Abstract
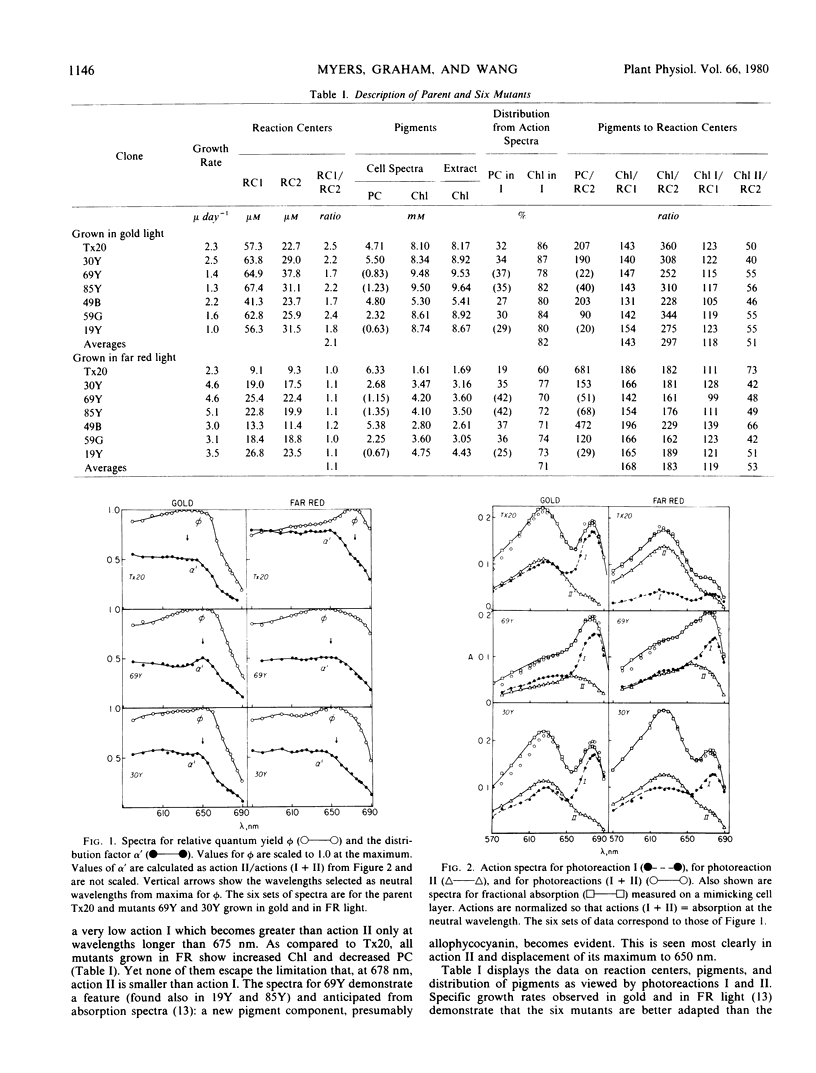
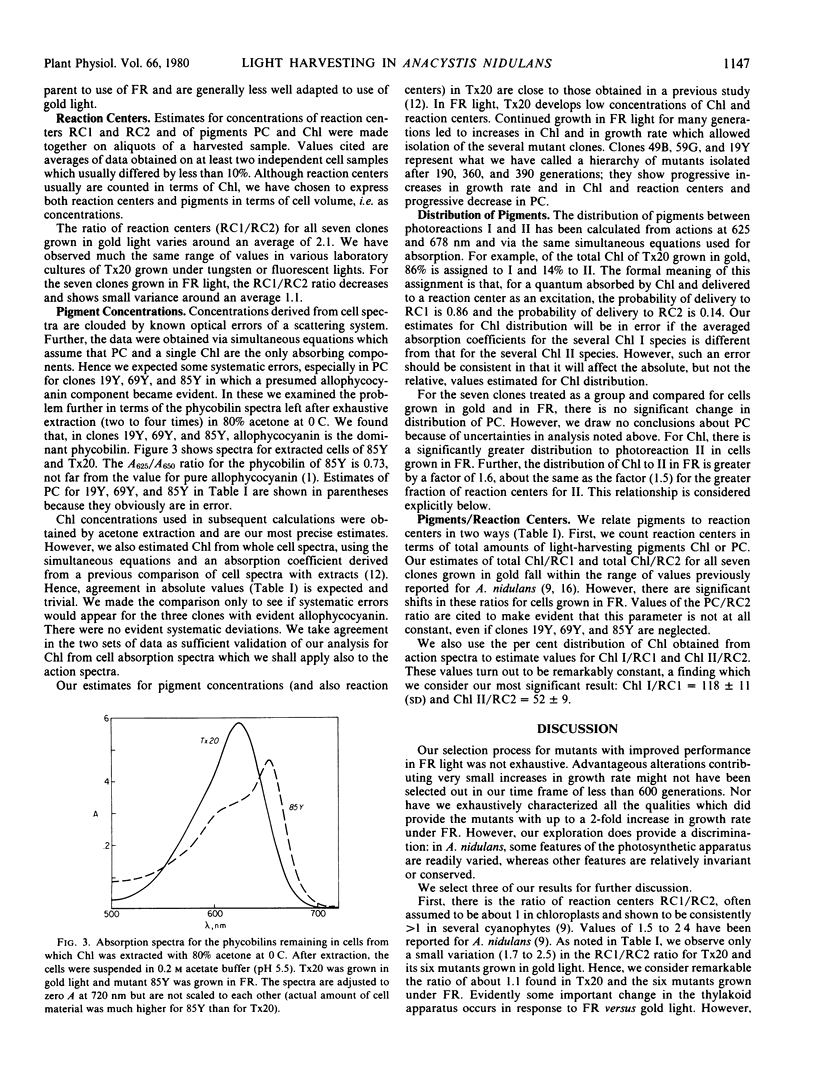
Spontaneous pigment mutants of Anacystis nidulans were self-selected for improved growth in far red light (> 650 nanometers). Questions were asked about those features of the light-harvesting mechanism which altered to give the mutants improved photosynthetic performance in far red. Answers were sought by comparing pigment and reaction center concentrations for the parent and six mutants grown in gold fluorescent and in far red light. Three significant results emerged. The ratio of reaction centers for photoreactions I and II (RC1/RC2) varied by a value of about 2.1 for all clones grown in gold and a value of about 1.1 for all clones grown in far red. Alteration of the ratio was not evident in any of the mutants.
Phycobilisome alterations were evident as decreased phycocyanin content in all mutants. In three mutants, allophycocyanin became the major remaining phycobilisome component. Action spectra for photoreactions I and II allowed estimates of chlorophylls serving each of the two reaction centers. Ratios of chlorophylls to reaction centers within each photosystem were chlorophyll I/RC1 = 118 ± 11 and chlorophyll II/RC2 = 52 ± 9 for all seven clones grown in both gold and far red light. Remarkable constancy of these ratios, in spite of wide variation in cell material, supports an hypothesis that in A. nidulans there are two chlorophyll proteins, each bearing a reaction center and chlorophylls in fixed ratio.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glazer A. N., Fang S., Brown D. M. Spectroscopic properties of C-phycocyanin and of its alpha and beta subunits. J Biol Chem. 1973 Aug 25;248(16):5679–5685. [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- HAXO F. T., BLINKS L. R. Photosynthetic action spectra of marine algae. J Gen Physiol. 1950 Mar;33(4):389–422. doi: 10.1085/jgp.33.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Joliot P., Joliot A., Kok B. Analysis of the interactions between the two photosystems in isolated chloroplasts. Biochim Biophys Acta. 1968 Apr 2;153(3):635–652. doi: 10.1016/0005-2728(68)90191-6. [DOI] [PubMed] [Google Scholar]
- Mimuro M., Fujita Y. Estimation of chlorophyll a distribution in the photosynthetic pigment systems I and II of the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1977 Mar 11;459(3):376–389. doi: 10.1016/0005-2728(77)90039-1. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Spontaneous pigment mutants of Anacystis nidulans selected by growth under far-red light. Arch Microbiol. 1980 Feb;124(2-3):143–148. doi: 10.1007/BF00427719. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Sherman L. A. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978 Aug 8;503(2):343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Vernon L. P. A fraction from Anabaena variabilis enriched in the reaction center chlorophyll P700. Biochim Biophys Acta. 1969 Jun 24;180(2):334–346. doi: 10.1016/0005-2728(69)90118-2. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Rusckowski M., Zilinskas B. A. Chlorophyll-Protein Complexes of the Cyanophyte, Nostoc sp. Plant Physiol. 1980 Feb;65(2):392–396. doi: 10.1104/pp.65.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Alberte R. S., Hunter F. A., Shiozawa J. A., Kan K. S. The organization of chlorophyll in the plant photosynthetic unit. Brookhaven Symp Biol. 1976 Jun 7;(28):132–148. [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]



