Abstract
Roadside grasslands undergoing secondary succession are abundant, and represent ecologically meaningful examples of novel, human-created ecosystems. Interactions between plant and soil communities (hereafter plant–soil interactions) are of major importance in understanding the role of biotic control in ecosystem functioning, but little is known about these links in the context of ecosystem restoration and succession. The assessment of the key biotic communities and interactions driving ecosystem development will help practitioners to better allocate the limited resources devoted to roadside grassland restoration. We surveyed roadside grasslands from three successional stages (0–2, 7–9 and > 20 years) in two Mediterranean regions of Spain. Structural equation modeling was used to evaluate how interactions between plants, biological soil crusts [BSCs], and soil microbial functional diversity [soil microorganisms] affect indicators of ecosystem development and restoration: plant similarity to the reference ecosystem, erosion control and soil C storage and N accumulation. Changes in plant community composition along the successional gradient exerted the strongest influence on these indicators. High BSC cover was associated with high soil stability, and high soil microbial functional diversity from late-successional stages was associated with high soil fertility. Contrary to our expectations, the indirect effects of plants, mediated by either BSCs or soil microorganisms, were very weak in both regions, suggesting a minor role for plant–soil interactions upon ecosystem development indicators over long periods. Our results suggest that natural vegetation dynamics effectively improved ecosystem development within a time frame of 20 years in the grasslands evaluated. They also indicate that this time could be shortened if management actions focus on: 1) maintain well-conserved natural areas close to roadsides to enhance plant compositional changes towards late-successional stages, 2) increase BSC cover in areas under strong erosion risk, to avoid soil loss, and 3) enhance soil microbial functional diversity in resource-limited areas, to enhance soil C and N accumulation.
Keywords: biological soil crusts, biotic control, ecosystem development, plant–soil interactions, restoration, roadside grasslands, secondary succession, soil microorganisms
Introduction
Land movements (roadside slopes, opencast mine sites or quarries) together with agricultural old-fields, usually result in grasslands undergoing secondary succession. These areas are among the most abundant and ecologically meaningful examples of novel, human-created ecosystems worldwide (Forman and Alexander 1998, Cramer et al. 2008). Specifically, roadside margins cover approximately 1% of most developed countries (Forman 2000), which in the United States represents an area equal to Austria or South Carolina (Forman and Alexander 1998). In addition, road construction is a major driver of grassland diversity change (Spellerberg 1998), with negative ecological consequences (Forman and Alexander 1998, Gelbard and Harrison 2005), but also opportunities as a potential new habitat for the conservation of biodiversity (National Research Council 2005, Hopwood 2008). It is critical to understand whether secondary successional processes in roadside grasslands suffice to guarantee ecosystem functioning recovery, since these mechanisms have direct implications for managing and restoring areas affected by road construction (Walker et al. 2007).
The contribution of above- and belowground biota to ecosystem development along successional gradients is one of the hottest emerging debates in ecology (Kardol et al. 2006, Bever et al. 2010, Kardol and Wardle 2010). A better understanding of the interactions between plant and soil communities (hereafter plant–soil interactions) can help to take a step forward on both basic and applied ecological knowledge regarding secondary succession and restoration of human-created grasslands (Walker et al. 2007, Van der Putten et al. 2009). The assessment of the key biotic communities and interactions driving ecosystem development will help practitioners to better allocate the limited resources devoted to restoration (Kardol and Wardle 2010). However, restoration ecology studies that have explicitly considered these interactions are currently scarce (Eviner and Hawkes 2008).
Important research efforts are being devoted to incorporate the effects of plant communities, soil biota and their interactions when evaluating the rate and outcome of plant species replacement along secondary successional gradients (Van der Putten 2003, Kardol et al. 2006, 2007). These interactions are of major importance in understanding the role of biotic control in ecosystem functioning (Van der Putten et al. 2009), including nutrient cycling or soil stabilization (Smith et al. 2003, Chaudhary et al. 2009). However, plant–soil interaction studies have rarely examined the relative influence of each biotic community, and their interactions, on processes other than plant species replacement (Ehrenfeld et al. 2005, Casper and Castelli 2007). On the other hand, the potential importance of plant–soil interactions to long-term plant succession has received strong support from microcosm experiments (Kardol et al. 2006, 2007). However, there is limited support for their importance under field conditions (Kulmatiski et al. 2008, Bever et al. 2010) where multiple plant species interact with each other to affect soil biota. Among the soil biota components, biological soil crusts (BSCs), which are constituted by a complex community of organisms (mosses, lichens, liverworts, cyanobacteria, fungi, bacteria), have been traditionally excluded from plant– soil interaction studies (Belnap et al. 2003). BSCs occur particularly in drylands, but also to some degree in most ecosystems (Bowker et al. 2010), and have strong influence on ecosystem services such as C and N fixing (Belnap and Lange 2003) or erosion control (Chaudhary et al. 2009). However, these organisms have been seldom addressed in the applied literature, representing an under exploited opportunity for restorationists (Bowker et al. 2008, Sedia and Ehrenfeld 2005).
We studied the relative roles and importance of different biotic communities (vascular plants, BSCs and soil microbial communities), and their interactions, as drivers of ecosystem development in roadside grasslands undergoing secondary succession. We also assessed the role of propagule dispersion from nearby areas on this process. The following indicators of ecosystem development, which are also key targets for roadside grassland restoration, were used: plant similarity to the reference ecosystem, and ecosystem services such as erosion control and soil C storage and N accumulation (Rentch et al. 2005). The reference ecosystem was selected as the dominant ecosystem surrounding the roadside grasslands with a late-successional plant community, low erosion rates and high soil fertility. The main purpose of this study was to identify the hurdles for ecosystem development and restoration in such environments (Pywell et al. 2002). Specifically, we addressed the following questions: 1) what is the relative importance of age, dispersal limitations, and biotic communities (plants, BSCs and soil microorganisms) in determining ecosystem development?, and 2) which portion of the effects of plant succession on ecosystem development is indirectly mediated by BSCs and soil microorganisms?
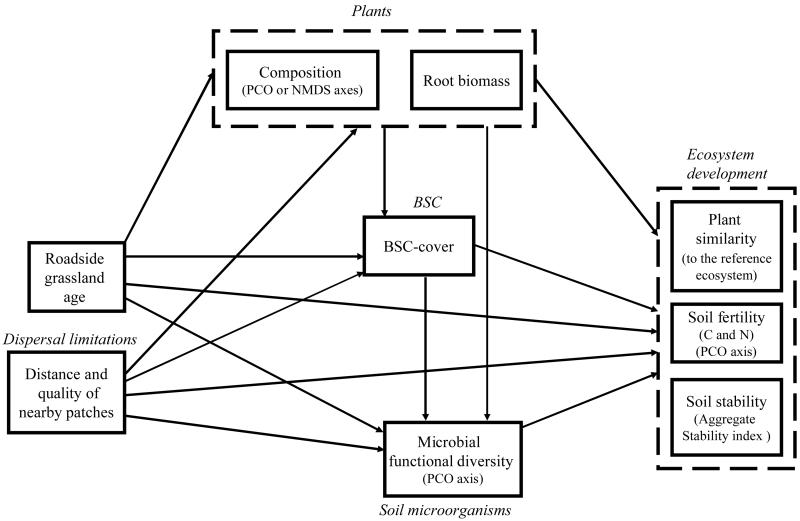
To answer these questions, we gathered data from a chronosequence of roadside grasslands (0–2, 7–9 and > 20 years since road construction works) in two Mediterranean regions of Spain, and analyzed them using structural equation modeling (Grace 2006). Following current ecological knowledge, we hypothesized a hierarchy of above-belowground causal relationships between the three biotic communities in a path diagram (Fig. 1): plants and BSCs directly affect soil microorganisms, while the former also affect BSCs. Although soil communities can establish independently of plants (Bardgett et al. 2007), they mainly develop with the plant community. This seems to be the case in the nutrient-poor and non-structured soils of roadside grasslands (Bochet et al. 2007), where plant functional traits associated with plant composition changes may substantially contribute to the build-up phase of soil development (Garnier et al. 2004). The three biotic communities affected indicators of ecosystem development, directly or indirectly through age and dispersal limitations. Structural equation models test the probability that this hypothesized model is a reasonable explanation of the data; but they do not rule out that other equally good or better models exist, such as feedbacks between plants and soil microorganisms (Kardol et al. 2006) or particular BSC effects upon plant establishment (Escudero et al. 2007). The test of this conceptual model will assess whether it is appropriate to describe secondary successional dynamics and to provide new ecosystem targets to be used in the restoration of human-created ecosystems such as roadside grasslands.
Figure 1.
Generalized a priori conceptual model depicting pathways by which age and dispersal limitations may influence plants, biological soil crusts, soil microorganisms and their interactions, and three indicators of ecosystem development. Dashed boxes depict conceptual variables that may be represented by real data measurements (solid boxes). Single-headed arrows signify a hypothesized causal influence of one variable upon another.
Methods
Study area and experimental design
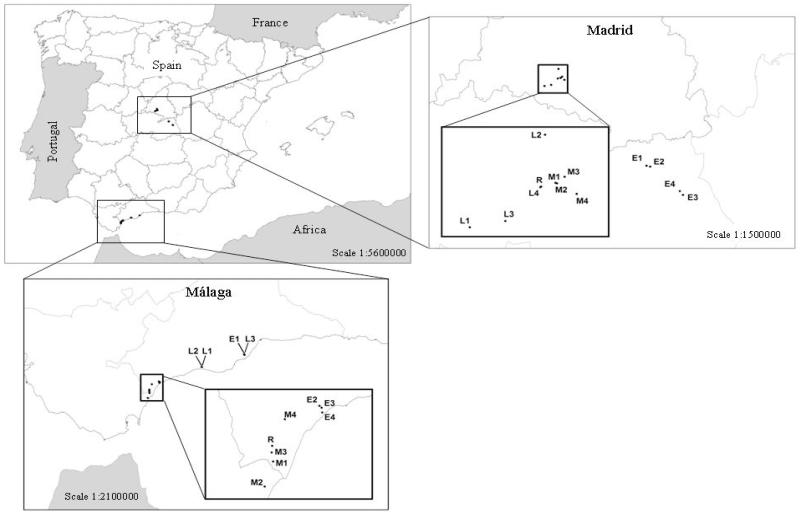
Two climatically contrasting regions were selected for this study (Fig. 2). The first was selected in Madrid, in the centre of the Iberian Peninsula. This area has a continental semiarid climate, with cold winters and a severe summer drought; mean temperature and annual precipitation are 15°C and 450 mm, respectively (Getafe Air Base climatic station 40°18′N, 3°44′W, 710 m.a.s.l., 1971-2000). The second region was located in Málaga (southern Spain). It has a maritime Mediterranean subhumid climate, with warmer winters than Madrid. Mean temperature and annual precipitation in this area are 18°C and 1,017 mm, respectively (Casares climatic station 36°26′N, 5°16′W, 380 m.a.s.l., 1990-2006).
Figure 2.
Location of the study regions: Madrid and Málaga, in the centre and south coast of Spain, respectively. E1– E4, M1– M4, L1– L4, represent the four roadside slope grasslands belonging to the early-, mid- and late-successional stages, respectively. R represents the reference ecosystem in both regions.
Roadside grasslands differing in the road construction age were selected to represent different successional stages: 0–2 years (early stage), 7–9 years (mid stage) and > 20 years (late stage). The purpose of this classification was to identify different temporal stages within our chronosequence (Kardol et al. 2006). However, we acknowledge that more time would be needed to talk about real late-successional stages in Mediterranean ecosystems. Information on construction ages was supplied by the road building-management company (Cintra Ltd.) or obtained by using aerial photographs (www.madrid.org/cartografia/planea/). The establishment and management of these roadside grasslands were similar between the three ages and in both regions. In Spain, general prescriptions are commonly applied irrespective of the local constraints for roadside grassland restoration (Matesanz et al. 2006). Since all the roadside slopes selected were embankments, neither topsoil nor organic matter was added in any of the study sites (information supplied by the road building-management company). Unfortunately, we ignore if the selected roadside grasslands were seeded or not. However, commercial seed mixtures in roadside restoration are composed by exogenous species, mainly herbs (grasses and legumes), which are not adapted to the harsh environmental conditions of Mediterranean roadside grasslands (e.g. nutrient-limited soils and summer drought, Matesanz et al. 2006). These seedings have poor establishment rates and are quickly replaced by native species (Matesanz et al. 2006, Bochet et al. 2007). Hence, we do not expect potential previous seedings to importantly affect succesional dynamics in the grasslands evaluated. Furthermore, to deal with spatial heterogeneity of non-age-related variation among sites (Walker et al. 2010), four different roadside slopes were initially selected within each age (Fig. 2). To homogenize the selection of grasslands and to minimize main sources of plant community structure and composition variation among roadside slopes, we selected in all cases embankments with short-time stockpiled surface calcareous soil and similar size (greater than 15 m long from top to bottom of the slope and 30 m wide), aspect and angle (Appendix A). After a visual inspection, only three late-successional roadside slopes were surveyed in Málaga due to the low availability of homogeneous grasslands from this age. The total roadside grasslands surveyed was 12 in Madrid (four grasslands per successional stage) and 11 in Málaga (four grasslands per successional stage, except for the late-successional grasslands). The design of our natural experiment was the same in both regions, and included two factors: age (three levels) as a fixed factor and roadside slope (four levels) as a random factor nested within age. Ten 1 × 1 m quadrats were surveyed in each grassland, giving a total of 120 plots (3 ages × 4 roadside slopes × 10 quadrats or replicates) per region (110 in Málaga).
Soils and vegetation were surveyed in each slope using the ten randomly selected 1 × 1 m quadrats. In addition, five randomly placed 1 × 1 m quadrats were surveyed in a nearby ecosystem in each region, which we used as the reference ecosystem. The selection of a reference ecosystem is obligated to guide restoration towards target communities (SER 2004). However, in regions such as the Mediterranean Basin, where the ecosystems have been intensively transformed by humans for centuries (Naveh and Dan 1973), the selection of a nearby natural ecosystem as a reference site is not an easy task (Hobbs et al. 2006). Recovering ecosystem services by promoting ecosystem development could be an appropriate restoration objective (Hobbs et al. 2006). Therefore, the reference condition was chosen in each region according to both plant community (dominated by late-successional species) and ecosystem services such as erosion control and soil C storage and N accumulation. The vegetation of the reference ecosystem consisted of a savanna-like system with scattered Retama sphaerocarpa L. among a grassy understory, and a shrubland dominated by Chamaerops humilis L. and Ulex parviflorus L. in Madrid and Málaga, respectively (Appendix B).
Assessing dispersal limitations
The arrival of propagules from external species pools is one of the most important ecological constraints for plant and soil community assembly (Zobel et al. 2000, Kardol et al. 2009), and for the restoration of degraded ecosystems (Novák and Prach 2003). Although potential past perturbations in the roadside slopes selected were avoided using information provided by the road construction company, variation in landscape context, and thus in propagule dispersion –an important limitation of most chronosequence studies (Walker et al. 2010)– was unknown. This variation may determine the species of the regional pool that can potentially colonize each studied grassland. Therefore, we assessed the dispersal limitations with a semi-qualitative landscape index to account for this variation. The distance and quality of the nearest four landscape patches surrounding each roadside slope, two of the main dispersal limitations (Novák and Prach 2003), were measured along the four cardinal points. Distance was measured as the orthogonal distance (in m) from the roadside slope border to the landscape patch border. Quality was measured with a simple nominal scale attending to the increasing potential of each patch as a source of propagule availability for dispersion, in terms of both propagule and species number: industrial-urban, farmland, old-field, roadside grassland, grass-shrubland mosaic and forest. This nominal scale was chosen according to the dominant land-use types in both study regions. Distance values were standardized as , where dk is the distance measured in the field between the roadside slope and the landscape patch evaluated (k), Dk is the standardized distance, and n = 4 because, for each roadside slope, one landscape patch was assessed along each cardinal point. To get the final dispersal limitation index, the quality of all the four patches was weighted by its standardized distance to the roadside slope and summed. Higher values indicate closer landscape patches of higher quality as propagule sources.
Sampling of plants, biological soil crusts and soils
The cover of each vascular plant species was visually determined in each 1 × 1 m plot by the same person between April–May 2009, the optimal phenological moment to measure the herbaceous communities studied (García-Palacios et al. 2010). Two randomly selected soil cores (5 × 10 cm) were removed in each plot and bulked to sample root biomass. In the laboratory, we washed the roots using a 500 μm sieve to retrieve fine roots. All roots were dried at 60°C to constant weight. We did not attempt to distinguish between live and dead roots. Three extra soil cores (5 × 7.5 cm) were removed in each plot. These soil cores were bulked by plot, homogenized and kept cold in the field until laboratory preparation. In the laboratory, the samples were sieved (2 mm mesh) and separated into two fractions. One fraction was immediately frozen at −80°C for microbial analysis and the other was air-dried for 1 month for biogeochemical analyses. Four 1cm diameter natural soil aggregates were sampled from random locations in each 1 × 1 m plot to measure the total cover of BSCs. Cover of visible BSC components, such as mosses and cyanobacteria, was used to visually determine total BSC cover, which is considered a suitable surrogate of BSC biomass (Bowker et al. 2008). Such small–scale surveys have been employed to study the effects of BSCs on several soil functions (Bowker et al. 2010, Maestre et al. 2010).
Assessing of soil microbial functional diversity
We analyzed the functional diversity of soil heterotrophic microbial communities with the MicroResp™ system (Campbell et al. 2003). This is a whole-soil method based on community level physiological profiles obtained by testing ecologically meaningful carbon sources of different chemical recalcitrance (Oren and Steinberger 2008). Here we used 14 carbon sources belonging to various chemical groups: amino acids, carbohydrates, carboxylic acids and fatty acid ester polymers (all Sigma Aldrich, UK). In functional terms, the substrate utilization rates of the carbon sources correspond to the catabolic attributes of different soil microbial functional groups. MicroResp is appropriate for plant–soil interaction studies aiming to evaluate how changes in plant composition, and therefore in resource inputs quality, modify the functional composition of soil microbial communities (Oren and Steinberger 2008). Prior to carrying out the MicroResp™ method, defrosted soils were introduced into the plates and pre-incubated for five days at 25°C and 40% of their water holding capacity. After that, the plates were incubated for 6 h and read at 595 nm. See Appendix C for a detailed description of the methodology employed.
Processes associated with ecosystem development
Plant community similarity (in percentage) of each 1 × 1 m plot to the average species composition of the reference ecosystem was calculated as a surrogate of plant compositional changes along the secondary successional gradient. Community similarity was calculated using the Bray-Curtis distance. Square root transformations were used to decrease the influence of the most abundant plant species (Lepš and Šmilauer 2003).
Soil aggregate stability was estimated as an indicator of erosion control because it is correlated with soil susceptibility to runoff, and with water infiltration capacity (Mueller 2007). Soil aggregates often slake and/or disperse in contact with water (Field et al. 1997). However, most soil stability tests are based only on slake tests. These tests do not take into account that soil micro-aggregates resulting from slaking can disintegrate to a massive structure, a process called dispersion (Dexter 1988). This disintegration or dispersion is also important since it can result in hard–setting dense soils and lead to gully and tunnel erosion, adversely affecting water and air movement, root penetration and function, and seedling establishment (Field et al. 1997). Here, soil stability was assessed in four 1 cm-diameter and 0.5 cm depth natural soil aggregates taken out from random locations in each plot with the aggregate stability in water test (ASWAT, Field et al. 1997). This field-based test submits soil aggregates to several timed water immersions and assesses cohesion using an ordinal scale ranging from 0 to 16. This test is divided into two parts, the first one is a slake test that estimates the physical stability of soil fragments in water. The second part assesses the chemical dispersion character of soil. High scores point to low soil stability, indicating a poor ability of soils to provide adequate aeration for plant growth and drainage (Field et al. 1997).
Total soil N and organic C were measured as indicators of N accumulation and C storage, respectively. Both variables have been broadly used in herbaceous ecosystems to study the build-up of nutrient pools during the course of secondary succession (Baer et al. 2002, Garnier et al. 2004). Total N was obtained on a SKALAR San++ Analyzer (Skalar, Breda, The Netherlands) after digestion with sulphuric acid. Organic C was determined by potassium dichromate oxidation using the Walkley–Black method (Nelson and Sommers 1982).
Statistical analyses
The one-dimensional biotic variables (root biomass and BSC cover) did not follow ANOVA assumptions, even after data transformation. Thus, the effects of age in all the biotic variables, either one- or multi-dimensional (plant composition and soil microbial functional diversity) were evaluated using a two-way permutational ANOVA-type test. We used age as a fixed factor and roadside slope as a random factor nested within age. Separate analyses were conducted in each region. All permutational ANOVA-type tests were conducted using the semi-parametric PERMANOVA approach (Anderson 2001, McArdle and Anderson 2001). It does not make distributional assumptions and is compatible with any distance measure. We used the Bray-Curtis distance for the plant community data and the Euclidean distance for the rest of the variables, and 9999 permutations of the raw data in all cases. Additionally, square root transformations were used to decrease the influence of the most abundant plant species (Lepš and Smilauer 2003), which shifts the focus of the analysis from primarily the dominants to the entire community. To aid in the interpretation of the analyses of plant and microbial functional diversity data, we also performed a principal coordinate analysis (PCO; Anderson et al. 2008). Significant differences between ages were evaluated with the PERMANOVA post-hoc pairwise t-test (Anderson 2001).
To assess the extent to which plant community composition explained differences in soil fertility, we used a distance-based linear model (DISTLM, McArdle and Anderson 2001). This approach is analogous to a traditional regression, but allows the use of data matrices as either dependent or independent variables. Our two matrices were plant community composition (a predictor matrix containing the percent cover data of all plant species) and soil fertility (a response matrix of soil C and N). To reduce the probability of identifying spurious predictors as important, we removed very rare plant species (those having less than three occurrences) from the plant community matrix. We selected parsimonious models using Akaike’s information criterion and the forward selection procedure to determine which matrix components were the most influential in the predictor matrix. The model with the lowest AIC value and P < 0.05 was selected as the best model in each region.
We modelled the network of relationships hypothesized in the a priori model of Fig. 1 between age, dispersal limitations, biotic communities (plants, BSCs and soil microorganisms) and the indicators of ecosystem development (plant similarity, soil fertility and stability) using structural equation modeling (SEM, Grace 2006). A detailed description of the approach followed can be found in Appendix D. Plant–soil interaction effects on ecosystem development were evaluated as the indirect effects of plants on ecosystem development indicators mediated by BSCs and/or soil microorganisms. Because of the high dimensionality of our dataset, we conducted some data reduction prior to analyses. Variables were derived to represent plant community composition, microbial community functional composition (named soil microorganisms), and soil fertility. All were based on ordination techniques. To the greatest extent possible, we used axes from the PCO analyses (above) to summarize community composition, although we encountered some difficulty with multicollinearity when later introducing these PCO axes of plant community composition in the Madrid models. In this case, we used a one-dimensional non-metric multidimensional scaling (NMDS) axis, also based on Bray-Curtis distance, which did not suffer from multicollinearity. Soil fertility was also a synthetic variable derived from the first axis of a PCO analysis of soil N and C, based upon Euclidean distance. The categorical predictor “successional state” was modelled using a composite variable. Composite variables have multiple uses, but here it functions to sum together the effects of the levels of a categorical variable, which are represented by dummy variables.
Using the variables either measured or derived above, we constructed our model and tested its fit. To better elucidate the main effects and interactions modulating each ecosystem development variable, and to simplify the model interpretation, we constructed a different model for each of the three processes associated with ecosystem development (plant similarity with the reference ecosystem, soil stability and fertility) in the two regions studied, totaling six models. Plant community composition was omitted from the plant similarity models because it was exceedingly difficult to simultaneously introduce age, plant community composition, and plant community distance from reference site without also introducing effects of multicollinearity. We used the traditional χ2 goodness-of-fit test, but because of its sensitivity to sample size, the NFI and RMSEA indices were also considered as alternative measures of model fit (Grace 2006). Low probability values are not desired in these statistical tests, unlike many others. When a satisfactorily fitting model is developed, path coefficients estimates are obtained, using the maximum likelihood estimation technique (Grace 2006). The path coefficient is directly analogous to a partial correlation coefficient of regression weight, and is interpreted as the size of an effect that one variable exerts upon another.
Our final step in the SEM analyses conducted was to introduce “conceptual” composite variables. They simply sum together the effects of multiple conceptually related variables upon another, collapsing the effects into a single path coefficient. In the soil stability and fertility models for both regions, plants were introduced as a composite variable to allow an additive combination of the effects of the plant community composition and the root biomass upon BSCs, soil microorganisms and the indicators of ecosystem development. The exceptions were in the plant similarity models, wherein we combined the direct effects of age with root biomass into a composite variable to estimate plant effects on plant similarity to the reference ecosystem. We adopted this strategy because plant composition had been omitted from these models (above). This simplified model structure accomplishes the basic objective of separating the effects of BSCs and soil microorganisms on distance from reference site from those directly attributable to plants, but cannot separate the effects of plant community composition from those of age.
Distance-based linear models, PERMANOVA, PCO and NMDS analyses were carried out using the PERMANOVA+ module for the PRIMER software (PRIMER-E Ltd., Plymouth Marine Laboratory, UK; Anderson et al. 2008). SEM analyses were performed with SPSS version 14.0 and AMOS (SPSS Inc., Chicago, IL, USA).
Results
Changes in biotic communities along the successional gradient
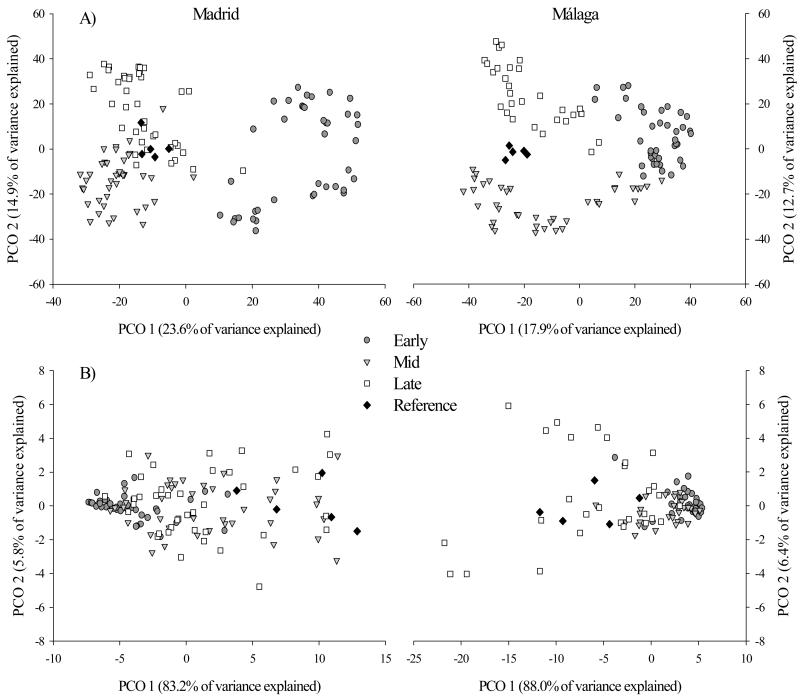
The age of the roadside slopes significantly affected plant community composition in both Madrid (F2,9 = 3.73, P < 0.001) and Málaga (F2,8 = 2.85, P < 0.001). The post-hoc pairwise t-test indicated significant differences (P < 0.05) between all ages in both regions (Fig. 3A). In the Madrid sites, plant community similarity with the reference ecosystem significantly increased along the successional gradient evaluated, from 13.6% in the early-successional stage to 31.6% in the late stage. Plant community compositional changes did not follow the same trend in the Málaga sites. In this case, the highest similarity with the reference ecosystem was found in the mid-successional stage (23.5%). Root biomass experienced a monotonic significant increase with age in the Madrid sites (F2,9 = 4.58, P = 0.044), where the late-successional stage reached root biomass values (117 g · m−2) similar to the reference ecosystem (129 g · m−2). Alternatively, in the Málaga sites, the root biomass was not significantly affected by roadside slope age (F2,8 = 2.92, P = 0.092). BSC cover was similar (45–48%) in all the ages in the Madrid sites (F2,9 = 7.53, P = 0.951), but increased with age in the Málaga sites (F2,8 = 71.35, P = 0.016). The functional diversity of soil microbial communities was significantly affected by age in Madrid and Málaga (F2,9 = 5.65, P = 0.018 and F2,8 = 12.11, P = 0.004). The post-hoc pairwise t-test indicated that the early- was different from the mid- and late-successional stages (P < 0.05) in Madrid (Fig. 3B). In Málaga (Fig. 3B), the microbial communities of the late- were different from the early- and midsucccessional stages (P < 0.05).
Figure 3.
Results of the canonical analysis of principal coordinates (PCO), showing the effects of age (early-, mid- and late-successional stages) on plant community composition (A) and soil microbial functional diversity (B) of Madrid and Málaga. Each data point represents a 1 × 1 m plot. PCO 1 and PCO 2 indicate both ordination axes. The reference ecosystem was introduced in each region only for interpretation purposes.
Relative importance of biotic control, dispersal limitations and age for ecosystem development
The different goodness-of-fit statistics (χ2, NFI and RMSEA) indicated that all the ecosystem development models evaluated fitted the data satisfactorily (Figs. 4, 5 and 6). The amounts of variance explained by the SEM models for all dependent variables, excepting BSCs in Madrid, were statistically significant (P < 0.001). These models were able to explain ca. two-thirds of the plant composition variance, and more than one third of the variance of the soil microorganisms in both Madrid and Málaga. However, they only had a similar explanatory power for the BSCs in Málaga.
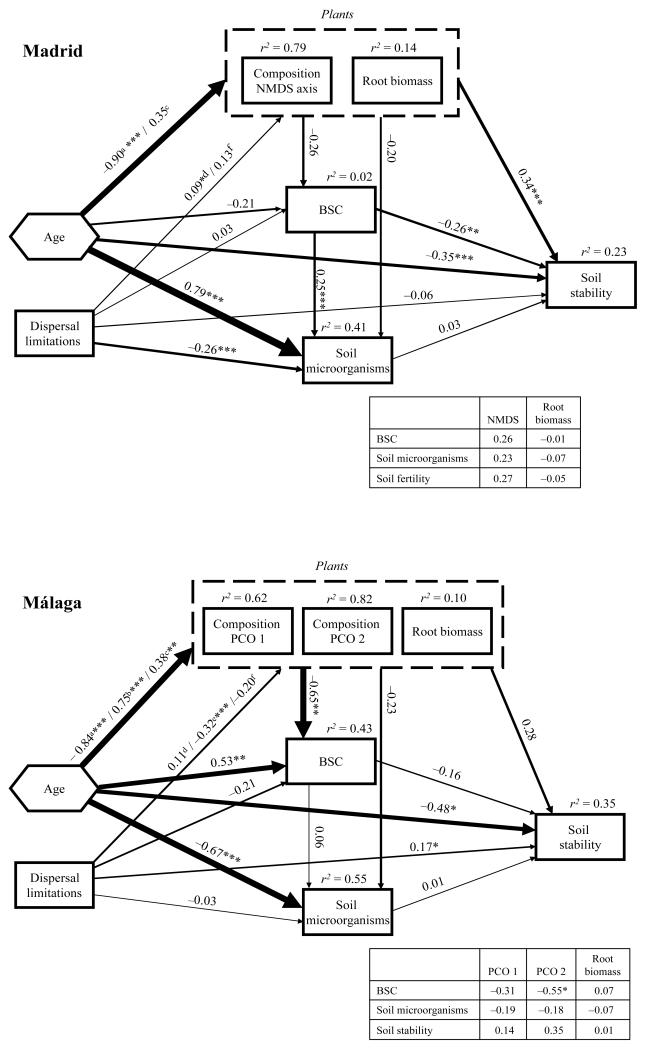
Figure 4.
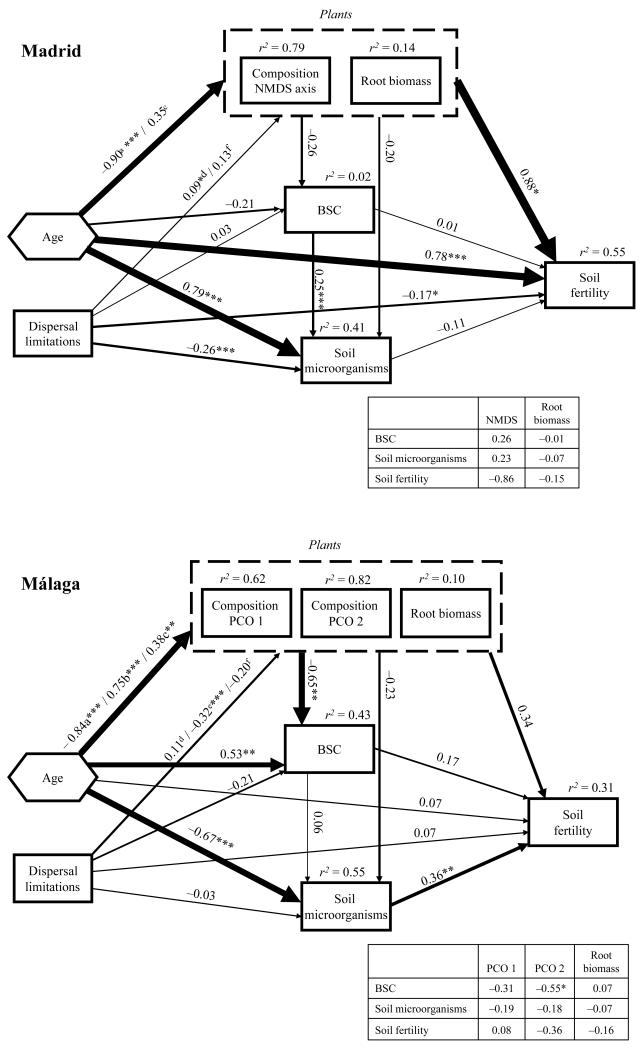
Final structural equation models for soil stability (aggregate stability test; ASWAT) in Madrid and Málaga. Hexagon represents the effects of roadside slope age (early, mid and late) on endogenous variables, which were introduced in the models as two-indicator latent variables. Numbers adjacent to arrows are path coefficients, analogous to regression weights and indicative of the effect size of the relationship. Width of arrows is proportional to the strength of path coefficients. As in other linear models, r2 signifies proportion of variance explained and appears above every response variable in the model. The inset table reflects the individual path coefficients from variables that compose the vegetation composite variable to BSC, soil microorganisms and soil stability. a, b and c denote path coefficients from age to NMDS or PCO 1 axis (Madrid or Málaga), PCO 2 and root biomass, respectively, and d, e and f from dispersal limitations to NMDS or PCO 1 axis (Madrid or Málaga), PCO 2 and root biomass, respectively. Goodness-of-fit statistics for the Madrid (χ2 = 2.075, P = 0.354; NFI = 0.994; RMSEA = 0.018, P = 0.456) and Málaga (χ2 = 4.379, P = 0.223; NFI = 0.992; RMSEA = 0.065, P = 0.331) models. NFI = Normed Fit Index. RMSEA = Root Mean Square Error of Approximation Index. Significance levels are as follows: *P < 0.05, **P < 0.01 and ***P < 0.001.
Figure 5.
Final structural equation models for soil fertility (the first axis of a principal component analysis for organic carbon and total nitrogen) in Madrid and Málaga. In the Madrid model, plant community composition was evaluated with a one-dimensional ordination (performed with non-metric multidimensional scaling; NMDS axis a, b and c denote path coefficients from age to NMDS or PCO 1 axis (Madrid or Málaga), PCO 2 and root biomass, respectively, and d, e and f from dispersal limitations to NMDS or PCO 1 axis (Madrid or Málaga), PCO 2 and root biomass, respectively. Goodness-of-fit statistics for the Madrid (χ2 = 0.014, P = 0.907; NFI = 1; RMSEA = 0, P = 0.920) and Málaga (χ2 = 4.379, P = 0.223; NFI = 0.992; RMSEA = 0.065, P = 0.331) models. Rest of legend as in Fig. 4.
Figure 6.
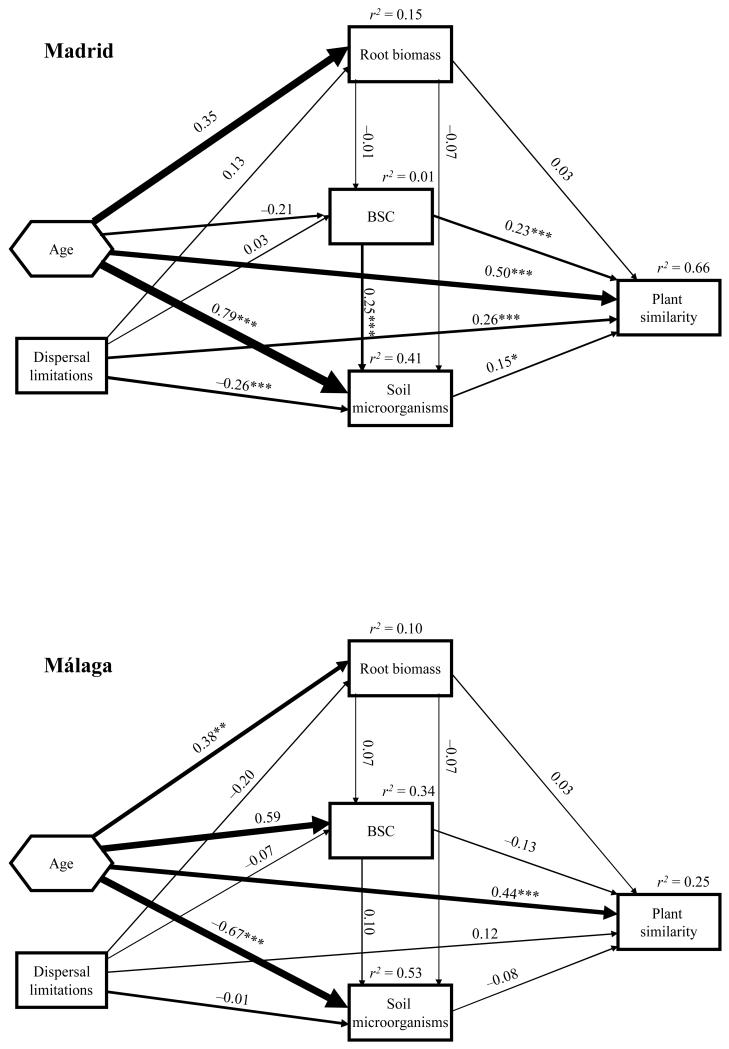
Final structural equation models for plant similarity (percentage of plant community similarity to the average species composition of the reference ecosystem) in Madrid and Málaga. The effects of age and root biomass were composite to estimate plant effects on plant succession. Goodness-of-fit statistics for the Madrid (χ2 = 0.716, P = 0.397; NFI = 0.997; RMSEA = 0, P = 0.464) and Málaga (χ2 = 1.587, P = 0.452; NFI = 0.994; RMSEA = 0, P = 0.542) models. Rest of legend as in Fig. 4.
Our SEM models (Fig. 4) were able to explain 23% and 35% of the variance in soil stability in Madrid and Málaga, respectively. The largest contribution to this variance was the direct effects of age in both regions. Age also exerted important indirect effects on soil stability via its strong influence upon the plant compositional axes. Higher values of the plant composition axes in Madrid (NMDS 1, see Appendix E) and Málaga (PCO 1 and 2, see Fig. 3A) were related to higher ASWAT score in both regions. These results, together with the previously mentioned age effects, indicated a soil stability increase mediated by plant composition in the mid- and late-successional stages in Madrid and Málaga. The relative contribution of BSCs and soil microorganisms upon soil stability was weaker than that of plants. However, BSC cover exerted a moderate direct effect in both regions, although this was only significant in Madrid. Dispersal limitations had a moderate effect on soil stability in Málaga.
Our SEM models were able to explain 55% and 31% of the variance in soil fertility in Madrid and Málaga, respectively (Fig. 5). Most of this variance was accounted for by a direct effect of age in Madrid, and by indirect effects of this variable via its influence upon plant composite variable in both regions. Lower values of the plant composition axes in Madrid (NMDS 1, see Appendix E) and Málaga (PCO 2, see Fig. 3A), and increases in root biomass in both regions, were associated with lower values of the soil fertility PCO 1 axis. Low values of this axis mean low soil C and N in Madrid, and high soil C and N in Málaga (Appendix F). These results, together with the previously mentioned age effects, indicated a soil fertility increase in the late-successional stages mediated by plant composition and root biomass in both regions. Soil microorganisms showed an important effect on soil fertility in Málaga (r = 0.36). Dispersal limitations had a significant, but of secondary importance, effect in Madrid.
The variance explained in the SEM models for plant community similarity to the reference ecosystem (Fig. 6) was relatively high in Madrid (r2 = 0.66), but not in Málaga (r2 = 0.25). Most of the plant similarity variance was accounted for by age direct effects in Madrid and Málaga. As explained in the Methods section, these direct effects indicate plant effects on plant similarity without separating the individual effect of plant composition and age. The relative importance of BSCs and soil microorganisms upon plant similarity was significant in Madrid but not in Málaga. Dispersal limitations significantly enhanced plant similarity in Madrid.
Plant–soil interactions associated with ecosystem development
We only found two significant plant–soil interactions. BSCs moderately influenced soil microorganisms in Madrid (r = 0.25), and age exerted important indirect effects on BSC via its influence upon the plant compositional axes in Málaga (Figs. 4 and 5). However, and most importantly, the effects of plant–soil interactions on ecosystem development were very weak. The significant BSCs effects upon soil stability (Fig. 4), and that of BSCs and soil microorganisms upon plant similarity (r = 0.23 and 0.15, Fig. 6) in Madrid, were all direct effects. The significant soil microorganism effects on soil fertility in Málaga (Fig. 5) were also the result of direct effects. Therefore, we did not find plant indirect effects on the indicators of ecosystem development (plant similarity, soil fertility and stability) mediated by BSCs or soil microorganisms in either region.
Discussion
Plant–soil interactions are known to drive the rate and outcome of plant species replacement in grasslands undergoing secondary succession (Kardol et al. 2006, 2007). However, their effect on other ecosystem properties that change through succession, such as soil stability and fertility, has been barely examined (Ehrenfeld et al. 2005, Casper and Castelli 2007). Our study contributes to fill this gap with a robust chronosequence design (Walker et al. 2010) coupled with a structural equation modeling approach. We found little evidence to suggest that plant–soil interactions play a major role as modulators of ecosystem development, rather plants and soil biota contribute to these functions in a largely independent fashion Our study shows that ecosystem development along secondary successional gradients in roadside grasslands is ultimately regulated by plants (Baer et al. 2002, Smith et al. 2003). Plant community compositional shifts, which depend mainly on time rather than on soil biota or local climatic conditions, and to a lesser extent root biomass, exerted the strongest biotic control on plant similarity to the reference ecosystem, soil stability and fertility.
Plant direct and indirect effects on ecosystem development
The indirect effects of plants upon ecosystem development, indirectly mediated by BSCs and/or soil microorganisms, were very weak in both climatically contrasting regions (Figs. 4, 5 and 6). Although these interactions might be crucial in the early stages of roadside development (García-Palacios 2010), in our study, neither soil fertility nor stability were affected by them. Plants and soil microorganisms developed towards the reference ecosystem (Fig. 3), and soil fertility and stability increased along the successional gradient. Together these results suggest that plant–soil interactions, as described by the hypothesized causal relationships tested here, play a minor role to control ecosystem development in roadside grasslands when a longer time scale is considered.
Our results show that plants, BSCs and soil microorganisms interacted to some degree, but did not influence the indicators of ecosystem development evaluated. Soil microorganisms from late-successional stages were related to higher BSC cover in Madrid. The successional development of BSCs is known to promote an increase of bacterial biomass and abundance (Yeager et al. 2003). The other significant plant–soil interaction found in Málaga upon BSCs was indirectly caused by age, not by plants, as the effects of plants on BSCs were mainly controlled by the strong effects of age upon plants. The direct effects of BSCs and soil microorganisms found upon plant similarity to the reference ecosystem in Madrid suggest that plant species replacement along the succession is directly promoted by these soil communities. The temporary persistence of late-successional plant species has been hypothesized to be promoted by positive plant–soil feedbacks with soil biota (Van der Putten 2003, Kardol et al. 2006). Although the effects found in our study were moderate in terms of variance explained in plant similarity, they may represent a field-based contribution to the amount of empirical plant–soil feedbacks studies carried out to date (Kulmatiski et al. 2008).
Our SEM models were satisfactorily fitted to our data, although the amount of variance explained was low in some of them, especially in the soil stability models. Two possible weaknesses may explain this pattern: 1) the use of 1 cm diameter soil aggregates to measure BSC cover in 1 × 1 m plots, and 2) additional unmeasured factors that contribute to soil stabilization. The use of more detailed measures of BSCs (e.g. richness and evenness, Maestre et al. 2005), soil microorganisms (e.g. AM fungi, Chaudhary et al. 2009 and/or invertebrates, De Deyn et al. 2003) and dispersal limitations (e.g. randomization tests supported by vegetation surveys in the nearby landscape patches, Bochet et al. 2007) would likely increase the strength of biotic communities ’ effects upon ecosystem development, and should be included in future studies.
Biotic control on ecosystem development along successional gradients
Our DISTLM models helped us to unravel the complexity of the relationships between age and plant community, and to assess the extent to which plant community composition explained differences in soil fertility in each region. Plant community composition explained more than half of the soil C and N variation at both regions (Appendix G). Soil C storage and N accumulation are important ecosystem services of grasslands worldwide (Scurlock and Hall 1998), and are largely determined by certain functional traits that covary with plant composition (De Deyn et al. 2008). Our results indicate the importance of above- and belowground plant functional traits, such as litter and root C:N, in modulating soil C and N pools. Late-successional microbial communities were directly linked to higher soil C storage and N accumulation in Málaga. Soil microbial functional diversity changed along the succession towards the reference ecosystem in Madrid and Málaga (Fig. 3B), suggesting that the microbial energy pathway changed from simple carbohydrate decomposers to ligninolytic microbes (Maharning et al. 2009). Litter and root quality from grasslands undergoing secondary succession have been found to change from low to high C:N rates (Garnier et al. 2004, Maharning et al. 2009). In this successional context, soil microbial communities respond to this increase in plant inputs recalcitrance (Bardgett 2005, Maharning et al. 2009). Although plant nutrients were not evaluated in this study, the change in microbial functional diversity towards the reference ecosystem found in both regions suggests a microbial response to hypothetical shifts in plant litter quality along the succession. Increases in soil C and N along the successional gradient (35% increase in the late-successional stages in both regions), promoted by the dominance of late-successional microbial communities specialized in decomposing recalcitrant plant litter, highlight the potential role of roadside grasslands as an effective C sink in a broad range of Mediterranean climates.
Biological soil crusts have been found to maintain soil stability and increase erosion resistance (Chaudhary et al. 2009), which is of major importance during early-successional stages in roadside grasslands (Rentch et al. 2005). Our results support these findings, as high BSC cover was associated with low ASWAT scores in both regions, and thus with high soil stability (Fig. 4). Therefore, BSC should be considered a plant–soil interaction of major importance affecting erosivity in terrestrial ecosystems. BSC cover was positively related to plant similarity to the reference ecosystem in Madrid but not in Málaga. Bowker (2007) suggested that in low abiotic stress systems (e.g. subhumid climate in Málaga) BSCs are a successional component, whereas in high abiotic stress systems (e.g. semiarid climate in Madrid) they become a permanent feature of the late-successional stages. Our results suggest an increasing importance for BSCs in plant succession, from low to high abiotic stress systems.
Anthropogenically induced successional gradients, such as old-fields or roadside grasslands, are very helpful to infer the mechanisms behind natural dynamics because covarying factors, such as site history, are often more clearly understood than in natural systems (Fukami and Wardle 2005). Well designed chronosequences can be a reasonable template for the study of the different drivers of secondary succession (Walker et al. 2010). The similarities in soil type, climate and topographic features make the studied roadside grasslands a good chronosequence model to study successional patterns (Garnier et al. 2004). Our chronosequence approach clearly identified different plant communities in each age in both regions (Fig. 3A). This change was directional and linear towards the reference ecosystem in the case of Madrid, probably because of biotic colonization processes from surrounding patches of high quality as seed sources (Novák and Prach 2003). A divergent trajectory is more plausible for the Málaga region, where the presence of woody species introduced by practitioners in some of the late-successional grasslands (Matesanz et al. 2006) may have promoted this pattern (Walker et al. 2010). The high relative importance of plant composition confronted with dispersal limitations, BSCs and soil microorganisms highlights the key role played by this biotic community to control ecosystem development.
Management implications
Our causal models have identified which biotic communities and interactions play important roles as drivers of ecosystem development in roadside grasslands undergoing secondary succession. Practitioners can use this information to prioritize the allocation of resources during ecosystem-level restoration (Chaudhary et al. 2009). When the aim of restoration is to promote plant similarity to the reference ecosystem, the colonization of propagules adapted to local site conditions from naturally vegetated areas (Pywell et al. 2002) is an effective mechanism that can be manipulated during road construction. Bochet et al. (2007) established a threshold of 150 m from natural areas to guarantee efficient seed dispersal to roadside grasslands in Mediterranean environments. However, only anemochorous plants (60% of plant species in these systems, Bochet et al. 2007) will be able to colonize. Hence, the plantation of late-successional woody seedlings is also desirable to further foster secondary succession in human-created ecosystems (Booth et al. 1999, Badia et al. 2007). Plant community compositional shifts towards late-successional stages had the largest influence upon soil C and N pools. Plant–soil feedbacks can also help to accelerate vegetation succession (De Deyn et al. 2003, Kardol et al. 2006), although the necessity of soil organisms introduction is confronted with their passive establishment following vegetation development (Kardol and Wardle 2010). However, the outstanding role played by late-successional microbial communities to increase soil fertility highlights the necessity to evaluate microbial inoculation in roadside grassland restoration. Although not evaluated in our models, the creation of nutrient-rich organic patches has a potential major influence to enhance soil nutrient cycling and plant productivity in the early-successional stages of roadside grasslands (García-Palacios et al. 2011), and should be also considered as an option to enhance the build-up of nutrient pools. If the management aim is to control soil erosion, special attention should be paid to BSC. Bowker (2007) pointed to propagule availability as one of the main limitations to restore these communities. Inoculation is the best-studied approach to overcome this limitation, but is considered suitable only on small scales due to culturing problems and the need of a “sacrifice area” providing the inoculum (Bowker 2007). Watering and fertilization can effectively promote the recovery of BSC in laboratory trials (Maestre et al. 2006), and could also be locally applied to promote BSC development.
In conclusion, our results provide evidence for a strong biotic control on ecosystem development in Mediterranean grasslands undergoing secondary succession, and have major implications for disentangling the complexity of above-belowground biota linkages and for improving the restoration of these grasslands. They indicate that natural vegetation dynamics can be used as an effective passive restoration tool in roadside grasslands, but this may take up to 20 years under Mediterranean climatic conditions. However, this period could be reduced, and the success of long-term ecosystem restoration actions maximized if these focus on: 1) promoting plant compositional changes towards late-successional stages, 2) increasing BSC cover, and 3) enhancing soil microbial functional diversity.
Supplementary Material
Acknowledgements
We thank Patricia Alonso, Victoria Ochoa, Yolanda Valiñani, Rebecca Mau and Arturo Vizcaíno for their help in the laboratory, and all the Cintra personnel in Madrid and Málaga for the facilities and support given during all the stages of this research. PGP and SS were supported by PhD fellowships from the EXPERTAL project, funded by Fundación Biodiversidad and Cintra Ltd. FTM is supported by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n° 242658 (BIOCOM). This research was supported by the EXPERTAL and REMEDINAL 2 projects, funded by Fundación Biodiversidad-Cintra S.A. and the Comunidad de Madrid, respectively.
References
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to software and statistical methods. PRIMER-E; Plymouth, UK: 2008. [Google Scholar]
- Badia D, Valero R, Gracia A, Martí C. Ten-year growth of woody species planted in reclaimed banks with different slopes. Arid Land Research and Management. 2007;21:67–79. [Google Scholar]
- Baer SG, Kitchen DJ, Blair JM, Rice CW. Changes in ecosystem structure and function along a chronosequence of restored grasslands. Ecological Applications. 2002;12:1688–1701. [Google Scholar]
- Bardgett R. The biology of soils: a community and ecosystem approach. Oxford University Press; 2005. [Google Scholar]
- Bardgett RD, Richter A, Bol R, Garnett MH, Bäumler R, Xu X, Lopez-Capel E, Manning D, Hobbs PJ, Hartley IR, Wanek W. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biology Letters. 2007;3:487–490. doi: 10.1098/rsbl.2007.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnap J, Hawkes CV, Firestone MK. Boundaries in miniature: Two examples from soil. BioScience. 2003;53:737–794. [Google Scholar]
- Belnap J, Lange OL. Biological soil crusts: structure, function and nanagement. Springer-Verlag; Berlin: 2003. [Google Scholar]
- Bever JD, Dickie ID, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M. Rooting theories of plant community ecology in microbial interactions. Trends in Ecology and Evolution. 2010;25:468–478. doi: 10.1016/j.tree.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet E, García-Fayos P, Tormo J. Roadslope revegetation in semiarid Mediterranean environments. Part I: Seed dispersal and spontaneous colonization. Restoration Ecology. 2007;15:88–96. [Google Scholar]
- Booth DT, Gores JK, Schuman GE, Olson RA. Shrub densities on pre-1985 reclaimed mine lands in Wyoming. Restoration Ecology. 1999;7:24–32. [Google Scholar]
- Bowker MA. Biological soil crust rehabilitation in theory and practice: an underexploited opportunity. Restoration Ecology. 2007;15:13–23. [Google Scholar]
- Bowker MA, Belnap J, Chaudhary VB, Johnson NC. Revisiting classic erosion models: the strong influence of biological soil crusts. Soil Biology and Biochemistry. 2008;40:2309–2316. [Google Scholar]
- Bowker MA, Maestre FT, Escolar C. Biological crusts as a model system for examining the biodiversity-ecosystem function relationship in soils. Soil Biology and Biochemistry. 2010;42:405–417. [Google Scholar]
- Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Applied and Environmental Microbiology. 2003;69:3593–3599. doi: 10.1128/AEM.69.6.3593-3599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper BB, Castelli JP. Evaluating plant–soil feedback together with competition in a serpentine grassland. Ecology Letters. 2007;10:394–400. doi: 10.1111/j.1461-0248.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary VB, Bowker MA, O’Dell TE, Grace JB, Redman AE, Rillig MC, Johnson NC. Untangling the biological contributions to soil stability in semiarid shrublands. Ecological Applications. 2009;19:110–122. doi: 10.1890/07-2076.1. [DOI] [PubMed] [Google Scholar]
- Cramer VA, Hobbs RJ, Standish RJ. What’s new about old fields? Land abandonment and ecosystem assembly. Trends in Ecology and Evolution. 2008;23:104–112. doi: 10.1016/j.tree.2007.10.005. [DOI] [PubMed] [Google Scholar]
- De Deyn GB, Raaijmakers CE, Zoomer HR, Berg MP, de Ruiter PC, Verhoef HA, Bezemer TM, van der Putten WH. Soil invertebrate fauna enhances grassland succession and diversity. Nature. 2003;422:711–713. doi: 10.1038/nature01548. [DOI] [PubMed] [Google Scholar]
- De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- Dexter AR. Advances in characterisation of soil structure. Soil and Tillage Research. 1988;11:199–238. [Google Scholar]
- Ehrenfeld JG, Ravit B, Elgersma K. Feedback in the plant–soil system. Annual Review of Environment and Resources. 2005;30:75–115. [Google Scholar]
- Escudero A, Martínez I, de la Cruz A, Otálora MG, Maestre FT. Species specific effects of soil lichens on the seedling emergence of three plant species from semiarid gypsum habitats. Journal of Arid Environments. 2007;70:18–28. [Google Scholar]
- Eviner VT, Hawkes CV. Embracing variability in the application of plant-soil interactions to the restoration of communities and ecosystems. Restoration Ecology. 2008;16:713–729. [Google Scholar]
- Field DJ, McKenzie DC, Koppia AJ. Development of an improved Vertisol stability test for SOILpak. Australian Journal of Soil Research. 1997;35:843–852. [Google Scholar]
- Forman RTT. Estimate of the Area Affected Ecologically by the Road System in the United States. Conservation Biology. 2000;14:31–35. [Google Scholar]
- Forman RTT, Alexander LE. Roads and their major ecological effects. Annual Review of Ecology and Systematics. 1998;29:207–231. [Google Scholar]
- Fukami T, Wardle DA. Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients. Proceedings of the Royal Society of London Series B: Biological Sciences. 2005;272:2105–2115. doi: 10.1098/rspb.2005.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Palacios P. Plant-soil interactions in roadside slopes: implications for restoration. Universidad Rey Juan Carlos; Madrid, Spain: 2010. Ph.D. Thesis. [Google Scholar]
- García-Palacios P, Soliveres S, Maestre FT, Escudero A, Castillo-Monroy AP, Valladares F. Dominant plant species modulate responses to hydroseeding, irrigation and fertilization during the restoration of motorway slopes. Ecological Engineering. 2010;36:1290–1298. [Google Scholar]
- García-Palacios P, Maestre FT, Gallardo A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. Journal of Ecology. 2011;99:551–562. doi: 10.1111/j.1365-2745.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Gelbard JL, Harrison S. Invasibility of roadless grasslands: an experimental study of yellow starthistle. Ecological Applications. 2005;15:1570–1580. [Google Scholar]
- Grace JB. Structural equation modeling and natural systems. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, et al. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography. 2006;15:1–7. [Google Scholar]
- Hopwood JL. The contribution of roadside grassland restorations to native bee conservation. Biological Conservation. 2008;141:2632–2640. [Google Scholar]
- Kardol P, Bezemer TM, van der Putten WH. Temporal variation in plant–soil feedback controls succession. Ecology Letters. 2006;9:1080–1088. doi: 10.1111/j.1461-0248.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- Kardol P, Cornips NJ, van Kempen ML, Bakx-Shotman JM, van der Putten WH. Microbe-mediated plant–soil feedback causes historical contingency effects in plant community assembly. Ecological Monographs. 2007;77:147–162. [Google Scholar]
- Kardol P, Newton JS, Bezemer TM, Maraun M, van der Putten WH. Contrasting diversity patterns of soil mites and nematodes in secondary succession. Acta Oecologica. 2009;35:603–609. [Google Scholar]
- Kardol P, Wardle DA. How understanding aboveground-belowground linkages can assist restoration ecology. Trends in Ecology and Evolution. 2010;25:670–679. doi: 10.1016/j.tree.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Kulmatiski A, Beard KH, Stevens JR, Cobbold SM. Plant–soil feedbacks: a meta-analytical review. Ecology Letters. 2008;11:980–992. doi: 10.1111/j.1461-0248.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- Lepš J, Šmilauer P. Multivariate Analysis of Ecological Data using CANOCO. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Maestre FT, Escudero A, Martínez I, Guerrero C, Rubio A. Does spatial pattern matter to ecosystem functioning? Insights from biological soil crusts. Functional Ecology. 2005;19:566–573. [Google Scholar]
- Maestre FT, Martín N, Díez B, López-Poma R, Santos F, Luque I, Cortina J. Watering, fertilization, and slurry inoculation promote recovery of biological crust function in degraded soils. Microbial Ecology. 2006;52:365–377. doi: 10.1007/s00248-006-9017-0. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Bowker MA, Escolar C, Puche MD, Soliveres S, Mouro S, García-Palacios P, Castillo-Monroy AP, Martínez I, Escudero A. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Philosophical Transactions of the Royal Society B. 2010;365:2057–2070. doi: 10.1098/rstb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharning AR, Mills ASS, Adl SM. Soil community changes during secondary succession to naturalized grasslands. Applied Soil Ecology. 2009;41:137–147. [Google Scholar]
- Matesanz S, Valladares F, Tena D, Costa-Tenorio M, Bote D. Early dynamics of plant communities on revegetated motorway slopes from southern Spain: Is hydroseeding always needed? Restoration Ecology. 2006;14:297–307. [Google Scholar]
- McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- Mueller EN. Scaling approaches to the modeling of water, sediment and nutrient fluxes within semi-arid landscapes, Jornada Basin, New Mexico. Logos Verlag; Berlin, Germany: 2007. [Google Scholar]
- National Research Council . Assessing and Managing the Ecological Impacts of Paved Roads. The National Academy of Sciences; Washington DC: 2005. [Google Scholar]
- Naveh Z, Dan J. The human degradation of Mediterranean landscapes in Israel. In: di Castri F, Mooney HA, editors. Mediterranean-type ecosystems: origin and structure. Springer; Berlin: 1973. pp. 373–390. [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy; Madison, USA: 1982. pp. 539–577. [Google Scholar]
- Novák J, Prach K. Vegetation succession in basalt quarries: pattern on a landscape scale. Applied Vegetation Science. 2003;6:111–116. [Google Scholar]
- Oren A, Steinberger Y. Catabolic profiles of soil fungal communities along a geographic climatic gradient in Israel. Soil Biology and Biochemistry. 2008;40:2578–2587. [Google Scholar]
- Pywell RF, Bullock JM, Hopkins A, Walker KJ, Sparks TH, Burke MJW, Peel S. Restoration of species-rich grassland on arable land: assessing the limiting processes using a multi-site experiment. Journal of Applied Ecology. 2002;39:294–309. [Google Scholar]
- Rentch JS, Fortney RH, Stephenson SL, Adams HS, Grafton WN, Anderson JT. Vegetation-site relationships of roadside plant communities in West Virginia, USA. Journal of Applied Ecology. 2005;42:129–138. [Google Scholar]
- Scurlock JMO, Hall DO. The global carbon sink: a grassland perspective. Global Change Biology. 1998;4:229–233. [Google Scholar]
- Sedia EG, Ehrenfeld JG. Differential effects of lichens, mosses and grasses on respiration and nitrogen mineralization in soils of the New Jersey Pinelands. Oecologia. 2005;144:137–147. doi: 10.1007/s00442-005-0037-0. [DOI] [PubMed] [Google Scholar]
- SER . The SER international primer on ecological restoration. Society for Ecological Restoration International; Tucson: 2004. www.ser.org. http://www.ser.org/pdf/primer3.pdf. [Google Scholar]
- Smith RS, Shiel RS, Bardgett RD, Millward D, Corkhill P, Rolph G, Hobbs PJ, Peacock S. Soil microbial community, fertility, vegetation and diversity as targets in the restoration management of a meadow grassland. Journal of Applied Ecology. 2003;40:51–64. [Google Scholar]
- Spellerberg IF. Ecological effects of roads and traffic: a literature review. Global Ecology and Biogeography Letters. 1998;7:317–333. [Google Scholar]
- Van der Putten WH. Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology. 2003;84:2269–2280. [Google Scholar]
- Van der Putten WH, Bardgett RD, de Ruiter PC, Hol WHG, Meyer KM, Bezemer TM, Bradford MA, Christensen S, Eppinga MB, Fukami T, Hemerik L, Molofsky J, Schädler M, Scherber C, Strauss SY, Vos M, Wardle DA. Empirical and theoretical challenges in aboveground–belowground ecology. Oecologia. 2009;161:1–14. doi: 10.1007/s00442-009-1351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LR, Walker J, Hobbs RJ. Linking restoration and ecological succession. Springer Science; New York: 2007. [Google Scholar]
- Walker LR, Wardle DA, Bardgett RD, Clarkson BD. The use of chronosequences in studies of ecological succession and soil development. Journal of Ecology. 2010;98:725–736. [Google Scholar]
- Yeager CM, Kornosky JL, Housman DC, Grote EE, Belnap J, Kuske CR. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Applied and Environmental Microbiology. 2004;70:973–983. doi: 10.1128/AEM.70.2.973-983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel M, Otsus M, Liira J, Moora M, Möls T. Is small-scale species richness limited by seed availability or microsite availability? Ecology. 2000;81:3274–3282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.