Abstract
Background
The current standard of care for burns requiring operative treatment consists of early burn excision and autologous split-thickness skin grafting. However, in large burns, sufficient donor sites may not be available to achieve total coverage, necessitating temporary coverage with allogeneic human cadaver skin grafts or synthetic skin substitutes. A previous study from this laboratory demonstrated that skin grafts from alpha-1,3 galactosyltransferase knockout (GalT-KO) miniature swine enjoyed survival comparable to that of allogeneic skin grafts in baboons.
Methods
In the present study, we have evaluated the immune response against sequential GalT-KO and allogeneic skin grafts to determine whether such serial grafts could extend the period of temporary wound coverage before definitive grafting with autologous skin.
Results
We report that rejection of primary GalT-KO skin grafts led to an anti-xenogeneic humoral response with no evidence for sensitization to alloantigens nor acceleration of rejection of allogeneic skin grafts. Similarly, presensitization with allogeneic skin did not lead to accelerated rejection of xenogeneic skin.
Conclusions
These data suggest that GalT-KO skin grafts could provide an early first-line treatment in the management of severe burns that would not preclude subsequent use of allografts, and that serial grafting of GalT-KO skin and allogeneic skin could potentially be used to provide an extended period of temporary burn wound coverage.
Keywords: Xenotransplantation, Burns, Skin grafting
Approximately 500,000 burn injuries occur per year in the United States, of which 40,000 require admission to a burn center (1). In addition to the local injury inflicted, large burns, covering more than 30% total body surface, carry a significant risk of a severe systemic insult, with maintenance of temperature homeostasis after substantial skin loss requiring elevation of the metabolic rate up to three times above baseline. Additionally, activation of pro-inflammatory cytokine cascades can lead to a systemic inflammatory response, adult respiratory distress syndrome, and shock, while nonspecific down-regulation of the immune response, coupled with loss of the skin’s natural barrier, renders the patient susceptible to opportunistic infections (2).
The current standard of care for burns requiring operative treatment is early burn excision and split-thickness skin grafting (3, 4). Autologous skin, harvested from nonburned regions of the patient’s own body, is preferred; however, in large burns, sufficient donor sites may not be available to achieve the necessary coverage even when meshed grafts are utilized (5, 6). In cases where sufficient autologous skin is not available, allogeneic skin from deceased donors may be grafted to provide temporary coverage. Although this allows for rapid coverage of the burn wound, allogeneic skin is eventually rejected and therefore does not provide definitive closure. Issues such as cost, limited availability, and the potential for transmission of pathogens must also be considered when deceased-donor allografts are used. A number of alternative synthetic and biological dressings have been developed, but all share a susceptibility to infection and high cost (7, 8).
Porcine skin is recognized to share many of the characteristics of human skin (9–13). Glutaraldehyde-fixed porcine skin has been used for temporary coverage of third-degree burns (14); however, fixed skin compares poorly to vital skin, as it fails to vascularize and functions only as a biological dressing. Vital porcine skin cannot readily be used in this role because of its susceptibility to rapid rejection mediated by naturally circulating, preformed antibodies (15). The major cell surface target for these antibodies is the alpha-galactosyl epitope, which is present in all mammals except for Old World primates and humans (16).
This laboratory has recently developed genetically modified alpha-1,3 galactosyltransferase knockout inbred (GalT-KO) miniature swine, which lack the alpha-galactosyl epitope. We have previously reported prolonged survival of skin from these animals transplanted across a pig-to-baboon barrier (17). In those studies, we have shown that skin grafts from these GalT-KO swine enjoy comparable survival to allogeneic skin in baboons and thus might provide a new source of vital skin grafts for the acute treatment of severe burns.
In this current study, we have confirmed the original results showing comparable survival of allogeneic and xenogeneic skin grafts and further characterized the humoral response to these grafts. In addition, we have investigated the potential use of GalT-KO xenogeneic and allogeneic skin grafts in series, in an attempt to provide an extended period of temporary wound coverage before definitive closure with autologous skin.
RESULTS
Xenogeneic Skin Grafts Survive in a Similar Manner to Allogeneic Skin Grafts
GalT-KO skin grafts were placed over full-thickness defects on the dorsum of recipient baboons (n=4). Grafts rapidly adhered to the wound bed and showed signs of vascularization by postoperative day (POD) 4. All grafts remained viable, but with early signs of rejection at POD 10. Rejection was complete by POD 12 or 13 in all cases. Representative pictures of the grafts are shown in Figure 1, 1°GalT-KO. After rejection of the GalT-KO grafts, subsequent grafts from allogeneic donors were placed on new wound beds. In similar fashion to the primary GalT-KO grafts, the allografts were rapidly adherent with signs of vascularization evident between POD 2 and 4, and the grafts remained intact and well vascularized until POD 10, after which rejection progressed to completion between POD 11 and 14. Representative pictures of the grafts are shown in Figure 1, 2°Allo. The graft survival data for all animals is summarized in Table 1, group 1. These results demonstrated that rejection of primary xenograft skin did not lead to accelerated rejection of a subsequent allograft.
FIGURE 1.
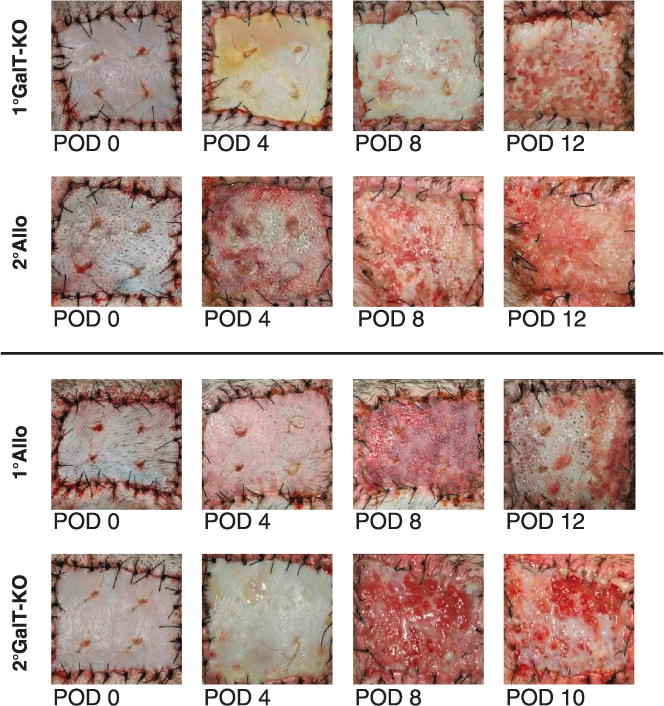
First set grafting with GalT-KO skin does not cause accelerated rejection of a secondary set allograft and vice versa, a first set allograft does not accelerate rejection of subsequent GalT-KO skin. Representative gross clinical pictures from serially grafted baboons are shown here. The top set show primary GalT-KO (1°G) then secondary allogeneic (2°A) grafts and the bottom set show primary allogeneic (1°A) then secondary GalT-KO grafts (2°G). Data shown is representative.
TABLE 1.
First set grafting with GalT-KO skin does not cause accelerated rejection of a secondary set allograft and vice versa, and a first set allograft does not accelerate rejection of subsequent GalT-KO skin
| Baboon ID | Primary graft (donor) | Survival, d | Secondary graft (donor) | Survival, d |
|---|---|---|---|---|
| Group 1: Primary allo; secondary xeno | ||||
| B321 | Allo (B322) | 13 | Xeno GalT-KO | 12 |
| B322 | Allo (B321) | 13 | Xeno GalT-KO | 13 |
| B3411 | Allo (B3511) | 12 | Xeno GalT-KO | 12 |
| B3511 | Allo (B3411) | 13 | Xeno GalT-KO | 10 |
| Group 2: Primary xeno; secondary allo | ||||
| B297 | Xeno GalT-KO* | 11 | Allo (B293) | 10 |
| B1011 | Xeno GalT-KO* | 13 | Allo (B0911) | 11 |
| B3811 | Xeno GalT-KO | 12 | Allo (B3911) | 12 |
| B3911 | Xeno GalT-KO | 13 | Allo (B3811) | 14 |
| Group 3: Primary xeno and allo; secondary xeno and allo | ||||
| B266 | Xeno GalT-KO | 7 | Xeno GalT-KO | 1 |
| Allo (B267) | 7 | Allo (B267) | 4 | |
| B267 | Xeno GalT-KO | 11 | Xeno GalT-KO | 1 |
| Allo (B267) | 11 | Allo (B267) | 4 |
Skin graft survival data for three groups of animals is summarized in this table. All allografts were fresh, whereas all xeno were frozen (except for recipient B297* and B1011*).
A second group of baboons (n=4) received primary allogeneic skin grafts over full-thickness defects. Similar to the primary GalT-KO grafts above, the primary allografts were adherent to the wound bed, demonstrated signs of vascularization between POD 2 and 4, and were viable at POD 10 but rejected between POD 12 and 13 (Fig. 1, 1°Allo). After rejection of the primary allografts, these recipients then received xenogeneic GalT-KO grafts. Similar to the results observed for primary xenogeneic skin grafts, these secondary GalT-KO grafts underwent a time course of rejection that was comparable, with complete rejection by POD 10 to 13 (Fig. 1, 2°GalT-KO). The graft survival results for all animals are summarized in Table 1, group 2. These results demonstrated that rejection of subsequent GalT-KO xenografts was not accelerated after rejection of primary allografts. These results stand in contrast to the survival of second set grafts from the same donor as the first set. Animals that received first set allografts had a survival of 11 days, whereas same donor second set allografts rejected in 4 days (n=2). Animals that received first set xenografts rejected in 11 days whereas the second set xenografts became white grafts, failing to vascularize and thus never becoming viable (n=2). The graft survival data for these animals is summarized in Table 1, group 3.
Rejection of Xenogeneic GalT-KO Skin Does Not Elicit a Cross-Sensitized Humoral Response to Subsequent Allografts
Whereas both T- and B-cell responses have been shown to play roles in the rejection of skin grafts, the lack of accelerated rejection of subsequent skin grafts in the xenogeneic or allogeneic, or allogeneic or xenogeneic, series suggested that cross-sensitization was not occurring at a level sufficient to significantly affect the clinical outcome of the grafts. To assess the production of antibody to both xenogeneic and allogeneic antigens after skin grafts, binding of recipient serum IgM and IgG to peripheral blood mononuclear cell (PBMC) targets from GalT-KO and allogeneic skin donors was measured by flow cytometry. Serum antibody levels were analyzed at multiple time points, including pregrafting and post-rejection of first and second grafts. As illustrated by the representative data in Figure 2A, an increase in anti-xenogeneic IgM and IgG above pre-existing levels was detected (as shown by the increase in fluorescence of cells incubated with recipient serum) in all animals after rejection of primary GalT-KO xenogeneic skin grafts (summarized in Table 2 for IgG) and persisted for the duration of the study. These data correlated with the increase in anti-xenogeneic antibody-mediated cytotoxicity that was also observed after rejection of the GalT-KO skin graft as shown by representative data in Figure 3A. Cytotoxicity data for all animals are summarized in Table 2.
FIGURE 2.
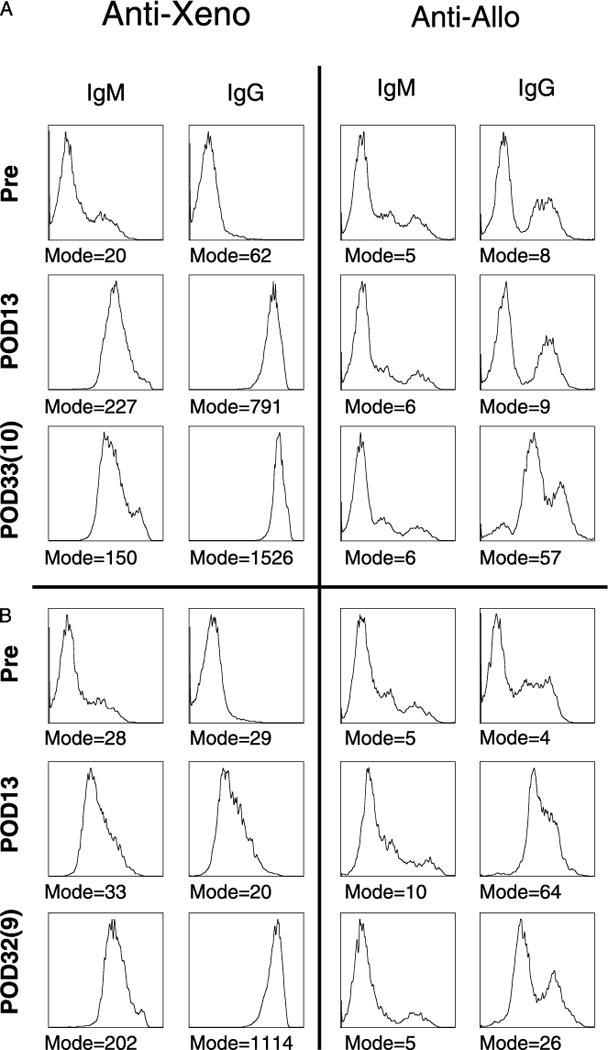
A and B, Primary grafting with GalT-KO skin elicits a strong anti-xenogeneic antibody response but no anti-allogeneic response and vice versa; primary allogeneic skin elicits an anti-allogeneic antibody response but no anti-xenogeneic antibody response. Serum collection time points are designated by postoperative date (POD); the first number is days since primary grafting, and the number in parentheses is days since secondary graft. Presence of anti-xenogeneic and anti-allogeneic IgM and IgG antibodies, in the recipients’ serum, was indirectly assayed by FACS analysis using donor PBMC. The mode of florescence is given for each plot. Data shown is representative.
TABLE 2.
Anti-xenogeneic and anti-allogeneic lgG antibodya, and anti-xenogeneic and anti-allogeneic cytotoxicityb
| Baboon ID | Pre-MFI Anti-Xeno |
Pre-MFI Anti-Allo |
Anti-Xeno Post 1° |
Anti-Allo Post 1° |
Anti-Xeno Post 2° |
Anti- Alio Post 2° |
Precytotox Anti-Xeno |
Precytotox Anti-Allo |
Anti-Xeno Post 1° |
Anti-Allo Post 1° |
Anti-Xeno Post 2° |
Anti-Allo Post 2° |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: primary alio; secondary xeno | ||||||||||||
| 8321 | 1 | 1 | 16 | 1 | 225 | 55 | 1:2 | 1:2 | 1:512 | 1:512 | >1:1024 | 1:512 |
| 8322 | 183 | 1 | 202 | 1 | 1,275 | 22 | 1:8 | 1:2 | 1:16 | 1:128 | 1:256 | 1:128 |
| 83411 | 149 | 4 | 158 | 7 | 1,252 | 40 | 1:32 | 1:2 | 1:64 | >1:1024 | >1:1024 | 1:512 |
| 83511 | 29 | 4 | 20 | 69 | 1,114 | 28 | 1:64 | 1:2 | 1:64 | 1:64 | >1:1024 | 1:512 |
| Group 2: primary xeno; secondary alio | ||||||||||||
| 8297 | 3 | 4 | 17 | 6 | 10 | 4 | 1:2 | ND | 1:64 | ND | 1:16 | ND |
| 81011 | 305 | 15 | 1,286 | 11 | 1,433 | 14 | 1:16 | 1:2 | 1:512 | 1:2 | 1:256 | 1:32 |
| 83811 | 9 | 4 | 426 | 5 | 552 | 5 | 1:32 | 1:2 | >1:1024 | 1:2 | 1:256 | 1:1024 |
| 83911 | 62 | 8 | 791 | 9 | 1,526 | 57 | 1:64 | 1:2 | >1:1024 | 1:16 | >1:1024 | 1:1024 |
Anti-xenogeneic and allogeneic antibody binding data is shown as the mode of fluorescence intensity (MFI) of IgG antibody binding.
The cytotoxicity titer is defined as the dilution after which percentage cytotoxicity falls below 50% of maximum. ND designates that the assay was not done. B297* had an abnormal antibody response, in comparison to other animals, producing IgM (MFI=169) but low IgG (MFI=14).
FIGURE 3.
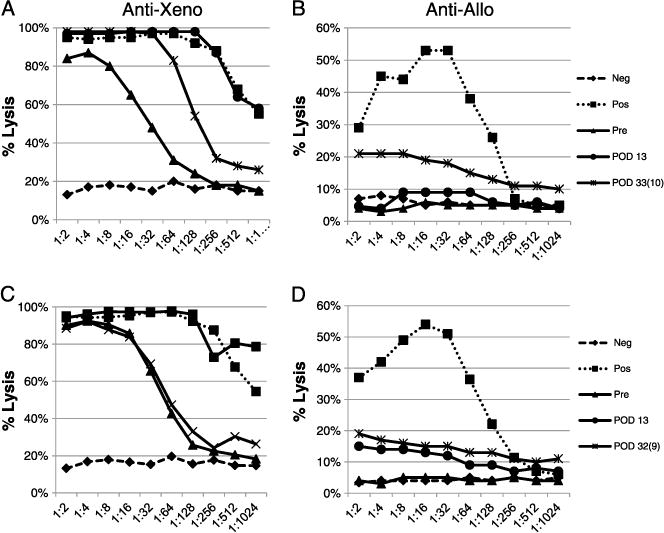
Reactivity of recipients’ serum against xeno- and allo-PBMC was assayed by complement-dependent cytotoxicity assay for the recipients who received a primary xenogeneic graft and a secondary allogeneic graft (A, B) and for the recipients who received a primary allogeneic graft and secondary xenogeneic graft (C, D). Data shown is representative.
After subsequent allograft rejection, anti-allogeneic antibody binding to target PBMC could not be detected reliably by flow cytometry, with only one of four animals demonstrating an increase in anti-allogeneic antibodies in the IgG fraction (representative data Fig. 2A). However, antibody-mediated anti-allogeneic cytotoxicity was demonstrated in all animals after rejection of these allografts, indicating that a response was mounted and had the capability to kill allogeneic cells. This anti-allogeneic antibody was not observed after rejection of the primary xenogeneic GalT-KO skin grafts until after rejection of the subsequent allogeneic grafts (representative data Fig. 3B), further demonstrating that the rejection of the xenogeneic skin grafts did not lead to a cross-reactive anti-allogeneic response. Cytotoxicity data for all animals is summarized in Table 2.
Rejection of Primary Allogeneic Skin Does Not Induce an Anti-Xenogeneic Humoral Response
In the clinical setting, patients who may potentially require GalT-KO grafts for wound coverage may present with preformed anti-allogeneic antibodies resulting from previous exposures. As shown above, although we did not observe accelerated rejection of GalT-KO skin grafts after rejection of primary allogeneic grafts, we wished to further characterize whether recipients demonstrated any detectable production of anti-xenogeneic antibodies after rejection of the primary allogeneic graft. Anti-xenogeneic and anti-allogeneic IgM and IgG serum antibodies were assessed by both flow cytometry and cytotoxicity assays from baboons that received primary allografts and secondary xenografts. As shown by data from representative animals in Figure 2B (and summarized in Table 2 for IgG), we observed no increase in anti-xenogeneic antibodies above pre-existing levels in the IgM or IgG subclasses of antibody after rejection of the primary allogeneic skin grafts, but a significant increase in both, after rejection of the subsequent GalT-KO grafts, and an increase in the antibody-mediated cytotoxicity to xenogeneic targets (representative data Fig. 3C). Cytotoxicity data for all animals are summarized in Table 2.
Similar to the results seen in recipients of primary xenogeneic and subsequent allogeneic grafts, serum IgM and IgG antibodies that specifically bound to allogeneic cells could only be detected by flow cytometry in one of four animals after rejection of primary allografts. However, all recipients demonstrated a significant increase in allogeneic cell lysis after allograft rejection (representative data Fig. 3D). Cytotoxicity data for all animals are summarized in Table 2. Taken together, these results suggest that the rejection of secondary GalT-KO xenogeneic skin grafts was a result of a specific anti-xenogeneic response, and not a result of cross-reactivity of anti-allogeneic antibodies produced as a result of primary allogeneic skin graft rejection.
DISCUSSION
The results reported in this study demonstrate that GalT-KO xenogeneic skin grafts can provide temporary coverage of full-thickness defects in baboons comparable to that provided by allogeneic skin. In addition to the similar clinical time course for rejection, the rejection of primary GalT-KO skin grafts leads to an anti-xenogeneic humoral response with no evidence for cross-sensitization to alloantigens, suggesting that GalT-KO skin grafts could provide an early first-line treatment in the management of severe burns that would not preclude subsequent use of allografts. It is of note that the robust anti-xenogeneic IgM and IgG antibody responses observed were against non-Gal epitopes, as both the skin grafts as well as target cells used in the various in vitro assays came from GalT-KO animals, deficient in the alpha-1,3 galactosyltransferase gene, and thus not subject to recognition by circulating natural anti-Gal antibodies. It was previously shown by Baertschiger et al. that there is no cross-reactivity between anti-allogeneic and anti-xenogeneic baboon immune responses in vitro (18). We have verified these in vitro findings, as well as demonstrated in vivo that recipients pre-sensitized to alloantigens do not have an increased response or accelerated rejection of GalT-KO xenogeneic skin grafts on subsequent exposure to xenogeneic antigens, indicating that the presence of traditional donor-specific alloantibodies that could be a barrier to the use of allogeneic skin grafts would not pose such a barrier in the use of GalT-KO skin. Furthermore, although it was previously shown that broadly HLA reactive monoclonal antibodies are able to induce complement dependent cytotoxicity against pig cells (19), whole serum from baboons that had rejected allogeneic skin grafts demonstrated no cytotoxicity against GalT-KO pig cells. The lack of accelerated rejection of secondary grafts, allogeneic or xenogeneic, was not caused by the inability to mount a memory response, as once a recipient has rejected an allogeneic or xenogeneic graft, subsequent donor-specific grafts are rejected in an accelerated manner (Table 1).
Although it has been shown previously that xenogeneic antibody responses include production of antibodies to MHC antigens (20), the more robust response observed against xenogeneic versus allogeneic cell-surface antigens suggests reactivity to additional kinds of antigens in the xenogeneic situation. Nevertheless, the presence of these additional antigens did not lead to an accelerated rejection of the xenogeneic grafts, suggesting that these additional antigens are of less importance for the cellular than for the humoral immune response. Thus, in the absence of Gal antigens, to which the most potent natural antibody reactivities are seen (17), it would appear that primary skin graft rejection is primarily a result of the cellular immune response and is comparable for allogeneic and xenogeneic skin grafts as indicated by similar graft survivals.
From a clinical perspective, the use of GalT-KO xenogeneic skin could avoid many of the disadvantages associated with the use of deceased-donor allogeneic skin, including high cost, limited availability, and the risk of human pathogen transmission. The potential risk of transmission of pathogenic organisms as a result of xenotransplantation has been studied extensively and addressed by the FDA (21, 22). Although there remains a theoretical concern about potential retroviral transmission, this risk now appears to be very small and, unlike human skin, swine skin can be assured to be free of the more common zoonotic pathogens by the use of animals from a specific pathogen-free colony (22) and can be produced in essentially unlimited quantities. In addition, we have previously demonstrated that swine skin can be cryopreserved and thawed with no significant effect on outcome in comparison to fresh skin (Table 1). The present demonstration that serial grafting of GalT-KO and allogeneic skin is possible in either order could potentially double the temporary coverage time for severely burned patients, providing an extended period for healing of autologous graft donor sites or the cultivation of suitable autologous skin for long-term coverage. All of these factors could be of significant benefit in the treatment of severe burns.
MATERIALS AND METHODS
Animals
Baboons were obtained from an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)–approved breeding facility (Mannheimer Institute, FL) and were between 1 and 2 years of age. Allogeneic donors were intentionally chosen to be MHC mismatched, as indicated by pretransplant mixed lymphocyte reactions. Xenogeneic donors were selected from the herd of MHC-defined GalT-KO MGH Miniature Swine.
Surgery
Under general anesthesia, 4×5 cm full-thickness skin wounds were created on the dorsum of recipient baboons by excision of skin, subcutaneous tissue, and fascia to reveal the underlying dorsal muscles. Hemostasis was achieved with electrocautery and a split-thickness skin graft (allogeneic, xenogeneic, or autologous) was placed. Grafts were sutured to the wound with interrupted 3-0 nylon sutures, which were removed between 7 and 12 days postoperatively. Xenogeneic skin grafts were harvested from a GalT-KO swine donor under general anesthesia, using an air-driven dermatome (Zimmer UK Ltd., Wiltshire, UK) set to 0.022 of an inch and were cryopreserved or placed immediately on recipient. Frozen grafts were thawed no more than 6 hr before use. Allogeneic and autologous grafts were similarly harvested from baboons under general anesthesia and placed directly on the recipient wound without cryopreservation. All grafts were dressed with a nonadherent pressure dressing, secured under a special primate jacket.
Isolation of Peripheral Blood Mononuclear Cells
Blood was drawn for pretransplant and post-rejection mixed lymphocyte reactions, and analysis of serum antibodies. PBMC were isolated by gradient centrifugation in lymphocyte separation medium (Organon Teknika, Durham, NC) and resuspended in AIM-V media (Invitrogen, Carlsbad, CA).
Serum Antibody Binding Assay
Swine and baboon PBMC were isolated from peripheral blood as described and counted by trypan blue exclusion. Cells where diluted to a concentration of 10×106 cells/mL in Flow Activated Cell Sorting media (1× Hanks Balanced Salt Solution with Ca+ and Mg+, 0.1% BSA, and 0.1% sodium azide) and 100 μL added to each FACS tube. Serum samples were de-complemented in a 56°C water bath for 30 min and serially diluted, starting at 1:10 and going out to 1:10,000, in FACS media. Volumes of 10 μL each of neat and diluted serum sample were added to the cells and incubated for 30 min at 4°C. Cells were washed twice before addition of 10 μL of a secondary antibody (FITC-conjugated goat anti-human or goat anti-swine IgM and IgG). Cells were incubated with the secondary antibody for 15 min at 4°C and washed once with FACS media before acquisition on a FACScan (Becton Dickson, Franklin Lake, NJ) using a propidium iodide gate. Flow cytometry data was analyzed using FlowJo (Tree Star Inc., Ashland, OR).
Antibody-Mediated, Complement-Dependent Cytotoxicity Assay
Antibody-mediated, complement-dependent cytotoxicity of serum from transplant recipients was measured using a modified version of the trypan blue dye exclusion lymphocytotoxicity technique of the National Institutes of Health (23) Baboon sera, in twofold dilutions from 1:2 to 1:1,024, were tested against baboon (skin donor) and GalT-KO porcine PBMC. Positive control serum samples were selected from animals that had rejected an allogeneic or xenogeneic skin graft and had previously shown a high level of killing in the assay. Detection of permeabilized cells was performed using the florescent viability stain 7-actinoaminomycin D (7-AAD; Sigma). Samples were acquired using a FACScalibur (Becton Dickson) and data analyzed in FlowJo. The cytotoxicity titer is defined as the dilution before the percentage cytotoxicity falls below 50% of maximum.
Acknowledgments
The authors wish to thank Jim Winter for anesthesia and operating room support, Dr. Robert Hawley and Dr. Melissa Mastroianni for internal review of the manuscript, and Rebecca A. Brophy and Deatrice Moore for administrative support.
Support from CO6RR020135-01 was provided for construction of the facility utilized for production and maintenance of miniature swine. Experimental funding was provided by Department of Defense, W81XWH-09-1-0419/DoDDR080729.
Footnotes
The authors declare no conflicts of interest.
A.A. contributed to the performance of the study, analysis of data, and preparation of the article. D.L. contributed to the performance of the study, design of the experiment, analysis of data, and preparation of the article. A.L.B. contributed to the performance of the study and data analysis. J.K. contributed to the performance of in vitro assays and data analysis. C.M. contributed to the performance of the study. D.H.S. contributed to the design of the experiments, data analysis, and preparation of the article. J.M.K. contributed to the design of experiments, data analysis, and preparation of the article. C.L.C. contributed to the design of the experiments, data analysis, and preparation of the article.
References
- 1.Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360:893. doi: 10.1056/NEJMct0804451. [DOI] [PubMed] [Google Scholar]
- 2.Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328:1427. doi: 10.1136/bmj.328.7453.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YB, Ogawa Y, Kakudo N, et al. Survival and wound contraction of full-thickness skin grafts are associated with the degree of tissue edema of the graft bed in immediate excision and early wound excision and grafting in a rabbit model. J Burn Care Res. 2007;28:182. doi: 10.1097/BCR.0B013E31802C8980. [DOI] [PubMed] [Google Scholar]
- 4.Desai MH, Herndon DN, Broemeling L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753. doi: 10.1097/00000658-199006000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lari AR, Gang RK. Expansion technique for skin grafts (Meek technique) in the treatment of severely burned patients. Burns. 2001;27:61. doi: 10.1016/s0305-4179(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 6.Vandeput J, Nelissen M, Tanner JC, et al. A review of skin meshers. Burns. 1995;21:364. doi: 10.1016/0305-4179(94)00008-5. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17:124. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wang HJ, Chou TD, Tsou TL, et al. The application of new biosynthetic artificial skin for long-term temporary wound coverage. Burns. 2005;31:991. doi: 10.1016/j.burns.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations—an overview. Skin Pharmacol Appl Skin Physiol. 2000;13:229. doi: 10.1159/000029928. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan TP, Eaglstein WH, Davis SC, et al. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 12.Vodicka P, Smetana K, Jr, Dvorankova B, et al. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005;1049:161. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhu KQ, Engrav LH, Tamura RN, et al. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burns. 2004;30:518. doi: 10.1016/j.burns.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Schechter I. Prolonged retention of glutaraldehyde-treated skin allografts and xenografts: immunological and histological studies. Ann Surg. 1975;182:699. doi: 10.1097/00000658-197512000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu A, Yamada K. Histopathology of xenografts in pig to non-human primate discordant xenotransplantation. Clin Transplant. 2010;24(Suppl 22):11. doi: 10.1111/j.1399-0012.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 16.Galili U, Shohet SB, Kobrin E, et al. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755. [PubMed] [Google Scholar]
- 17.Weiner J, Yamada K, Ishikawa Y, et al. Prolonged survival of GalT-KO swine skin on baboons. Xenotransplantation. 2010;17:147. doi: 10.1111/j.1399-3089.2010.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baertschiger RM, Dor FJ, Prabharasuth D, et al. Absence of humoral and cellular alloreactivity in baboons sensitized to pig antigens. Xenotransplant. 2004;11:27. doi: 10.1111/j.1399-3089.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Mulder A, Kardol MJ, Arn JS, et al. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol Immunol. 2010;47:809. doi: 10.1016/j.molimm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Sachs DH, Winn HJ, Russell PS. The immunologic response to xenografts. Recognition of mouse H-2 histocompatibility antigens by the rat. J Immunol. 1971;107:481. [PubMed] [Google Scholar]
- 21.Levy MF, Crippin J, Sutton S, et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000;69:272. doi: 10.1097/00007890-200001270-00013. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation in Humans. FDA/CBER/DCGT/LCI (HFM-40); 1401 Rockville Pike, Rockville, MD 20852, USA: http://www.fda.gov/cber/guidelines.htm. Accessed June 2013. [Google Scholar]
- 23.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]


