009;12:930–941. doi: 10.1111/j.1461-0248.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens SN, Breshears DD, Meyer CW. Spatial distributions of understory light along the grassland/forest continuum: effects of cover, height, and spatial pattern of tree canopies. Ecological Modeling. 2000;126:79–93. [Google Scholar]
- Millenium Ecosystem Assessment . Ecosystems and Human Well-Being: Biodiversity Synthesis. World Resources Institute; Island Press; 2005. p. 100. [Google Scholar]
- Moro MJ, Pugnaire FI, Haase P, Puigdefábregas F. Mechanisms of interaction between a leguminous shrub and its understorey in a semi-arid environment. Ecography. 1997;20:175–184. [Google Scholar]
- Pendleton RM, Hoeinghaus DJ, Gomes LC, Agostinho AA. Loss of rare fish species from tropical floodplain food webs affects community structure and ecosystem multifunctionality in a mesocosm experiment. PLoS ONE. 2014;9:e84568. doi: 10.1371/journal.pone.0084568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quero JL, Maestre FT, Ochoa V, García-Gómez M, Delgado-Baquerizo M. On the importance of shrub encroachment by sprouters, climate, species richness and anthropic factors for ecosystem multifunctionality in semi-arid Mediterranean ecosystems. Ecosystems. 2013;16:1248–1261. doi: 10.1007/-s10021-013-9683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak Z, Nippert JB, Collins SL. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology. 2012;93:697–703. doi: 10.1890/11-1199.1. [DOI] [PubMed] [Google Scholar]
- Reich PB, Peterson DW, Wedin DA, Wrage K. Fire and vegetation effects on productivity and nitrogen cycling across a forest–grassland continuum. Ecology. 2001;82:1703–1719. [Google Scholar]
- Riginos C, Grace JB, Augustine DJ, Young TP. Local versus landscape-scale effects of savanna trees on grasses. Journal of Ecology. 2009;97:1337–1345. [Google Scholar]
- Sankaran M, Ratnam J, Hanan N. Woody cover in African savannas: the role of resources, fire and herbivory. Global Ecology and Biogeography. 2008;17:236–245. [Google Scholar]
- Schlesinger WH, Reynolds JF, Cunningham GL, Huenneke LF, Harrel WM, Virginia RA, et al. Biological feedbacks in global desertification. Science. 1990;247:1043–1048. doi: 10.1126/science.247.4946.1043. [DOI] [PubMed] [Google Scholar]
- Scholes RJ, Archer SR. Tree-grass interactions in savannas. Annual Review in Ecology and Systematics. 1997;28:517–544. [Google Scholar]
- Sirami C, Seymour C, Midgley G, Barnard P. The impact of shrub encroachment on savanna bird diversity from local to regional scale. Diversity and Distributions. 2009;15:948–957. [Google Scholar]
- Soliveres S, Eldridge DJ. Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Functional Ecology. 2013;28:530–537. doi: 10.1111/1365-2435.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliveres S, Eldridge DJ, Hemmings F, Maestre FT. Nurse plant effects on plant species richness in drylands: The role of grazing, rainfall and species specificity. Perspectives in Plant Ecology, Evolution and Systematics. 2012;14:402–410. doi: 10.1016/j.ppees.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague WR, Borchard R, Ansley J, Pinchak B, Cox J, Foy JK, et al. Sustainable management strategies for mesquite rangeland: the Waggoner Kite project. Rangelands. 1997;19:4–8. [Google Scholar]
- Throop HL, Archer SR. Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecological Applications. 2007;17:1809–1823. doi: 10.1890/06-0889.1. [DOI] [PubMed] [Google Scholar]
- Van Auken OW. Shrub invasions of North American semiarid grasslands. Annual Review in Ecology and Systematics. 2000;31:197–215. [Google Scholar]
- Wagg C, Bender SF, Widmer F, van der Heijden MGA. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proceedings of the National Academy of Sciences USA. 2014 doi: 10.1073/pnas.1320054111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CW, Archer SR, Asner GP, McMurtry CR. Climatic/edaphic controls on soil carbon/nitrogen response to shrub encroachment in desert grassland. Ecological Applications. 2007;17:1911–1928. doi: 10.1890/06-1580.1. [DOI] [PubMed] [Google Scholar]
- Zavaleta ES, Pasari JR, Hulvey KV, Tilman GD. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proceedings of the National Academy of Sciences USA. 2010;107:1443–1446. doi: 10.1073/pnas.0906829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.