Summary
Recent evidence suggests that soil nutrient heterogeneity, a ubiquitous feature of terrestrial ecosystems, modulates plant responses to ongoing global change (GC). However, we know little about the overall trends of such responses, the GC drivers involved, and the plant attributes affected.
We synthesized literature to answer the question: Does soil heterogeneity significantly affect plant responses to main GC drivers, such as elevated atmospheric carbon dioxide concentration (CO2), nitrogen (N) enrichment and changes in rainfall regime?
Overall, most studies have addressed short-term effects of N enrichment on the performance of model plant communities using experiments conducted under controlled conditions. The role of soil heterogeneity as a modulator of plant responses to elevated CO2 may depend on the plasticity in nutrient uptake patterns. Soil heterogeneity does interact with N enrichment to determine plant growth and nutrient status, but the outcome of this interaction has been found to be both synergistic and inhibitory. The very few studies published on interactive effects of soil heterogeneity and changes in rainfall regime prevented us from identifying any general pattern.
We identify the long-term consequences of soil heterogeneity on plant community dynamics in the field, and the ecosystem level responses of the soil heterogeneity × GC driver interaction, as the main knowledge gaps in this area of research.
In order to fill these gaps and take soil heterogeneity and GC research a step forward, we propose the following research guidelines: 1) combining morphological and physiological plant responses to soil heterogeneity with field observations of community composition and predictions from simulation models; and 2) incorporating soil heterogeneity into a trait-based response-effect framework, where plant resource-use traits are used as both response variables to this heterogeneity and GC, and predictors of ecosystem functioning.
Synthesis. There is enough evidence to affirm that soil heterogeneity modulates plant responses to elevated atmospheric CO2 and N enrichment. Our synthesis indicates that we must explicitly consider soil heterogeneity to accurately predict plant responses to GC drivers.
Keywords: CO2, changes in rainfall regime; community-level studies; ecosystem functioning; global change; N enrichment; soil nutrient heterogeneity; root foraging; plant performance; plant-soil interactions
Introduction
An intrinsic feature of most terrestrial ecosystems is the spatial heterogeneity in the distribution of soil nutrients (hereafter referred as soil heterogeneity), which are supplied to plant individuals, populations and communities in a patchy manner at different spatial scales (e.g. Robertson et al. 1988; Jackson & Caldwell 1993; Farley & Fitter 1999). The way that plants respond to this heterogeneity has for many years intrigued plant scientists, botanists, ecologists and agronomists, and since the early studies of Gleason (1926) and Clark & Evans (1954) a large number of papers have been published on this topic (reviewed, among others, by Robinson 1994; Hutchings, Wijesinghe & John 2000; Huber-Sannwald & Jackson 2001; Hodge 2004). This large body of research has revealed the profound consequences of soil heterogeneity for plants. For example, at large spatial scales, this heterogeneity has been found to affect the distribution and productivity of plants in agroecosystems (Stein et al. 1997), forests (Mou et al. 1995), grasslands (Robertson et al. 1988) and salt marshes (Pan et al. 1998). Also, at smaller spatial scales, such as those affecting individual roots and whole root systems, soil heterogeneity promotes a variety of morphological and physiological plant responses. In order to enhance resource capture, plant roots have developed exceptional morphological plasticity, such as changes in biomass allocation, root morphology and longevity, to increase root proliferation into nutrient-rich patches (Hutchings & de Kroon 1994; Hodge 2004, 2006). Although physiological responses (e.g. changes in nutrient uptake kinetics) are under-studied, they also play decisive roles for root foraging both in the short (Fransen, Blijjenberg & de Kroon 1999; Jansen et al. 2006) and the long (Fransen, de Kroon & Berendse 2001; García-Palacios, Maestre & Gallardo 2011) term. The importance of these responses goes beyond the functioning of plant modules and individuals, as they may determine the competitive ability in two-species mixtures (Robinson et al. 1999), the survival and size inequality patterns within plant populations (Maestre & Reynolds 2006a), and the composition, productivity and functioning of plant assemblages (Bliss et al. 2002; Maestre, Bradford & Reynolds 2005; Wijesinghe, John & Hutchings 2005; but see Casper & Cahill 1996).
Morphological and physiological responses to soil heterogeneity occur mainly at the level of individual roots and whole root systems (Hodge 2004, 2006). Root responses to soil heterogeneity are likely to play key functional roles if we consider the importance of root foraging behavior, and root turnover and exudation on crucial ecosystem processes, such as soil stability, carbon (C) sequestration, N-fixation and organic matter decomposition (Wardle 2002; De Deyn, Cornelissen & Bardgett 2008). Roots account for more than half of the total plant biomass in terrestrial ecosystems (Mokany, Raison & Prokushkin 2006; Poorter et al. 2012), are involved in the transport of C, N, phosphorus (P) and water in the soil (Smith et al. 1999), and participate in the weathering of soil minerals (Bormann et al. 1998). Both the roots themselves and the exudates that they produce support an extremely diverse and complex network of symbiotic and free-living soil organisms, whose activities drive many ecosystem functions and feedback processes with plants (Kardol, Bezemer & van der Putten 2006; van der Heijden, Bardgett & van Straalen 2008). As a result, we expect that roots will play an important role in determining the community- and ecosystem-level responses to soil heterogeneity.
The potential importance of soil heterogeneity as a modulator of ecosystem responses to changes in plant diversity is highlighted by recent biodiversity-ecosystem functioning research. Most of this research has focused on the role of changes in plant species richness and, to a smaller degree, of composition and evenness on ecosystem functions such as aboveground biomass, decomposition and nutrient cycling (see Hooper et al. 2005 for a review). However, despite the significance of roots for observed productivity responses to modifications in these biotic attributes (de Kroon et al. 2012), it is unknown how these changes are modified by soil heterogeneity and the root responses it promotes. The potential for roots to do so is great: this heterogeneity is both ubiquitous and, as discussed above, known to have multiple effects on the performance of individual plants.
How soil heterogeneity modulates the effects of plant community attributes on productivity depends largely on the traits of the component species (Hooper et al. 2005), such as root foraging precision (Rajaniemi & Reynolds 2004; de Kroon & Mommer 2006; Kembel et al. 2008). Recent studies have found that root proliferation in response to soil heterogeneity interacts with species composition to determine the productivity of model plant communities. Maestre, Bradford & Reynolds (2006) found that shoot biomass in three-species model communities was less responsive to soil heterogeneity when Poa pratensis was present, which was related with a low root foraging precision to nutrient patches for this species. An analogous result was found by Maestre & Reynolds (2007a) in similar three-species model communities. In this case, plant assemblages with Holcus lanatus did not show an increase in root biomass when nutrients were heterogeneously distributed, although this species experienced a high root proliferation into nutrient patches. These results provide evidence that soil heterogeneity affects the composition of plant assemblages (Bliss et al. 2002; Wijesinghe, John & Hutchings 2005) by affecting the resource-use strategies of individual species. Thus, soil heterogeneity will likely interact with potential changes in plant community composition induced by global change (GC) drivers. Although this interaction is modulated by changes in the resource-use strategies of the plant species forming the community, its effects may scale up to influence community and ecosystem dynamics.
In nature, plants occur in complex communities, whose response is shaped by multiple biotic and abiotic variables, including animals, nutrient availability and climatic conditions, which are likely to interact with soil heterogeneity to modify the way that plants respond to it (Maestre, Bradford & Reynolds 2005). However, most ecological research on soil heterogeneity has evaluated the root responses of isolated plants growing in artificial and highly controlled experimental settings, without considering variables other than this factor (Robinson 1994; Hodge 2004; Kembel & Cahill 2005). Thus, our understanding on the ecological consequences of soil heterogeneity in natural conditions is clearly hindered by the simplicity of the experimental systems employed to date, and by the low number of multispecies studies evaluating the joint role of this heterogeneity and other factors, especially at the population and community levels.
Among abiotic factors likely to interact with soil heterogeneity to drive plant responses, atmospheric concentration of carbon dioxide (CO2), and nutrient and water availability, deserve special attention because they are major drivers of the ongoing GC (elevated atmospheric CO2, N enrichment and changes in rainfall regime) being faced by terrestrial ecosystems worldwide (Vitousek et al. 1997; IPCC 2007). Increasing concern with respect to the ecological consequences of such change has boosted research on the topic, and nowadays there is a plethora of studies evaluating the effects of GC drivers on key ecosystem components, such as plants and soil organisms, and processes, such as net primary productivity and nutrient cycling (e.g. Körner & Bazzaz 1996; Poorter & Navas 2003; Bardgett, Freeman & Ostle 2008). Most of the research conducted on this topic has evaluated the ecological effects of the different GC drivers in isolation from each other (Luo et al. 1999; Körner 2001). During the last decade, however, research has developed towards more complex experiments that evaluate the simultaneous effects of several GC drivers on organism performance and ecosystem processes, such as elevated atmospheric CO2 and nutrient availability (He, Bazzaz & Schmid 2002; Reich et al. 2001), elevated atmospheric CO2, nutrient availability, temperature and rainfall (Shaw et al. 2002), and elevated atmospheric CO2, temperature, rainfall and ozone (Hanson et al. 2005). These complex experiments have demonstrated that different drivers act simultaneously, and often interactively (but see Zavaleta et al. 2003), to determine the responses of ecosystem components and processes to GC, emphasizing the need for multifactorial approaches when evaluating them (Norby & Luo 2004). Furthermore, much uncertainty remains as how the traits and attributes of plant species and communities interact with abiotic factors to modulate terrestrial ecosystem responses to GC (Tylianakis et al. 2008a).
Over the last 20 years, many reviews have synthesized the literature on plant responses to soil heterogeneity. These have tackled issues like the type of individual plant responses to this phenomenon (Robinson 1994; Hutchings & de Kroon 1994; Hutchings, Wijesinghe & John 2000), the variation in these responses among species and functional groups (Robinson & Van Vuuren 1998; Kembel & Cahill 2005; Kembel et al. 2008), the mechanisms underlying responses to soil heterogeneity (Hodge 2004; de Kroon et al. 2009), the role of mycorrhizae on nutrient acquisition from nutrient patches (Hodge 2006), the effects of soil heterogeneity upon plant competition belowground (Casper & Jackson 1997), and the implications of soil heterogeneity for populations and communities (Hutchings, John & Wijesinghe 2003; Huber-Sannwald & Jackson 2001). However, and to our knowledge, no review so far has synthesized studies evaluating the joint effects of soil heterogeneity and GC drivers on plant individuals, populations, communities, and ecosystems. We redress this by asking the question: Does soil heterogeneity significantly affect plant responses to global change? First, we review available literature to explore whether soil heterogeneity modulates plant responses to some of the main GC drivers, including elevated atmospheric CO2, N enrichment and changes in rainfall regime. The interaction of soil heterogeneity with other GC drivers, such as warming, invasive species or land use changes, has not been addressed in the literature, and thus it is not approached in this review. Second, we discuss the potential of plant-mediated effects of soil heterogeneity and GC on ecosystem functioning. Finally, we identify knowledge gaps and future research directions that should advance our understanding of the ecological consequences of soil heterogeneity in a changing world.
Looking for evidence: Is soil heterogeneity affecting plant responses to global change?
At the species level, interactions between GC drivers and soil heterogeneity may occur because the latter promotes plant responses (e.g. changes in biomass allocation and nutrient uptake patterns) that are also a consequence of drivers such as elevated atmospheric CO2 and N enrichment (Poorter & Nagel 2000; Poorter & Navas 2003; Hodge 2004). At the assemblage level, such interactions may take place because co-occurring plants often differ in their ability to profit from this heterogeneity (Bliss et al. 2002; Kembel & Cahill 2005; Wijesinghe, John & Hutchings 2005), and in the direction and magnitude of their responses to GC drivers (Grünzweig & Körner 2001). Multifactorial experiments have demonstrated that plant responses to elevated CO2 at both species and assemblage levels are often dependent on the availability of soil nutrients such as N and P (Berntson & Bazzaz 1997; Stöcklin, Schweizer & Körner 1998). However, it is unknown whether such plant responses are modified by other nutrient attributes, including their spatial distribution, because interactions between soil nutrient heterogeneity and drivers such as elevated atmospheric CO2 on plant assemblages have barely begun to be explored (Arnone 1997; Maestre, Bradford & Reynolds 2005; Maestre & Reynolds 2006b, 2007a).
A search on the ISI® Web of Science using the keywords “heterogeneity” or “homogenous” or “heterogeneous” or “patch” and “soil” or “nutrient” with a timespan between 1990 and 2011 yields 9,630 references (search conducted on 7th February 2012). However, when evaluated carefully, only 13 of them (Table 1) explicitly addressed the interaction between soil heterogeneity (including both homogeneous and heterogeneous distributions of soil resources with the same nutrient availability) and a GC driver (elevated atmospheric CO2, N enrichment and changes in rainfall regime). Over 75% of these studies used N enrichment as the common GC driver (although not interpreted in the same manner in most of the studies, we considered treatments promoting an increase in nutrient availability as proxy of a N enrichment treatment). Therefore, the relatively low sample size (13 studies), and the bias towards a unique GC driver prevented us to perform a quantitative review of the literature using meta-analysis, a statistical approach that has been used to summarise published results in a wide variety of ecological topics (Gurevitch, Morrow & Walsh 1992; Gurevitch, Morrison & Hedges 2000), including root responses to soil heterogeneity (Kembel & Cahill 2005). Despite this, the number of studies found is enough to conduct a qualitative assessment of the plant responses observed, and to identify overall trends and current gaps in our understanding. Such appraisal is presented below.
Table 1.
Summary features and results of studies addressing interactions between global change (GC) drivers and soil nutrient heterogeneity (NH).
| Study | Global change driver | Study type | Study level | Nutrient patch | Duration | Response variable | Interaction GC × NH |
|---|---|---|---|---|---|---|---|
| Arnone (1997) | CO2 | CC | Com | I | 20-69 D | Fine root lenght | * |
| CO2 | CC | Com | I | 20-69 D | Root biomass | - | |
| Baer et al. (2004) | N enr | F | Com | I | 3 Y | Richness | - |
| N enr | F | Com | I | 3 Y | Diversity | - | |
| Day, Hutchings & John (2003) | N enr | G | Pop | I | 60 D | Shoot biomass | * |
| N enr | G | Pop | I | 60 D | Root biomass | * | |
| Fransen & de Kroon (2001) | N enr | G | Sp | O | 2 Y | Shoot biomass | * |
| N enr | G | Sp | O | 2 Y | Root biomass | * | |
| García-Palacios, Maestre & Gallardo (2011) | N enr | CG | Com | O | 1.5 Y | Shoot biomass | - |
| N enr | CG | Com | O | 1.5 Y | Root biomass | - | |
| Lamb, Haag & Cahill (2004) | N enr | CC | Sp | O | 60 D | Shoot biomass | - |
| Maestre & Reynolds (2006a) | CO2 | CC | Pop | O | 90 D | Shoot biomass | *, -, - |
| N enr | CC | Pop | O | 90 D | Shoot biomass | *, -, * | |
| Maestre & Reynolds (2006b) | CO2 | CC | Com | O | 90 D | Shoot biomass | - |
| CO2 | CC | Com | O | 90 D | Root biomass | - | |
| N enr | CC | Com | O | 90 D | Shoot biomass | * | |
| N enr | CC | Com | O | 90 D | Root biomass | * | |
| Maestre & Reynolds (2007a) | CO2 | CC | Com | O | 93 D | Shoot biomass | - |
| CO2 | CC | Com | O | 94 D | Root biomass | - | |
| Maestre & Reynolds (2007b) | N enr | CC | Com | O | 100 D | Shoot biomass | - |
| N enr | CC | Com | O | 100 D | Root biomass | - | |
| CRR | CC | Com | O | 100 D | Shoot biomass | * | |
| CRR | CC | Com | O | 100 D | Root biomass | * | |
| Maestre, Bradford & Reynolds (2005) | CO2 | CC | Com | O | 120 D | Shoot biomass | * |
| CO2 | CC | Com | O | 120 D | Root biomass | - | |
| N enr | CC | Com | O | 120 D | Shoot biomass | - | |
| N enr | CC | Com | O | 120 D | Root biomass | - | |
| Maestre et al. (2007) | CO2 | CC | Pop | O | 90 D | Shoot biomass | -, -, - |
| N enr | CC | Pop | O | 90 D | Shoot biomass | -, *, * | |
| Wang, de Kroon & Smits (2007) | CRR | G | Sp | I | 60 D | Shoot biomass | - |
| CRR | G | Sp | I | 60 D | Root biomass | - |
CO2 = elevated atmospheric CO2, N enr = N enrichment, CRR = changes in rainfall regime, CC = climate chamber, F = field, G = glasshouse, CG = common garden, Com = community, Sp = species, Pop = population, I = inorganic, O = organic, D = days, and Y = years.
significant GC × NH interaction (P < 0.05).
As commented before, although we found representation of all the three GC drivers considered, N enrichment was the most frequently assessed. Secondly, only one study (Baer et al. 2004) was performed in the field (the remaining were either glasshouse or climate chamber experiments). Seven of the studies were performed at the community level, three at the population level and three at the individual level. We found nine studies using organic patches and four using inorganic. More than 75% of the studies were short-term, with durations between two and three months. Practically all the studies used surrogates of plant performance like root and shoot biomass as the response variables to evaluate the effects and interactions between soil heterogeneity and GC drivers.
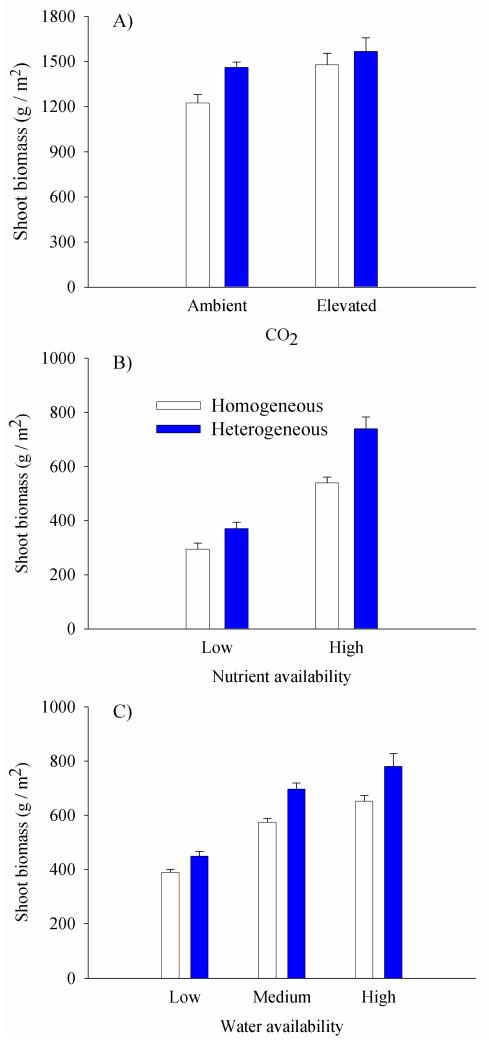
We found the following results regarding the interactions between soil heterogeneity and the three GC drivers assessed. Elevated atmospheric CO2 significantly interacted with soil heterogeneity in three of the six studies evaluated. Arnone (1997) found that precision of root foraging measured with fine root length (his response variable) increased under elevated atmospheric CO2 69 days after the addition of the nutrient patches. Maestre, Bradford & Reynolds (2005) found a significant interaction when evaluating the effect of these factors upon shoot biomass (Fig. 1A). The positive effects of elevated atmospheric CO2 on shoot biomass were observed only under homogeneous conditions. Although not included in Table 1, significant interactions between soil heterogeneity and elevated atmospheric CO2 have been also found when evaluating plant nutrient uptake. For instance, Maestre, Bradford & Reynolds (2005) and Maestre & Reynolds (2006b) found that soil heterogeneity modulated the effects of elevated atmospheric CO2 on the nutrient use efficiency of experimental grassland communities. Both studies found that the positive effect of elevated atmospheric CO2 on nutrient use efficiency was reduced by soil heterogeneity. This suggests that plant plasticity in N uptake may determine the role of soil heterogeneity as a modulator of plant responses to elevated atmospheric CO2.
Figure 1.
Shoot biomass of experimental grassland communities compared across soil nutrient heterogeneity levels and atmospheric CO2 (A), nutrient availability (B) as a measure of N enrichment, and water availability (C) as a measure of changes in rainfall regime. (A) Consisted of two levels of atmospheric CO2: ambient (37.5 Pa) and elevated (70 Pa). (B) Consisted of two levels of nutrient availability: low (40 mg N) and high (120 mg N). (C) Consisted of three levels of water availability: low, medium and high (15.92, 31.84 and 47.76 L / m2 of water per week, respectively). Data are means + SE (n = 18 for data in A, n = 32 for data in B and n = 24 for data in C). Redrawn with permission from the New Phytologist Trust (A: Maestre, Bradford & Reynolds 2005), Global Change Biology (B: Maestre & Reynolds 2006b) and Ecology (C: Maestre & Reynolds 2007b).
Five out of nine studies included in Table 1 found a significant interaction between soil heterogeneity and N enrichment. The positive effect of soil heterogeneity upon plant performance was always higher in conditions of high nutrient availability, suggesting non-additive effects when N enrichment enhances the nutrient availability of soil patches. For example, Maestre & Reynolds (2006b) found a significant interaction between soil heterogeneity and nutrient availability in experimental grassland communities, with a notable increase in shoot biomass when nutrients were supplied heterogeneously and at high availability levels (Fig. 1B). This effect has been found previously in nutrient-limited plants, where root proliferation under heterogeneous conditions increases with increasing contrast between the patch and the background soil (Lamb, Haag & Cahill 2004). On the other hand, ecosystems thought as being not limited by N, such as tropical and subtropical systems, may experience a negative interaction between these two factors, because heterogeneity in N availability may be reduced by N enrichment (Bobbink et al. 2010). The N homogeneity hypothesis (Gilliam 2006) predicts declines in the diversity of forest understory species as a result of excess N enrichment, decreasing the naturally high spatial heterogeneity in soil N availability (Hutchings, John & Wijesinghe 2003), which contributes to the maintenance of understory diversity. Interestingly, the soil heterogeneity × N enrichment interaction affected the performance of species, populations and communities in the same manner above- and belowground (Table 1). The responses of shoot and root biomass to this interaction in each study was either significant or non-significant for both variables. This common above-belowground pattern in plant responses to soil heterogeneity × N enrichment interaction supports the hypothesis of resource heterogeneity as a key driving force of within-community trait variation (Ackerly & Cornwell 2007). Soil heterogeneity has been identified as an important selective pressure on trait evolution above- and belowground (Kembel & Cahill 2011).
The interaction of soil heterogeneity with changes in rainfall regime promoting alterations in water availability was especially underrepresented, with only two studies. Of particular note is the study of Maestre & Reynolds (2007b) who found a significant change in the rainfall regime × soil heterogeneity interaction when analysing shoot biomass in five-species experimental assemblages (Fig. 1C). These authors found that shoot biomass was considerably higher at medium and high water availability levels when nutrients were heterogeneously supplied.
In summary, current evidence indicates that soil heterogeneity does affect plant responses to GC drivers, but we must interpret this affirmation with some cautions because the relatively low number of studies carried out to date. The role of soil heterogeneity in modulating plant responses to elevated atmospheric CO2 may depend on the plasticity of species to change their nutrient uptake patterns (Maestre, Bradford & Reynolds 2005). Spatial patterning of soil nutrients does interact with N enrichment to determine plant responses, but the outcome of this interaction has been found to be both synergistic (Lamb, Haag & Cahill 2004; García-Palacios, Maestre & Gallardo 2011) and inhibitory (Giliam 2006). There are not enough studies published addressing the interaction between soil heterogeneity and changes in rainfall regime to elucidate any general pattern. Although we focused on two-way interactions between soil heterogeneity and any GC driver, multifactorial studies assessing, in the same experimental design, the joint effects of more than one driver together with soil heterogeneity do exist. Indeed, there is evidence of multifactorial interactions between soil heterogeneity and multiple GC drivers. For example, Maestre & Reynolds (2006a) found a significant three-way interaction between soil heterogeneity, N enrichment and elevated atmospheric CO2 on the shoot biomass of Plantago lanceolata populations. The nutrient use efficiency of experimental temperate grasslands has been also found to be significantly affected by the same three-way interaction (Maestre, Bradford & Reynolds 2005; Maestre & Reynolds 2007a). In addition, Maestre & Reynolds (2007b) found a significant soil heterogeneity × N enrichment × changes in rainfall regime interaction on the root: shoot biomass ratio of plant assemblages. Given that multifactorial GC experiments have frequently found non-additive effects on ecosystem components and processes (Norby & Luo 2004; Reich et al. 2001; Dukes et al. 2005; but see Zavaleta et al. 2003), these complex experiments are needed to elucidate potentially counterintuitive effects of GC drivers and soil heterogeneity on plant performance and ecosystem functioning.
From plants to ecosystems: plant-mediated effects of soil heterogeneity and global change on ecosystem functioning
If soil heterogeneity has profound effects on plant processes, such as changes in biomass allocation (Hutchings, John & Wijesinghe 2003), productivity (Maestre, Bradford and Reynolds 2005), or species replacement (Bliss et al. 2002; Wijesinghe, John & Hutchings 2005), we may expect potential effects of soil heterogeneity upon those ecosystem processes tightly linked with them, such as C and N cycling. However, there are always compromises associated with the studied ecosystem responses to soil heterogeneity, because factors such as disturbance, nutrient availability or inter-annual variation in the supply of key resources such as water may override the effects of soil heterogeneity (Huber-Sannwald & Jackson 2001). Therefore, it is difficult to distinguish between the effects of GC drivers and those of soil heterogeneity on ecosystem functioning (Huber-Sanwald & Jackson 2001). Nevertheless, increasing our knowledge on how plant responses to soil heterogeneity modulate plant and ecosystem responses to GC drivers can undoubtedly help improve our ability to predict impacts of GC on plant assemblages and ecosystem functioning (Körner 2003).
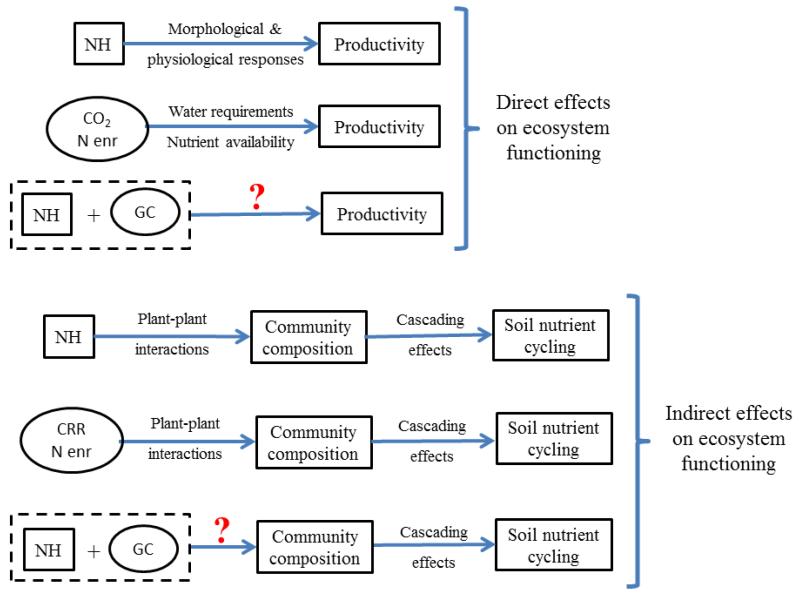
Plant-mediated effects of soil heterogeneity on ecosystem responses to GC may arise directly and indirectly (Fig. 2). The most intuitive direct effect is that related with the stimulation of primary productivity by soil heterogeneity (Day, Hutchings & John 2003; Wijesinghe, John & Hutchings 2005; Maestre & Reynolds 2007a). Such biomass responses are usually associated with precise root foraging (Maestre, Bradford & Reynolds 2005; García-Palacios, Maestre & Gallardo 2011) and physiological changes in nutrient uptake (Hodge 2004) in response to heterogeneous nutrient supply. There is ample evidence on the individual effects and interactions of GC drivers such as elevated atmospheric CO2 and N enrichment on plant productivity (Navas et al. 1999; Jones et al. 2000; He, Bazzaz & Schmid 2002). Therefore, we may expect the benefits that plants obtain when growing in a CO2-enriched atmosphere or nutrient-rich soil, such as reduced water requirements and increases in nutrient availability (Poorter 1993; Niklaus & Körner 2004), to interact with those that plants obtain from soil heterogeneity.
Figure 2.
Schematic representation showing the direct and indirect effects of soil heterogeneity and global change on ecosystem functioning that are mediated by plants. NH = soil nutrient heterogeneity, CO2 = elevated atmospheric CO2, N enr = N enrichment, GC = global change, and CRR = changes in rainfall regime. Blue arrows represent direct effects. Square boxes depict soil nutrient heterogeneity. Ellipse boxes depict global change. Rectangle boxes depict both plant and ecosystem effects. Dashed boxes depict NH × GC interactions. Interrogation marks represent unknown or understudied effects.
The indirect role played by plants on the linkages between soil heterogeneity, GC and ecosystem responses are related mainly to changes in plant community composition, which promote cascading effects on processes such as soil nutrient cycling (Wardle 2002; De Deyn et al. 2008; Bardgett 2011). Two main mechanisms may enhance these compositional changes. First, it is known that soil heterogeneity influences community composition because it changes the nature of competition between coexisting species (Kembel & Cahill 2005). This effect is directly linked with the ability of soil heterogeneity to alter belowground neighborhood structure (Casper & Jackson 1997), competitive interactions (Fransen, de Kroon & Berendse 2001), and species relative abundances (Wijesinghe, John & Hutchings 2005). In addition, if soil heterogeneity is able to induce interactions between root and shoot competition (Rajaniemi 2011), it would have a variety of significant consequences for community composition (Rajaniemi 2003). However, most of the studies addressing this issue have studied single plant species or populations, but not entire communities (but see Wijesinghe, John & Hutchings 2005; Rajaniemi 2011; García-Palacios, Maestre & Gallardo 2011). Second, plant-plant interactions are modified by GC drivers such as changes in rainfall regime (Maestre et al. 2003) or N enrichment (Klanderud & Totland 2005). Given that the outcome of these interactions is expected to have major effects on ecosystem processes such as productivity or nutrient cycling (Mulder, Uliassi & Doak 2001; Hooper et al. 2005, but see Maestre et al. 2010), the interplay between soil heterogeneity and GC may affect ecosystem functioning through their effects upon plant-plant interactions. Both direct and indirect effects of changes in plant composition promoted by soil heterogeneity may suggest that the effects of plant diversity on ecosystem functioning are dependent on this heterogeneity. This suggestion is supported by previous studies from Tylianakis et al. (2008b) and García-Palacios, Maestre & Gallardo (2011) who found that soil heterogeneity modulated the diversity-ecosystem function relationship.
Although not plant-mediated, the functional effects of other biotic communities on ecosystem functioning, such as soil microbes and fauna, are also likely to be influenced by soil heterogeneity. The most important factor promoting soil nutrient heterogeneity is the seasonal accumulation of soil organic matter (Facelli & Pickett 1991). However, other factors, such as the burrowing activity and enrichment of excreta of soil animals (Stark 1994), and the exploitation of readily available root exudates by microbial communities and their consumers (Hodge et al. 1998), are also responsible for creating and depleting soil nutrient patches. Accordingly, we would expect that plant-soil organism interactions change in response to such heterogeneity. For example, differences in nutrient uptake strategies between plant species and functional groups may affect the degree and rate of depletion of nutrient-rich patches, thereby affecting nutrients otherwise available for soil microbes (Wardle 2002). As reviewed by Hodge, Robinson & Fitter (2000), spatial differences in N availability is a key determinant of success in N competition between plants and soil microorganisms. Thus, plant-microorganisms competition for nutrients cannot be fully understood without an explicit consideration of soil heterogeneity. On the other hand, mutualistic interactions such as between plant roots and mycorrhizal fungi have been found to promote the productivity increase by soil heterogeneity (Hodge, Campbell & Fitter 2001), and hence they may act as a determinant of the soil heterogeneity effects on plant diversity-nutrient cycling relationships. Taking this information into account, and the fact that plant-soil interactions are known to be affected by elevated atmospheric CO2 or climate change (reviewed in Bardgett & Wardle 2010), we may expect an important role for soil heterogeneity as a modulator of these linkages under GC. However, we are only beginning to address how soil biota may influence, either directly or indirectly, nutrient capture from heterogeneous nutrient supplies (Hodge et al. 1998; Hodge & Fitter 2010), and hence the potential role of soil heterogeneity as a modulator of plant-soil interaction responses to GC is not known.
What next? Confounding factors, knowledge gaps and future research directions
CONFOUNDING FACTORS IN SOIL HETEROGENEITY STUDIES
As suggested by Huber-Sannwald & Jackson (2001), it is essential to separate resource availability and heterogeneity, because they frequently interact to determine plant and ecosystem responses (Maestre, Bradford & Reynolds 2005; Maestre & Reynolds 2007a,b; García-Palacios, Maestre & Gallardo 2011). To decide whether a plant compensated for a localized supply of nutrients, and to separate this effect from a pure increase in soil nutrients, the study must include a homogeneously distributed nutrient control arranged in multiple levels of availability (low, intermediate and high). The patch treatment should also have the same nutrient availability levels of the control. Only in this way can responses to local cues be distinguished from systemic effects from the rest of the plant (de Kroon et al. 2009). Such comparisons, however, are not the norm in soil heterogeneity studies where homogenous controls usually have nutrient availability intermediate to patch and background, rendering the same overall nutrient availability for homogenous and heterogeneous treatments.
Most previous research has concentrated upon the addition of inorganic patches of phosphate or nitrate, because such sources are immediately available to the plant. In natural soils, however, complex organic patches are the norm, and the decomposition of, and nutrient release from, these complex patches will be vastly different from that resulting from the addition of inorganic nutrient patches to soil (Fransen & de Kroon 2001). In addition, there is ample evidence that plants can use a range of chemical forms of N, including a range of amino acids and peptides (Weigelt, Bol & Bardgett 2005; Hill et al. 2011), and, in some situations, outcompete soil microorganisms for organic N (see Schimel & Bennet 2004 for a review). Thus, to simulate realistic plant responses in soil heterogeneity experiments, organic patches should be used in order to allow the interaction with species-specific soil biota, such as pathogen-mediated root overproduction (de Kroon et al. 2012). The trend seems to be changing, since we found more studies using organic than inorganic nutrient patches (Table 1). Last but not least, experiments should always be conducted with real, as opposed to artificial, soils, as they allow the full complement of biological, chemical and physical interactions to occur with roots, and plants grown with them experience more realistic conditions than those raised in artificial growing medium or sand (Robinson 1994).
KNOWLEDGE GAPS AND GUIDELINES FOR FUTURE EXPERIMENTAL AND THEORETICAL RESEARCH
Morphological and physiological responses to soil heterogeneity are quite well addressed in the literature (Jackson & Caldwell 1992; Fransen, Blijjenberg & de Kroon 1999, Fransen, de Kroon & Berendse 2001; Hodge 2004; Cahill & McNickle 2011), but other important factors remain understudied. The long-term benefits of soil heterogeneity and consequent root foraging into nutrient patches for both individual plants and communities are unclear, as most studies are typically carried out over a very short-time span (e.g. Hodge et al. 1998; Fransen, Blijjenberg & de Kroon 1999). The short duration of most studies means that effects of patch depletion and root turnover are overlooked, which may limit the long-term rewards of root foraging for perennial plants (Fransen & de Kroon 2001). It is important to know about such longer-term effects because most plant species are perennial, and demographic processes are expressed over longer time frames than those used in most experiments so far. Fransen & de Kroon (2001) found that the initial increase in Holcus lanatus performance experienced under a heterogeneous nutrient supply, promoted by precise root foraging and high nutrient uptake, disappeared in the second growing season. This rapid decline in plant performance was probably due to patch depletion and high C and nutrient losses due to a limited root life span. However, García-Palacios, Maestre & Gallardo (2011) found that soil heterogeneity increased shoot and root biomass even in the second growing season. However, the plant functional groups evaluated by these authors showed different mechanisms to consistently increase their performance along the two growing seasons. Whereas legumes experienced higher nutrient use efficiency in the first growing season, they likely took advantage from bacterial N fixation and/or mycorrhizal fungi activity in the second. The non-legume forbs, despite having the lowest morphological plasticity in the first growing season (as measured by root foraging precision), showed enough physiological plasticity to acquire more N from the organic patches during the second growing season. In summary, the long-term consequences of soil heterogeneity are far from being understood, and more research is needed to fully approach the relative importance of this intrinsic ecosystem feature for mid- and long-term plant dynamics. In particular, the interactive effects of root foraging and root mortality in heterogeneous soils remain to be studied.
Kembel & Cahill (2005) suggested that there were not enough data available to determine whether Tilman & Pacala (1993) were correct in their argument that small-scale of nutrient heterogeneity was unimportant for plant communities. The main problem is that soil heterogeneity has a more subtle impact on plant communities than on individuals or monocultures (Hodge 2004). Although we also believe that the consequences of soil heterogeneity upon plant community structure remain largely unexplored, some specifications should be made. Over 50% of studies listed in Table 1 used model communities, suggesting that soil heterogeneity research is beginning to approach community-level issues. However, most of them have focused on a limited number of species in controlled conditions (Maestre & Reynolds 2006a,b, 2007a,b; but see García-Palacios, Maestre & Gallardo 2011). Therefore, to fully understand the role of soil heterogeneity as a key driver of plant responses to GC, current research must move beyond the study of root responses of isolated plants in controlled conditions, and focus on the plant community consequences of soil heterogeneity in the field.
Important insights could be obtained by using field observations to test predictions from modeling studies, where community processes are simulating from individual responses obtained with plant monocultures (e.g. Jongejans, Huber & de Kroon 2010). An example of this approach, although focused on the topic of plant-soil feedback, can be found in Mangan et al. (2010). They found an interesting matching in the sign of the feedback between tree species and soil biota, the performance of tree seedlings was reduced when grown in the presence of enemies associated with adult trees, by means of greenhouse experiments, field observations and simulation models. This study provides an interesting methodological framework to take soil heterogeneity research a step forward. First, the most dominant plants species within an ecosystem should be identified to assess their individual responses to soil heterogeneity in common garden experiments. Morphological (e.g. selective root placement to nutrient patches relative to shoot biomass; Fransen & de Kroon 2001) and physiological (e.g. nutrient capture from 15N-labelled organic patches; Fransen, Blijjenberg & de Kroon 1999; Maestre, Bradford & Reynolds 2005) responses are needed to fully evaluate the phenotypic plasticity of each species (García-Palacios et al. 2011). Second, these individual plant responses should be correlated with the observed species abundance and community composition in the field. At the same time, soil nutrient heterogeneity could be evaluated in the field at different spatial scales using metrics such as the coefficient of variation of nutrient contents (Baer et al. 2004) or the mean absolute difference in nutrient availability between pairs of sampling plots (Bakker, Blair & Knapp 2003). Whether the plant community structure matches the individual responses to soil heterogeneity and correlates with soil heterogeneity metrics in the field, soil heterogeneity will be playing an important role in plant community assembly (Wijesinghe, John & Hutchings 2005). Finally, community dynamics could be simulated by means of stochastic spatially explicit cellular automata models (Balzter, Braun & Köhler 1998). By testing whether simulations including the strength of individual plant responses to soil heterogeneity measured in the experiments generate community species abundance of similar rank order as those found in the field, we could assess the long-term importance of soil heterogeneity at the community level in natural conditions.
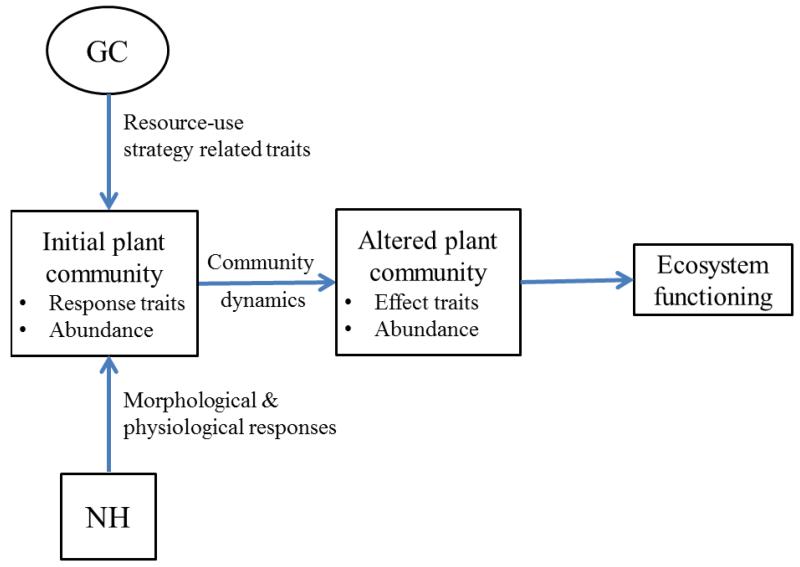
Scaling from soil heterogeneity-plant responses to the ecosystem level is, however, more complicated. Even though ecosystem processes such as C and N cycling are expected to be affected simultaneously by soil heterogeneity and GC, as previously discussed, we are aware of only one study assessing the interactive role of soil heterogeneity and GC (N enrichment) on C, N and P cycling (García-Palacios, Maestre & Gallardo 2011), which did not find any significant interaction. With this aim, we propose that soil heterogeneity should be introduced into the trait-based response-effect framework (Fig. 3). This framework was first approach by Goldberg (1990), and then was applied to ecosystem science by Lavorel & Garnier (2002) and Naeem & Wright (2003). The necessity of placing foraging ability in the broader context of plant traits was previously suggested by Kembel et al. (2008). They found significant correlations between root foraging precision and a range of functional traits involved in plant growth, which suggest a potential effect of soil heterogeneity on ecosystem functioning via modification of plant traits.
Figure 3.
Conceptual model proposed to include plant responses to soil heterogeneity (NH) and global change (GC) into a trait-based response-effect framework. Both NH and GC may affect the abundance and representation of a suite of response traits related with the plant-resource use strategy, with further effects on community dynamics. The subsequent and altered plant community may impact ecosystem functioning in a different way than the initial community via changes in the abundance and representation of ecosystem-effect traits. If GC and NH affect the same suite of response and effect traits, this conceptual framework will allow us to include soil heterogeneity when scaling plant responses to global change to the ecosystem level.
In the trait-based response-effect framework, plant community-level dynamics can be scaled to the ecosystem level with a two-step procedure to: 1) assess community responses to GC, predicted by response traits; and 2) evaluate the concomitant effects of community changes on ecosystem processes, predicted by effect traits (see Suding et al. 2008 for both theoretical description and examples of application). The abundance of some plant traits may respond to GC drivers, with further changes in community dynamics, as usually all species do not behave similarly (Grime et al. 2000). The altered plant community will impact ecosystem functioning via changes in the type, range and abundance of ecosystem-effect traits. Response traits will have a similar reaction to a particular GC driver such as N enrichment or elevated atmospheric CO2, and effect traits will exert similar effects on one or several ecosystem functions. Traits related with resource-use strategies that allow elucidating between conservation and acquisition strategies may suffice as both response and effect traits (Suding et al. 2008). GC is expected to alter plant-resource use strategies via either a change in resource availability and/or a shift in community composition (Lee et al. 2001; Maestre, Bradford & Reynolds 2005). As outlined in Fig. 3, this framework offers a unique way to link plant and ecosystem effects to soil heterogeneity and GC because the traits determining the plant resource-use strategy, that are affected by GC, are also involved in the morphological and physiological plant responses to soil heterogeneity (Hutchings, John & Wijesinghe 2003). However, this framework will work only if the plant traits affected by soil heterogeneity are both relevant response traits to GC and significant effect traits affecting key ecosystem processes, such as the case of specific root length found by P. García-Palacios, F.T. Maestre & R. Milla (unpubl. data). Future experimental studies should evaluate the joint effects of soil heterogeneity and multiple GC drivers on resource-use strategy traits and community composition at a first step, and then assess how the altered plant community affects surrogates of ecosystem functioning such as productivity and nutrient cycling.
Concluding remarks
Our review highlights how soil heterogeneity modulates plant responses to GC drivers, with potential cascading effects on ecosystem functioning. As a result, we argue that plant response predictions to GC drivers, including elevated atmospheric CO2, N enrichment and changes in rainfall regime, will not be informative if they do not explicitly consider the role played by soil heterogeneity. Despite the important knowledge gaps found, such as the long-term consequences on plant dynamics or the effects on plant communities in the field, soil heterogeneity does interact with GC. However, further research is needed to assess under what conditions soil heterogeneity will have synergistic (Maestre & Reynolds 2006b; García-Palacios, Maestre & Gallardo 2011) or inhibitory (Stampfli & Fuhrer 2010; Fridley et al. 2011) interactions with GC drivers. Scaling plant responses to soil heterogeneity to the ecosystem level is also a promising avenue to identify the functional consequences of the linkages between soil heterogeneity, GC and plant communities. We propose two different approaches to overcome these gaps and advance understanding of the role of soil heterogeneity: 1) to combine morphological and physiological plant responses with field observations of community composition and simulation models, in order to assess the long-term importance of soil heterogeneity at the community level in natural conditions; and 2) to include soil heterogeneity into a trait-based response-effect framework (Kembel et al. 2008), in order to scale from plants responses to such heterogeneity to the ecosystem level.
Acknowledgements
This review was made possible thanks to the support of the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n° 242658 (BIOCOM), awarded to FTM. FTM acknowledges support from the Spanish Ministerio de Educación (Salvador de Madariaga program) during the writing of the manuscript. PGP was supported by a postdoctoral Fulbright fellowship from the Spanish Ministerio de Educación.
References
- Ackerly DD, Cornwell WK. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecology Letters. 2007;10:135–145. doi: 10.1111/j.1461-0248.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- Arnone JA., III Temporal responses of community fine root populations to long-term elevated atmospheric CO2 and soil nutrient patches in model tropical ecosystems. Acta Oecologica. 1997;18:367–376. [Google Scholar]
- Baer SG, Blair JM, Collins SL, Knapp AK. Plant community responses to resource availability and heterogeneity during restoration. Oecologia. 2004;139:617–639. doi: 10.1007/s00442-004-1541-3. [DOI] [PubMed] [Google Scholar]
- Bakker C, Blair JM, Knapp AK. A comparative assessment of potential mechanisms influencing plant species richness in grazed grasslands. Oecologia. 2003;137:385–391. doi: 10.1007/s00442-003-1360-y. [DOI] [PubMed] [Google Scholar]
- Balzter H, Braun PW, Köhler W. Cellular automata models for vegetation dynamics. Ecological Modelling. 1998;107:113–125. [Google Scholar]
- Bardgett RD, Freeman C, Ostle NJ. Microbial contributions to climate change through carbon-cycle feedbacks. The ISME Journal. 2008;2:805–814. doi: 10.1038/ismej.2008.58. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Wardle DA. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change. Oxford Series in Ecology and Evolution, Oxford University Press; UK: 2010. [Google Scholar]
- Bardgett RD. Plant-soil interactions in a changing world. F1000 Biology Reports. 2011;3:16. doi: 10.3410/B3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GM, Bazzaz FA. Nitrogen cycling in microcosms of yellow birch exposed to elevated CO2: simultaneous positive and negative below-ground feedbacks. Global Change Biology. 1997;3:247–258. [Google Scholar]
- Bliss KM, Jones RH, Mitchell R, Mou P. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytologist. 2002;154:409–417. doi: 10.1046/j.1469-8137.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Cahill JF, Jr., McNickle GG. The behavioral ecology of nutrient foraging by plants. Annual Review of Ecology, Evolution, and Systematics. 2011;42:289–311. [Google Scholar]
- Casper BB, Cahill JF., Jr. Limited effects of soil nutrient heterogeneity on populations of Abutilon theophrasti (Malvaceae) American Journal of Botany. 1996;83:333–341. [PubMed] [Google Scholar]
- Casper BB, Jackson RB. Plant competition underground. Annual Review of Ecology and Systematics. 1997;28:545–570. [Google Scholar]
- Clark PJ, Evans FC. Distance to nearest neighbour as a measure of spatial relationships in populations. Ecology. 1954;35:445–453. [Google Scholar]
- Day KJ, Hutchings MJ, John EA. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. Journal of Ecology. 2003;91:541–553. [Google Scholar]
- De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Mommer L. Root foraging theory put to the test. Trends in Ecology and Evolution. 2006;21:113–116. doi: 10.1016/j.tree.2005.11.021. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ. A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell and Environment. 2009;32:704–712. doi: 10.1111/j.1365-3040.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Hendriks M, van Ruijven J, Ravenek J, Padilla FM, Jongejans E, Visser EJW, Mommer L. Root responses to nutrients and soil biota: drivers of species coexistence and ecosystem productivity. Journal of Ecology. 2012;100:6–15. [Google Scholar]
- Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, Thayer S, Tobeck T, Mooney HA, Field CB. Responses of grassland production to single and multiple global environmental changes. PLoS Biology. 2005;3:e319. doi: 10.1371/journal.pbio.0030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facelli JM, Pickett STA. Plant litter: its dynamics and effects on plant community structure. The Botanical Review. 1991;57:1–32. [Google Scholar]
- Farley RA, Fitter AH. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology. 1999;87:688–696. [Google Scholar]
- Fransen B, Blijjenberg J, de Kroon H. Root morphological and physiological plasticity of perennial grass species and the exploitation of spatial and temporal heterogeneous nutrient patches. Plant and Soil. 1999;211:179–189. [Google Scholar]
- Fransen B, de Kroon H. Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a two-year experiment. Journal of Ecology. 2001;89:711–722. [Google Scholar]
- Fransen B, de Kroon H, Berendse F. Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology. 2001;82:2534–2546. [Google Scholar]
- Fridley JD, Grime JP, Askew AP, Moser B, Stevens CJ. Soil heterogeneity buffers community response to climate change in species-rich grassland. Global Change Biology. 2011;17:2002–2011. [Google Scholar]
- García-Palacios P, Maestre FT, Gallardo A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. Journal of Ecology. 2011;99:551–562. doi: 10.1111/j.1365-2745.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam FS. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. Journal of Ecology. 2006;94:1176–1191. [Google Scholar]
- Gleason HA. The individualistic concept of the plant association. Bulletin of the Torrey Botanical Club. 1926;53:7–26. [Google Scholar]
- Grime JP, Brown VK, Thompson K, Masters GJ, Hillier SH, Clarke IP, Askew AP, Corker D, Kielty JP. The response of two contrasting limestone grasslands to simulated climate change. Science. 2000;289:762–765. doi: 10.1126/science.289.5480.762. [DOI] [PubMed] [Google Scholar]
- Grünzweig JM, Körner Ch. Growth, water and nitrogen relations in grassland model ecosystems of the semi-arid Negev of Israel exposed to elevated CO2. Oecologia. 2001;128:251–262. doi: 10.1007/s004420100657. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Morrow LL, Walsh J. A meta-analysis of competition in field experiments. The American Naturalist. 1992;140:539–572. [Google Scholar]
- Gurevitch J, Morrison JA, Hedges LV. The interaction between competition and predation: a meta-analysis of field experiments. Amercian Naturalist. 2000;155:435–453. doi: 10.1086/303337. [DOI] [PubMed] [Google Scholar]
- Hanson PJ, Wullschleger SD, Norby RJ, Tschaplinski TJ, Gunderson CA. Importance of changing CO2, temperature, precipitation, and ozone on carbon and water cycles of an upland-oak forest: incorporating experimental results into model simulations. Global Change Biology. 2005;11:1402–1423. [Google Scholar]
- He JS, Bazzaz FA, Schmid B. Interactive effects of diversity, nutrients and elevated CO2 on experimental plant communities. Oikos. 2002;97:337–348. [Google Scholar]
- Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Climate Change. 2011;1:50–53. [Google Scholar]
- Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytologist. 1998;139:479–494. doi: 10.1046/j.1469-8137.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Fitter AH. Are microorganisms more effective than plants at competing for nitrogen? Trends in Plant Science. 2000;5:304–308. doi: 10.1016/s1360-1385(00)01656-3. [DOI] [PubMed] [Google Scholar]
- Hodge A, Campbell CD, Fitter AH. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature. 2001;413:297–299. doi: 10.1038/35095041. [DOI] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Hodge A. Plastic plants and patchy soils. Journal of Experimental Botany. 2006;57:401–441. doi: 10.1093/jxb/eri280. [DOI] [PubMed] [Google Scholar]
- Hodge A, Fitter AH. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences. 2010;107:13754–13759. doi: 10.1073/pnas.1005874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- Huber-Sannwald E, Jackson RB. Heterogeneous soil-resource distribution and plant responses-from individual-plant growth to ecosystem functioning. Progress in Botany. 2001;62:451–476. [Google Scholar]
- Hutchings MJ, de Kroon H. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research. 1994;25:159–238. [Google Scholar]
- Hutchings MJ, Wijesinghe DK, John EA. The effects of heterogeneous nutrient supply on plant performance: a survey of responses, with special reference to clonal herbs. In: Hutchings MJ, John EA, Stewart AJA, editors. The Ecological Consequences of Environmental Heterogeneity. Blackwell Science; Oxford, UK: 2000. pp. 91–110. [Google Scholar]
- Hutchings MJ, John EA, Wijesinghe DK. Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology. 2003;84:2322–2334. [Google Scholar]
- IPCC . Climate Change 2001: Impacts, Adaptation and Vulnerability. International Panel of Climate Change; Geneva, Switzerland: 2007. [Google Scholar]
- Jackson RB, Caldwell MM. Shading and the capture of localized soil nutrients: nutrient contents, carbohydrates, and root uptake kinetics of a perennial tussock grass. Oecologia. 1992;91:457–462. doi: 10.1007/BF00650316. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Caldwell MM. Geostatistical patterns of soil heterogeneity around individual perennial plants. Journal of Ecology. 1993;81:683–692. [Google Scholar]
- Jansen C, van Kempen MML, Bogemann GM, Bouma TJ, de Kroon H. Limited costs of wrong root placement in Rumex palustris in heterogeneous soils. New Phytologist. 2006;171:117–126. doi: 10.1111/j.1469-8137.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Jones TH, Bezemer TM, Körner Ch., Lawton JH, Thompson LJ. Comparing studies of artificial and natural ecosystem responses to CO2 enrichments. Biotronics. 2000;29:1–7. [Google Scholar]
- Jongejans E, Huber H, de Kroon H. Scaling up phenotypic plasticity with hierarchical population models. Evolutionary Ecology. 2010;24:585–599. [Google Scholar]
- Kardol P, Bezemer TM, van der Putten WH. Temporal variation in plant–soil feedback controls succession. Ecology Letters. 2006;9:1080–1088. doi: 10.1111/j.1461-0248.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- Kembel SW, Cahill JF., Jr. Plant phenotypic plasticity belowground: a phylogenetic perspective on root foraging trade-offs. American Naturalist. 2005;166:216–230. doi: 10.1086/431287. [DOI] [PubMed] [Google Scholar]
- Kembel SW, de Kroon H, Cahill JF, Jr., Mommer L. Improving the scale and precision of hypotheses to explain root foraging ability. Annals of Botany. 2008;101:1295–1301. doi: 10.1093/aob/mcn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cahill JF., Jr. Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE. 2011;6:e19992. doi: 10.1371/journal.pone.0019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanderud K, Totland Ø. Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology. 2005;86:2047–205. [Google Scholar]
- Körner Ch. Experimental plant ecology: some lessons from global change research. In: Press MC, Huntly NJ, Levin S, editors. Ecology: Achievement and Challenge. Blackwell Science; Oxford, UK: 2001. pp. 227–248. [Google Scholar]
- Körner Ch. Nutrients and sink activity drive plant CO2 responses: Caution with literature-based analysis. New Phytologist. 2003;159:537–538. doi: 10.1046/j.1469-8137.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- Körner Ch., Bazzaz FA. Carbon Dioxide, Population, and Community. Academic Press; San Diego, USA: 1996. [Google Scholar]
- Lamb EG, Haag JJ, Cahill JF., Jr. Patch-background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti. Functional Ecology. 2004;18:836–843. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Lee TD, Tjoelker MG, Ellsworth DS, Reich PB. Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytologist. 2001;150:405–418. [Google Scholar]
- Luo Y, Reynolds JF, Wang Y, Wolfe D. A search for predictive understanding of plant responses to elevated [CO2] Global Change Biology. 1999;5:143–156. [Google Scholar]
- Maestre FT, Bautista S, Cortina J. Positive, negative and net effects in grass-shrub interactions in Mediterranean semiarid grasslands. Ecology. 2003;84:3186–3197. [Google Scholar]
- Maestre FT, Bradford MA, Reynolds JF. Soil nutrient heterogeneity interacts with elevated CO2 and nutrient availability to determine species and assemblage responses in a model grassland community. New Phytologist. 2005;168:637–650. doi: 10.1111/j.1469-8137.2005.01547.x. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Reynolds JF. Nutrient availability and atmospheric CO2 partial pressure modulate the effects of nutrient heterogeneity on the size structure of populations in grassland species. Annals of Botany. 2006a;98:227–235. doi: 10.1093/aob/mcl093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Reynolds JF. Spatial heterogeneity in nutrient supply modulates plant nutrient and biomass responses to multiple global change drivers in model grassland communities. Global Change Biology. 2006b;12:2431–2441. [Google Scholar]
- Maestre FT, Bradford MA, Reynolds JF. Soil heterogeneity and community composition jointly influence grassland biomass. Journal of Vegetation Science. 2006;17:261–270. [Google Scholar]
- Maestre FT, Reynolds JF. Biomass responses to elevated CO2, soil heterogeneity and diversity: an experimental assessment with grassland assemblages. Oecologia. 2007a;151:512–520. doi: 10.1007/s00442-006-0577-y. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Reynolds JF. Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology. 2007b;88:501–511. doi: 10.1890/06-0421. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Valladares F, Reynolds JF. Individual vs. population plastic responses to elevated CO2, nutrient availability and heterogeneity: a microcosm experiment with co-occurring species. Plant and Soil. 2007;296:53–64. [Google Scholar]
- Maestre FT, Bowker MA, Escolar C, Puche MD, Soliveres S, Mouro S, García-Palacios P, Castillo-Monroy AP, Martínez I, Escudero A. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Philosophical Transactions of the Royal Society B. 2010;365:2057–2070. doi: 10.1098/rstb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan SA, Schnitzer SA, Herre EA, Mack KM, Valencia MC, Sánchez EI, Bever JD. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature. 2010;466:752–755. doi: 10.1038/nature09273. [DOI] [PubMed] [Google Scholar]
- Mokany K, Raison RJ, Prokushkin AS. Critical analysis of root: shoot ratios in terrestrial biomes. Global Change Biology. 2006;12:84–96. [Google Scholar]
- Mou P, Jones RH, Mitchell RJ, Zutter B. Spatial distribution of roots in Sweetgum and Loblolly Pine monocultures and relations with above-ground biomass and soil nutrients. Functional Ecology. 1995;9:689–699. [Google Scholar]
- Mulder CPH, Uliassi DD, Doak DF. Physical stress and diversity-productivity relationships: the role of positive interactions. Proceedings of the National Academy of Sciences USA. 2001;98:6704–6708. doi: 10.1073/pnas.111055298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecology Letters. 2003;6:567–579. [Google Scholar]
- Navas ML, Garnier E, Austin MP, Gifford RM. Effect of competition on the responses of grasses and legumes to elevated atmospheric CO2 along a nitrogen gradient: differences between isolated plants, monocultures and multi-species mixtures. New Phytologist. 1999;143:323–331. [Google Scholar]
- Niklaus PA, Körner Ch. Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecological Monographs. 2004;74:491–511. [Google Scholar]
- Norby RJ, Luo Y. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytologist. 2004;162:281–293. [Google Scholar]
- Pan D, Bouchard A, Legendre P, Domon G. Influence of edaphic factors on the spatial structure of inland halophytic communities: A case study in China. Journal of Vegetation Science. 1998;9:797–804. [Google Scholar]
- Poorter H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio. 1993;104:77–97. [Google Scholar]
- Poorter H, Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology. 2000;27:595–607. [Google Scholar]
- Poorter H, Navas ML. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist. 2003;157:175–198. doi: 10.1046/j.1469-8137.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Rajaniemi TK. Evidence for size asymmetry of belowground competition. Basic and Applied Ecology. 2003;4:239–24. [Google Scholar]
- Rajaniemi T, Reynolds HL. Root foraging for patchy resources in eight herbaceous plant species. Oecologia. 2004;141:514–525. doi: 10.1007/s00442-004-1666-4. [DOI] [PubMed] [Google Scholar]
- Rajaniemi TK. Competition for patchy soil resources reduces community evenness. Oecologia. 2011;165:169–174. doi: 10.1007/s00442-010-1710-5. [DOI] [PubMed] [Google Scholar]
- Reich PB, Knops J, Tilman D, Craine J, Ellsworth D, Tjoelker M, Lee T, Wedin D, Naeem S, Bahauddin D, et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature. 2001;410:809–812. doi: 10.1038/35071062. [DOI] [PubMed] [Google Scholar]
- Philip RG, Klingensmith KM, Klug MJ, Paul EA, Crum JR, Ellis BG. Soil resources, microbial activity, and primary production across an agricultural ecosystem. Ecological Applications. 1997;7:158–170. [Google Scholar]
- Robertson GP, Huston MA, Evans FC, Tiedge JM. Spatial variability in a successional plant community: patterns of nitrogen availability. Ecology. 1988;68:744–748. [Google Scholar]
- Robinson D. The responses of plants to non-uniform supplies of nutrients. New Phytologist. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society of London B. 1999;266:431–435. [Google Scholar]
- Robinson D, Van Vuuren MMI. Responses of wild plants to nutrient patches in relation to growth rate and life-form. In: Lambers H, Poorter H, van Vuuren MMI, editors. Variation in Plant Growth. Backhuys; Leiden, The Netherlands: 1998. pp. 237–257. [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB. Grassland responses to global environmental changes suppressed by elevated CO2. Science. 2002;298:1987–1990. doi: 10.1126/science.1075312. [DOI] [PubMed] [Google Scholar]
- Smith DM, Jackson NA, Roberts JM, Ong CK. Reverse flow of sap in tree roots and downward siphoning of water by Grevillea robusta. Functional Ecology. 1999;13:256–264. [Google Scholar]
- Stampfli A, Fuhrer J. Spatial heterogeneity confounded ozone-exposure experiment in semi-natural grassland. Oecologia. 2010;162:515–522. doi: 10.1007/s00442-009-1462-2. [DOI] [PubMed] [Google Scholar]
- Stark JM. Causes of soil nutrient heterogeneity at different scales. In: Caldwell MM, Pearcy RW, editors. Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above- and Belowground. Academic Press; San Diego, USA: 1994. pp. 255–284. [Google Scholar]
- Stein A, Brouwer J, Bouma J. Methods for comparing spatial variability patterns of millet yield and soil data. Soil Science Society of America Journal. 1997;61:861–870. [Google Scholar]
- Stöcklin J, Schweizer K, Körner Ch. Effects of elevated CO2 and phosphorus addition on productivity and community composition of intact monoliths from calcareous grasslands. Oecologia. 1998;116:50–56. doi: 10.1007/s004420050562. [DOI] [PubMed] [Google Scholar]
- Suding KN, Lavorel S, Chapin FS, Cornelissen H, Diaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas ML. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Global Change Biology. 2008;14:1125–1140. [Google Scholar]
- Tilman D, Pacala SW. The maintenance of species richness in plant communities. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities. University of Chicago Press; Chicago, USA: 1993. pp. 13–25. [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecology Letters. 2008a;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- Tylianakis T, Rand TA, Kahmen A, Klein AM, Buchmann N, Perner J, Tscharntke T. Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLOS Biology. 2008b;6:947–956. [Google Scholar]
- van der Heijden MGA, Bardgett RD, van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchencko S, Melillo JM. Human domination of the Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Wang L, de Kroon H, Smits AJM. Combined effects of partial rootzone drying and patchy fertilizer placement on nutrient acquisition and growth of oilseed rape (Brassica napus) Plant and Soil. 2007;295:207–216. [Google Scholar]
- Wardle D. Communities and Ecosystems: Linking the aboveground and Belowground Components. Princeton University Press; Madison, USA: 2002. Monographs in population biology v. 34. [Google Scholar]
- Weigelt A, Bol R, Bardgett RD. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- Wijesinghe DK, John EA, Hutchings MJ. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology. 2005;93:99–112. [Google Scholar]
- Zavaleta ES, Shaw MR, Chiariello NR, Mooney HA, Field CB. Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proceedings of the National Academy of Sciences USA. 2003;100:7650–7654. doi: 10.1073/pnas.0932734100. [DOI] [PMC free article] [PubMed] [Google Scholar]