Abstract
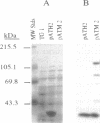
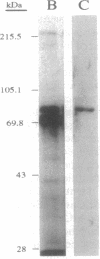
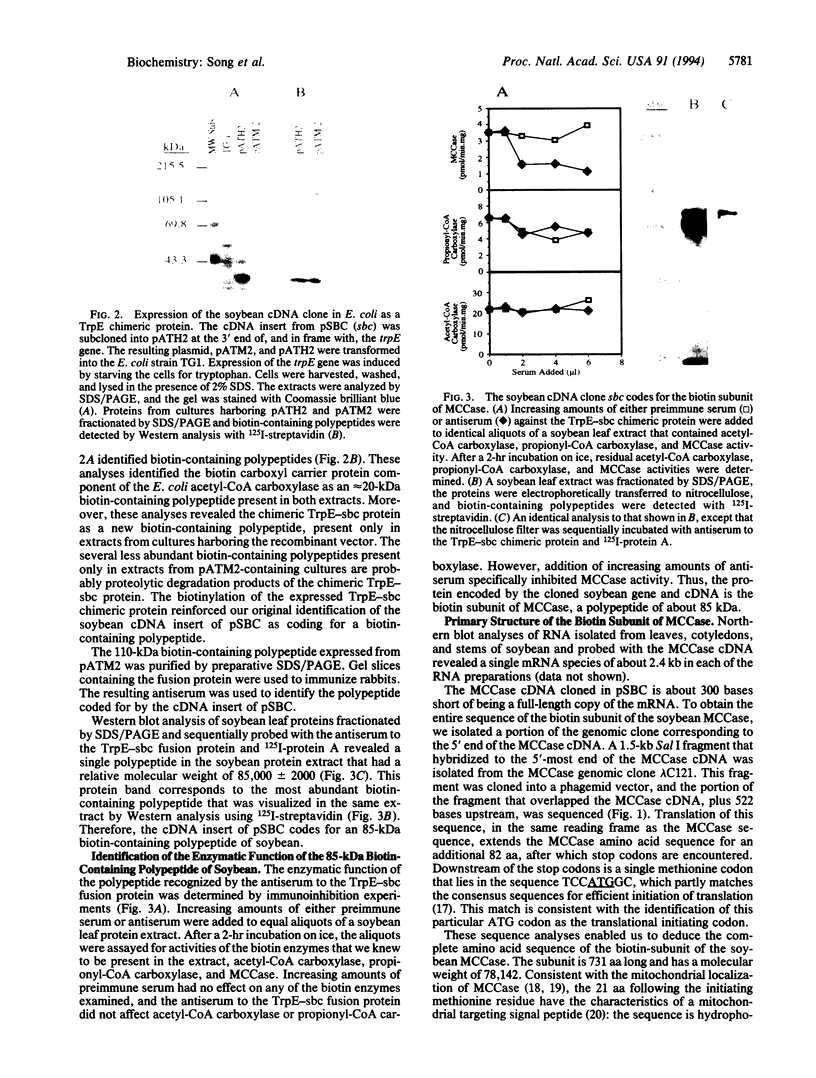
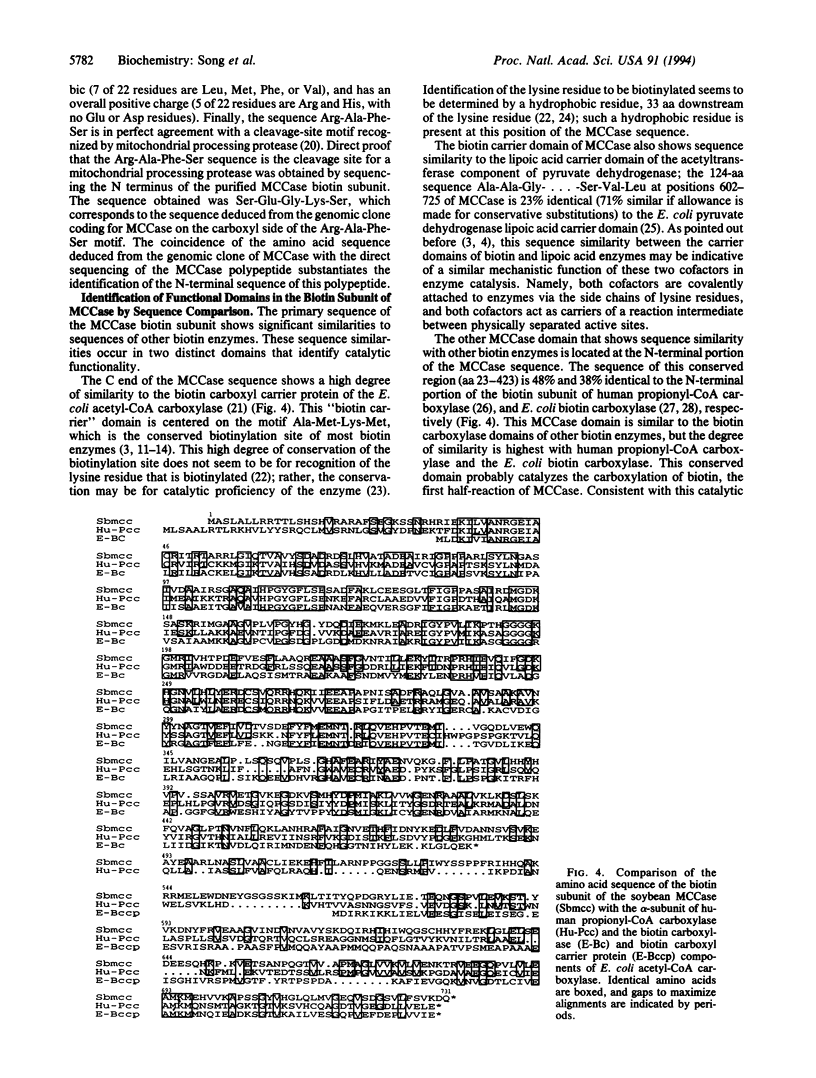
Soybean genomic clones were isolated based on hybridization to probes that code for the conserved biotinylation domain of biotin-containing enzymes. The corresponding cDNA was isolated and expressed in Escherichia coli through fusion to the bacterial trpE gene. The resulting chimeric protein was biotinylated in E. coli. Antibodies raised against the chimeric protein reacted specifically with an 85-kDa biotin-containing polypeptide from soybean and inhibited 3-methylcrotonoyl-CoA carboxylase (EC 6.4.1.4) activity in cell-free extracts of soybean leaves. Thus, the isolated soybean gene and corresponding cDNA code for the 85-kDa biotin-containing subunit of 3-methylcrotonoyl-CoA carboxylase. The nucleotide sequence of the cDNA and portions of the genomic clones was determined. Comparison of the deduced amino acid sequence of the biotin-containing subunit of 3-methylcrotonoyl-CoA carboxylase with sequences of other biotin enzymes suggests that this subunit contains the functional domains for the first half-reaction catalyzed by all biotin-dependent carboxylases--namely, the carboxylation of biotin. These domains are arranged serially on the polypeptide, with the biotin carboxylase domain at the amino terminus and the biotin-carboxyl carrier domain at the carboxyl terminus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Feel W., Chirala S. S., Wakil S. J. Cloning of the yeast FAS3 gene and primary structure of yeast acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4534–4538. doi: 10.1073/pnas.89.10.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldet P., Alban C., Axiotis S., Douce R. Characterization of biotin and 3-methylcrotonyl-coenzyme a carboxylase in higher plant mitochondria. Plant Physiol. 1992 Jun;99(2):450–455. doi: 10.1104/pp.99.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wurtele E. S., Wang X., Nikolau B. J. Purification and characterization of 3-methylcrotonyl-CoA carboxylase from somatic embryos of Daucus carota. Arch Biochem Biophys. 1993 Aug 15;305(1):103–109. doi: 10.1006/abbi.1993.1398. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem. 1990 Jun 25;265(18):10327–10333. [PubMed] [Google Scholar]
- Genbauffe F. S., Cooper T. G. The urea amidolyase (DUR1,2) gene of Saccharomyces cerevisiae. DNA Seq. 1991;2(1):19–32. doi: 10.3109/10425179109008435. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Lewis H. M., Graham L. D., Packman L. C., Perham R. N. Genetic reconstruction and functional analysis of the repeating lipoyl domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Mol Biol. 1985 Oct 20;185(4):743–754. doi: 10.1016/0022-2836(85)90059-2. [DOI] [PubMed] [Google Scholar]
- Hector M. L., Cochran B. C., Logue E. A., Fall R. R. Subcellular localization of 3-methylcrotonyl-coenzyme A carboxylase in bovine kidney. Arch Biochem Biophys. 1980 Jan;199(1):28–36. doi: 10.1016/0003-9861(80)90252-0. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Pichersky E., Cashmore A. R. A tomato cDNA encoding a biotin-binding protein. Nucleic Acids Res. 1987 May 11;15(9):3928–3928. doi: 10.1093/nar/15.9.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. The mechanism of biotin-dependent enzymes. Annu Rev Biochem. 1989;58:195–221. doi: 10.1146/annurev.bi.58.070189.001211. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Kondo H., Shiratsuchi K., Yoshimoto T., Masuda T., Kitazono A., Tsuru D., Anai M., Sekiguchi M., Tanabe T. Acetyl-CoA carboxylase from Escherichia coli: gene organization and nucleotide sequence of the biotin carboxylase subunit. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9730–9733. doi: 10.1073/pnas.88.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lamhonwah A. M., Mahuran D., Gravel R. A. Human mitochondrial propionyl-CoA carboxylase: localization of the N-terminus of the pro- and mature alpha chains in the deduced primary sequence of a full-length cDNA. Nucleic Acids Res. 1989 Jun 12;17(11):4396–4396. doi: 10.1093/nar/17.11.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Jan 15;267(2):855–863. [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Reeve J. N. Conservation of structure in the human gene encoding argininosuccinate synthetase and the argG genes of the archaebacteria Methanosarcina barkeri MS and Methanococcus vannielii. J Bacteriol. 1988 Jul;170(7):3125–3130. doi: 10.1128/jb.170.7.3125-3130.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the fabE gene and flanking regions containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 May 25;17(10):3982–3982. doi: 10.1093/nar/17.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtif V. L., Samols D. Mutagenesis affecting the carboxyl terminus of the biotinyl subunit of transcarboxylase. Effects on biotination. J Biol Chem. 1987 Aug 25;262(24):11813–11816. [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Use of streptavidin to detect biotin-containing proteins in plants. Anal Biochem. 1985 Sep;149(2):448–453. doi: 10.1016/0003-2697(85)90596-2. [DOI] [PubMed] [Google Scholar]
- Quinn C. L., Stephenson B. T., Switzer R. L. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J Biol Chem. 1991 May 15;266(14):9113–9127. [PubMed] [Google Scholar]
- Samols D., Thornton C. G., Murtif V. L., Kumar G. K., Haase F. C., Wood H. G. Evolutionary conservation among biotin enzymes. J Biol Chem. 1988 May 15;263(14):6461–6464. [PubMed] [Google Scholar]
- Schiele U., Niedermeier R., Stürzer M., Lynen F. Investigations of the structure of 3-methylcrotonyl-CoA carboxylase from Achromobacter. Eur J Biochem. 1975 Dec 1;60(1):259–266. doi: 10.1111/j.1432-1033.1975.tb20998.x. [DOI] [PubMed] [Google Scholar]
- Shenoy B. C., Xie Y., Park V. L., Kumar G. K., Beegen H., Wood H. G., Samols D. The importance of methionine residues for the catalysis of the biotin enzyme, transcarboxylase. Analysis by site-directed mutagenesis. J Biol Chem. 1992 Sep 15;267(26):18407–18412. [PubMed] [Google Scholar]
- Sternberg M. J., Taylor W. R. Modelling the ATP-binding site of oncogene products, the epidermal growth factor receptor and related proteins. FEBS Lett. 1984 Oct 1;175(2):387–392. doi: 10.1016/0014-5793(84)80774-7. [DOI] [PubMed] [Google Scholar]
- Takai T., Yokoyama C., Wada K., Tanabe T. Primary structure of chicken liver acetyl-CoA carboxylase deduced from cDNA sequence. J Biol Chem. 1988 Feb 25;263(6):2651–2657. [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Differential Accumulation of Biotin Enzymes during Carrot Somatic Embryogenesis. Plant Physiol. 1992 Aug;99(4):1699–1703. doi: 10.1104/pp.99.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Plants contain multiple biotin enzymes: discovery of 3-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase and pyruvate carboxylase in the plant kingdom. Arch Biochem Biophys. 1990 Apr;278(1):179–186. doi: 10.1016/0003-9861(90)90246-u. [DOI] [PubMed] [Google Scholar]