Abstract
Purpose Of Review
Optimizing hemostasis with antifibrinolytics is becoming a common surgical practice. Large clinical studies have demonstrated efficacy and safety of tranexamic acid (TXA) in the trauma population to reduce blood loss and transfusions. Its use in patients without preexisting coagulopathies is debated, as thromboembolic events are a concern. In this review, perioperative administration of TXA is examined in non-trauma surgical populations. Additionally, risk of thromboembolism, dosing regimens, and timing of dosing are assessed.
Recent Findings
Perioperative use of tranexamic acid is associated with reduced blood loss and transfusions. Thromboembolic effects do not appear to be increased. However, optimal dosing and timing of TXA administration is still under investigation for non-trauma surgical populations.
Summary
As part of a perioperative blood management program, tranexamic acid can be used to help reduce blood loss and mitigate exposure to blood transfusion.
Keywords: Tranexamic Acid, Perioperative, Transfusion, Thromboembolism, Fibrinolysis
INTRODUCTION
First, stop the bleeding! As anesthesiologists on the front line of trauma and acutely bleeding surgical patients, we must continuously seek mechanisms to promote hemostasis. The problem: severely injured trauma patients in shock develop an acute coagulopathy manifested by reduced clot strength and hyperfibrinolysis. The CRASH II and MATTERs trials addressed this problem and provided evidence that antifibrinolytics such as tranexamic acid reduce all cause mortality in the trauma population.[1, 2] Intuitively, this makes sense. Bleeding to death in the first 48 hours after trauma accounts for approximately 50% of all deaths.[3] By blocking clot breakdown, we promote hemostasis and reduce death due to bleeding. In recent years, however, the results of the CRASH II and MATTERs trials have been extrapolated to non-trauma surgery. The problem: surgical patients with normal hemostatic function preoperatively may excessively bleed due to the surgery itself thus requiring transfusion. It is well known that antifibrinolytics have been used for years in surgery where the risk of bleeding is severe. Cardiac surgery and liver transplantation have provided a wealth of data on antifibrinolytic use. However, the risk of concurrent coagulopathies is great in both of these populations.[4, 5] In this article, we will discuss regulation of the fibrinolytic pathway and how it is altered in trauma. Next, we will review and summarize the use of antifibrinolytics, specifically TXA, in recent literature. Finally, we will summarize recent antifibrinolytic use in non-trauma populations and conclude with recommendations.
METHODS
EMBASE, MEDLINE, PUBMED, and Google Scholar databases were examined for perioperative use of tranexamic acid in non-trauma surgical populations from late 2012 to 2014.
REGULATION OF FIBRINOLYSIS
Fibrinolysis is the process of clot degradation that promotes fluidity, effectively recanalizing a vessel occluded by thrombus.[6] During normal coagulation, fibrinogen is converted to fibrin, which incorporates into bound platelets and forms a hemostatic clot at the point of vascular injury. After hemostasis is achieved, the clot is enzymatically broken down. This process requires the conversion of inactive single-chain plasminogen (PLG) to an active two-chain protease. Fibrinolysis is therefore initiated when plasminogen is converted to plasmin by tissue plasminogen activator (t-PA). Tissue plasminogen activator is the principal PLG-activator in plasma and has a relatively weak affinity for PLG. However, in the presence of fibrin, t-PA's affinity for PLG is increased several fold.[7] Tissue plasminogen activator is released due to venous occlusion, stress, physical activity, or vasoactive drugs such as epinephrine. Figure 1 graphically depicts fibrinolysis.
Figure 1.
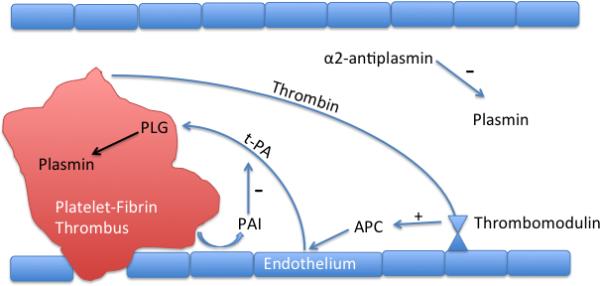
The thrombus releases thrombin into circulation activating protein C. Tissue plasminogen activator cleaves plasminogen into plasmin to initiate fibrinolysis. PLG-activator inhibitors are stimulated by platelets to slow fibrinolysis. Antiplasmins inhibit plasmin released into circulation.
While plasmin cannot circulate freely in plasma due to rapid inactivation by antiplasmin, plasminogen can. Plasminogen incorporates into the evolving thrombus due to its high affinity for fibrin, mediated by lysine-binding domains.[7] During clot evolution, thrombin is shed into circulation where it binds thrombomodulin located on the endothelial surface. This complex activates Protein C that in turn blocks inactivation of t-PA by inhibiting plasminogen activator inhibitor.[8] Locally synthesized t-PA binds plasminogen within the thrombus to form plasmin and begins enzymatic breakdown into fibrin degradation products. As fibrin is broken down, more lysine binding sites are exposed, binding more PLG thus enhancing plasmin formation. Lysine analogues such as tranexamic acid can bind these lysine cites and block plasmin formation.
To prevent widespread fibrinolysis, the system has several checks or inhibitors. First, t-PA binding is enhanced in the presence of fibrin, thus only binds locally to plasminogen within thrombus. Next, platelets and thrombin stimulate the release of plasminogen activator inhibitor that slows plasmin formation. Antiplasmins circulating in the plasma also quickly inactivate any plasmin released from the thrombus.
COAGULOPATHY IN TRAUMA
In approximately one-third of all trauma patients, the degree of shock and hypoperfusion will be sufficient to induce an endogenous biological process resulting in coagulopathy.[9] It is important to realize that injury severity and resulting shock is an important instigator in Trauma Induced Coagulopathy (TIC) and carries a 50% mortality when diagnosed.[10] Multiple mechanisms contribute to the hypocoagulable state after severe trauma (Table 1). The discovery of TIC as an endogenous process that occurs outside of hemodilution, hypothermia, and acidemia spurred the development of new resuscitation strategies. Damage control or hemostatic resuscitation (DCR) consists of limiting crystalloid administration, rapid rewarming, time-limited hypotension during hemorrhage, and emphasis on ratio based blood components to mimic whole blood administration.[11, 12] A central tenet of DCR is to treat the altered biology manifested during trauma. One important alteration appreciated in TIC is hyperfibrinolysis. For this reason, the CRASH 2 trial studied administration of Tranexamic Acid, an antifibrinolytic, and found that early administration of this drug decreased all cause mortality after severe trauma.[1]
Table 1.
Pathophysiology of Trauma Induced Coagulopathy
| Protein C Activation | Blocks thrombin formation, enhances fibrinolysis, Antiinflammatory |
| Glycocalyx Disruption | Autoheparinization, enhanced fibrinolysis |
| Platelet Exhaustion | Decreased thrombin generation, decreased clot strength, reduced cellular signaling |
| Fibrinogen Depletion | Decreased clot strength, decreased speed of clot formation |
Protein C Activation
Two prospective studies have shown conclusively that Protein C is activated early after severe trauma and acts as a driver of TIC.[13, 14] After severe traumatic injury and in the presence of hypoperfusion, thrombin is released systemically into the circulation and binds with thrombomodulin and the endothelial protein C Receptor. This complex then activates circulating Protein C and results in a “thrombin switch” where thrombin paradoxically becomes an initiator of anticoagulation.[15, 16] Activated Protein C (APC) has multiple anticoagulant functions. First, by inhibiting Factors V and VIII, thrombin generation is impaired, ultimately halting clot formation. Next, APC inhibits Plasminogen Activator Inhibitor resulting in increased fibrinolysis. Finally, activated Protein C exerts profound antiinflammatory effects by modulating transcription factors essential to cellular signaling.[8] High circulating levels of APC upon admission were found to be predictors of poor outcomes such as increased mortality, organ injury, increased blood transfusion requirements, and reduced ICU ventilator-free days.[17] Table 2 lists studies that demonstrate APC's role in TIC.[14, 17-19]
Table 2.
Activated Protein C Studies
| Brohi et al. [18] | Journal of Trauma 2008, 64(5): 1211-1217. | Prospective cohort, 208 patients | Single Trauma Center | Thrombin-thrombomodulin leads to hyperfibrinolysis via activated protein C consumption of PAI-1 |
| Chesebro et al. [19] | Shock. Dec 2009; 32(6): 659-665. | Mouse Model of Traumatic Shock | NA | Endogenous acute coagulopathy after trauma is mediated by the activation of the protein C pathway |
| Cohen et al. PROMMT Study [14] | Journal of Trauma and Acute Care Surgery: 2013, 75: S40-S47. | Prospective Observational, 1198 patients | Multi-Center | ATC is associated with the depletion of factors II, V, VII, VIII, IX, X, and I and is driven by the activation of the protein C system. |
| Cohen et al. [17] | Ann Surg. 2012 Feb; 255(2): 379-85. | Prospective cohort, 203 patients | Single Center | Higher plasma levels of aPC upon admission are predictive of poor clinical outcomes after major trauma. |
Endothelial Injury
Perhaps as important as activation of Protein C, injury to the endothelium is an important factor in the development of acute traumatic coagulopathy. The endothelium is lined with an anticoagulant rich, negatively charged surface layer called the glycocalyx. This 1-micron thick layer contains approximately 1 liter of non-circulating heparin like substances.[20] Additionally, the endothelium is comprised of Weibel-Palade bodies that contain concentrated levels of tissue plasminogen activator (tPA) and angiopoetin 2 (Ang-2). When damaged, endothelial components such as syndecan-1, soluble thrombomodulin, Ang2, and t-PA are released into the circulation resulting in autoheparinization and hyperfibrinolysis.[21] Ostrowski et al. observed that about 5% of severely injured trauma patients demonstrate autoheparinization and increased fibrinolysis on thromboelastometry.[21] Two independent studies have shown that high circulating levels of syndecan-1 or Ang-2, both markers of endothelial damage, are predictors of increased mortality.[22, 23] Table 3 lists studies demonstrating the importance of endothelial injury in trauma.[21, 23, 24]
Table 3.
Endothelial Injury Studies
| Johansson et al. [23] | Ann Surg 2011; 254:194-200 | Prospective Clinical, 75 trauma patients | Single Center | High circulating syndecan-1, a marker of endothelial glycocalyx degradation, is associated with inflammation, coagulopathy and increased mortality |
| Ostrowski [21] | J Trauma Acute Care Surg. 2012 Jul; 73(1): 60-6. | Prospective Observational, 77 patients | Single Center | Acute endogenous coagulopathy with autoheparinization by TEG appeared mechanistically linked to endothelial glycocalyx degradation. |
| Ostrowski [24] | Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2012, 20:27 | Prospective Observational, 80 trauma patients | Single Center | Sympathoadrenal activation, shock and inflammation may be critical drivers of endothelial activation and damage early after trauma |
Fibrinogen Depletion
Fibrinogen is an acute phase reactant with limited stores in the human body. Thus, depletion is rapid after severe trauma due to hemorrhagic loss. Additionally, enhanced fibrinolysis due to inactivation of Plasminogen Activator Inhibitor caused by APC also results in depleted fibrinogen due to consumption. Two studies by Rourke and Kornblith, demonstrate the significance of fibrinogen depletion. First, using different thromboelastograph assays, Kornblith showed convincingly that fibrinogen contributes about 30% to total clot strength. In trauma, patients with lower functional fibrinogen had greater had greater transfusion requirements and higher mortality.[25] Rourke demonstrated that fibrinogen is rapidly depleted after trauma and that low fibrinogen levels predicted increased mortality at 24 hours.[26] Table 4 lists studies of fibrinogen depletion in trauma.[26-28]
Table 4.
Fibrinogen Depletion Studies
| Hagemo et al. [27] | Scandinavian journal of trauma, resuscitation, and emergency medicine. Jul 17 2013; 21(1): 56. | Pig Model | Single Center | Early increase in fibrinogen attenuates hypocoagulable state in trauma. |
| Rourke et al. [26] | Journal of thrombosis and hemostasis: JTH. Jul 2012; 10(7): 1342-1351. | Prospective Cohort, 517 patients | Single Center | Low admission fibrinogen level was independently associated with injury severity score. Fibrinogen level was an independent predictor of mortality at 24 h and 28 days. |
| Schlimp et al. [28] | Critical care. Jul 12 2013; 17(4): R137. | Retrospective, 675 patients | Single Center | Fibrinogen upon admission correlated strongly with Hb, BE and ISS. |
Platelet Dysfunction
Though not directly contributing to hyperfibrinolysis, platelet exhaustion during trauma and its ensuing effects on cellular activation are important in the pathogenesis of TIC. Hoffman's cell-based model of hemostasis provides and excellent description of the integral role platelets plays in coagulation.[29] Acting as the principal site of thrombin formation and as a key signaler of cellular activation, the platelet is instrumental in normal coagulation. Several studies have demonstrated significant inactivation of platelets after trauma despite normal platelet levels.[30] Table 5 summarizes these studies.[25, 30-32]
Table 5.
Platelet Dysfunction Studies
| Wohlauer et al. [31] | J Am Coll Surg. 2012 May; 214(5): 739-746. | Prospective, 51 patient samples | Single Center | Impairment of platelet function in response to AA was 44.9% (IQR 26.6-59.3%), compared to 0.5% (IQR 0-3.02%) in volunteers |
| Donahue et al.[32] | Journal of Neurotrauma. 2014 Feb; 31:404-410 | Rodent Model | Single Center | Large decrease in platelet stimulation toward ADP, AA, and collagen after blunt force head trauma |
| Kutcher et al. [30] | J Trauma Acute Care Surg. 2012 July; 73(1): 13-19. | Prospective, 101 patients | Single Center | Clinically significant platelet dysfunction after trauma in the presence of an otherwise reassuring platelet count and standard clotting studies, with profound implications for mortality. |
| Kornblith et al. [25] | J Trauma Acute Care Surg. 2014 Feb; 76(2): 255-6; discussion 262-3. | Prospective, 251 patients | Single Center | Coagulopathic patients (international normalized ratio ≥ 1.3) had significantly lower admission %MA (FF) than noncoagulopathic patients (24.7% vs. 31.2%, p < 0.05). Despite this importance of fibrinogen, platelets had a greater contribution to clot strength at all time points after injury. |
USE OF TRANEXAMIC ACID IN TRAUMA PATIENTS
It is well understood that the cause of death in nearly half of all trauma patients is life-threatening hemorrhage. Nearly all trauma patients suffer some form of coagulopathy.[33] Trauma Induced Coagulopathy (TIC), which begins as early as at the time of injury,[34] is present in up to 40% percent of patients following major traumatic injury[3]. Early recognition and treatment of TIC may improve outcome in trauma resuscitation. [35] Historically, plasma based testing (aPTT, PT, INR) has been used to monitor coagulation. Plasma based testing is used extensively to monitor patients receiving anti-coagulation but has not been validated in monitoring the hemorrhaging trauma patient.[36, 37]
Viscoelastic monitoring (TEG, rTEG, ROTEM, SONOCLOT), cell based testing, shows promise in diagnosing trauma related coagulopathy and fibrinolysis.[38-40] Viscoelastic testing is still more common in European centers, but is gaining acceptance in American ones. Coagulopathy, requiring transfusion, remains a significant entity in critically ill bleeding patients. Transfusion of red blood cells and blood components are accompanied by the risks of allergic and hemolytic reactions. In addition blood group mismatch, transfusion related acute lung injury (TRALI), transfusion associated circulatory overload (TACO), bacterial transmission and errors such as the mismatching of blood groups add to the risk of transfusion.[41] The pathophysiology of traumatic coagulopathy is very different from post operative bleeding, however, there exist similarities between the two such as defects in thrombin generation, plasmin generation, activation of the inflammatory response, as well as an imbalance between fibrin generation and breakdown.[42] The use of antifibrinolytics is encouraging and has been shown to reduce transfusion needs and bleeding, and temper hyperfibrinolysis although more large prospective randomized trials are needed.
CRASH 2 Trial
The CRASH-2 trial (Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2) is the largest multi-national randomized placebo controlled trial of the effects of the early administration of TXA on death (28 day mortality), thromboembolic events, and blood use in adult trauma patients. Thromboembolic events were defined as myocardial infarction, stroke, and pulmonary embolism. This randomized controlled trial was carried out in 274 hospitals in 40 countries and enrolled 20,211 adult trauma patients. Patients were randomized within 8 hours of injury to either the TXA group (1 gram loading dose over 10 minutes followed by an infusion of 1 gram over 8 hours) or a matching placebo.[43] Eligibility were those adult trauma patients with systolic BP < 90 mmHg or heart rate > 110 beats per minute (defined as significant hemorrhage) and within 8 hours of injury.
10,096 patients were allocated to the TXA group and 10,115 patients were allocated to the placebo group. 19,944 patients (99.1%) were known to have completed the loading dose and 18,965 (94.2%) the 8 h maintenance dose. 3076 (15.3%) patients died, of those 1086 (35.3%) died on the day of randomization. There were 1063 deaths from bleeding, of which 637 (59.9%) were on the day of randomization. All-cause mortality at four weeks was significantly reduced with RR (relative risk) of death for those treated with tranexamic acid of 0.91 (95% CI 0.85– 0.97, p=0.0035). [44] The risk of death due to bleeding was also significantly reduced (TXA 489 [4.9%] vs. placebo 574 [5.7%]) RR 0.85 (95% CI 0.76-0.96, p = 0.0077).
There was no statistically significant difference in the incidence of thromboembolic events including PE, DVT, stroke or myocardial infarction, multi-organ failure and head injury between the two groups. Head injury (TBI) was the leading cause of death with TXA group 603 (6.0%) and 621 placebo group 621 (6.2%) RR 0.97 (95% CI 0.87 – 1.08, p = 0.60) There was also no statistical difference for blood products transfused. The authors concluded that TXA safely reduced the risk of death from bleeding in the hemorrhaging trauma patient. In their opinion, TXA should be considered for administration in bleeding trauma patients.
There were significant limitations to the CRASH 2 Trial such as a lack of massive transfusion protocol, the lack of information regarding additional blood product use, the approach to randomization and the lack of data regarding laboratory data and the extent of acute traumatic coagulopathy.[44]
CRASH 2 Exploratory Analysis: Early TXA Administration
This exploratory analysis of the CRASH 2 Trial was carried out in order to determine the effects of TXA administration on death due to bleeding according to the time to treatment, type of injury (penetrating only, blunt, blunt plus penetrating), Glasgow Coma Score (GCS), and systolic blood pressure as an indication of severity of hemorrhage. There was convincing evidence that the rate of death due to hemorrhage varied according to the time from injury until treatment. Early treatment with TXA reduced the risk of death by bleeding if given within an hour of injury (Table 6). [1] There was no significant increase in the risk of death from all other non-bleeding causes combined. The authors noted that caution should be taken when administering TXA to patients greater than 3 hours out from time of injury. These may be two different patient populations. Those further out from injury (> 3hours) may be more acidotic, hypothermic and in a more prothrombotic phase of their traumatic injury.[45, 46]
Table 6.
Time to Initial Dose of TXA related to mortality.
| N | All Causes Of Death | Bleeding Death | Non-Bleeding Death | |
|---|---|---|---|---|
| Overall | 20127 | 0.91 (0.85-0.97) p = 0.0035 | 0.85 (0.76-0.96) p = 0.0077 | 0.94 (0.86-1.02) p = 0.13 |
| Time to Treatment (hours) | ||||
| ≤ 1 | 7451 | 0.87 (0.76-0.97) | 0.68 (0.57-0.82) | 1.04 (0.89-1.21) |
| > 1-3 | 6033 | 0.87(0.77-0.97) | 0.79 (0.64-0.97) | 0.91 (0.78-1.02) |
| > 3 | 6634 | 1.00 (0.90-1.13) | 1.44 (1.12-1.84) | 0.89 (0.78-1.02) |
Total number treated, relative risk (95% CI) of death in those treated with tranexamic acid, overall and time to treatment. Adapted from CRASH 2 Exploratory analysis
MATTERs Study
The MATTERs (Military Application of Tranexamic Acid in Trauma Emergency Resuscitation study) was designed to study the efficacy of TXA administration on mortality, total blood product use, and complications due thromboembolic events in combat related injuries. It is a retrospective observational study comparing TXA administration to no TXA in patients receiving at least one unit of packed red blood cells. A subgroup of patients who received a massive transfusion (≥ 10 units of packed red blood cells) was examined in addition. A total of 896 consecutive trauma admissions (combat) were identified prospectively from the US and UK trauma registries. Two hundred ninety-three patients received TXA.
Despite being more severely injured (mean ISS 25.2 [TXA] vs. 22.5 [no TXA] p < 0.001), the TXA group had a lower unadjusted mortality than the no TXA group (17.4% vs. 23.9%, p =0.03). This benefit was greater in the group receiving massive transfusion. (14.4% vs. 28.1%, p = 0.004). In this group, an independent association with survival (odds ratio = 7.228; 95% CI, 3.016-17.322) was noted as well as less coagulopathy (p = 0.003). Thirty-day survival was higher in the TXA group (p = 0.004) in both the overall cohort as well as the group receiving massive transfusion. In the overall cohort, hypotension, a GCS ≤ 8, and the presence of coagulopathy were associated with a higher mortality. In the massive transfusion group a GCS of ≤ 8 and an ISS of ≥ 15 was associated with mortality but TXA use demonstrated an independent survival benefit. No clinical parameters had an association with DVT or PE in either cohort in a separate analysis. The authors concluded that TXA use, with component-based resuscitation, results in the improvement of coagulopathy and survival post combat injury. This result was more significant in those requiring a massive transfusion.[2]
MATTERs II Study
This retrospective observational study of 1,332 patients was designed to determine the association of cryoprecipitate and survival after combat injury. A secondary, but unforeseen, observation of the MATTERs study demonstrated an increase volume of cryoprecipitate given to the cohort who received TXA. The study population consisted of four cohorts: casualties that received TXA but not cryoprecipitate, casualties that received cryoprecipitate but not TXA, casualties that received both cryoprecipitate and TXA and casualties that received neither. The no TXA-no cryoprecipitate cohort demonstrated the highest mortality (23.6%) and the TXA-cryoprecipitate cohort had the lowest mortality (11.6%), (p = 0.001).
The effect of TXA was examined in order to determine if there was an interaction with cryoprecipitate. In a Synergy model there was found to be no interaction between TXA and cryoprecipitate (p = 0.21). The independent additive effect of TXA and cryoprecipitate in combination was found to have an OR of 0.34 (95% CI 0.20-0.58) (p < 0.001). There appears to exist and additive effect between TXA and cryoprecipitate that is beneficial in terms of a survivor benefit when given to severely injured combat casualties requiring transfusion. [47]
USE OF TRANEXAMIC ACID IN NON-TRAUMA PATIENTS
A very thorough review of the use of TXA in anesthetic practice was written in the British Journal of Anaesthesia in 2013.[41] This review examined the use of antifibrinolytics in multiple types of surgical populations. In summary, TXA use was associated with reduced allogenic blood transfusion without increased thromboembolic events. Benefits were seen in neurosurgical, obstetric, orthopedic, liver, and cardiac surgery. Limiting the dose of TXA due to its seizure inducing properties was suggested.
The use of TXA in cardiac surgery (Table 7) continues to gain popularity since the removal of Aprotinin from the market but remains more costly than ε- aminocaproic acid. [48-54] The population pharmacokinetics of TXA in adults has been investigated with recommendations on dosing. The safety of antifibrinolytics in children requires further investigation. The question of increased thromboembolic events remains an important concern requiring more adequately powered randomized control trials (RCT).[55]
Table 7.
Vascular/Cardiac Surgery
| Aoki and Suczawa [48] | Retrospective, 2014 | Endovascular Abdominal Aortic Repair, 187 patients. TXA dose (1500 mg/day p.o. on day of EVAR until 6 months after) Aneurysm shrinkage in TXA group (80%) no TXA group (45%). |
| Faraoni and Goobie [49] | Randomized Trial, 2014 | Compared two doses of TXA (30 mg/kg bolus + 16 mg/kg/hr infusion)(HIGH) or 5 mg/kg bolus + 5 mg/kg/hr (LOW) to NaCl (PLACEBO) in patients undergoing CPB. No difference in TXA dose on fibrinolysis or clinical outcomes (33 patients) |
| Siguat and Tremey [50] | Multicenter, RCT, 2013 | Two dosing regimens were compared with the primary endpoint being the incidence of blood product transfusion to day 7. LOW dose (10 mg/kg bolus + 1 mg/kg/hr infusion) vs. HIGH dose (30 mg/kg bolus+ 16 mg/kg/hr infusion). High dose is more effective than low dose in decreasing transfusion needs but does not reduce the incidence of blood product transfusion to day 7. (569 patients) |
| Falana and Patel [51] | Retrospective, Observational Cohort, 2014 | Efficacy and safety of TXA vs. Amicar. 120 patients undergoing cardiovascular surgery who received at least one dose of TXA or Amicar. Efficacy outcome was perioperative hemorrhage defined as, chest tube drainage >1500ml in any 8 hr period, 10 or more units of RBCs, re-op for bleeding or death from hemorrhage within 30days. No difference in safety or efficacy was found. Amicar is cheaper and perhaps a better economic choice. (120 patients) |
| Hasegawa and Oshima [52] | Retrospective database review, 2014 | Children <20 kg underwent bloodless cardiac surgery for simple procedures. TXA group compared to the no TXA group. TXA dose of 100 mg/kg in bolus + 10mg/kg/hr infusion. There were significant reductions in operative time, dopamine dose, peak serum lactate, intubation time, chest tube drainage, duration, and hospital stay. (71 patients) |
| Grassin-Delyle and Tremey [53] | Investigative Study, 2013 | TXA LOW dose group (10 mg/kg+1 mg/kg/hr for the operation and 1 mg/kg into CPB pump). TXA HIGH dose grp (30 mg/kg + 16 mg/kg/hr and 2 mg/kg into CPB). Recommended dosing to maintain therapeutic plasma levels is 46 mg/kg infusion over 1 hour + 11 mg/kg hr (50-75kg patients) 10 mg/kg/hr (75-100kg patients) or 9 mg/kg/hr (100-125 kg patients) infusion over 3 hrs. (61 patients) |
| Wang and Zie [54] | Prospective, RCT, 2012 | Off Pump Coronary Bypass. Patients received TXA (1 gm bolus followed by 400 mg/hr during surgery). Primary outcome: post-op chest tube drainage. TXA patients had a significant reduction in chest tube drainage, transfusion of blood and fresh frozen plasma. No difference in mortality morbidity or resources used. (231 patients) |
Tranexamic acid in orthopedic surgery (Table 8) has shown great promise in reducing allogenic blood transfusions and is becoming integral to blood management strategies. [56-61] Recent retrospective studies have not shown evidence of increased thromboembolic events. Larger randomized control trials are still needed to determine the ideal dosing and timing of TXA administration in this population. Likewise, head and neck surgery and obstetrics have found benefit in perioperative TXA administration. Table 9 [62-66] Recent studies support TXA use to reduce blood loss while larger powered studies are needed to ascertain thromboembolic event risk. Absolute and relative contraindications to TXA administration are listed in Table 10.
Table 8.
Orthopedic Surgery
| Gandhi and Evans [56] | Meta-analysis | Patients underwent primary unilateral TKA or THA. Study comparisons of TXA to placebo or no treatment. Mean overall blood loss favored TXA over control for TKA (−1.149)(p < 0.001) and THA (−0.504)(p <0.001). Transfusion requirements were decreased as well as no increased incidence of DVT in TKA or THA with TXA use. Authors conclude routine use of TXA in TKA and THA to decrease blood loss |
| Wind and Barfield [57] | Retrospective database review, 2013 | Patients received TXA via infusion, topical application, or neither and the need for transfusion. TXA infusion produced a statistically significant decrease in transfusion rate (p < 0.001). Transfusion rate with TXA (4.39%) without TXA (19.86%) and topical TXA (12.86%) (1494 patients) |
| Poeran and Rasul [58] | Retrospective cohort, 2014 | Intervention of TXA by dose (none, ≤ 1000 mg, 2000 mg, ≥ 3000 mg.) Patients receiving TXA had significantly lower transfusion rates while not increasing the rate of thromboembolic events and acute renal failure across all dosing ranges. (872,416 patients) |
| Yang and Li [59] | Meta-analysis or RCTs, 2013 | 581 Patients undergoing mainly scoliosis surgery according to the Cochrane Collaboration Guidelines. TXA was given IV in various dosing regimens. Patients also received Amicar as a comparator as well as placebo. Overall, those treated with TXA who required blood transfusions were decreased by 35% than those treated with Amicar and placebo. A dose independent effect of TXA was also observed. They found blood loss was significantly decreased as well as transfusion requirement in the TXA treated group. |
| Oremus and Sustaric [60] | RCT, 2014 | 98 Patients underwent TKA or THA. Patients randomized to TXA (1 gram followed by 1 gram 3 hours later) or saline placebo. Proportion of patients receiving autologous blood reinfusion was lower in the TXA group than placebo, absolute difference of −75.5%. No differences in homologous transfusions and hematologic variables between groups. Authors conclude that with a blood salvage protocol and the addition of TXA a post-operative blood salvage system in unnecessary. |
| Dahuja and Dahuja [61] | Prospective cohort, 2014 | 60 Patients with primary osteoarthritis of both knees undergoing staged bilateral TKA at an interval of 3 weeks that had previously received TXA for the first procedure. Used as own controls. TXA 15 mg/kg given before tourniquet and then every 8 hours for 2 days. Total post-operative drain output lower in the TXA group. Hematocrit and platelet count were similar. No thromboembolic events in both groups. TXA treated patients showed no bleeding tendency and increased fibrinogen levels. |
Table 9.
Other Surgical Sites
| Christabel [62] | RCT, 2014 | 49 Patients requiring a LeFort I osteotomy. Evaluated the efficacy of hypotensive anesthesia in combination with TXA on intraoperative blood loss. The TXA group received 10 mg/kg in a bolus pre-operatively. Placebo group received saline 5 ml. The TXA group showed a statically significant reduction in blood loss (45%). Authors conclude a lower need for postoperative transfusion and a better operative field. |
| Robb [63] | Review Article, 2014 | The use of TXA in ENT surgery needs further controlled randomized trials. Two recent pilot studies looked at primary hemorrhage in children and secondary hemorrhage in adults post tonsillectomy suggesting further RCTs needed. |
| Dakir [64] | Randomized Pilot, 2014 | 12 Patients 20-40 years of age with multiple facial fractures. 6 received TXA 10 mg/kg before induction of anesthesia and 6 were allocated to the saline placebo group. The TXA group had a significantly reduced blood loss compared with placebo (489.17 ± 106.7 ml vs. 900.83 ± 113.7 ml.) None of the TXA group required a blood transfusion. |
| Heesen [65] | Meta-analysis, 2014 | Seven trials with low risk of bias comparing TXA to placebo in 1760 parturients. Blood loss was significantly lower in the TXA group as was the needs for blood transfusion (RR 0.34, p= 0.0001). Uterotonics were needed more in the placebo group and more gastrointestinal adverse events were noted in the TXA group. Thromboembolic events were not significant in either group. |
| Perel [66] | Cochrane Review, 2013 | Five trials were identified (372 people) and 3 trials (260 people) contributed to the data. The effect of TXA on mortality was uncertain (RR 1.01; 95% CI 0.14 – 7.3). TXA reduced the probability of blood transfusion. The effects on DVT (RR 2.29; 95% CI 0.68 to 7.66) and stroke (RR 2.79; 95% CI 0.12 to 67.10) were uncertain. |
Table 10.
Contraindications To Tranexamic Acid
| Exclusion: |
| 1. DVT or PE within 12 months of surgery |
| 2. History of DVT or PE being treated with anticoagulation |
| 3. Known congenital thrombophilia |
| 4. Cardiac stent or ischemic stroke within 1 year |
| Relative Contraindications: |
| 1. Renal impairment |
| 2. Severe ischemic heart disease |
| 3. History of thromboembolic or vascular disease |
| 4. Disseminated intravascular coagulation (DIC) |
| 5. History of seizures |
Adapted from manufacturer monograph.
CONCLUSION
Tranexamic acid has gained much popularity in surgery due to its promotion of hemostasis. Though cardiac surgery, liver transplantation, and trauma have traditionally been the areas of antifibrinolytic use, other surgical venues are now exploring TXA benefits. The concern over using antifibrinolytics routinely to reduce intraoperative blood loss in patients without known coagulopathies is valid. Several studies have now shown reduced blood transfusion and reduced intraoperative blood loss when administering TXA. Importantly, there does not appear to be a morbidity burden from increased thromboembolic events. More randomized control trials need to be conducted to determine appropriate dosing regimens for different surgical populations. In addition, timing of administration appears to be critical for optimal effect of hemostasis without increasing risk of thromboembolism. At this time, it appears that using TXA in non-trauma surgery to promote hemostasis reduces blood loss and does not increase thromboembolic phenomenon.
Key Points.
In severe trauma with shock, tranexamic acid is used to treat hyperfibrinolysis and is part of a damage control resuscitation strategy.
Large clinical trials have demonstrated reduction in blood transfusions without increased incidence of thromboembolic events in the trauma population when TXA is administered.
Ideal dosing regimens for trauma and non-trauma surgeries are still under investigation.
In non-trauma surgery, perioperative use of TXA is associated with improved surgical field views, reduced blood loss, and fewer blood transfusions.
Tranexamic acid has not been shown to statistically increase thromboembolic events in the perioperative period.
Acknowledgements
None
Disclosure of Funding For This Work: NIH R01 GM086416 (JFP)
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Contributor Information
Jeff Simmons, Anesthesia Services Division, Trauma Section UAB Department of Anesthesiology 804 Jefferson Tower 619 South 19th Street Birmingham AL 35249.
Robert A. Sikorski, Department of Anesthesiology University of Maryland School of Medicine Division of Trauma Anesthesiology Medical Director of Perfusion and Cell Salvage Services R Adams Cowley Shock Trauma Center Baltimore, Maryland (410) 328-2630 rsikorski@umm.edu.
Jean-Francois Pittet, Critical Care Division, Department of Anesthesiology Professor of Surgery and Cell Biology Investigator, Center for Lung Injury and Repair University of Alabama at Birmingham Phone: 205-996-4755 pittetj@uab.edu.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
◆of special interest
◆◆ of outstanding interest
- 1.Collaborators C, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–101, 101 e1-2. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 2.Morrison JJ, Dubose JJ, Rasmussen TE, et al. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Archives of surgery. 2012;147(2):113–9. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 3.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. The Journal of trauma. 2008;65(4):748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 4.Sabate A, Dalmau A, Koo M, et al. Coagulopathy management in liver transplantation. Transplantation proceedings. 2012;44(6):1523–5. doi: 10.1016/j.transproceed.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Solbeck S, Genet G, et al. Coagulopathy and hemostatic monitoring in cardiac surgery: an update. Scandinavian cardiovascular journal : SCJ. 2012;46(4):194–202. doi: 10.3109/14017431.2012.671487. [DOI] [PubMed] [Google Scholar]
- 6.Barash PG. Clinical anesthesia. 6th ed. xviii. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2009. p. 1640. [Google Scholar]
- 7.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. British journal of haematology. 2005;129(3):307–21. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 8◆◆.Noel P, Cashen S, Patel B. Trauma-induced coagulopathy: from biology to therapy. Seminars in hematology. 2013;50(3):259–69. doi: 10.1053/j.seminhematol.2013.06.009. [Well written, succient article detailing mechanisms involved in trauma coagulopathy as well as current therapies.] [DOI] [PubMed] [Google Scholar]
- 9.Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013;53(Suppl 1):28S–37S. doi: 10.1111/trf.12033. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. The Journal of trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 11◆.Kaafarani HM, Velmahos GC. Damage Control Resuscitation In Trauma. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2014;103(2):81–8. doi: 10.1177/1457496914524388. [This article reviews multimodal resuscitation strategies in trauma.] [DOI] [PubMed] [Google Scholar]
- 12.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA surgery. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of surgery. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14◆◆.Cohen MJ, Kutcher M, Redick B, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. The journal of trauma and acute care surgery. 2013;75(1 Suppl 1):S40–7. doi: 10.1097/TA.0b013e31828fa43d. [This article explains the pathogenesis of traumatic coagulopathy describing the importance of correcting hyperfibrinolysis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion in critical care. 2007;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 16.Davenport R. Pathogenesis of acute traumatic coagulopathy. Transfusion. 2013;53(Suppl 1):23S–7S. doi: 10.1111/trf.12032. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Annals of surgery. 2012;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. The Journal of trauma. 2008;64(5):1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 19.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–65. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv : European journal of physiology. 2007;454(3):345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. The journal of trauma and acute care surgery. 2012;73(1):60–6. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 22.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PloS one. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson PI, Stensballe J, Rasmussen LS, et al. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Annals of surgery. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski SR, Sorensen AM, Windelov NA, et al. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scandinavian journal of trauma, resuscitation and emergency medicine. 2012;20:27. doi: 10.1186/1757-7241-20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25◆.Kornblith LZ, Kutcher ME, Redick BJ, et al. Fibrinogen and platelet contributions to clot formation: Implications for trauma resuscitation and thromboprophylaxis. The journal of trauma and acute care surgery. 2014;76(2):255–63. doi: 10.1097/TA.0000000000000108. [This article illustrates components of clot formation and the relative importance of fibrinogen and platelets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. Journal of thrombosis and haemostasis : JTH. 2012;10(7):1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 27◆.Hagemo JS, Jorgensen JJ, Ostrowski SR, et al. Changes in fibrinogen availability and utilization in an animal model of traumatic coagulopathy. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21(1):56. doi: 10.1186/1757-7241-21-56. [This article explains how fibrinogen availabilty is critical to proper trauma resuscitation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlimp CJ, Voelckel W, Inaba K, et al. Estimation of plasma fibrinogen levels based on hemoglobin, base excess and Injury Severity Score upon emergency room admission. Critical care. 2013;17(4):R137. doi: 10.1186/cc12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thrombosis and haemostasis. 2001;85(6):958–65. [PubMed] [Google Scholar]
- 30.Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. The journal of trauma and acute care surgery. 2012;73(1):13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. Journal of the American College of Surgeons. 2012;214(5):739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donahue DL, Beck J, Fritz B, et al. Early platelet dysfunction in a rodent model of blunt traumatic brain injury reflects the acute traumatic coagulopathy found in humans. Journal of neurotrauma. 2014;31(4):404–10. doi: 10.1089/neu.2013.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. The Surgical clinics of North America. 2012;92(4):877–91, viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Current opinion in anaesthesiology. 2012;25(2):229–34. doi: 10.1097/ACO.0b013e3283509675. [DOI] [PubMed] [Google Scholar]
- 35.Mitra B, Cameron PA, Mori A, et al. Acute coagulopathy and early deaths post major trauma. Injury. 2012;43(1):22–5. doi: 10.1016/j.injury.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Grottke O. Coagulation management. Current opinion in critical care. 2012;18(6):641–6. doi: 10.1097/MCC.0b013e328358e254. [DOI] [PubMed] [Google Scholar]
- 37.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arteriosclerosis, thrombosis, and vascular biology. 2006;26(1):41–8. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 38.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfusion medicine reviews. 2012;26(1):1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Schochl H, Forster L, Woidke R, et al. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia. 2010;65(2):199–203. doi: 10.1111/j.1365-2044.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 40.Jeger V, Willi S, Liu T, et al. The Rapid TEG alpha-Angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. TheScientificWorldJournal. 2012;2012:821794. doi: 10.1100/2012/821794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41◆◆.Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. British journal of anaesthesia. 2013;111(4):549–63. doi: 10.1093/bja/aet154. [This aricle reviews the use of antifibrinolytics in multiple surgical specialties.] [DOI] [PubMed] [Google Scholar]
- 42.Faraoni DVDLP. 2014 A Sytsematic Review of antifibrinolytics and massive injury - Copy.pdf. Minerva Anestesiologica. 2014;80(10):1115–22. [PubMed] [Google Scholar]
- 43.Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 44.Napolitano LM, Cohen MJ, Cotton BA, et al. Tranexamic acid in trauma: how should we use it? The journal of trauma and acute care surgery. 2013;74(6):1575–86. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 45.Johansson PI, Ostrowski SR. Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Medical hypotheses. 2010;75(6):564–7. doi: 10.1016/j.mehy.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 46.Pär I, Johansson AMS, Perner Anders. DIC or Acute Coagulopathy.pdf. Critical care. 2011;15(R272):1–10. doi: 10.1186/cc10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47◆◆.Morrison JJ, Ross JD, Dubose JJ, et al. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II Study. JAMA surgery. 2013;148(3):218–25. doi: 10.1001/jamasurg.2013.764. [This study depicts the added mortality benefit when cryoprecipitate is used with tranexamic acid.] [DOI] [PubMed] [Google Scholar]
- 48.Aoki A, Suezawa T, Yamamoto S, et al. Effect of antifibrinolytic therapy with tranexamic acid on abdominal aortic aneurysm shrinkage after endovascular repair. Journal of vascular surgery. 2014;59(5):1203–8. doi: 10.1016/j.jvs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Faraoni D, Goobie SM. New insights about the use of tranexamic acid in children undergoing cardiac surgery: from pharmacokinetics to pharmacodynamics. Anesthesia and analgesia. 2013;117(4):760–2. doi: 10.1213/ANE.0b013e3182a22278. [DOI] [PubMed] [Google Scholar]
- 50.Sigaut S TB. 2014 Comparison of Two Doses of Tranexamic Acid in Adults.pdf. Anesthesiology. 2014;120:590–600. doi: 10.1097/ALN.0b013e3182a443e8. [DOI] [PubMed] [Google Scholar]
- 51.Falana O, Patel G. Efficacy and Safety of Tranexamic Acid Versus -Aminocaproic Acid in Cardiovascular Surgery. The Annals of pharmacotherapy. 2014 doi: 10.1177/1060028014549558. [DOI] [PubMed] [Google Scholar]
- 52.Hasegawa T, Oshima Y, Maruo A, et al. Intraoperative tranexamic acid in pediatric bloodless cardiac surgery. Asian cardiovascular & thoracic annals. 2014;22(9):1039–45. doi: 10.1177/0218492314527991. [DOI] [PubMed] [Google Scholar]
- 53.Grassin-Delyle S, Tremey B, Abe E, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. British journal of anaesthesia. 2013;111(6):916–24. doi: 10.1093/bja/aet255. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Xie G, Jiang T, et al. Tranexamic acid reduces blood loss after off-pump coronary surgery: a prospective, randomized, double-blind, placebo-controlled study. Anesthesia and analgesia. 2012;115(2):239–43. doi: 10.1213/ANE.0b013e3182264a11. [DOI] [PubMed] [Google Scholar]
- 55.Ker K, Roberts I. Tranexamic acid for surgical bleeding. Bmj. 2014;349:g4934. doi: 10.1136/bmj.g4934. [DOI] [PubMed] [Google Scholar]
- 56.Ganhi R EH, Mahomed S. 2013 Tranexamic acid and the reduction of blood loss.pdf. BMC Research Notes. 2013;6(184):1–14. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. The Journal of arthroplasty. 2014;29(2):387–9. doi: 10.1016/j.arth.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 58.Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. Bmj. 2014;349:g4829. doi: 10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PloS one. 2013;8(2):e55436. doi: 10.1371/journal.pone.0055436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oremus K, Sostaric S, Trkulja V, et al. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54(1):31–41. doi: 10.1111/trf.12224. [DOI] [PubMed] [Google Scholar]
- 61.Dahuja A, Dahuja G, Jaswal V, et al. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. The Journal of arthroplasty. 2014;29(4):733–5. doi: 10.1016/j.arth.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Christabel A, Muthusekhar MR, Narayanan V, et al. Effectiveness of tranexamic acid on intraoperative blood loss in isolated Le Fort I osteotomies - A prospective, triple blinded randomized clinical trial. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2014 doi: 10.1016/j.jcms.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Robb PJ. Tranexamic acid - a useful drug in ENT surgery? The Journal of laryngology and otology. 2014;128(7):574–9. doi: 10.1017/S0022215114001285. [DOI] [PubMed] [Google Scholar]
- 64.Dakir A, Ramalingam B, Ebenezer V, et al. Efficacy of Tranexamic Acid in Reducing Blood Loss during Maxillofacial Trauma Surgery-A Pilot Study. Journal of clinical and diagnostic research : JCDR. 2014;8(5):ZC06–8. doi: 10.7860/JCDR/2014/8680.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heesen M, Bohmer J, Klohr S, et al. Prophylactic tranexamic acid in parturients at low risk for post-partum haemorrhage: systematic review and meta-analysis. Acta anaesthesiologica Scandinavica. 2014;58(9):1075–85. doi: 10.1111/aas.12341. [DOI] [PubMed] [Google Scholar]
- 66.Perel P Kk. Cochrane Review Tranexamic acid for reducing mortality in emergency and.pdf. Cochrane Collaboration. 2013;2013(1) doi: 10.1002/14651858.CD010245.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


