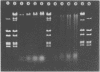
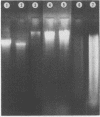
Abstract
A procedure is reported for the isolation of high molecular weight maize DNA from whole plant tissue. Nuclei are isolated in the presence of ethidium bromide from leaf, node, and tassel or endosperm tissues and the DNA is extracted and purified. The resulting DNA has a double-strand molecular weight of about 125 kilobase pairs and a single-strand molecular weight of about 125 kilobases. The DNA is cleavable by a number of common restriction endonucleases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blamire J., Cryer D. R., Finkelstein D. B., Marmur J. Sedimentation properties of yeast nuclear and mitochondrial DNA. J Mol Biol. 1972 Jun 14;67(1):11–24. doi: 10.1016/0022-2836(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman M. Purification of DNA by affinity chromatography removal of polysaccharide contaminants. Anal Biochem. 1975 May 12;65(1-2):293–297. doi: 10.1016/0003-2697(75)90512-6. [DOI] [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. E. The isolation of high molecular weight DNA from whole organisms or large tissue masses. Anal Biochem. 1978 Apr;85(2):609–613. doi: 10.1016/0003-2697(78)90262-2. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- HOTTA Y., BASSEL A. MOLECULAR SIZE AND CIRCULARITY OF DNA IN CELLS OF MAMMALS AND HIGHER PLANTS. Proc Natl Acad Sci U S A. 1965 Feb;53:356–362. doi: 10.1073/pnas.53.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty D. M., Scheif R. F. Location in bacteriophage lamdba DNA of cleavage sites of the site-specific endonuclease from Bacillus amyloliquefaciens H. J Virol. 1976 May;18(2):659–663. doi: 10.1128/jvi.18.2.659-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Künsch U., Temperli A. Simple rapid procedures for isolation of tobacco leaf nuclei. Anal Biochem. 1972 Sep;49(1):48–57. doi: 10.1016/0003-2697(72)90241-2. [DOI] [PubMed] [Google Scholar]
- Hamlett N. V., Lange-Gufstafson B., Rhoades M. Physical map of the bacteriophage T5 genome based on the cleavage products of the restriction endonucleases SalI, SmaI, BamI, and HpaI. J Virol. 1977 Oct;24(1):249–260. doi: 10.1128/jvi.24.1.249-260.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzmanowski A., Staroń K., Tyniec B., Toczko K. Effect of ethidium bromide on the digestion of chromatin DNA with micrococcal nuclease. Biochim Biophys Acta. 1978 Dec 21;521(2):493–501. doi: 10.1016/0005-2787(78)90291-5. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Davidson N. Electron microscope heteroduplex study of sequence relations of T2, T4, and T6 bacteriophage DNAs. Virology. 1974 Jan;57(1):93–111. doi: 10.1016/0042-6822(74)90111-1. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. P., Berman-Kurtz M., Kulikowski R. R. Isolation of plant nuclei. Methods Enzymol. 1974;31:558–565. doi: 10.1016/0076-6879(74)31060-9. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Picciano D. J., Schaad J. W., 4th Dominant lethality in frog embryos after paternal treatment with triethylenemelamine: cytogenetics, morphology, and swimming capability. Environ Mutagen. 1979;1(3):221–231. doi: 10.1002/em.2860010304. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Gamborg O. L., Miller R. A. Isolation and Properties of Deoxyribonucleic Acid from Protoplasts of Cell Suspension Cultures of Ammi visnaga and Carrot (Daucus carota). Plant Physiol. 1972 Sep;50(3):319–321. doi: 10.1104/pp.50.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P., Kramer R. A., Davis R. W. Cloning of the yeast ribosomal DNA repeat unit in SstI and HindIII lambda vectors using genetic and physical size selections. J Mol Biol. 1978 Aug 15;123(3):371–386. doi: 10.1016/0022-2836(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Pitout M. J., Potgieter D. J. The isolation of highly polymerized deoxyribonucleic acid from maize. Biochim Biophys Acta. 1968 Jun 18;161(1):188–196. doi: 10.1016/0005-2787(68)90308-0. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tautvydas K. J. Mass isolation of pea nuclei. Plant Physiol. 1971 Apr;47(4):499–503. doi: 10.1104/pp.47.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]