Abstract
Previous attempts of α-1,3-galactocyltransferase knockout (GalTKO) pig bone marrow (BM) transplantation (Tx) into baboons have demonstrated a loss of macro-chimerism within 24 h in most cases. In order to achieve improved engraftment with persistence of peripheral chimerism, we have developed a new strategy of intra-bone BM (IBBM) Tx. Six baboons received GalTKO BM cells, with one-half of the cells transplanted into the bilateral tibiae directly and the remaining cells injected intravenously (IBBM/BM-Tx) with a conditioning immunosuppressive regimen. In order to assess immune responses induced by the combined IBBM/BM-Tx, three recipients received donor SLA-matched GalTKO kidneys in the peri-operative period of IBBM/BM-Tx (Group 1), and the others received kidneys 2 months after IBBM/BM-Tx (Group 2). Peripheral macro-chimerism was continuously detectable for up to 13 days (mean 7.7 days; range 3–13) post-IBBM/BM-Tx and in three animals, macro-chimerism reappeared at days 10, 14 and 21. Pig CFUs, indicating porcine progenitor cell engraftment, were detected in the host BM in four of six recipients on days 14, 15, 19 and 28. In addition, anti-pig unresponsiveness was observed by in vitro assays. GalTKO/pCMV-kidneys survived for extended periods (47 and 60 days). This strategy may provide a potent adjunct for inducing xenogeneic tolerance through BM-Tx.
Introduction
Two major obstacles in clinical transplantation are the shortage of available organs and the lifelong necessity for immunosuppressive drugs. A potential strategy for solving both of these obstacles is the use of organs from pigs and the induction of immunologic tolerance across this xenogeneic barrier. Bone marrow transplantation (BM-Tx) has been demonstrated to induce donor-specific tolerance in rodent (1), porcine (2), non-human primate (3), and, most recently, human clinical cases (4,5). It has also been successful in concordant rodent (6) and pig-to-NOD/SCID mouse (7) xenogeneic models. Despite promising results in rodent models, xenogeneic BM-Tx in preclinical pig-to-nonhuman primate models has yet to be successful (8–12). Previous studies using porcine BM cells infused intravenously following ex vivo immunoadsorption of natural anti-Gal antibodies (Nab) have only demonstrated transient macro-chimerism, where most of the infused cells were undetectable within 24 h (8,9). Although the Nab were considered likely to be the major obstacle in this model, the use of α-1,3-galactocyltransferase gene knock-out (GalTKO) pigs (13) as BM donors had only limited effects on prolonging peripheral macro-chimerism (11,12). Two of 10 animals had transient donor-specific hyporesponsiveness in vitro following BM-Tx, while none of the animals showed detectable pig cells by flow cytometry for more than 12 h post-BM intravenous infusion (IV BM-Tx) ((12) and a subsequent unpublished study).
Intravenously injected BM cells must travel throughout the circulatory system, which can lead to a significant loss of cells (14). Recent data in allogeneic models demonstrated that direct injection of donor BM cells into recipient BM spaces (intra-bone bone marrow transplantation: IBBM-Tx) produced rapid reconstitution and a higher survival rate compared to IV injection (15). Therefore, we applied a modified IBBM-Tx procedure to our preclinical pig-to-baboon model to assess whether this would allow us to achieve improved, persistent macro-chimerism as well as engraftment of BM across a xenogeneic barrier. We demonstrate here that this new strategy leads to (i) markedly prolonged detectable peripheral macro-chimerism, (ii) higher incidence of BM engraftment both at the injection site (local engraftment) and systemically, and (iii) prolonged survival of life-supporting GalTKO pig kidney grafts up to 60 days without co-transplantation of a pig thymic graft (16).
Materials and Methods
Details of materials and methods are described separately in the Supporting Information.
Animals
Recipients were Papio hamadryas baboons (n = 6) of known ABO blood type and with body weights of 4–7 kg (Mannheimer Foundation, Homestead, FL). BM cell (n = 6) and kidney (n = 7) donors were Massachusetts General Hospital (MGH) inbred GalTKO miniature swine (13). All swine for BM cell donors were of SLAdd (Class Id, Class IId) swine leukocyte antigen haplotype, hereafter referred to as DD. Most of the kidney donors, with two exceptions, were DD GalTKO pigs that were SLA-matched to the BM donors. Baboons B336 and B344 received kidneys from HH GalTKO donors (Class Ia, Class IId) (17–19) due to a shortage of DD GalTKO pigs. All animal care was performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication no. 86–23, revised 1996).
Surgical procedures
All surgical procedures, including kidney transplantation, BM-Tx, splenectomy, intravenous or intra-arterial line insertions, and BM biopsies were performed under general anesthesia as previously described (8–12,20).
IBBM-Tx Combined with BM-Tx (IBBM/BM-Tx)
Collagen gel matrix was used for the IBBM-Tx to retain BM graft cells in the recipient BM cavity. Cellmatrix, a purified collagen solution for cell culture, was purchased from Nitta Gelatin, Inc. For IBBM-Tx, half of the BM cells were mixed with the collagen gel matrix solution and transplanted directly into the bone cavities of the recipient tibiae as well as the iliac crest for one animal using an 18-gauge needle. The remaining BM cells were infused intravenously on the same day.
Kidney Tx
The baboons received an orthotopic kidney transplant from GalTKO swine donors using a previously reported procedure (16) either within 1 week (Group 1) or 2 months (Group 2) after IBBM/BM-Tx to assess whether this strategy could induce tolerance of swine tissues. The right native kidney was removed from the baboon recipients on the day of the kidney transplantation (K-Tx). The left native kidney was not removed but the left ureter was completely ligated. In the event of loss of graft function with high serum creatinine (S-Cr) within 2 weeks, the left ureter was untied to keep the recipients alive in order to continue the subsequent immunological assays if the animal was not seriously ill.
Conditioning regimen for IBBM/BM-Tx
All animals were subjected to a non-myeloablative conditioning regimen with minor modifications from the regimens that have previously been reported from this laboratory (11,12).
Clinical laboratory studies
Blood cell counts, chemistries, coagulation assays, and blood levels of immunosuppressive drugs were carried out at regular intervals.
Colony forming unit assay
In vitro progenitor cell assays were performed according to methods previously described (21). Following 10–14 days of incubation in 5% CO2 at 37°C, each culture dish was visually scored through an inverted microscope and evaluated for the presence and frequency of colony-forming units of granulocytes and/or macrophages (CFU-GM), colony-forming units of granulocytes, erythroid cells, macrophages, and/or megakaryocytes (CFU-GEMM) and burst-forming unit-erythroid (BFU-E). Pools of 10 CFU-GM and BFU-E colonies were isolated for PCR analysis to detect the presence of porcine cytochrome b DNA (see below).
DNA assays
A polymerase chain reaction (PCR) assay that specifically amplifies the porcine cytochrome b gene was used to detect porcine DNA in bulk BM samples and from CFUs grown from BM aspirates from baboon recipients (11). Samples were considered negative for porcine DNA if a strong signal for baboon GAPDH, indicated a sufficient quantity and quality of DNA template. Porcine BM aspirate-derived CFUs were used as the positive control and naïve baboon BM aspirate-derived CFUs were used as the negative control.
Mixed lymphocyte reaction
The mixed lymphocyte reaction (MLR) in vitro assay has been previously described (22). The number of counts per minute (CPM) could be normalized into the stimulation index (SI) by dividing the CPM in all wells by the CPM within the self-stimulator wells.
Flow cytometry for detection of chimerism and quantification of T cell depletion
Monoclonal antibodies to specific cell subsets were used to determine the phenotype of pig or baboon cells during immunologic recovery or during subsequent stages of peripheral macro-chimerism and BM engraftment. To characterize baboon cell populations, we used antibodies against CD3, CD4/CD8 for T cell subsets, CD20 for B cells, CD45 for all baboon leukocytes, and Class I. In order to detect pig cells specifically (macro-chimerism), we used 1030H1-19 (mouse anti-pan-pig tissues, IgM) followed by PE-conjugated anti-mouse IgM antibody.
Assays of humoral immunity
BM recipient baboons were tested for antibody production and binding to BM donor cells. Serum titers of induced anti-non-Gal baboon IgG and IgM antibodies were measured by flow cytometry and analyzed using WinList mode analysis software for detection of cell-bound antibody.
ELISPOT assay
Baboon cytokine ELISPOT assay was performed to detect interferon gamma (IFNγ) after transplantation. Ninety six-well polyvinylidene difluoride plates (Millipore) were coated overnight at 4°C with anti-IFNγ capture antibody. Baboon PBMCs (2.0 × 105 cells/well) were incubated with medium or irradiated PBMCs (self, donor and third party) or phytohemagglutinin (PHA). After culture, biotinylated anti-IFNγ Ab were used to detect the presence of cells secreting IFNγ.
Histology examination
Tissue samples were fixed in 1% formaldehyde, embedded in paraffin and subsequently sectioned. Tissues were stained using either hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS).
Results
Simultaneous IBBM/BM-Tx with K-Tx (Group 1) and delayed K-Tx (Group 2)
Six baboons received IBBM-Tx combined with intravenous BM-Tx (IBBM/BM-Tx) from MGH GalTKO pigs with a slightly modified previously described conditioning regimen (11). Baboons B318, B324 and B331 (Group 1) received kidneys from GalTKO pigs that were SLA-matched to the BM donor pigs. B324 and B331 received a K-Tx on the day of IBBM/BM-Tx, while B318 underwent a K-Tx 7 days after IBBM/BM-Tx. The remaining three baboons, B296, B336 and B344 (Group 2), received a GalTKO K-Tx 2 months after IBBM/BM-Tx. Details of the outcomes of the GalTKO kidneys transplants will be described later.
CD3+ T cells were depleted to <100 cells/μL for 3 weeks after IBBM/BM-Tx, and CD20+ B cells were also substantially reduced for approximately 1 month after IBBM/BM-Tx. B318 (Group 1) and B296 (Group 2) required platelet transfusions on days 23–26 and 0, respectively, due to platelet counts (PLT) below 20 K/μL as a result of TBI.
BM cells from GalTKO pig donors were processed to attain concentrations of 4.1–8.3 × 109 cells/kg. Approximately half of the isolated BM cells (1.8–5.9 × 109 cells/kg) were injected directly into host tibiae with collagen gel matrix (see Materials and Methods Section; Table 1). One baboon (B344) also received BM cells in the right iliac crest (0.9 × 109 cells/kg). The remaining BM cells were infused intravenously on the same day.
Table 1.
Summary of intra-bone bone marrow transplantation
| Animal | Group 1
|
Group 2
|
||||
|---|---|---|---|---|---|---|
| B318 | B324 | B331 | B296 | B336 | B344 | |
| BM cells for IBBM Tx1 (×109 cells/kg) | 4.0 | 5.9 | 3.3 | 1.8 | 3.5 | 3.6 |
| Sites of IBBM Tx | Both tibias | Both tibias | Both tibias | Both tibias | Both tibias | Both tibias, right iliac |
| Percentage of peripheral macrochimerism on POD 2 after IBBM Tx | 5.0 | 4.1 | 1.6 | 0.1 | 29.1 | 13.2 |
| Duration of detectable peripheral macrochimerism (days) | 13 | 6 (Reappeared at day 14) | 10 | 3 (Reappeared at day 10) | 10 | 13 (Reappeared at day 21) |
| BM engraftment (day of biopsy) | – | Positive (19) | Positive (14) | Positive (28) | – | Positive (15) |
| Day of KTx2 | 7 and 13 | 0 | 0 | 73 | 70 and 204 | 62 |
| Excision of kidney graft (days) and serum creatinine (Cre) | 133 and 7 (3.3/1.5) | 19 (1.0) | 14 (1.7) | 173 (3.8) | 133 and 60 (2.3/6.7) | 47 (5.4) |
| Baboon’s survival after IBBM Tx (days) | 27 | 19 | 14 | 90 | 264 | 109 |
| Cause of death | Cardiac death | Pneumonia | Gastrointestinal bleeding | Sacrificed | Sacrificed | Sacrificed |
Intra-bone bone marrow transplantation.
Days after IBBM Tx.
pCMV positive kidneys.
One baboon in Group 1 died at day 27 from cardiac death (B318), another at day 19 from pneumonia (B324), and the third on day 14 from gastrointestinal bleeding (B331), while baboons in Group 2 survived for over 90 days (Table 1). These results suggest that, although the current conditioning regimen for the IBBM/BM-Tx is safe as demonstrated by the prolonged survival of animals in Group 2, the combination of the IBBM/BM-Tx and the invasive surgical procedure (K-Tx) during the induction period (Group 1) is not well tolerated in this xenotransplantation model.
Level and durability of donor cell macro-chimerism in peripheral blood in the induction period following IBBM/BM-Tx
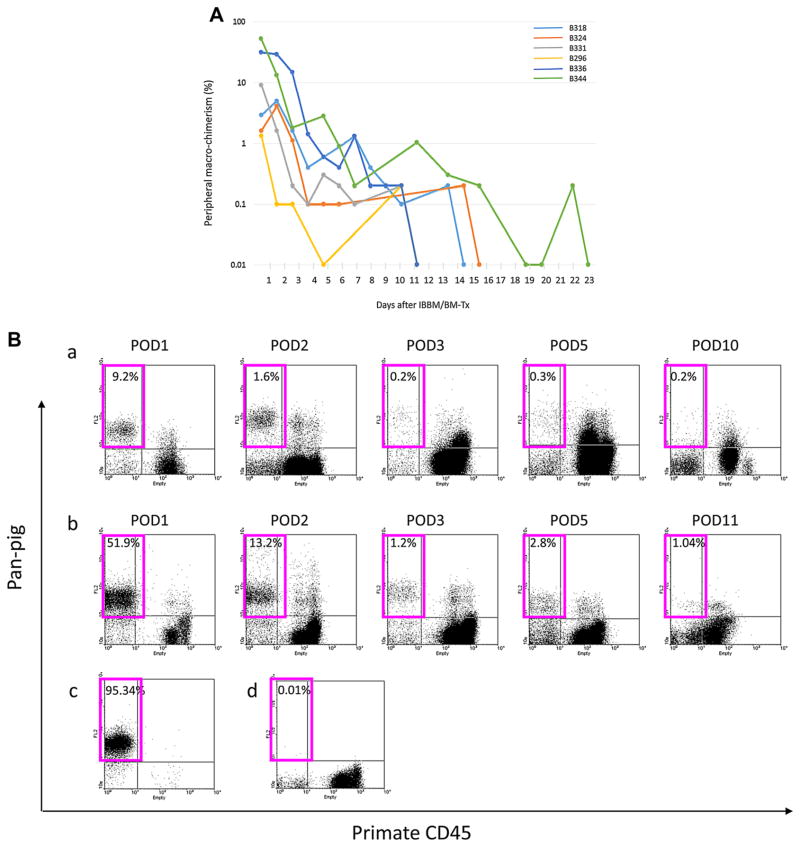
Flow cytometric data of baboon blood indicating pig cell chimerism are shown in Figure 1. In contrast to previous findings in our GalTKO IV BM-Tx model (12), high levels of peripheral pig cells were present in the circulation in the induction period in all of the baboon recipients (Figure 1A). FACS profiles clearly demonstrated porcine macro-chimerism (pan-pig Ab positive but primate CD45 negative, Figure 1B). Two of three recipients had higher macro-chimerisms at day 2 in Group 2, but there was no difference in the duration of macro-chimerism between Groups 1 and 2. In three cases, B324, 296 and B344, macro-chimerim was less than 0.2% at days 6, 3 and 13, respectively, but subsequently reappeared at days 14, 10 and 21, suggesting either a slow release of donor cells from recipient bones or BM engraftment. Although the animals were tested at later time points, chimerism was not detected beyond day 28.
Figure 1.
(A) Peripheral macro-chimerism after IBBM/BM-Tx. (B) Facs profiles of peripheral macrochimerism. (a) B331 (Group 1), (b) B344 (Group 2), (c) Positive control (normal pig blood), (d) Negative control (naive baboon blood). Pink squares showed pig cells (pan-pig antigen positive cells) in baboon recipient.
Evidence for BM engraftment
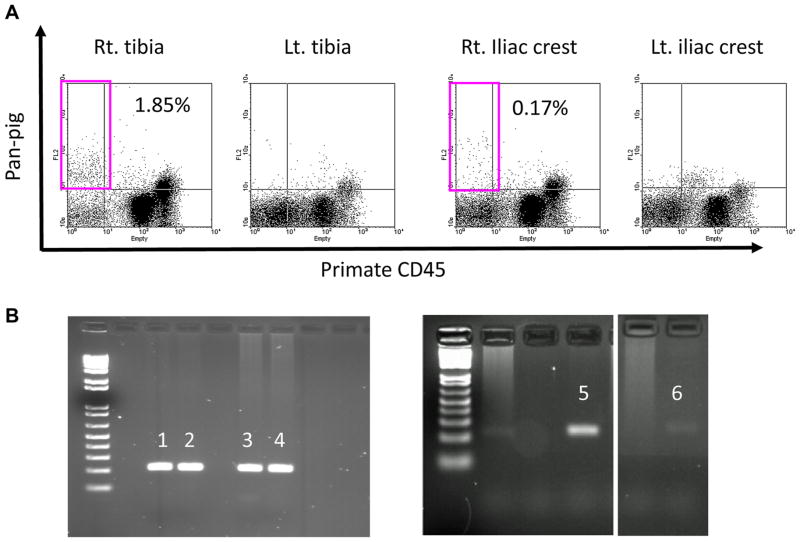
BM biopsies were performed in B296, B318, B336 and B344 on PODs 28, 27, 27 and 15, respectively. In the remaining two baboons, B324 and B331, BM samples were obtained when they were sacrificed at days 19 and 14, respectively. BM engraftment was confirmed by CFU assay followed by PCR for porcine cytochrome b. Four of six baboons showed engraftment of pig BM at remarkably higher levels than what was observed in our previous report with only intravenous BM-Tx from GalTKO pig (11,12). Notably, BM macro-chimerism was also detected at the injection sites by flow cytometry (Figure 2A). Micro-chimerism was confirmed by CFU assay in the tested animals (B324 and B296), not only at the site of BM injection but also at a separate site (Figure 2B), indicating systemic BM engraftment in these animals.
Figure 2. Bone marrow macro- and micro-chimerism after IBBM/BM-Tx.
(A) Pan-pig positive cells were detected in right tibia and right iliac crest from the bone marrow aspiration samples on POD15 (B344). (B) Positive bands of porcine cytochrome b were detected in the tibia (3) and sternum bone (4) of B324, and in the right tibia (5) and right iliac crest (6) of B296. 1 and 2 were positive control.
Donor-specific hyporesponsiveness and absence of anti-pig antibody development following IBBM/BM-Tx
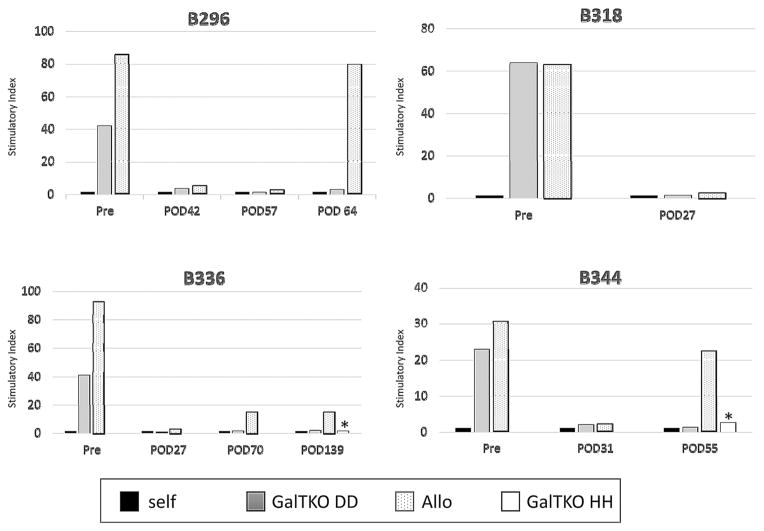
Immune responses after IBBM/BM-Tx were assessed by MLR, ELISPOT and flow cytometry. Pretransplant MLR showed robust proliferative responses to GalTKO DD (BM donor haplotype) pig and allogeneic cell stimulators (Figure 3). During the first 2 months following IBBM/BM-Tx with the conditioning regimen, the MLRs showed general hyporesponsiveness in all of the animals. However, allogeneic responses slowly returned with reconstitution of T/B cells, while anti-GalTKO DD pig cell responses remained very low (see POD 64 for B296, POD 70 for B336 and POD 55 for B344 in Figure 3). Similar to the anti-DD responses, B336 and B344 which received kidney grafts from GalTKO HH pigs, were hyporesponsive to their kidney donor before K-Tx (see POD139 for B336 and POD55 for B344 in Figure 3).
Figure 3. Mixed lymphocyte reaction (MLR).
Pretransplant MLR showed a robust proliferative response to GalTKO pig and allogeneic cell stimulators. These animals showed general hyporesponsiveness until around 30 days after IBBM/BM-Tx. However, allogeneic responses began to recover while stimulation by GalTKO DD pig cells (BM donor type) remained very low. B336 and B344 showed hyporesponsiveness against kidney donor (the different haplotype GalTKO HH pig) cells on POD139 and POD55, respectively (asterisk shows response against haplotype HH).
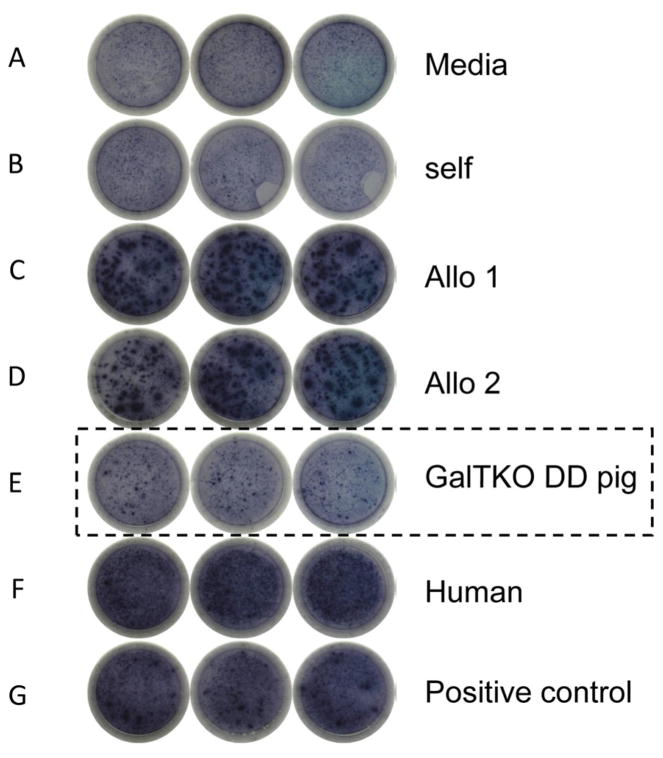
Since MLR primarily assess CD4 T cell responses, we assessed CD8 T cell responses by IFNγ ELISPOT assays. B336 showed numerous IFNγ spots at day 183 in wells with allogeneic and human cells, while only minimal reactivity, similar to the response to self, was observed with GalTKO DD pig cells (Figure 4).
Figure 4. ELISPOT assay for INFγ.
Many spots could be seen in the wells stimulated by allogeneic cells (C and D), human cells (F) and PHA (G) on POD183 (B336), while the spots were few in the well stimulated by GalTKO DD pig cells (E). This was similar to what was observed in wells stimulated by media (A) and autologous PBMC (B).
Moreover, none of the animals in Group 2 developed anti-pig IgM or IgG following IBBM/BM-Tx with delayed K-Tx (Figure 5A). B318 and B331, which both received a K-Tx in the induction period, died early with stable renal graft function (see below) and no elicited anti-pig IgM or IgG in the sera.
Figure 5.
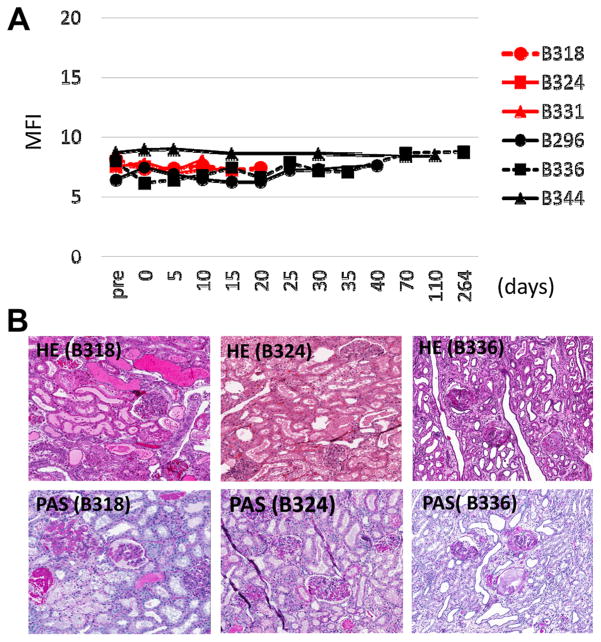
(A) Changes in pig IgG antibody against GalTKO pig cells in sera of recipients after IBBM/BM-Tx assessed by flow cytometry. No animals developed anti-pig antibodies. (B) Histology (HE and PAS staining) of the kidney grafts. Kidney samples were taken at day 20 for B318 (primary graft), at day 19 for B324 and day 264 (60 days after 2nd K-Tx) for B336.
Outcome of K-Tx
In Group 2 (delayed K-Tx), no drugs for T cell depletion or B cell depletion were given before K-Tx. However, chimeric anti-CD154 mAb (once a week) and low-dose MMF (less than 50 mg/kg/day) were continued both before and following the delayed K-Tx. We have recently reported that latent porcine cytomegalovirus (pCMV) in donor animals can lead to activated pCMV in the xeno-kidneys, likely leading to rejection (23) (See more details in Discussion Section). Three animals in this study (B318, B296 and B336) received pCMV positive kidneys before we were aware of these findings concerning pCMV. Primary kidneys in the others (B324, B331 and B344) as well as the second kidney of B336 were from pCMV negative GalTKO donors.
As described above, B318, B324 and B331 which all received a K-Tx within 1 week post IBBM/BM-Tx (Group 1) died at day 27, 19 and 14, respectively; B324 and B331 had s-Cr levels less than 1.7 mg/dL at the time of death. After B318 received a primary K-Tx, the platelet count decreased to 27 K/μL and the animal became severely anemic. Because multiple blood transfusions were required and the s-Cr increased to 3.3 mg/dl, the first kidney was replaced with an SLA-matched GalTKO kidney at day 20 (13 days following the primary K-Tx). Histology of the excised kidneys showed no rejection in Group 1 (B324 in Figure 5B). However, the primary graft from B318 had congested glomeruli with apparent thrombi and fragmented red blood cells without neutrophils or cellular infiltrates (B318 in Figure 5B) suggesting pCMV associated graft loss (23). Unfortunately, the animal died from cardiac failure 7 days after the second kidney transplantation at day 27. Neither acute nor humoral rejection was observed in the second kidney.
All baboons in Group 2 survived over 90 days and two of the three kidney grafts (second kidney graft of B336 and primary kidney graft of B344) had stable function. B336 lost the primary kidney graft (pCMV positive) 13 days after K-Tx. We excised the kidney, untied the animal’s native ureter and immunosuppression was terminated. The excised primary graft was hemorrhagic, similar to the primary graft of B318. However, the animal remained hyporesponsive in cellular assays (see above) and did not develop elicited antibodies even 60 days after the primary kidney graftectomy. The second kidney of B336 and a primary kidney of B344, which were proven pCMV negative by qPCR, functioned well but eventually lost function at days 60 (B336) and 47 (B344), respectively. S-Cr levels were 6.7 mg/dL (B336) and 5.4 mg/dL (B344). The excised kidneys had glomerular and tubular damage but no hemorrhagic humoral rejection was observed (B336 in Figure 5B). B296 received a pCMV positive kidney. Before K-Tx, the MLR showed donor specific unresponsiveness at day 64 (Figure 3) and no anti-donor antibody was detected (Figure 5A). B296 was sacrificed at day 17 due to decreased platelets and severe anemia, likely due to disseminated intravascular coagulation (DIC). Unfortunately, no pCMV negative kidney graft was available for B296 at this time.
Discussion
Xenotransplantation, or transplantation between different species, offers the tremendous benefit of an inexhaustible supply of organs. Pigs are generally considered to be the best candidate for xenotransplantation donors (17,24) as they are physiologically and anatomically similar to humans. Because pigs constitutively express the cell surface α-1,3-Gal antigen, to which humans and non-human primates (NHP) have pre-formed antibody, pig-to-NHP grafts undergo hyperacute rejection (25,26). Our lab has recently demonstrated that utilizing GalTKO pigs as donors (13) prolongs life-supporting renal grafts for up to 3 months when a T cell tolerance strategy, involving co-transplantation of a vascularized GalTKO thymus, is included (16).
Despite promising results in a GalTKO rodent model, which demonstrated successful engraftment of GalT+/+ mouse BM cells into GalTKO mouse recipients (27), GalTKO porcine BM-Tx in a pig-to-NHP model has yet to be successful. Our lab recently demonstrated transient micro-chimerism in 2 of 10 baboons receiving BM that was infused intravenously, however, macro-chimerism was only detected transiently in the periphery (less than 2 days) and not at all in the BM. Moreover, 8 of 10 baboons were sensitized after BM-Tx with evidence of elicited anti-pig antibodies ((12) and a subsequent unpublished study). Two major causes of early BM graft loss with BM-Tx in a GalTKO pig-to-NHP may be responsible for the different outcome observed in the BM-Tx in the GalT+/+ mouse to GalTKO mouse model: pre-formed anti-non-Gal Nab (28) and phagocytosis due to CD47 incompatibility (29,30).
A previous study performed in mice showed a smaller proportion of donor BM cells (1–2%) localized to the BM in recipients that were preconditioned by irradiation (14), indicating that the localization of donor BM cells at the host BM is a critical component of both BM engraftment and the reconstitution of host hematopoiesis. Therefore, we hypothesized that IBBM-Tx could increase the engraftment of porcine BM cells. Our results demonstrated that our new strategy including IBBM-Tx showed (i) a high percent and durable macro-chimerism and (ii) a high incidence (4 of 6 animals) of BM engraftment with hyporesponsiveness across xenogeneic barriers in our pig-to-baboon model. Furthermore, although chimerism eventually disappeared after IBBM-Tx, (iii) baboons retained donor specific hyporesponsiveness and long-term xeno-kidney graft survival was achieved in both recipients in Group 2 that received pCMV negative GalTKO kidneys (more details in a later section).
We included IV BM infusion (i) as a marker of the initial (day 0–2) peripheral chimerism and (ii) to decrease circulating Nab through binding to IV infused BM cells and thereby protect the “slow release” BM cells that were injected with the collagen gel matrix solution directly into the bone. We used a four- to eightfold higher number of BM cells in this study than in previous studies (11), but this dose was split between intra-bone and IV administration with half going to each. Thus, the amount of BM that was administered IV was increased by only a factor of two to four. This higher number of cells infused might be a part of high percent of peripheral chimerism at day 2, although we consider this unlikely because macro-chimerism markedly decreased or disappeared within 2 days (most of donor cells were gone in 12 h) in previous studies (11,12). We also included anti-CD154 mAb treatment to the conditioning regimen, which likewise may have played a role in the improved chimierism and engraftment we have observed (11), although this too had been attempted previously without major improvement of chimerism or evidence of engraftment (11). To our knowledge, this is the first report of a high incidence of BM engraftment, durable porcine BM cell survival within the host BM and donor-specific unrespnosiveness in this preclinical xenotransplantation model. This innovative strategy may also be applicable to clinical allogeneic tolerance protocols.
Injecting porcine BM cells into the recipient’s BM spaces could minimize the exposure of pig BM cells to preformed anti-non-Gal Nab and macrophages. This could result in a much greater survival of donor hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) after inoculation into the recipient’s BM compared to conventional intravenous infusion of BM cells (15). Since the BM possesses an extensive vascular network, injected BM cells are at a high risk of leaving the BM and entering the peripheral circulation. In order to retain the injected BM cells at the site of injection, we used a collagen gel, Cellmatrix, that is typically used as a three-dimensional gel matrix to promote the growth and differentiation of isolated cell cultures (31,32). In this study, donor cells reappeared after day 7 in three of six baboons. As described above, at the time of IBBM/BM-Tx, some of the intravenously infused BM cells may eliminate pre-formed circulating anti-non-Gal Nab and, therefore, increase the survival of donor BM cells slowly released from injected sites of bones.
Baboons in Group 1 demonstrated a short period of survival that was likely a result of the conditioning regimen’s toxicity for the K-Tx that was performed in the peri-transplant period of IBBM/BM-Tx. In addition, percent macro-chimerism at day 2 was relatively lower than that in Group 2. Inflammatory responses associated with kidney transplantation at the same time as BM-Tx might have negative effects on peripheral chimerisism in peri-operative period. In Group 2, B336 lost its primary renal graft at day 13 without evidence of sensitization (see Results section), while its second graft was accepted up to day 60, confirming no immunologic sensitization at the T or B cell levels. The early loss of the first graft was likely due to the presence of pCMV in the graft. We have recently found a strong association between the presence of pCMV in GalTKO porcine kidneys and markedly decreased graft survival with evidence of early activation of endothelial cells in our pig-to-baboon xeno-kidney transplantation model (23). In fact, qPCR confirmed the presence of pCMV in the first graft, while the second graft, which was intentionally prepared to be pCMV-negative, was accepted up to day 60. Notably, immunosuppression of B336 was terminated after removal of the primary kidney until the second kidney graft. The primary graft of B344, which was pCMV-negative, also functioned up to day 47. Loss of kidneys may be due to either innate responses (macrophages, etc) or low grade T/B cell responses that are undetectable in vitro. We have previously shown that kidneys transplanted with vascularized thymic tissue demonstrated prolonged renal graft survival at an average of 51 days with a T cell depletion induction regimen (33), while recipients of kidneys transplanted alone, but otherwise treated similarly, rejected grafts by day 34 (16). These results were superior to those of other research centers, where most kidney transplants were rejected within 16 days (34,35) even with T cell depletion in the induction period (34). Although it is in an admittedly small number of animals, the survivals reported here, without T cell depletion at the time of K-Tx, are similar to the average survival that we have reported with co-transplantation of thymus with T cell depletion (33).
Another strategy to avoid phagocytosis that is currently under investigation in this laboratory is the use of our recently developed GalTKO/human CD47 (hCD47) transgenic (Tg) pigs (36). Recent work, specifically focused on CD47, showed that the interaction between CD47 and signal-regulatory protein alpha (SIRPα) downregulates phagocytosis by macrophages and that there is interspecies incompatibility of the CD47-SIRPα interaction (29,30). Because it appears that the innate immunologic responses, specifically those of macrophages, are associated with xenogeneic rejection, the use of BM cells transfected with hCD47 or BM cells from GalTKO/hCD47 Tg animals holds significant potential. Our future plans include the use of GalTKO/hCD47 Tg pigs for thymokidney/IBBM-Tx into baboons.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Isabel Hanekamp for her helpful advice and review of this manuscript. We thank Dr. Keith Reimann for anti-CD154 mAb (5C8 chimeric Ab) for this research. This research was supported by the Project 1 of the NIH/NIAID 2P01AI45897 (KY), the core facility of NIH/NIAID 2P01AI45897 (JAF and RAW), and the MGH Swine Facility grant C06 RR020135-01 (DHS).
Abbreviations
- BM
bone marrow
- CFU
colony-forming units
- CPM
counts per minute
- GalTKO
alpha-1,3-galactosyltransferase knockout
- HE
hematoxylin and eosin
- IBBM
intra-bone bone marrow
- IV
intravenous
- MGH
Massachusetts General Hospital
- MLR
mixed lymphocyte reaction
- Nab
natural antibodies
- PAS
periodic acid-Schiff
- PBMC
peripheral blood mononuclear cells
- pCMV
porcine cytomegalovirus
- PLT
platelet
- POD
post-operative day
- S-Cre
serum creatinine
- SI
stimulation index
- SLA
swine leukocyte antigen
- TBI
total body irradiation
- TI
thymic irradiation
- TM
thrombotic microangiopathy
- Tx
transplantation
Footnotes
Authors’ Contribution
Masayuki Tasaki (First author): Primarily performed the experimental procedures in vivo and in vitro, analyzed data and wrote this manuscript with Kazuhiko Yamada. Isaac Wamala: Participated in in vivo experimental procedures. Aseda Tena: Performed the molecular assays. Vincenzo Villani: Participated in in vivo experimental procedures and in analyzing data. Mitsuhiro Sekijima: Participated in in vivo experimental procedures. Vimukthi Pathiraja: Participated in in vivo experimental procedures. Jay Fishman and Robert Wilkinson: Designed and performed the molecular assays for viral infection. Shannon G. Moran: Participated in in vivo experimental procedures. Taylor A. Cormack: Participated in in vitro experimental procedures and in analyzing data. Eric Clayman: Participated in analyzing data and writing this manuscript. J. Scott Arn: Maintained MGH GalT-knockout swine colony and participated in analyzing data. Akira Shimizu: Examined the histopathology. David H. Sachs: Participated in analyzing data. Kazuhiko Yamada (Corresponding author): Primarily designed the experimental protocol, performed the experimental procedures, performed the experimental procedures, analyzed data, and wrote this manuscript.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchimoto Y, Huang CA, Yamada K, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 4.Spitzer TR, Delmonico F, Tolkoff-Rubin N, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: The induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480–484. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharabi Y, Aksentijevich I, Sundt TM, III, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: Production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe M, Qi J, Sykes M, Yang YG. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood. 2002;99:3823–3829. doi: 10.1182/blood.v99.10.3823. [DOI] [PubMed] [Google Scholar]
- 8.Sablinski T, Gianello PR, Bailin M, et al. Pig to monkey bone marrow and kidney xenotransplantation. Surgery. 1997;121:381–391. doi: 10.1016/s0039-6060(97)90307-x. [DOI] [PubMed] [Google Scholar]
- 9.Sablinski T, Emery DW, Monroy R, et al. Long-term discordant xenogeneic (porcine-to-primate) bone marrow engraftment in a monkey treated with porcine-specific growth factors. Transplantation. 1999;67:972–977. doi: 10.1097/00007890-199904150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Buhler L, Awwad M, Treter S, et al. Pig hematopoietic cell chimerism in baboons conditioned with a non-myeloablative regimen and CD154 blockade. Transplantation. 2002;73:12–22. doi: 10.1097/00007890-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Tseng YL, Dor FJ, Kuwaki K, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 2004;11:361–370. doi: 10.1111/j.1399-3089.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 12.Griesemer A, Liang F, Hirakata A, et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation. 2010;17:300–312. doi: 10.1111/j.1399-3089.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 14.Cui J, Wahl RL, Shen T, et al. Bone marrow cell trafficking following intravenous administration. Br J Haematol. 1999;107:895–902. doi: 10.1046/j.1365-2141.1999.01779.x. [DOI] [PubMed] [Google Scholar]
- 15.Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: A powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–3299. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 17.Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 18.Sachs DH. MHC homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as models in biomedical research. Ames, Iowa: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 19.Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: a study in miniature swine. Hum Immunol. 1996;50:1–10. doi: 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- 20.Pennington LR, Sakamoto K, Popitz-Bergez FA, et al. Bone marrow transplantation in miniature swine. I. Development of the model. Transplantation. 1988;45:21–26. doi: 10.1097/00007890-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowski T, Monroy R, Giovino M, et al. Effect of pig-specific cytokines on mobilization of hematopoietic progenitor cells in pigs and on pig bone marrow engraftment in baboons. Xenotransplantation. 1999;6:17–27. doi: 10.1034/j.1399-3089.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekijima M, Waki S, Sahara H, et al. Results of life-supporting galactosyltransferase knockout kidneys in cynomolgus monkeys using two different sources of galactosyltransferase knockout swine. Transplantation. 2014;98:419–426. doi: 10.1097/TP.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs DH, Galli C. Genetic manipulation in pigs. Curr Opin Organ Transplant. 2009;14:148–153. doi: 10.1097/mot.0b013e3283292549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels LJ, Platt JL. Hyperacute xenograft rejection as an immunologic barrier to xenotransplantation. Kidney Int Suppl. 1997;58:S28–S35. [PubMed] [Google Scholar]
- 26.Good AH, Cooper DK, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: Implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 27.Yang YG, deGoma E, Ohdan H, et al. Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187:1335–1342. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galili U. Interacti on of the natural anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, VerHalen J, Madariaga ML, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi M, Adachi Y, Shigematsu A, et al. Intra-bone marrow injection of donor bone marrow cells suspended in collagen gel retains injected cells in bone marrow, resulting in rapid hemopoietic recovery in mice. Stem Cells. 2008;26:2211–2216. doi: 10.1634/stemcells.2008-0035. [DOI] [PubMed] [Google Scholar]
- 33.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyl-transferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tena A, Kurtz J, Leonard DA, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014;14:2713–2722. doi: 10.1111/ajt.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.