Abstract
Background
Hyponatremia is the most common electrolyte disorder and it is associated with increased morbidity and mortality. However, there is no clear demonstration that the improvement of serum sodium concentration ([Na+]) counteracts the increased risk of mortality associated with hyponatremia. Thus, we performed a meta-analysis that included the published studies that addressed the effect of hyponatremia improvement on mortality.
Methods and Findings
A Medline, Embase and Cochrane search was performed to retrieve all English-language studies of human subjects published up to June 30th 2014, using the following words: “hyponatremia”, “hyponatraemia”, “mortality”, “morbidity” and “sodium”. Fifteen studies satisfied inclusion criteria encompassing a total of 13,816 patients. The identification of relevant abstracts, the selection of studies and the subsequent data extraction were performed independently by two of the authors, and conflicts resolved by a third investigator. Across all fifteen studies, any improvement of hyponatremia was associated with a reduced risk of overall mortality (OR=0.57[0.40-0.81]). The association was even stronger when only those studies (n=8) reporting a threshold for serum [Na+] improvement to >130 mmol/L were considered (OR=0.51[0.31-0.86]). The reduced mortality rate persisted at follow-up (OR=0.55[0.36-0.84] at 12 months). Meta-regression analyses showed that the reduced mortality associated with hyponatremia improvement was more evident in older subjects and in those with lower serum [Na+] at enrollment.
Conclusions
This meta-analysis documents for the first time that improvement in serum [Na+] in hyponatremic patients is associated with a reduction of overall mortality.
Introduction
Hyponatremia is the most common electrolyte disorder encountered in clinical practice, and in its mild presentation (i.e. serum [Na+] 130–134 mmol/L) occurs in up to 30% of hospitalized patients [1, 2]. It is well known that acute severe hyponatremia may have dramatic consequences caused by cerebral edema and may lead to death [3]. There is now convincing evidence that also mild chronic hyponatremia, traditionally not considered as a potentially harmful condition, may actually be associated with adverse effects, such as gait alterations and falls, attention deficits [4], bone loss and fractures [5–8]. Interestingly, it has been demonstrated that chronic hyponatremia exacerbates multiple signs of senescence in aged rats, such as sarcopenia, osteoporosis, cardiac fibrosis, and hypogonadism [9]. These manifestations are somewhat unexpected, considering that in chronic hyponatremia adaptation mechanisms should establish a new osmotic equilibrium across the plasma membrane; consequently, water moves out of the cells, thus reducing brain edema. However, experimental data showed that low extracellular [Na+] directly affects cell homeostasis in neuronal cells as well as in osteoclast precursors, independently of reduced osmolality [8, 10]. Therefore, these data demonstrated that the detrimental effects of hyponatremia extend beyond the “osmotic theory”, in agreement with clinical observations.
A number of studies have reported an association between hyponatremia, even when mild to moderate, and mortality across many diverse conditions (e.g., pneumonia, heart failure, acute myocardial infarction, cirrhosis and cancer [11–16]). We have recently reported an extensive meta-analysis to assess the relationship between hyponatremia and mortality. Data from 81 studies for a total of 147,948 hyponatremic subjects indicated that hyponatremia is associated with an increased risk of overall mortality (RR = 2.60[2.31–2.93]) [17]. Furthermore, hyponatremia appeared associated with an increased risk of death when patients were analyzed separately based on specific diseases, such as myocardial infarction, heart failure, cirrhosis and pulmonary infections. Interestingly, a quite small difference in serum [Na+] (mean 4.8 mmol/L) was detected in patients who died compared to survivors.
Whether the improvement of hyponatremia counteracts the increased risk of mortality associated with hyponatremia has not been clearly ascertained, so far. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) study, neither all-cause nor cardiovascular mortality significantly differed between patients with heart failure treated with the vasopressin type 2 receptor antagonist, tolvaptan, and those receiving placebo [18]. However, a subset analysis of those patients in EVEREST who were markedly hyponatremic (defined as a serum [Na+] <130 mEq/L) did show a significant reduction of cardiovascular morbidity and mortality after discharge in patients treated with tolvaptan [19]. Nonetheless, EVEREST was not powered to examine outcomes in the smaller subgroup of patients enrolled who had both heart failure and hyponatremia. A significant positive relationship between an increase in serum [Na+] and decreased mortality was found in 322 patients hospitalized for acute heart failure and followed for up to 3 years [20]. Conversely, a multicenter analysis of almost 3000 patients hospitalized for acute heart failure in Korea showed that patients admitted with hyponatremia had a worse prognosis compared to those with normonatremia, but this relation persisted regardless of whether the hyponatremia improved during the hospitalization [21]. However, this study presented some limitations, because it was a retrospective analysis from a registry and not a prospective randomized trial. In addition, the change in serum [Na+] was assessed only once, prior to or at discharge from the hospital. The aim of this study was to perform a meta-analysis based on published studies of hyponatremic patients that included data on the effect of correction of serum [Na+] on mortality.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (S1 File) (http://www.prisma-statement.org/).
Eligibility criteria
Studies specifically addressing the association between mortality rate and improved or normalized serum [Na+] were included in the analysis
Information source and Search strategy
An extensive Medline, Embase, and Cochrane search was performed including the following words: ("hyponatraemia"[All Fields] OR "hyponatremia"[MeSH Terms] OR "hyponatremia"[All Fields]) AND ("mortality"[Subheading] OR "mortality"[All Fields] OR "mortality"[MeSH Terms]) AND ("epidemiology"[Subheading] OR "epidemiology"[All Fields] OR "morbidity"[All Fields] OR "morbidity"[MeSH Terms]) AND ("sodium, dietary"[MeSH Terms] OR ("sodium"[All Fields] AND "dietary"[All Fields]) OR "dietary sodium"[All Fields] OR "sodium"[All Fields] OR "sodium"[MeSH Terms]). The search up to June 30th 2014 was restricted to English-language articles and studies of human participants. A hand-searched bibliography of retrieved papers for additional references was performed. Details of the literature search process are outlined in the flow chart. The identification of relevant abstracts, the selection of studies based on the criteria described above, and the subsequent data extraction were performed independently by two of the authors (G.C., C.G.), and conflicts resolved by a third investigator (A.P.).
Study selection
The meta-analysis was performed including all studies comparing mortality rate in subjects with or without improvement of hyponatremia (see Fig 1 and Table 1). Studies not specifically addressing the association between mortality rate and improved or normalized serum [Na+] were excluded from the analysis (see Table 2).
Fig 1. Trial flow diagram.
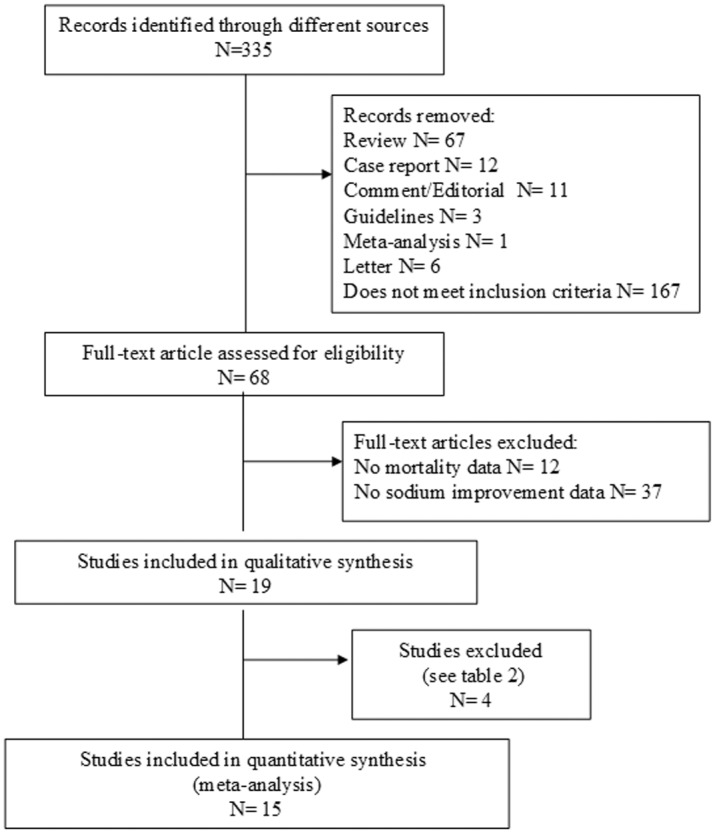
Table 1. Observational studies included in the meta-analysis.
| Source | Type of disease | Age (years) | Male % | Na+ cut-off (mEq/L) | Patients (n) | Persistent HN (n) | Improved HN (n) | Deaths Persistent HN (n) | Deaths Improved HN (n) |
|---|---|---|---|---|---|---|---|---|---|
| Licata et al., 2003 [25] | HF | 76.4 | 63.5 | 135 | 107 | 54 | 53 | 43 | 20 |
| Klein et al, 2005 [13] | HF | 68 | 68 | 135 | 244 | 151 | 93 | 11 | 12 |
| Hoorn et al, 2006 [2] | Hospitalized | 60.9+16.9 | 48.7 | 125 | 74 | 19 | 55 | 7 | 7 |
| Gheorghiade et al, 2007 [26] | HF | 53,6 | NA | 134 | 103 | 71 | 32 | 22 | 9 |
| Rossi et al, 2007 [27] | HF | 65 | 67 | 135 | 68 | 23 | 45 | 5 | 5 |
| Hackworth et al, 2009 [28] | Liver transplantation | 51.7+7.8 | 80.7 | 130 | 90 | 34 | 56* | 2 | 8 |
| Rusinaru et al, 2009 [29] | HF | 77.3+9.2 | 46 | 136 | 91 | 46 | 45* | 42 | 33 |
| Waikar et al, 2009 [14] | Hospitalized | 67 | NA | NA | 8318 | 4524 | 3794 | 1846 | 1461 |
| Hansen et al, 2010 [30] | SCLC | NA | NA | NA | 61 | 46 | 15* | NA | NA |
| Madan et al, 2011 [20] | HF | 65.9+15.8 | 55.2 | 135 | 279 | 57 | 222 | 53 | 208 |
| Lee et al, 2012 [21] | HF | 70.5 | NA | 135 | 464 | 190 | 274* | 185 | 263 |
| Vaishya et al, 2012 [31] | Emergency in patients | NA | 58.2 | 120 | 175 | 106 | 69* | 64 | 27 |
| Ng et al, 2013 [32] | APE | 73.5+12.7 | 48 | 135 | 114 | 56 | 58* | 55 | 56 |
| Qureshi et al, 2013 [33] | MI | 67.5+16.4 | 55.7 | 134 | 1798 | 280 | 1518* | 155 | 425 |
| Darmon et al, 2014 [34] | Intensive care unit patients | 63.9 | 60.6 | 135 | 1830 | 811 | 1019 | 163 | 179 |
* = threshold for serum [Na+] improvement >130 mmol/L.
HN: hyponatremia; HF: heart failure; SCLC: small cell lung cancer; APE: acute pulmonary embolism; MI; myocardial infarction.
Table 2. Studies that met inclusion criteria but did not provide data for meta-analysis.
| First author, year | Brief description of the study and main conclusions |
|---|---|
| Nzerue et al, 2003 [35] | Retrospective study of 168 hospitalized patients treated for severe hyponatremia. Mortality was higher in patients with slow correction rate but there were no data about mortality rate in patients with corrected or improved hyponatremia vs patients with persistent hyponatremia. |
| Doshi et al, 2012 [36] | Retrospective analysis of 4702 hospitalized patients with cancer, of which 47% were hyponatremic. Increase in serum sodium was associated with lower 90-day mortality, but there were no data about mortality rate in patients with corrected or improved hyponatremia vs patients with persistent hyponatremia. |
| Hauptman et al, 2013 [19] | Analysis of data from the EVEREST trial [18] to assess the clinical course and the outcomes of hospitalized patients with heart failure and hyponatremia and treated with tolvaptan. In patients with severe hyponatremia, tolvaptan therapy was associated with reduced cardiovascular mortality but it was not specified whether serum [Na+] was normalized or improved. |
| Lee et al, 2013 [37] | Retrospective review of the electronic medical records of 512 patients who received a liver transplant, of which 48% were hyponatremic. Delta sodium concentrations were associated with a higher in-hospital mortality, but were no data about mortality rate in patients with corrected, improved or overcorrected hyponatremia vs patients with persistent hyponatremia |
Outcome and quality assessment
The principal outcome of this analysis was the effect of serum [Na+] improvement (whatever obtained), on mortality rate. A secondary outcome included the effect of serum [Na+] >130 mmol/L at follow-up on mortality rate. The quality of the studies was assessed using the Cochrane criteria [22].
Statistical analysis
Heterogeneity on mortality rate was assessed by using I2 statistics. Even when a low heterogeneity was detected, a random-effects model was applied, because the validity of tests of heterogeneity can be limited with a small number of component studies.
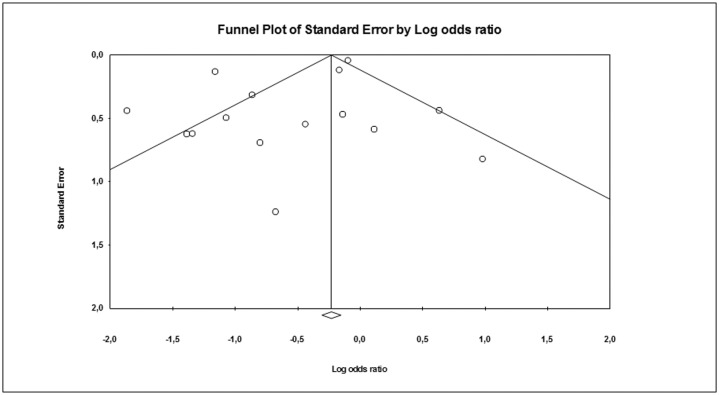
In order to estimate possible publication or disclosure bias, we used funnel plots, the Begg adjusted rank correlation test and Egger's [23, 24] (Fig 2). However, because these tests have low statistical power when the number of trials is small, undetected bias may still be present. Odds ratio with 95% Confidence Interval (OR) was calculated for mortality rate after sodium improvement. A sensitivity analysis was performed considering those studies reporting data on mortality rate after serum [Na+] improvement at 12 and 36 months of follow-up or after serum [Na+] ≥130 mmol/L at follow-up. A meta-regression analysis was performed to test the effect of age and serum [Na+] cut-off enrolment on overall mortality rate. In addition, a linear regression analysis model, weighting each study for the number of subjects enrolled, was performed to verify the independent effect of hyponatremia improvement on mortality after the adjustment for age and sex and serum [Na+] cut-off.
Fig 2. Funnel plot of the observational studies included in the meta-analysis.
All data were calculated using Comprehensive Meta-analysis Version 2, Biostat, and (Englewood, NJ, USA). Logistic multivariate analysis was performed on SPSS (Statistical Package for the Social Sciences; Chicago, USA) for Windows 20.1.
Results
Out of 335 retrieved articles, 320 articles were excluded for different reasons. The flow of the meta-analysis is summarized in Fig 1, and the characteristics of the trials included in the meta-analysis are summarized in Table 1 [2, 13, 14, 20, 21, 25–34]. Sixty-eight full-text articles were considered potentially eligible for the meta-analysis. However, 49 of them were excluded because they did not included information about serum [Na+] improvement (n = 37) or about mortality (n = 12) (see S2 file). Four additional studies were excluded because they did not specifically address the association between mortality rate and serum [Na+] improvement or normalization (Table 2) [19, 35–37]. Among the 15 selected studies, 7 studies evaluated the effect of hyponatremia improvement on overall mortality rate in subjects with heart failure, 2 in hospitalized series of subjects, one each including patients subjected to orthopic liver transplantation, admitted to an emergency room or to an intensive care unit, affected by lung cancer, myocardial infarction or pulmonary acute embolism (Table 1). All the studies included in the analysis were observational ones.
Overall, 13,816 hyponatremic subjects with a mean age of 66.1 years and a mean follow-up of 33.6 months were included in the meta-analysis. Hyponatremia was defined according to varying cut-off definitions in the included studies (Table 1). In particular, 14 studies enrolled only hyponatremic subjects, whereas in one case [25] a mixed cohort of hyponatremic/eunatremic patients was considered. Among the studies that were evaluated, an improvement of serum [Na+] of any degree was obtained in 53.2% of patients through different approaches. However, about 1 out of 4 patients (27%) did not reach a threshold of 130 mmol/L, after improvement (Table 1). I2 was 85.5 (p<0.0001) and the Begg-adjusted rank correlation test, calculated on the basis of overall mortality rate for hyponatremia improvement, suggested no major publication bias (Kendall tau 0.00; p = 1.0). Similar results were obtained by applying Egger’s regression (Intercept -1.24±0.79; p = 0.14).
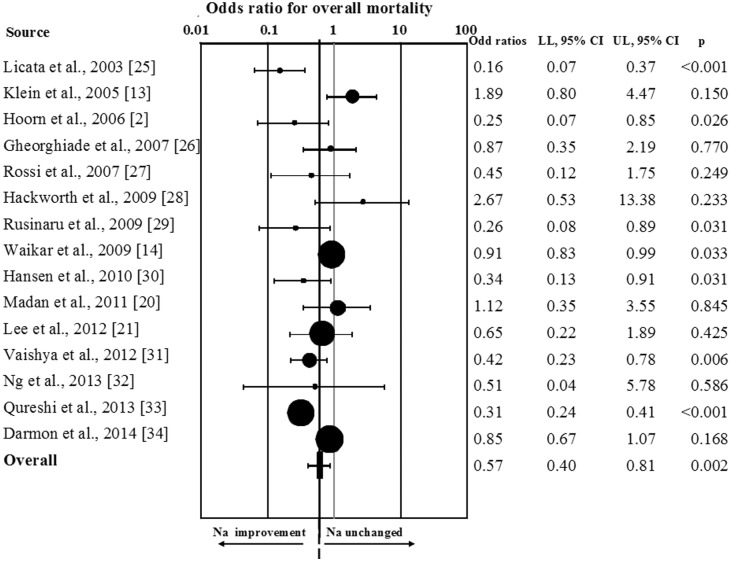
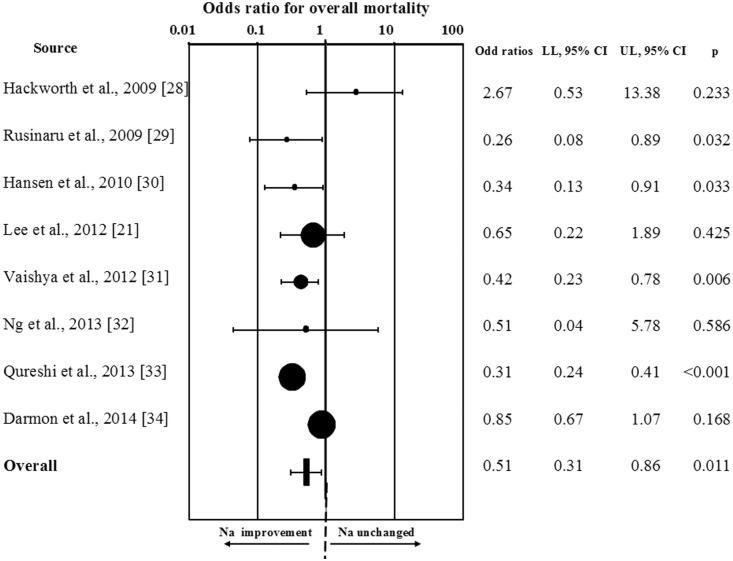
When all studies were considered, hyponatremia improvement was significantly associated with a reduction of overall mortality (OR = 0.57[0.40;0.81]; p = 0.002; Fig 3). Similar results were observed when the study enrolling a mixed population of hyponatremic/eunatremic subjects was excluded from the analysis (OR = 0.63[0.44;0.89]; p = 0.001). Interestingly, the results were even more impressive by performing a sensitivity analysis, which considered only those studies (n = 8) reporting a threshold for serum [Na+] improvement >130 mmol/L (RR = 0.51[0.31;0.86]; p<0.001; (Fig 4). The favorable effect of hyponatremia improvement on mortality rate was confirmed in those studies (n = 4) reporting data at 12 months of follow-up (OR = 0.55[0.36;0.84];p = 0.006), and a trend toward a reduction of mortality rate was observed at 36 months (n = 3, OR = 0.67[0.45;1.02];p = 0.06).
Fig 3. Odds ratio for overall mortality rate in patients with any increase of serum [Na+].
Fig 4. Odds ratio for overall mortality rate in patients from studies in which a threshold for serum [Na+] improvement >130 mmol/L was reported.
Interestingly, a meta-regression analysis showed that the reduced mortality rate in patients in whom hyponatremia improved was more evident in older subjects (S = −0.06[−0.010;−0.02];p = 0.004;I = 3.62[1.01;6.24];p = 0.01) and in those with lower serum [Na+] at enrollment (S = 0.07[0.03;0.001];p = 0.001; I = −9.10[−14.37;−3.84];p = 0.001). The association between reduced mortality rate after serum [Na+] improvement and [Na+] cut-off at enrollment was confirmed in a multiple regression model even after adjustment for age, gender and follow-up (adj.r = 0.39; p<0.0001).
Discussion
There is evidence from the literature that hyponatremia is associated with an increased risk of mortality in patients with diverse clinical conditions, and in a recent meta-analysis we definitively confirmed this finding [17]. On the other hand, it has not been clearly established, so far, whether an improvement of hyponatremia is able to revert or decrease the increased risk of death associated with hyponatremia.
Therefore, we performed a meta-analysis, in which we included all of the English-language published studies until June 30th 2014 that compared the mortality rate in human subjects with or without interval improvement of hyponatremia. Fifteen published studies were selected according to the specified inclusion criteria yielding a total of 13,816 hyponatremic patients with a mean follow-up of 33.6 months. We found that an increase of serum [Na+] was obtained in about half of the patients. This finding in principle suggests that the management of patients with hyponatremia is far from being optimal, in agreement with data from the literature [38, 39]. In those patients in whom serum [Na+] was improved, the overall mortality rate was reduced up to 60% compared to patients with no improvement of their hyponatremia. These data were obtained by considering any increase of serum [Na+]. However, the association between improvement of hyponatremia and reduced mortality was even stronger when we performed a sensitivity analysis based on those studies in which a threshold for improvement of at least 130 mmol/L was reported (up to 70% of mortality rate reduction); this result increases the strength of the relationship between significant increases in serum [Na+] and reduction of mortality rate.
We also observed that the reduced risk of mortality associated with hyponatremia improvement appears to last during prolonged follow-up. Specifically, a significantly reduced mortality persisted at 12 months of follow-up, and a similar trend was observed al 36 months. In the latter case, the absence of a statistically significant difference may very likely be due to the smaller number of studies in which a more prolonged follow-up was performed [14, 29, 32]. It might be also hypothesized that other factors might mitigate the effect of serum [Na+] improvement on mortality rate in the long term.
Another interesting finding from a meta-regression analysis was that the effect of the improvement of hyponatremia on the reduced risk of mortality increases as a function of the prevalence of patients with a more advanced age. This observation is of particular importance because hyponatremia occurs more commonly in elderly subjects [1]. Another meta-regression analysis also indicated that the beneficial effect of the improvement of hyponatremia is more evident in patients with lower serum [Na+] at enrollment. This finding implies that these patients deserve an even more thoughtful workup for the correction of hyponatremia, because the outcome may be dramatically more favorable compared to patients not properly treated.
Overall, this meta-analysis demonstrates that an improvement in serum [Na+] is associated with a decreased mortality rate observed in hyponatremic patients, although a cause-effect relationship cannot be extrapolated from the studies that have been analyzed. In addition, some limitations of our meta-analysis must be recognized. First of all, the present data cannot clarify whether hyponatremia and/or its lack of correction contribute directly to poor outcomes or are simply markers for severity of underlying co-morbidities, and whether improvements of the underlying co-morbidities influenced both the serum [Na+] and the mortality of the hyponatremic patients [40]. Accordingly, it should recognized that the data were adjusted only for age and gender, whereas the prevalence of associate morbidities was not considered as possible confounders, because they were not reported adequately in a sufficient number of studies. Hence, potential unmeasured confounders may have caused residual confounding effects, but the measured factors that are correlated with such confounders should have mitigated this bias. However, meta-analysis is particularly useful when there is a variety of reports with low statistical power; in this situation, pooling of data can improve power and provide a more convincing result. It should be also recognized that the duration of the available trials is relatively short. A further limitation is represented by incomplete reporting of the data on mortality rate in trials only marginally designed for the assessment of mortality endpoints after [Na+] improvement. In particular, all the data that were analyzed were from observational studies and none of them was originally designed to address clinical outcomes after serum [Na+] improvement. Accordingly, no placebo-controlled studies were available for inclusion in the present meta-analysis. Finally, the statistical analysis showed the presence of heterogeneity that was particularly evident in two studies [13, 28]. Finally, statistical analyses did not suggest any relevant publication bias, the possibility of selective reporting cannot be excluded. These considerations do not negate the value of the novel findings reported in our study, but rather highlight the need for additional, well-designed studies of clinical outcomes with effective therapies in patients with hyponatremia [41–43].
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006; 119:30–35. [DOI] [PubMed] [Google Scholar]
- 2. Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatremia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006; 28:70–76. [DOI] [PubMed] [Google Scholar]
- 3. Gill G, Huda B, Boyd A, Skagen K, Wile D, Watson I, et al. Characteristics and mortality of severe hyponatremia—a hospital-based study. Clin Endocrinol (Oxf). 2006; 65:246–249. [DOI] [PubMed] [Google Scholar]
- 4. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness and attention deficits. Am J Med. 2006; 119:71.e1–8. [DOI] [PubMed] [Google Scholar]
- 5. Gankam KF, Andres C, Sattar L, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008; 101:583–588. 10.1093/qjmed/hcn061 [DOI] [PubMed] [Google Scholar]
- 6. Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010; 5: 275–280. 10.2215/CJN.06120809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010; 25:554–563. 10.1359/jbmr.090827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2010; 286:10864–10875. 10.1074/jbc.M110.155002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barsony J, Manigrasso MB, Xu Q, Tam H, Verbalis JG. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age (Dordr). 2012; 35: 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benvenuti S, Deledda C, Luciani P, Modi G, Bossio A, Giuliani C, et al. Low extracellular sodium causes neuronal distress independently of reduced osmolality in an experimental model of chronic hyponatremia. Neuromolecular Med. 2013; 15:493–503. 10.1007/s12017-013-8235-0 [DOI] [PubMed] [Google Scholar]
- 11. Wald R, Jaber BL, Price LL. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010; 170:294–302. 10.1001/archinternmed.2009.513 [DOI] [PubMed] [Google Scholar]
- 12. Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, et al. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med. 2008; 8:16 10.1186/1471-2466-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Piña IL, Felker GM, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Study. Circulation. 2005; 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 14. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009; 122:857–865. 10.1016/j.amjmed.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008; 359:1018–1026. 10.1056/NEJMoa0801209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terzian C, Frye EB, Piotrowski ZH. Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med. 1994; 9:89–91. [DOI] [PubMed] [Google Scholar]
- 17. Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One. 2013; 8:e80451 10.1371/journal.pone.0080451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konstam MA, Gheorghiade M, Burnett JC Jr, Maggioni AP, Swedberg K, Udelson JE, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007; 297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 19. Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail. 2013; 19:390–397. 10.1016/j.cardfail.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 20. Madan VD, Novak E, Rich MW. Impact of change in serum sodium concentration on mortality in patients hospitalized with heart failure and hyponatremia. Circ Heart Fail. 2011; 4:637–643 10.1161/CIRCHEARTFAILURE.111.961011 [DOI] [PubMed] [Google Scholar]
- 21. Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, et al. Improvement of hyponatraemia during hospitalization for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry. Heart. 2012; 98:1798–1804. 10.1136/heartjnl-2012-302334 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 The Cochrane Collaboration. 2008. Available www.cochrane-handbook.org.
- 23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 24. Egger M, Davey SG, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003; 145:459–66. [DOI] [PubMed] [Google Scholar]
- 26. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Piña IL, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007; 167:1998–2005. [DOI] [PubMed] [Google Scholar]
- 27. Rossi J, Bayram M, Udelson JE, Lloyd-Jones D, Adams KF, Oconnor CM, et al. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care. 2007; 9:82–86. [DOI] [PubMed] [Google Scholar]
- 28. Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, Luketic VA, et al. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int. 2009; 29:1071–1077. 10.1111/j.1478-3231.2009.01982.x [DOI] [PubMed] [Google Scholar]
- 29. Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy A, et al. Relation of serum sodium level to long-term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol. 2009; 103:405–410. 10.1016/j.amjcard.2008.09.091 [DOI] [PubMed] [Google Scholar]
- 30. Hansen O, Sørensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer. 2010; 68:111–114. 10.1016/j.lungcan.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 31. Vaishya R, Kaur J, Seema, Chopra S, Jaswal S. Mortality predictors in severe hyponatraemia in emergency inpatients. J Indian Med Assoc. 2012; 110:94–97. [PubMed] [Google Scholar]
- 32. Ng AC, Chow V, Yong AS, Chung T, Kritharides L. Fluctuation of serum sodium and its impact on short and long-term mortality following acute pulmonary embolism. PLoS One. 2013; 8:e61966 10.1371/journal.pone.0061966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qureshi W, Hassan S, Khalid F, Almahmoud MF, Shah B, Tashman R, et al. Outcomes of correcting hyponatremia in patients with myocardial infarction. Clin Res Cardiol. 2013; 102:637–44. 10.1007/s00392-013-0576-z [DOI] [PubMed] [Google Scholar]
- 34. Darmon M, Pichon M, Schwebel C, Ruckly S, Adrie C, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014; 41:394–399. 10.1097/SHK.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 35. Nzerue CM, Baffoe-Bonnie H, You W, Falana B, Dai S. Predictors of outcome in hospitalized patients with severe hyponatremia. J Natl Med Assoc. 2003; 95:335–343. [PMC free article] [PubMed] [Google Scholar]
- 36. Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012; 59:222–228. 10.1053/j.ajkd.2011.08.029 [DOI] [PubMed] [Google Scholar]
- 37. Lee J, Kim DK, Lee JW, Oh KH, Oh YK, Han JS, et al. Rapid correction rate of hyponatremia as an independent risk factor for neurological complication following liver transplantation. Tohoku J Exp Med. 2013; 229:97–105. [DOI] [PubMed] [Google Scholar]
- 38. Huda MS, Boyd A, Skagen K, Wile D, Van Heyningen C, Watson I, et al. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J. 2006; 82:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giuliani C, Cangioli M, Beck-Peccoz P, Faustini-Fustini M, Fiaccadori E, Peri A. Awareness and management of hyponatraemia: the Italian Hyponatraemia Survey. J Endocrinol Invest. 2013; 36:693–698. 10.3275/8925 [DOI] [PubMed] [Google Scholar]
- 40. Konstam MA, Udelson JE. Hyponatraemia and vasopressin in heart failure: markers or mediators? Eur J Heart Fail. 2009; 13:242–244. [DOI] [PubMed] [Google Scholar]
- 41. Peri A, Pirozzi N, Parenti G, Festuccia F, Menè P. Hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J Endocrinol Invest. 2010; 33: 671–682. 10.3275/7290 [DOI] [PubMed] [Google Scholar]
- 42. Peri A. Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab. 2013; 98: 1321–1332. 10.1210/jc.2012-4082 [DOI] [PubMed] [Google Scholar]
- 43. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013; 12:S1–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.