Abstract
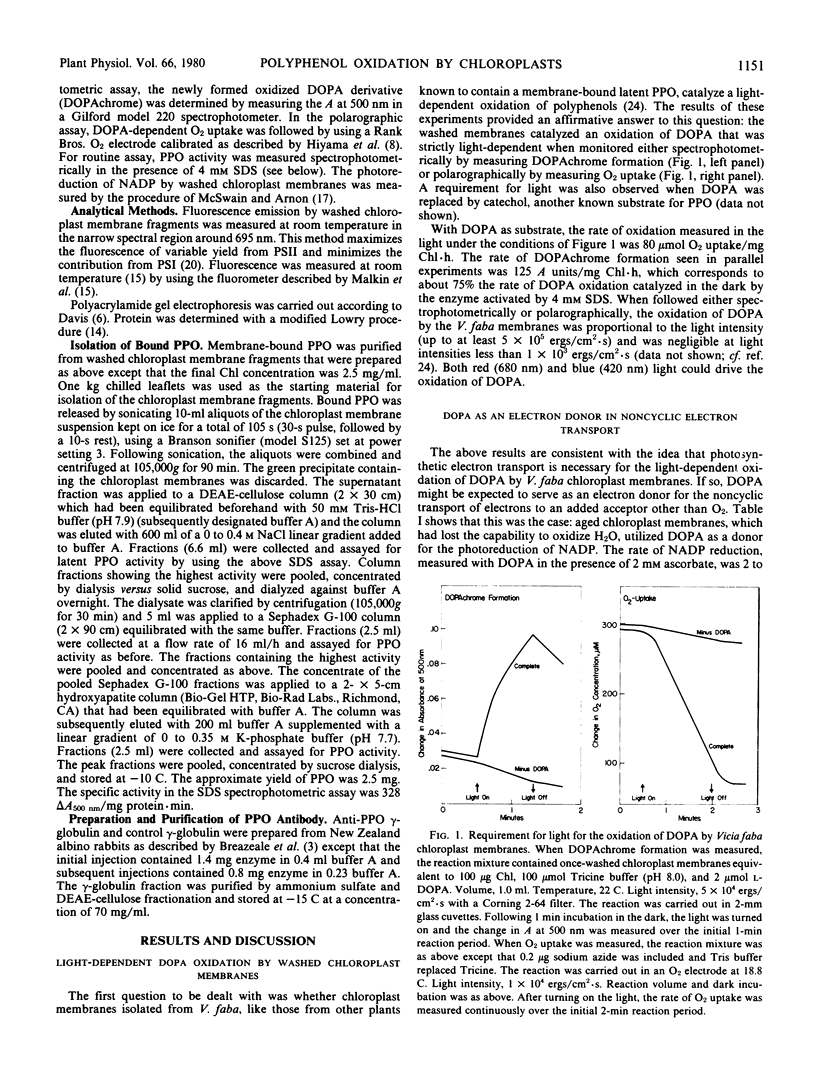
The mechanism whereby light effects polyphenol oxidation was examined with Vicia faba chloroplast membranes known to contain a bound latent polyphenol oxidase. Results obtained with the inhibitors 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-idopropyl-p-benzoquinone (DBMIB) indicated an involvement of the non-cyclic electron transport pathway in the light-dependent oxidation of polyphenols, such as dihydroxyphenylalanine (DOPA). Further evidence was provided by experiments in which (a) DOPA replaced H2O as electron donor for the photoreduction of NADP, (b) NADP replaced O2 as electron acceptor in the photochemical oxidation of DOPA, and (c) the variable fluorescence associated with photosystem II was increased by DOPA. The photochemical oxidation of DOPA by V. faba chloroplast membranes was insensitive to KCN and to antibodies against purified latent polyphenol oxidase. The results are consistent with the conclusion that the light-dependent oxidation of polyphenols by V. faba chloroplast membranes is achieved independently of the latent membrane-bound polyphenol oxidase. Electrons derived from polyphenols seem to enter the noncyclic electron transport chain on the oxidizing side of photosystem II and to react with O2 at an unidentified site on the photosystem I side of the DCMU/DBMIB blocks.
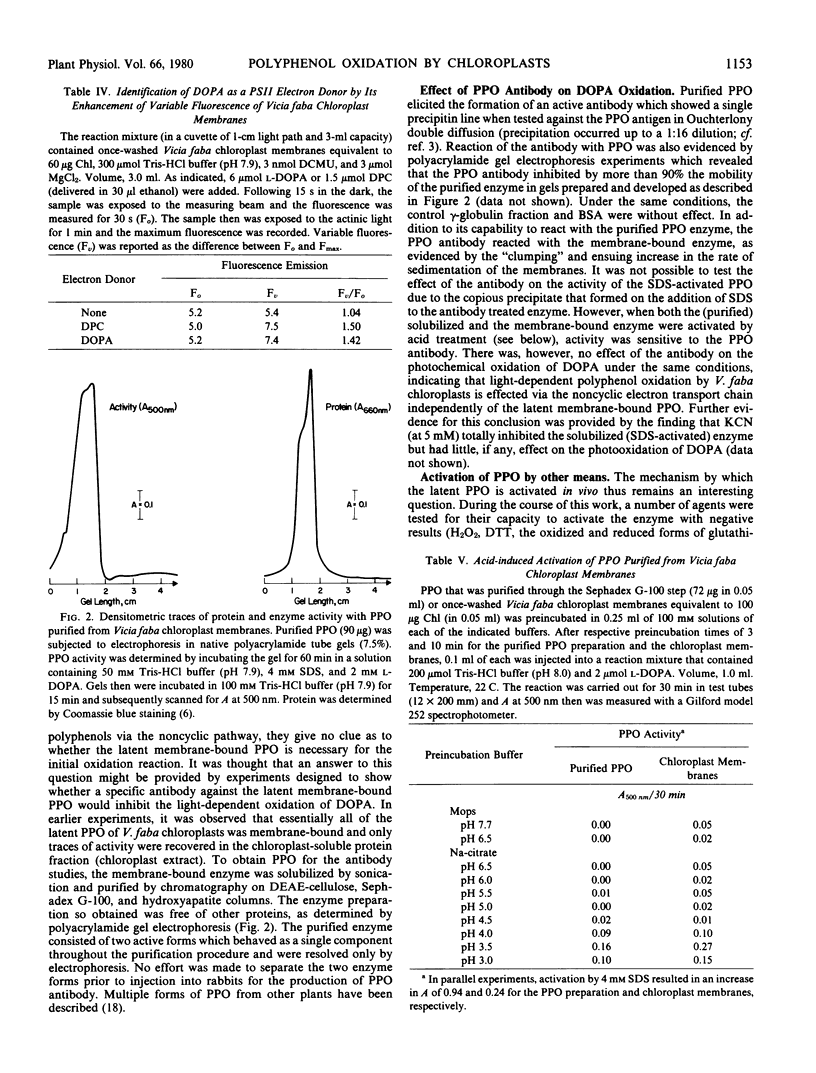
The physiological mechanism for the activation of latent polyphenol oxidase remains an unanswered question. Present results suggest that activation could occur through either acidification or the release of free fatty acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hiyama T., McSwain B. D., Ufert W. Simultaneous measurement of oxygen concentration and absorbance changes. Anal Biochem. 1976 Nov;76(50):365–368. doi: 10.1016/0003-2697(76)90296-7. [DOI] [PubMed] [Google Scholar]
- Izawa S., Ort D. R. Photooxidation of ferrocyanide and iodide ions and associated phosphorylation in NH2OH-treated chloroplasts. Biochim Biophys Acta. 1974 Jul 25;357(1):127–143. doi: 10.1016/0005-2728(74)90118-2. [DOI] [PubMed] [Google Scholar]
- KENTEN R. H. Latent phenolase in extracts of broad-bean (Vicia faba L.) leaves. I. Activation by acid and alkali. Biochem J. 1957 Oct;67(2):300–307. doi: 10.1042/bj0670300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H. Latent phenolase in extracts of broad-bean (Vicia fabaL.) leaves. 2. Activation by anionic wetting agents. Biochem J. 1958 Feb;68(2):244–251. doi: 10.1042/bj0680244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Malkin R., Barber J. On the function of the fluorescence quenchers in chloroplasts and their relation to the primary electron acceptor of photosystem II. Arch Biochem Biophys. 1979 Mar;193(1):169–178. doi: 10.1016/0003-9861(79)90020-1. [DOI] [PubMed] [Google Scholar]
- Malkin R., Knaff D. B., McSwain B. D. The effect of oxidation-reduction potential on the fluorescence yield of spinach chloroplasts at 77 degrees K. FEBS Lett. 1974 Oct 1;47(1):140–142. doi: 10.1016/0014-5793(74)80444-8. [DOI] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- SHIN M., TAGAWA K., ARNON D. I. CRYSTALLIZATION OF FERREDOXIN-TPN REDUCTASE AND ITS ROLE IN THE PHOTOSYNTHETIC APPARATUS OF CHLOROPLASTS. Biochem Z. 1963;338:84–96. [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


