Abstract
Recent findings in several organ systems show that cytoneme-mediated signaling transports signaling proteins along cellular extensions and targets cell-to-cell exchanges to synaptic contacts. This mechanism of paracrine signaling may be a general one that is used by many (or all) cell types in many (or all) organs. We briefly review these findings in this perspective. We also describe the properties of several signaling systems that have previously been interpreted to support a passive diffusion mechanism of signaling protein dispersion, but can now be understood in the context of the cytoneme mechanism.
Keywords: cytonemes, filopodia, morphogen, paracrine signaling, synapse, TGF-β
Introduction
Animal cells communicate over long distances in various ways. Endocrine cells signal systemically by releasing hormones that disseminate in the vasculature. Neurons also release signals, but they exchange information at synapses that form where their axons and dendrites contact target cells. Some axons extend over distances of several meters, effectively bridging distant cells so that signals are within only 15–20 nm of their target receptors when they are released. Paracrine signaling, the third general mechanism, may be considered to be a variant of endocrine signaling, functioning at relatively short range when secreted signals move limited distances by passive diffusion in extracellular fluid. The evidence that has supported this mechanism of paracrine signaling has been obtained over many years in many different experimental systems. However, recent work that we discuss here describes paracrine signaling that is instead contact-mediated and dependent on transient synapses that non-neuronal cells make. These synapses form at sites where specialized signaling filopodia called cytonemes extend to contact target cells.
The classical model of paracrine signaling assumes that signals disperse by passive diffusion
The many paracrine signaling proteins that have been characterized. They include the Fibroblast Growth Factors (FGFs) and other proteins that activate Receptor Tyrosine Kinases (RTKs), TGF-β family members, Wnt proteins, Hedgehog (Hh) proteins, chemokines, as well as cytokines and other ligands that activate the Jak-STAT pathway. All act at a distance, presumably by binding to receptors on target cells after diffusing from the producing cells that secrete them. In many cases, their distributions and patterns of activation indicate that they have spread out from source cells, generating concentration gradients that decline with increasing distance, and cells that are the targets of the signals respond in a concentration-dependent manner. The question we address is not whether developmental fields have concentration gradients of signaling proteins that regulate growth and pattern, but how these proteins disperse to distant cells.
The first report of non-autonomous instructive signaling described a region of the amphibian gastrula (the blastopore dorsal lip) that induced gastrulation and embryogenesis after transfer to ectopic locations in recipient embryos [1]. This inductive capacity was not a general property of embryo cells, suggesting that the cells at the blastopore lip are specially endowed to make something (an “inducer”) that instructs and patterns outlying cells. This group of cells has been branded a “developmental organizer”, and the simplest mechanism that has been considered is the synthesis and secretion of an inducer molecule that disseminates by diffusion. The search for such inducers took many forms and succeeded only after many decades. Although the inducers were assumed to be small organic molecules that diffuse rapidly and pass efficiently from cell to cell, the known inducers are proteins such as TGF-β, FGF, Wnt and Hh. Mathematical analyses and experimental studies show that diffusion may, within certain parameters of diffusion coefficient, geometric tortuosity, viscosity and extracellular volume, generate the observed gradients in the times available during development (reviewed in [2]). The discovery of filopodia that serve as conduits that move signaling proteins between source and target cells adds a new dimension and a radically different mechanism for dissemination ([3], and reviewed in [4]).
Cytonemes mediate paracrine signaling by bridging signaling cells
Filopodia are thin cellular extensions that have been observed in many cell types. As described in excellent reviews [5–8], they have been assigned different roles to account for their presence in various contexts (e.g. cell migration, cell adhesion, force generation, wound healing, environmental sensing, antigen presentation, and neuronal pathfinding). Although their physical properties vary (2–400 µm in length, 0.1–0.3 µm diameter), all are actin-based, they extend and retract at velocities that have been measured as much as 25 µm/minute, and their tips can contact other cells. Their different shapes and roles are reflected in the many names that have been coined (e.g. thick filopodia, thin filopodia, growth cone filopodia, dendritic spines, invadopodia, gliapodia, myopodia). Cytonemes are the specialized filopodia that have been shown to traffic signaling proteins such as morphogens, growth factors and cell determination factors. They have been observed in both vertebrate and invertebrate systems and have been characterized most extensively in Drosophila larval tissues [4].
In the Drosophila larval wing imaginal disc, cells in several signaling centers produce signaling proteins that activate signal transduction in cells that express receptors for these signaling proteins. One of the signaling centers expresses the TGF-β family member Decapentaplegic (Dpp); its Type 1 and Type 2 receptor subunits, Thickveins (Tkv) and Punt, are expressed by all wing disc cells (reviewed in [9]). The Dpp signaling center is a 8–10 cell-wide row that flanks the anterior side of the anterior/posterior (A/P) compartment border (Fig. 1A). The A/P border bisects the wing disc along the entire dorsoventral axis. Cells in the Dpp signaling center both produce and respond to Dpp, although it is not known whether their signal transduction responses are due to autocrine, juxtacrine or paracrine signaling. Outside of the signaling center and as far away as the disc flanks, cells also respond to Dpp, and evidence that Dpp moves from the cells that express it in order to bind to Tkv on distant cells include both GFP fluorescence and antibody staining in discs that overexpress Dpp:GFP in the signaling center [10–14]. Dpp distributes in concentration gradients that decline with increasing distance from the signaling center; the question how Dpp moves across the disc has been studied and discussed extensively [15–19].
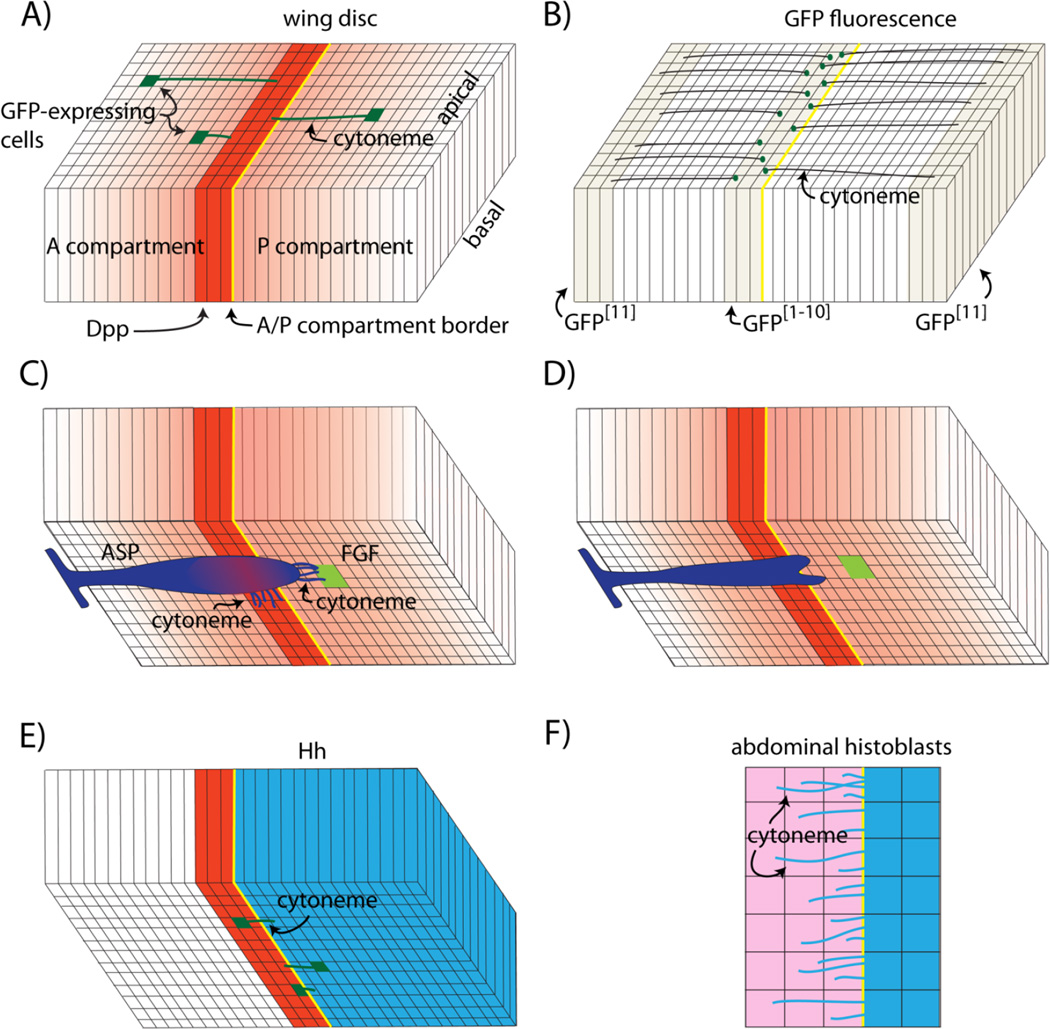
Figure 1.
Cytonemes of the Drosophila wing disc and histoblasts. A: The wing blade primordium is depicted as a columnar monolayer subdivided by the anterior/posterior compartment border (yellow) and a strip of Dpp-expressing cells (red). Concentration gradients of Dpp protein and Dpp signal transduction (red) spread from the Dpp expression domain and cell clones that express GFP have cytonemes (green) that orient along the apical surface toward the Dpp expressing cells. B: GFP reconstitution (GRASP) generates fluorescence (green dots) in the Dpp expression domain in wing discs that express complementary parts of GFP as membrane-tethered external proteins in the disc flanks and the Dpp expression domain. Fluorescence marks points of cell-cytoneme contact. C: The tracheal air sac primordium (ASP; purple) adjoins the basal surface of the wing disc and extends cytonemes toward both Dpp-expressing (red) and FGF-expressing (green) cells; the gradient of Dpp signal transduction in the ASP is shown in red. D: Mutant ASP cells that do not extend cytonemes that synapse with the disc cells do not activate Dpp signal transduction and the ASP is morphologically abnormal. E: Basal cytonemes (green) that carry Hh extend from both Hh-expressing cells (blue) in the posterior compartment and from Hh-receiving cells (red) in the anterior compartment. F: The rows of Hh-expressing cells (blue) in monolayered epithelium of abdominal histoblasts extend Hh-carrying cytonemes to Hh-receiving cells (pink) that express the Hh receptor Patched (Ptc).
The wing disc is a flattened sac composed of two connected epithelial sheets, each one cell deep. The cells of the columnar layer generate most of the adult wing and thorax, and numbering >40,000, are the most numerous. They are highly elongated along their apical/basal axis. The cells of the opposing peripodial layer are relatively flat, described as squamous, and number <2,000. Signaling studies have focused on the columnar cells, concentrating mostly on the region that produces the wing blade.
Columnar cells in the wing blade primordium that are outside of the Dpp signaling center and respond to Dpp extend cytonemes that orient toward the signaling center (Fig. 1A), and the properties of these cytonemes are consistent with the idea that they bridge the distance between Dpp producing and receiving cells, and move Dpp between them [3, 20–22]. The presence and orientation of these cytonemes depend on active Dpp signaling – under conditions of reduced Dpp expression (dppts at restrictive temperature), their number declines and they appear disordered and lack consistent orientation. They increase in number and appear to have random orientations under conditional activation of ubiquitous, uniform Dpp over-expression (Heat shock induced expression of Dpp), and they orient toward somatic clones that over-express Dpp ectopically. There is also evidence that they make direct contact and synapse with the cells in the Dpp signaling center. GRASP (GFP reconstitution across synaptic partners) is a technique that was developed to identify stable cell-cell contacts that juxtapose cell membranes at distances ≤20 nm, such as the synapses that neurons make. GRASP employs two extracellular, membrane-tethered fragments of GFP that can self-assemble to generate fluorescent protein. The points of GRASP fluorescence at the Dpp signaling center in discs that express one of the complementing GFP fragments in the dpp domain and the other in the disc flanks (Fig. 1B) indicates that cells in these distant locations make direct contact despite their separation by as much as 40 µm. The Dpp-dependent cytonemes contain Tkv that concentrates in motile puncta and appear to be specific for Dpp.
Although these studies establish a strong correlation between the Dpp-dependent disc cytonemes and Dpp signaling in the disc, direct evidence for a role in Dpp trafficking has been obtained for cytonemes that extend from a wing disc-associated tracheal branch called the Air Sac Primordium (ASP). Growth and differentiation of the ASP depends on Dpp that it receives from the Dpp signaling center of the disc (Fig. 1C) [22]. Its growth and differentiation also depends on Branchless/Fibroblast growth factor (FGF) received from a FGF signal source in the disc [23]. The ASP extends two types of cytonemes - one that contains Tkv and synapses with Dpp-producing disc cells and another that contains the FGF receptor Breathless (Btl) and synapses with FGF-producing disc cells [21, 22]. The signaling dependence and apparent plasticity of both types of cytonemes are similar to the Dpp-dependent cytonemes of the wing disc. Moreover, it has been possible to show that the Tkv-containing cytonemes pick up and traffic Dpp from the disc and that Dpp signal transduction in the ASP depends on the contact-dependent uptake (Fig. 1D). Although these studies cannot rule out a role for Dpp that might be secreted in a diffusible form from source cells, only cytoneme-associated Dpp has been detected in the space between Dpp producing and receiving cells, and the ASP does not take up and respond to Dpp or develop normally if its cytonemes are defective and unable to make functional contacts with Dpp source.
Hh signaling is cytoneme-dependent
Hedgehog (Hh) is another paracrine signaling system that regulates growth and differentiation of Drosophila epithelia and for which signal trafficking is cytoneme-mediated (Fig. 1E). In the wing disc, cytonemes with motile, Hh-containing vesicles that extend from the basal surface of Hh-expressing columnar cells orient toward Hh-receiving cells, and their properties are consistent with the idea that they deliver Hh to Hh-responding cells. Genetic conditions that reduce the number and length of the cytonemes also reduce the level and expanse of Hh signaling [24]. Cytonemes also extend from the basal surface of the columnar cells that receive and respond to Hh. These cytonemes have motile vesicles that contain the Hh receptor Patched (Ptc) as well as Hh and Interference hedgehog (Ihog), and Hh signal transduction is reduced if formation of these cytonemes are compromised [25]. Thus in the wing disc, Hh movement is mediated both by cytonemes that deliver Hh to Hh-receiving cells as well as cytonemes that take Hh up from producing cells. Again, these results implicate cytonemes as essential conduits for moving signaling proteins from source to target cells.
Hh-trafficking cytonemes have also been characterized in the histoblast cells that generate the abdominal epidermis [24]. Analysis of the histoblasts has taken advantage of the fact that the abdominal epithelium is visible in intact animals (through a window cut out of the pupal case), and growth and development continues over periods of observation that have exceeded 14 hours. In contrast, wing discs must be dissected and mounted in culture medium in order to image cytonemes for shorter period of time, and there is no evidence that isolated wing discs continue to develop ex vivo. Cytonemes carrying Hh emanate from Hh-expressing histoblasts (Fig. 1F). They are dynamic, extending across the entire domain of Hh-responding cells, they appear to contact the responding cells with their tip, and they rapidly retract.
Cytonemes or specialized signaling filopodia have been reported in several other Drosophila and vertebrate systems. They appear to mediate Hh signaling in Drosophila ovary germline cells [26] and larval lymph glands [27], to mediate Notch-dependent lateral inhibition in the wing disc [28], and to deliver Epidermal growth factor (EGF) in the leg disc [29]. In zebrafish embryos, Wnt8a has been observed localizing to cytoneme-like processes that extend from Wnt8a-expressing cells [30]; the best extant evidence that links cytonemes to signaling in vertebrates has been reported for the chick limb bud in which Sonic hedgehog (Shh) moves several hundred microns across the mesenchyme [31]. Shh is produced by a small group of cells at the posterior margin, but activates Hh signal transduction across the developmental field of the limb bud to specify the number and identity of digits. Dynamic cytonemes emanate from the Shh expressing cells and transport Shh along their length, which have been measured as much as 150 µm. The properties of these extensions are consistent with a trafficking role. Filopodia also appear to mediate long range Notch/Delta signaling between xanthophores and melanophores in zebrafish embryos [32].
Perspective
The findings described in this abbreviated summary provide strong experimental evidence for paracrine signaling that is cytoneme-mediated and contact-dependent. The most informative signaling systems have been Dpp in the Drosophila wing disc and the wing disc-associated ASP and Hh in the Drosophila wing disc and abdomen, all of which have genetic and physical attributes that are favorable for these studies [21, 22, 24]. Cytonemes are thin (<200 nm diameter) and only weakly fluorescent when marked with proteins such as membrane-tethered eGFP, are sensitive to physical manipulations, and most do not survive fixation. Imaging is therefore challenging and only possible if background fluorescence is low, if fluorescent markers are expressed in a mosaic fashion in a portion of the cells so that cytonemes extend over a non-fluorescent (dark) background, and if the cytonemes orient along a suitable optical plane. The ASP system has the additional feature that transcriptional enhancers are available that drive expression specifically in either of the two different organs (i.e. wing disc and trachea), making it possible to separately label and specifically tailor both cell types. The power of this system is evident in the study described above which showed that Dpp produced by the wing disc neither moves to nor activates signal transduction in the ASP if the ASP is incapable of extending cytonemes that synapse with the disc (Fig. 1D). Without Dpp reception and signaling in the ASP, the ASP does not develop normally, but the wing disc, which is dependent on Dpp, develops normally in the absence of a proper ASP. Dpp signaling in these discs is unaffected by the defective ASP, indicating that Dpp production is also normal.
This conclusion has fundamental implications: if the Dpp-producing disc cells signal to cells that make cytonemes (other disc cells) but not to cells that cannot make cytonemes (the ASP cells), then Dpp is not normally secreted except in the context of cytoneme contacts. There is ample evidence that cells that express receptors for a ligand respond when bound by the ligand whether the ligand arrives by its normal route or by exogenous administration. Neurons in culture or in ex vivo explants are activated by neurotransmitter added from a pipet. Cultured cells respond to recombinant ligand produced in bacteria and added to culture medium. Cells in developing tissues respond to recombinant ligand that emanates from beads that are impregnated with ligand. The point is that receptors discriminate ligand not route of administration, and the fact that cells respond to ligand that arrives by diffusion is not evidence that the ligand normally diffuses in extracellular space. The analysis of the ASP showing that cytoneme contacts are essential for Dpp uptake and signaling should therefore be understood in the context of ASP cells that express Tkv and are primed to respond to Dpp. Because Dpp moves from producing to receiving cell only at cytoneme synapses, the lack of response by ASP cells suggests that Dpp is not near their receptors in the absence of cytoneme contacts, that Dpp is not released constitutively, and that Dpp does not disperse across tissues by free diffusion.
The extracellular matrix has a critical role in signal dispersion
Passive diffusion is a conceptually simple gradient-generating mechanism, but it is not compatible with the morphogen gradients that form in anatomically complex tissues such as imaginal discs [33]. The discs are not flat and also have multiple deep folds, and although they have closely apposed epithelial layers, morphogen concentrations decline exponentially with distance across the epithelial layer in which they are produced and appear to disperse and signal only in that epithelial layer. These distributions suggest that morphogens are constrained to the plane of the epithelium that expresses them, and various mechanisms of “restricted diffusion” have been proposed to account for morphogen dispersal. One such mechanism is based on the extracellular matrix (ECM), whose constituent proteoglycans are proposed to sculpt the concentration gradients by binding to signaling proteins, co-receptors and other proteins that are involved in controlling the dispersion of signals (Fig. 2A) (reviewed in [34, 35]).
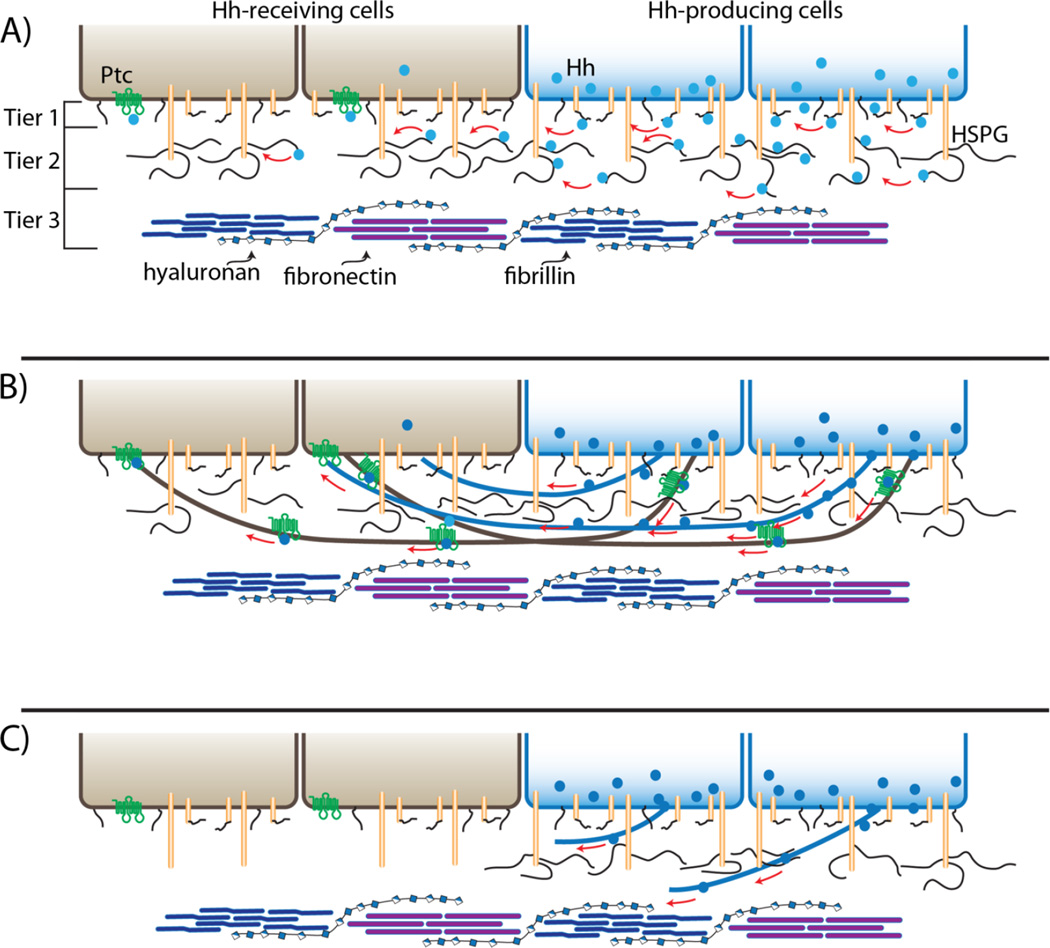
Figure 2.
Restricted diffusion and cytoneme models of Hh gradient formation. A: Drawings depicting two cells expressing and secreting Hh (blue) that interacts directly with HSPGs in the ECM and disperses to generate a concentration gradient across two receiving cells (brown; Ptc, green) by surface diffusion. The "conceptual” ECM is depicted as three tiers consisting of (1) glycolipids (black lines) and surface glycoproteins (tan cylinders with black lines), (2) HSPGs (tall tan cylinders with black lines) and (3) extracellular microfibils (blue), fibronectin (purple) and hyaluronan (beaded strand). B: This drawing depicts Hh dispersing along cytonemes (black) that extend from expressing (brown) and receiving (blue) cells. C: Mutant receiving cells (brown) that do not express HSPGs neither extend cytonemes nor are contacted by cytonemes that extend from Hh-expressing cells.
TGF-β signaling involves interactions with the ECM
The process that generates TGF-β and moves it from producing to receiving cells involves multiple steps and many proteins (reviewed in [36, 37]), and it will be described here in outline because the principles of TGF-β trafficking may be relevant to the way other paracrine signaling proteins move through extracellular space. The TGF-β translation product is an inactive preproprotein. Signal peptidase generates an inactive proprotein that has an N-terminal propeptide (predicted MW ~30 kDa) joined at a furin convertase site to the functional TGF-β peptide (MW 13 kDa). TGF-β proprotein dimerizes and is glycosylated in the endoplasmic reticulum, but the mature 13 kDa TGF-β peptide that furin hydrolysis subsequently generates remains bound to the prodomain and inactive. In different contexts, furin cleavage has been observed either before or after secretion; extracellular hydrolysis is regulated by a secreted glycoprotein called Emelin1. Irrespective of its cleavage state, TGF-β is secreted together with the prodomain (termed the latency-associated peptide, LAP) and a binding protein called LTBP (latent TGF-β binding protein). Non-covalent interactions between fibrillins, which are important structural components of the ECM, and LTBPs are thought to mediate placement of latent TGF-β in the extracellular matrix (ECM). TGF-β is inactive and cannot bind surface receptors until activated by an as yet uncharacterized process that dissociates it from LAP and LTBP (Fig. 3).
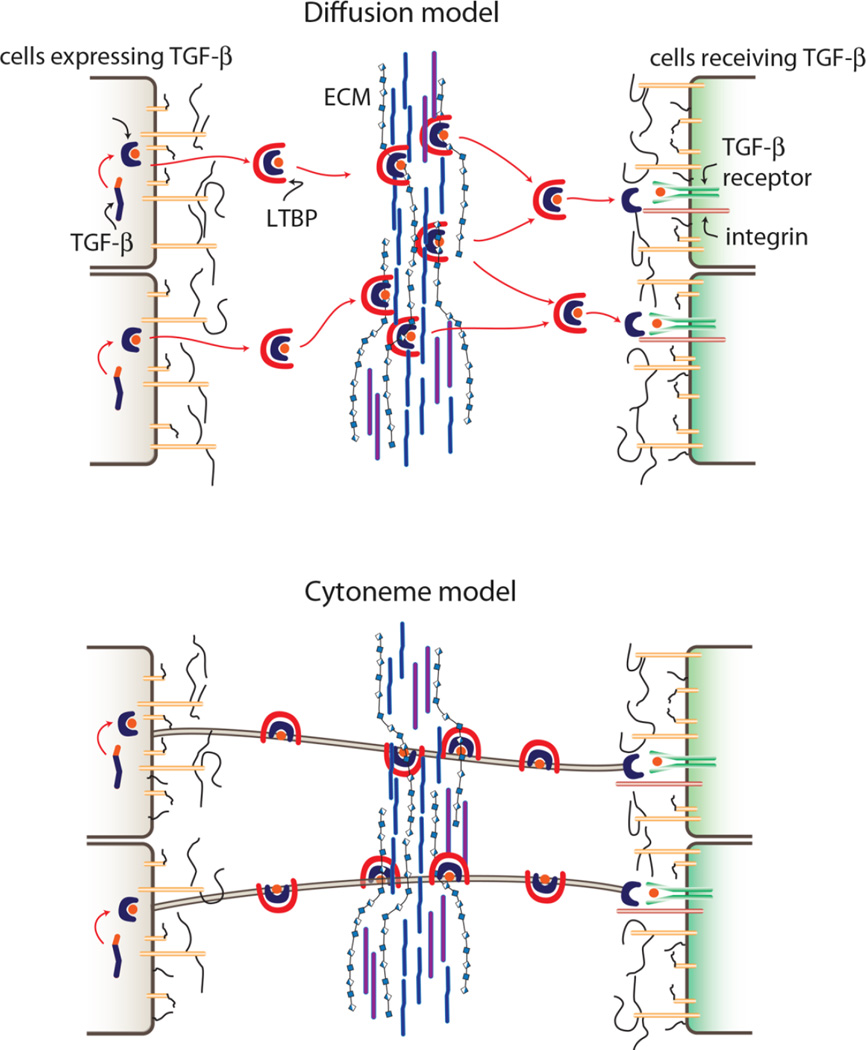
Figure 3.
Diffusion and cytoneme models of TGF-β dispersion. Drawings depict TGF-β (purple + orange cylinder) that is synthesized and processed into mature peptide (orange circle) that is inactive while bound with latent associated peptide (LAP, purple) and latent TGF-β binding protein (LTBP, red). A: Dispersion by diffusion predicts that inactive LAP-bound TGF-β that is secreted as extracellular protein binds to ECM and that release from the ECM initiates the process of activation that involves binding to integrin (red cylinder) and receptor (green cylinders). B: The cytoneme model predicts that LAP-bound TGF-β disperses as an external but not extracellular protein and that release occurs only upon binding to integrin and receptor.
The pathway that leads to paracrine TGF-β is regulated at its start (synthesis of the mRNA) and at its end (release of active signaling protein), and at least at one point in between (furin cleavage). There are clearly many more intermediate steps at which distinct functions are required, but here we apply the term “regulate” strictly, referring only to those steps whose rates are controlled. This definition is consistent with its usage in, for instance, glycolysis, in which the activities of only three of the ten enzymes vary in response to metabolic conditions, and it contrasts with the usage that encompasses all required functions. However, even with this more restrictive designation, we might argue that despite the multiple control points, the one critical step that defines the biological output of the pathway is the release of TGF-β from the producing cell. Because there is no biological activity while TGF-β is bound and no biological activity until it is released, we might consider that the TGF-β maturation process strictly maintains TGF-β in an inactive state while priming the system for signaling. TGF-β is a powerful and critically important regulator of growth and differentiation, so its temporal and spatial activity must be precisely controlled. The steps that prepare TGF-β for release ensure that it cannot signal until the process is complete and signaling is appropriate.
By analogy, the steps that regulate neurotransmitter synthesis and sequestration in neurosecretory vesicles are essential to endow a neuron with the capacity to signal, but they are without consequence until neurotransmitters are released from the presynaptic cell. This aspect of TGF-β signaling is similar, but neuronal signaling at synapses differs from the ECM-dependent model of TGF-β signaling in a fundamental way. A neuron is sensitive to neurotransmitter whether the signal arrives from a synaptic partner or from afar, because its receptors cannot distinguish the source, but the synaptic mechanism, which involves pre-selection of targets and regulation of both release and uptake of neurotransmitter, imparts tight temporal, positional and quantitative specificity. In the ECM-dependent model, however, release of TGF-β from the producing cells is of secondary importance to its liberation from the ECM, and binding sites in the ECM are a consequence of a random walk after release from the producing cell. Temporal, positional and quantitative control of signaling is therefore diminished by the spatial spread of TGF-β in the ECM. Cytoneme-mediated signaling at “morphogenetic synapses”, by contrast, endows paracrine signaling with the specificity characteristic of neurons [38]. The finding that signaling by the TGF-β family member Dpp is cytoneme-dependent raises the question of the role of the ECM in cytoneme-mediated signaling.
Cytoneme-mediated signaling is dependent on the ECM
Mutant Drosophila cells that cannot synthesize glycosaminoglycan (GAG)-modified proteoglycans are deficient for Hh, Wingless, Dpp and FGF signaling ([35, 39] and reviewed in [40, 41]). Whereas these signaling proteins normally activate signal transduction and are present in cells many cell diameters away from their source, most mutant cells activate signal transduction poorly and appear to take up signaling proteins at reduced levels. The only mutant cells that respond at high levels to the signaling proteins are those that are juxtaposed to producing cells. Moreover, signaling proteins are also significantly reduced in genetically normal cells that are separated from source cells by mutant territory, and these distal cells are also signaling-deficient. These observations have been interpreted as functional evidence for a GAG-dependent mechanism of dispersion and for models such as the one illustrated in Figure 2A. This model is based on surface diffusion and the idea that heparan sulfate proteoglycans (HSPGs) transiently bind signaling proteins, keeping them close to the cell surface as they diffuse away from their source. Surface diffusion is a well-characterized process that involves particles hopping between adjacent adsorption sites on a surface; its rates are dependent on a number of factors including the strength of adsorption, the structure and properties of the binding species on the surface and the chemical potential gradients at the surface. Although we lack any measures of these parameters for cells, we can make a rough estimate of the extracellular topography with respect to HSPGs.
A low-resolution model of a typical extracellular space might have three tiers (Fig. 2A). At the cell surface are numerous glycoproteins and glycolipids that have short, branched glycans. A second tier that may be thought of as a canopy that extends over the lower tier consists of proteoglycans that have linear transmembrane protein cores and extracellular domains to which branched and modified glycosaminoglycan (GAG) are attached. Above these two tiers is a network of “matrix” glycoproteins that are not directly attached to the cell. Signaling proteins such as TGF-β and FGF bind to the GAG chains of extracellular proteoglycans, and the journey they take to the receptors in the lower tier is dependent on interactions with glycoproteins in the upper tiers. Estimates of the number of cell surface HSPGs (105 – 106 per cell) are approximately an order of magnitude lower than the number of glycoproteins and glycolipids in the first tier (J. Esko, personal communication, and [42]). If a 10 µm diameter "average" cell (which has a surface area of 3×1010 sq. Å) distributes the 105 – 106 HSPGs evenly over its surface, then for purposes of a back-of-the-envelope calculation, each HSPG will occupy an average area of a square with sides of 170–550 Å. GAG chains are approximately 20–100 kDa, and because a 100 kDa GAG chain is predicted to have a radius of gyration of approximately 230 Å [43], we estimate that an HSPG with multiple GAG chains might occupy a square with 500 Å sides. Although this calculation is imprecise, it yields a model that is consistent with a surface topography that has full coverage by HSPGs, and therefore with a sliding, surface diffusion model for dispersion.
A different interpretation of the mutant HSPG phenotype focuses on the mutant cells that activate signal transduction at normal levels despite their lack of HSPGs. These are the cells that are juxtaposed to source cells. Their responsiveness suggests that direct contact with source cells may be sufficient to enable binding and uptake of the signaling proteins in an HSPG-independent manner, whereas long distance signaling, which is cytoneme-mediated and contact-dependent [22], may be HSPG-dependent. Recent results imaging cytonemes in the context of HSPG mutant cells indicates that this interpretation is correct [24].
As described above, cytonemes extend along the basal surface of the columnar cells of wing imaginal discs, and some carry Hh from posterior compartment Hh-producing cells across the anterior/posterior compartment border to anterior compartment cells [24, 44]. Wild type anterior cells at the border have many cytonemes crossing their basal surface. In contrast, clones of HSPG-deficient mutant cells on the anterior side of the compartment border, which do not activate Hh signal transduction normally and whose anterior neighbors also do not activate Hh signal transduction normally [39, 45], do not have cytonemes on their basal surface (Fig. 2B, C) [24]. This finding supports the idea that cytonemes mediate Hh transport between producing and receiving cells, and suggests that interactions with HSPGs are essential for cytoneme growth and/or stability. The essential role of the HSPGs for Hh signaling in the wing disc is therefore to provide a substrate for the cytonemes that track over the surface of the disc cells.
Discussion
The evidence for cytoneme-mediated and contact-dependent signaling in Drosophila larval tissues is strong. Although we do not yet understand how this mechanism of dispersion and transfer of signaling proteins generates concentration gradients across fields of cells, the gradients depend on it. One possibility takes into account the plasticity and transient nature of cytonemes and the apparent correlation between number of cytonemes, cytoneme length, proximity to source cells and abundance of signaling protein. Shorter cytonemes are more numerous than longer ones and are more numerous closest to source cells where signaling proteins are most abundant. Concentration gradients may therefore be a product of the frequency and duration of functional contacts where signaling proteins are released and taken up. The reason for proposing this model is not to champion it but simply to describe one that is consistent with the data and that is not based on diffusion. We do this in order to discount the notion that the role of cytonemes in gradient formation should be dismissed because our understanding is incomplete. Cytoneme-mediated dispersion is the only mechanism that is supported by direct observation and for which functional genetic evidence exists. The more important question relevant to this discussion is the role of cytonemes in contexts other than Drosophila larval tissues, and in particular the implications for the role of the ECM.
We first address two issues. The term “extracellular” is frequently used to describe secreted proteins, but without specifying their state. Extracellular can refer to the external face of proteins that are integral to the plasma membrane or are attached to the outside surface of a cell. It is also used to refer to proteins that cells secrete and release and that are not attached to the cell. The imprecision becomes problematic, for instance, with antibody staining protocols for fluorescence immunohistochemistry that detect extracellular protein. Fluorescence microscopy lacks sufficient resolution to distinguish cell-associated protein from protein that has been released but remains in close proximity to its source. It is also an issue for protein that is in a cytoneme and at a distance from the cell body if the cytoneme is not marked. Such protein will appear to be in exovesicles and not associated with a cell. Henceforth, we use the term “external” for cell-associated moieties on the outside of cells - “they have been externalized” - and reserve the term “extracellular” for those that are not bound to the cells that release them.
Both externalized and extracellular proteins are secreted, but distinguishing between these two states is critically important for mechanism. The lumen of the wing imaginal discs is a small space that separates the apical faces of the columnar and peripodial cells by as little as 6 µm. Although Dpp is expressed only by a narrow stripe of cells at the A/P compartment border in both the columnar and peripodial layers, fluorescence immunohistochemistry detects Dpp across the entire A/P expanse of the lumen [46]. If we assume that the Dpp is free in lumen, it would be reasonable to conclude that it has dispersed by diffusion. However, because apical cytonemes do not survive the fixation protocols that were used for immunohistochemistry and because cytonemes cannot be detected if their components are not marked, the relationship between Dpp and cytonemes in the lumen was not revealed in these studies and it is not clear whether the Dpp is external (and cytoneme-associated) or extracellular (and free). The presumption of the “simpler” mechanism of extracellular diffusion is justified in the absence of any knowledge of structural elements in the lumen that might be relevant to Dpp dispersion. The fact that cytonemes are present in the lumen invalidates such assumptions.
The issue of cell association is not resolved by the presence of protein in medium conditioned by cells grown in culture, by observations made of over-expression conditions, or by responses to exogenously supplied signaling protein (see [38]). The issue is also relevant to the interactions of secreted proteins with the ECM. Whereas studies of vertebrate TGF-β implicate proteoglycans in the upper tier of the ECM as critical for signaling, neither histochemical nor biochemical studies have the resolution or sensitivity to establish whether TGF-β that is bound to ECM is extracellular (free) or is external and cytoneme-associated. Indeed, it is possible that vertebrate TGF-β and Drosophila Dpp move between cells by similar mechanisms, that TGF-β is cytoneme-bound when passing between expressing and receiving cells and that ECM-associated TGF-β is not extracellular (Fig. 3). For both the vertebrate and Drosophila systems, a better understanding of the structure of the ECM, of the role and structure of cytonemes, and of the state of in transit signaling proteins is needed in order to know if the apparent differences reflect different mechanisms.
Conclusions
The structural and functional similarities of synaptic signaling by axons and cytonemes reveal an ancient kinship, and such evolutionary conservation might favor the idea of a universal mechanism for paracrine signaling – that “every cell is a neuron and a neuron is not alone” in the sense that all cells communicate at distance by reaching out with extensions to make direct contacts where signals are exchanged. The observations we made in Drosophila larval tissues that are consistent with this cytoneme model include EGF signaling in the eye imaginal disc, Hh and Dpp signaling in the wing imaginal disc, and FGF and Dpp signaling in the ASP. Indeed, we have not found any signaling system that lacks cytonemes linking signaling cells. Studies of the Drosophila ovary, wing disc and abdominal histoblasts also report cytonemes that correlate with signaling [24, 26, 44], and there are numerous reports of cytoneme-like structures that correlate with paracrine signaling in other invertebrate [47, 48] as well as vertebrate systems [31, 49, 50].
These observations favor a mechanism of paracrine signaling that is shared by all cells, including neurons, but they do not eliminate the possibility of non-contact dependent paracrine signaling. Certainly there are many well-characterized signaling systems for which the presence and role of cytonemes has not been explored. Guidance cues that steer neuronal pathfinding are examples, although the actual distributions have only been inferred and have not been directly observed (reviewed in [51]). Long distance Dpp signaling and Dpp dispersion in the early Drosophila embryo is another example [52], and studies of the Xenopus embryo also reveal long distance dispersion of signaling proteins [53]. Unfortunately, the conclusion these studies make - that diffusion disperses the signaling proteins – is based solely on the observed patterns of expression and dispersion.
The speeds at which Dpp and FGF spread through tissues (measured in various ways including FRAP (Fluorescence recovery after photobleaching), spatial FRAP, fluorescence correlation microscopy and pair correlation function microscopy [14, 54, 55] are consistent with free diffusion when certain values for relevant parameters are assumed, but it is important to recognize that diffusion is but one mechanism of dispersion and neither rates nor patterns of distribution distinguish between them. In the absence of evidence for or against the presence of signal protein-carrying cytonemes, conclusions should reflect the uncertainties that the state of understanding demands.
Acknowledgments
The authors acknowledge support from the National Institutes of Health (grants GM030637 and GM105987 to T.B.K. and K99HL114867 to S.R.).
Abbreviations
- ASP
air sac primordium
- A/P
anterior/posterior
- Btl
breathless
- Dpp
decapentaplegic
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- FRAP
fluorescence recovery after photobleaching
- GAG
glycosaminoglycan
- GFP
green fluorescent protein
- GRASP
GFP reconstitution across synaptic partners
- Hh
hedgehog
- HSPG
heparan sulfate proteoglycan
- Ihog
interference hedgehog
- LAP
latency-associated peptide
- LTBP
latent TGF-β binding protein
- Ptc
patched
- RTK
receptor tyrosine kinase
- Shh
sonic hedgehog
- TGF-β
Transforming growth factor-β
- Tkv
thickveins
References
- 1.Spemann H, Mangold H. Über Weckung organisatorischer Fähigkeiten durch Verplanzung in organisatorische Umgebung. Roux's Arch. 1924;109:557–577. doi: 10.1007/BF02079696. [DOI] [PubMed] [Google Scholar]
- 2.Lander AD, Lo WC, Nie Q, Wan FY. The measure of success: constraints, objectives, and tradeoffs in morphogen-mediated patterning. Cold Spring Harb Pperspect Biol. 2009;1:a002022. doi: 10.1101/cshperspect.a002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 4.Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development. 2014;141:729–736. doi: 10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faix J, Breitsprecher D, Stradal TE, Rottner K. Filopodia: Complex models for simple rods. Int J Biochem Cell Biol. 2009;41:1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Heckman CA, Plummer HK., 3rd Filopodia as sensors. Cell Signal. 2013;25:2298–2311. doi: 10.1016/j.cellsig.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. J Cell Sci. 2013;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellor H. The role of formins in filopodia formation. Biochim Biophys Acta. 2010;1803:191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Raftery LA, Umulis DM. Regulation of BMP activity and range in Drosophila wing development. Curr Opin Cell Biol. 2012;24:158–165. doi: 10.1016/j.ceb.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 11.Lecuit T, Cohen SM. Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development. 1998;125:4901–4907. doi: 10.1242/dev.125.24.4901. [DOI] [PubMed] [Google Scholar]
- 12.Schwank G, Dalessi S, Yang SF, Yagi R, et al. Formation of the long range Dpp morphogen gradient. PLoS Biol. 2011;9:e1001111. doi: 10.1371/journal.pbio.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Lo WC, Suhalim JL, Digman MA, et al. Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc. Curr Biol. 2012;22:668–675. doi: 10.1016/j.cub.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baena-Lopez LA, Nojima H, Vincent JP. Integration of morphogen signalling within the growth regulatory network. Curr Opin Cell Biol. 2012;24:166–172. doi: 10.1016/j.ceb.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Crozatier M, Glise B, Vincent A. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 2004;20:498–505. doi: 10.1016/j.tig.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Kornberg TB. The imperatives of context and contour for morphogen dispersion. Biophys J. 2012;103:2252–2256. doi: 10.1016/j.bpj.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwank G, Basler K. Regulation of organ growth by morphogen gradients. Cold Spring Harb Perspect Biol. 2010;2:a001669. doi: 10.1101/cshperspect.a001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wartlick O, Gonzalez-Gaitan M. The missing link: implementation of morphogenetic growth control on the cellular and molecular level. Curr Opin Genet Dev. 2011;21:690–695. doi: 10.1016/j.gde.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 21.Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 2014;343:1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff M, Gradilla AC, Seijo I, Andres G, et al. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Kornberg TB. The Hedgehog gradient of the Drosophila wing imaginal disc requires cytonemes. 55th Annual Drosophila Research Conference; San Diego, CA. 2014. [Google Scholar]
- 26.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, et al. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen M, Georgiou M, Stevenson NL, Miodownik M, et al. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev Cell. 2010;19:78–89. doi: 10.1016/j.devcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y, Han C, Axelrod JD. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev Cell. 2012;23:507–518. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luz M, Spannl-Muller S, Ozhan G, Kagermeier-Schenk B, et al. Dynamic association with donor cell filopodia and lipid-modification are essential features of Wnt8a during patterning of the zebrafish neuroectoderm. PLoS One. 2014;9:e84922. doi: 10.1371/journal.pone.0084922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamada H, Watanabe M, Lau HE, Nishida T, et al. Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development. 2014;141:318–324. doi: 10.1242/dev.099804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornberg TB, Guha A. Understanding morphogen gradients: a problem of dispersion and containment. Curr Opin Genet Dev. 2007;17:264–271. doi: 10.1016/j.gde.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Restrepo S, Zartman JJ, Basler K. Coordination of patterning and growth by the morphogen DPP. Curr Biol. 2014;24:R245–R255. doi: 10.1016/j.cub.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113:410–418. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornberg TB, Roy S. Communicating by touch - neurons are not alone. Trends Cell Biol. 2014;24:370–376. doi: 10.1016/j.tcb.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. 2000;19:303–307. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 41.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 42.Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bathe M, Rutledge GC, Grodzinsky AJ, Tidor B. A coarse-grained molecular model for glycosaminoglycans: application to chondroitin, chondroitin sulfate, and hyaluronic acid. Biophys J. 2005;88:3870–3887. doi: 10.1529/biophysj.104.058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callejo A, Bilioni A, Mollica E, Gorfinkiel N, et al. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci USA. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takei Y, Ozawa Y, Sato M, Watanabe A, et al. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- 46.Gibson MC, Lehman DA, Schubiger G. Lumenal transmission of decapentaplegic in Drosophila imaginal discs. Dev Cell. 2002;3:451–460. doi: 10.1016/s1534-5807(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama-Oda Y, Oda H. Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development. 2003;130:1735–1747. doi: 10.1242/dev.00390. [DOI] [PubMed] [Google Scholar]
- 48.McClay DR. The role of thin filopodia in motility and morphogenesis. Exp Cell Res. 1999;253:296–301. doi: 10.1006/excr.1999.4723. [DOI] [PubMed] [Google Scholar]
- 49.Danilchik M, Williams M, Brown E. Blastocoel-spanning filopodia in cleavage-stage Xenopus laevis: Potential roles in morphogen distribution and detection. Dev Biol. 2013;382:70–81. doi: 10.1016/j.ydbio.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–1433. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 51.Dickson BJ, Zou Y. Navigating intermediate targets: the nervous system midline. Cold Spring Harb Perspect Biol. 2010;2:a002055. doi: 10.1101/cshperspect.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 53.Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci USA. 2013;110:20372–20379. doi: 10.1073/pnas.1319745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kicheva A, Pantazis P, Bollenbach T, Kalaidzidis Y, et al. Kinetics of morphogen gradient formation. Science. 2007;315:521–525. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- 55.Yu SR, Burkhardt M, Nowak M, Ries J, et al. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–536. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]