Abstract
Immunoglobulin (Ig)G/IgM autoantibodies to phosphatidylserine/prothrombin (aPS/PT) were evaluated individually and in combination with criteria anti-phospholipid (aPL) tests in a prospectively ascertained cohort of patients at risk for anti-phospholipid syndrome (APS). One hundred and sixty (160) consecutive requests for lupus anti-coagulant (LAC) from the University of Utah Health Sciences Center were identified during 8 weeks. Of these, 104 unique patients had additional requests for cardiolipin (aCL) and/or beta2 glycoprotein I (aβ2GPI) IgG and/or IgM; samples were retained and analysed for aPS/PT, aCL and/or aβ2GPI IgG and IgM antibodies. Following testing, a comprehensive chart review was performed and patients categorized according to their clinical diagnosis. Individual and combined sensitivities, specificities, odd ratios (OR), diagnostic accuracy for specific tests or combinations by receiver operating characteristic (ROC), area under the curve (AUC) analyses and correlations between test results were determined. The sensitivities of aPS/PT IgG/IgM (54·6/45·5%) were lower than LAC (81·8%) but higher relative to aCL IgG/IgM (27·3/0%) or aβ2GPI IgG/IgM (27·3/0%). The best correlation between LAC and any aPL test was observed with aPS/PT (P = 0·002). There was no significant difference in the diagnostic accuracies for any panel with LAC: LAC/aβ2GPI IgG/aCL IgG [AUC 0·979, OR 475·4, 95% confidence interval (CI) 23·1–9056·5, P = 0·0001 and LAC/aβ2GPI IgG/aPS/PT IgG or LAC/aPS/PT IgG/aCL IgG (AUC 0·962, OR 265·3, 14·2–4958·2, P = 0·0001). The high correlation between LAC and aPS/PT IgG/IgM in this preliminary study suggest that this marker may be useful in the evaluation of APS. More studies to determine the optimal aPL antibody tests combination are needed.
Keywords: anti-phospholipid antibodies, anti-phospholipid syndrome, ELISA
Introduction
Anti-phospholipid syndrome (APS) is an autoimmune disease characterized by arterial and/or venous thrombosis, specific obstetric complications and persistent anti-phospholipid (aPL) antibodies, as defined by the revised Sapporo Sydney criteria 1,2. The laboratory test criteria for APS include lupus anti-coagulant (LAC), as well as immunoglobulin (Ig)G and IgM antibodies to cardiolipin (aCL) and beta2 glycoprotein I (aβ2GPI). Anti-phospholipid antibodies associated with APS are heterogeneous and target phospholipids, phospholipid-binding protein alone, or phospholipid-binding protein complexes. Of the aPL test criteria, the LAC assay has the strongest predictive value for both thrombosis and adverse pregnancy outcome in APS 3,4. However, the clotting-based nature of the LAC assay is fraught with pre-analytical limitations, the need for multiple reagents and diversity in platforms, as well as challenges with interpreting results 5–7. The aCL and aβ2GPI antibody assays are less specific for APS 8. The absence of reliable, robust diagnostic markers for APS thus limits patient identification and management. Not surprisingly, some investigators have introduced the concept of ‘seronegative’ APS, suggesting that pathogenic autoantibodies might target other proteins and/or protein-bound phospholipid complexes 2,3,9,10.
Antibodies against prothrombin (PT), a plasma protein that is essential in the coagulation cascade, have been reported in patients diagnosed with APS. A significant proportion of LAC activity is attributable to antibodies against PT (aPT) 11. In general, aPT antibodies have been reported to occur in 50–90% of APS patients, usually in association with other aPL autoantibody criteria 11–19. Some investigators have detected aPT in immunoassays by coating gamma (γ)-irradiated enzyme-linked immunosorbent assay (ELISA) plates with PT 12,14 or phosphatidylserine/prothrombin (PS/PT) complex 15. Thus, the prevalence of aPT antibodies in APS also appears to be dependent upon the type of plates used for detection, as well as the antigen formulation of the immunoassay 12,14,15. Several studies have shown correlation between aPT and aPS/PT antibodies; however, these antibody markers appear to possess different predictive values for disease 17–23. Compared to aPT IgG, IgG antibodies to PS/PT show better correlation with LAC results and predict thrombosis in APS 13,15,19,23. Despite these observations, the clinical relevance of aPS/PT in routine evaluation of APS and related diseases remains to be validated fully. Validation studies in APS are complicated by the absence of reliable reference materials, the inherent bias associated with correlating performance with poorly defined aPL tests and/or the study populations 15–22,24–28. Except for one study, which was limited by full access to clinical information 25, there are no published investigations that have examined prospectively the performance of aPS/PT antibodies in addition to all three aPL test criteria. Thus, the aim of this study was to investigate the diagnostic relevance of aPS/PT IgG and IgM in patients undergoing evaluation for thrombosis and/or pregnancy-related morbidity in a tertiary hospital setting. In addition, this investigation aimed to determine the best aPL antibody combination in the diagnostic work-up of patients with suspicion for APS or related disorders.
Materials and methods
Patient selection and sample collection
The Autoimmune Immunology and Coagulation Laboratories at ARUP Laboratories Inc., Salt Lake City receives specimens from the University of Utah Health Sciences Center facilities and Intermountain Healthcare facilities, as well as other hospitals and community clinics for laboratory evaluation of APS. For this study, 160 consecutive patient samples with requests for LAC testing from the University of Utah Health Sciences Center facilities were identified during a period of 8 weeks. Of these patient samples, 104 had additional orders for IgG and IgM aCL and/or aβ2GPI antibody evaluation. Plasma and/or serum samples obtained from these 104 patients were retained and evaluated for the presence of aPS/PT IgG and IgM antibodies. Test requisitions for these patients were not accompanied by clinical indications for disease evaluation. Following testing, a comprehensive chart review was performed and patients classified according to specific manifestations associated with APS, including venous or arterial thrombosis or pregnancy-related morbidity (PRM), defined as recurrent early miscarriage, fetal death or delivery prior to 34 weeks' gestation for pre-eclampsia or placental insufficiency 2. APS (primary APS, PAPS) or APS associated with systemic lupus erythematosus (SLE) (secondary APS, SAPS) was identified by positive laboratory test for either LAC, IgG and/or IgM aCL and/or aβ2GPI, with supporting clinical presentation that fulfilled the international consensus classification criteria 2. Diagnoses of SLE, non-SLE autoimmune diseases, thrombosis, inherited thrombophilias, PRM and other clinical conditions (malignancy, migraine, vasculitis, splenic infarction, myalgia, joint pain, septic shock, cervical spondylitis, osteogenesis imperfecta, biliary peritonitis) were obtained from the medical records. Patients with APS-associated clinical manifestations with aPL antibody test criteria inconsistent with current laboratory recommendations for diagnosis were included in the other clinical conditions category (Table 1). The non-SLE autoimmune diseases included multiple sclerosis, systemic sclerosis, rheumatoid arthritis, Sjögren's syndrome, Raynaud's disease, Hashimoto's thyroiditis, autoimmune hepatitis and bullous pemphigoid. The study was approved by the University of Utah institutional review board (IRB) under a study protocol for autoimmune disease research studies.
Table 1.
Patient characteristics and classification based on disease groups and anti-phospholipid syndrome (APS) clinical manifestations
| Patients | Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Number (n) of patients | 104 | |||||||
| Mean age in years | 40 (range 18–79) | |||||||
| Gender | Females (n) | 78 (75%) | ||||||
| Males (n) | 26 (25%) | |||||||
| Race | Caucasian (n) | 90 (79%) | ||||||
| Other* (n) | 14 (21%) | |||||||
| APS clinical manifestations | |||||||
|---|---|---|---|---|---|---|---|
| Disease groups (number, n) | VT (n = 19) | AT (n = 24) | Any TE (n = 40) | Toxaemia (n = 5) | Fetal demise (n = 5) | RPL (n = 8) | Any PRM (n = 14) |
| Primary APS (n = 8) | 5 | 4 | 8 | 1 | 1 | 0 | 1 |
| Secondary APS (n = 3) | 1 | 1 | 2 | 0 | 0 | 1 | 1 |
| SLE (n = 5) | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Non-SLE autoimmune diseases (n = 11) | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| Inherited thrombophilia (n = 2) | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| Idiopathic thrombosis (n = 15) | 5 | 10 | 15 | 0 | 0 | 0 | 0 |
| Equivocal LAC with APS manifestations (n = 8) | 2 | 3 | 5 | 0 | 1 | 3 | 3 |
| Any PRM with negative criteria laboratory tests for APS (n = 8) | 0 | 0 | 0 | 4 | 2 | 4 | 8 |
| Other clinical conditions (n = 44) | 4 | 3 | 6 | 0 | 1 | 0 | 1 |
Other included patients from diverse racial background or ethnicities with no definite majority. These include individuals of African American, Asian, Middle Eastern, non-Hispanic Latin American, Hawaiian/Pacific Island or undefined descent. VT = venous thrombosis; AT = arterial thrombosis; TE = any thrombotic event; RPL = recurrent pregnancy loss; any PRM = any pregnancy-related morbidity; SLE= systemic lupus erythematosus; LAC = lupus anti-coagulant.
Detection of lupus anti-coagulant and specific anti-phospholipid antibodies
LAC testing was performed according to the updated guidelines of the International Society on Thrombosis and Hemostasis (ISTH) 5 on a STA-R automated coagulation analyser. Screening on platelet-poor citrated plasma used two different clotting assay principles: a sensitive activated partial thromboplastin time (aPTT) and dilute Russell's viper venom time (dRVVT), each performed according to the manufacturer's recommendations. If one or both screening tests was prolonged, a mixing study was performed using a 1:1 mix of patient sample and pooled normal plasma (PNP), followed by repeat testing of the aPPT and/or the dRVVT clotting time without incubation. Results from mixing studies that failed to correct to within the local cut-off values, and thus suggested an inhibitory effect, underwent reflexive confirmation (neutralization) testing to demonstrate phospholipid dependency by increasing the concentration and/or composition of phospholipids (PL). Hexagonal phase assay with increased PL was used when mixing studies for aPPT or dRVVT showed an inhibitory effect, but the confirmatory steps were negative. LAC was considered present when one or more of the clotting assays was positive (i.e. prolonged aPTT or dRVVT in mixing studies with positive confirmatory testing or positive hexagonal phase in the case of negative confirmatory testing). Evaluation for confounding factors to distinguish LAC from other causes of prolonged clotting times that may mimic or co-exist with LAC, such as factor deficiency or inhibitor or anti-coagulant therapies, was performed using other assays, including thrombin time (TT), reptilase time (RT) and heparin neutralization. The results of LAC were considered equivocal if the clotting times were prolonged but corrected in the 1:1 mix. While this could indicate factor deficiency, it may also suggest a weak inhibitor or an inhibitor that exerts its activity only in an incubated mix for 1 h.
For the detection of aCL, aβ2GPI and aPS/PT IgG and IgM antibodies, patient samples were screened using kits from Inova Diagnostics (San Diego, CA, USA). All assays were performed following the manufacturer's protocol by an experienced technician who was blinded to previous results for these samples. For APS, the presence or absence of aβ2GPI and aPS/PT IgG and IgM antibodies was based on the manufacturer's suggested reference ranges. In the case of aβ2GPI antibodies, antibody levels greater than 20 SGU or SMU (standard IgG or standard IgM units, respectively) were considered positive. The cut-off determination for the aPS/PT IgG and IgM assays were set at 30 units. In the case of aCL IgG and/or IgM antibodies, only moderate to high levels (for this assay, greater than 40 GPL and/or greater than 40 MPL units) were considered in the diagnosis of APS, as per the revised Sapporo laboratory criteria 2. Thus, although the manufacturer's recommended reference interval for aCL IgG is greater than 20 GPL (with 15–19 GPL as indeterminate) or greater than 20 MPL (with 13–19 MPL as indeterminate), only antibody levels greater than or equal to 40 GPL or 40 MPL were considered clinically relevant.
Statistical analysis
Positive primary APS or secondary APS disease was defined by the presence of defined laboratory and clinical criteria for APS 2; all other diagnoses were defined as negative. Based on this categorization, sensitivity, specificity, area under the curve (AUC) and odds ratio (OR) were estimated. Sensitivity and specificity were calculated using a 2 × 2 table. The OR and P-values were estimated using Cochran Mantel–Haenszel statistics with logistic regression utilized where indicated. Comparison of aPS/PT IgG and/or IgM antibody levels between primary APS and/or secondary APS versus SLE, non-SLE autoimmune diseases and others (all non-autoimmune categories) was performed using a simple t-test to compare means. Correlation between any two analytes was assessed by Fisher's exact test. Results were considered statistically significant if P-values were less than 0·05. All statistical analyses were performed using sas software, version 9·3 (SAS Institute, Inc., Cary, NC, USA).
Results
Of the 104 patients evaluated, 78 (75%) were female and 26 (25%) were male. The mean age of the patients was 40 years (range = 18–79), and the majority of the patients were Caucasian. Venous and/or arterial thrombotic events were diagnosed in 40 cases (38·5%), while any PRM occurred in 14 of the 78 female patients (∼18·0%, Table 1). In this cohort, primary APS was identified in eight subjects, three had secondary APS, five SLE only, 11 non-SLE autoimmune diseases, two inherited thrombophilia (prothrombin mutation G20210A and FV Leiden) and 15 idiopathic thrombosis. An additional eight patients had equivocal LAC results, and eight had PRM but negative tests for APL antibodies. Finally, 44 of 104 (42·3%) patients had other clinical conditions or family histories associated with thrombosis which warranted laboratory evaluation of aPL antibodies. All the eight primary APS patients had thrombosis; only one had experienced both thrombosis and fetal demise.
Of the primary APS patients, LAC was detected in seven of the eight cases and was the most common aPL antibody. Of the secondary APS cases, LAC was detected in two of the three patients (Table 2). Autoantibodies to PS/PT were present in five of the eight primary APS patients (62·5%) and more prevalent than either the aCL or aβ2GPI IgG/IgM antibodies. Overall, in the patients with primary APS, the hierarchy of aPL antibodies was of the order: LAC>aPS/PT>aβ2GPI = aCL (Table 2). In the patients with non-SLE autoimmune diseases (n = 11), aPS/PT autoantibodies were the most common compared to the aPL antibody criteria. In this group, two of the three aPS/PT antibody-positive cases were of IgM isotype (31 and 93 units), and none of these patients had histories of either thrombosis or PRM. Unlike the aPS/PT antibody-positive cases in this group, a patient with VT was found to be positive for both aCL and aβ2GPI antibodies (data not shown). None of the patients with inherited thrombophilia or idiopathic thrombophilia was antibody-positive.
Table 2.
Prevalence of specific anti-phospholipid antibodies in the different study groups
| Study groups (n = 104) | % (Number of positive cases per group) | |||
|---|---|---|---|---|
| LAC | aCL IgG and/or IgM | aβ2GPI IgG and/or IgM | aPS/PT IgG and/or IgM | |
| Primary APS (n = 8) | 87·5 (7/8) | 25 (2/8) | 25 (2/8) | 62·5 (5/8) |
| Secondary APS (n = 3) | 66·7 (2/3) | 33·3 (1/3) | 33·3 (1/3) | 33·3 (1/3) |
| SLE (n = 5) | 0 | 0 | 0 | 0 |
| Non-SLE autoimmune diseases (n = 11) | 9·1 (1/11) | 9·1 (1/11) | 9·1 (1/11) | 27·3 (3/11) |
| Inherited thrombophilia (n = 2) | 0 | 0 | 0 | 0 |
| Idiopathic thrombosis (n = 15) | 0 | 0 | 0 | 0 |
| Equivocal LAC with APS manifestations (n = 8) | 0 | 0 | 0 | 12·5 (1/8) |
| Any PRM with negative criteria laboratory tests for APS (n = 8) | 0 | 0 | 0 | 0 |
| Other clinical conditions (n = 44) | 9·1 (4/44) | 9·1 (4/44) | 2·3 (1/44) | 13·7 (6/44) |
| Overall | 13·5 (14/104) | 7·7 (8/104) | 4·8 (5/104) | 15·4 (16/104) |
APS = anti-phospholipid syndrome; LAC = lupus anti-coagulant; aCL = anti-cardiolipin; aβ2GPI = anti-beta2 glycoprotein I; aPS/PT = anti-phosphatidylserine/prothrombin complex; PRM = pregnancy-related mortality; SLE = systemic lupus erythematosus; Ig = immunoglobulin.
To further understand the diagnostic relevance of aPS/PT IgG/IgM antibodies, the clinical and laboratory features of aPS/PT antibody-positive patients were investigated. Of the 16 aPS/PT IgG- and/or IgM-positive cases, three occurred independently of the criteria aPL antibodies. Six of the 16 had primary APS or secondary APS; all six were positive for IgG antibodies, with levels ranging from 59 to 205 units compared to five positive for IgM isotype, with a range from 64 to 210 units (Table 3 and Fig. 1). With the exception of one patient with a clinical manifestation of RPL, all had thrombosis and one had AT and fetal demise. None of the aPS/PT antibody-positive patients with non-SLE autoimmune disease had clinical manifestations associated with APS. Two of the three positive cases in that group had IgM isotype antibody (31 and 93 units); the third patient had an aPS/PT IgG level of 41. Only one of eight cases with equivocal LAC results with RPL (HD-029), who was negative for IgM and IgG aCL and aβ2GPI antibodies, had a positive aPS/PT IgG (31 units). This was the only aPS/PT IgG-positive patient who fulfilled the clinical criteria of PRM but was negative for the criteria aPL antibody tests. In the patients categorized as having other clinical conditions (n = 44, Table 1), chart reviews indicated that seven cases fulfilled the clinical criteria for APS with negative or inadequate (equivocal or low) aPL antibody criteria. Two of the patients with thrombosis (HD-041 and HD-070, Table 3) had positive aPS/PT IgM results of 79 and 34 units in addition to low levels of aCL IgM (26 MPL and 20 MPL, respectively). As only aCL antibodies above 40 GPL or 40 MPL met the consensus criteria for APS, these patients were categorized as having ‘probable’ APS (Table 3). Of the remaining 37 patients with other clinical conditions, four were positive for aPS/PT (one for both IgG and IgM; three for aPS/PT IgG only) (Table 3). Patient HD-083, dual-positive for aPS/PT IgG (124 units) and IgM (209 units), is a 31-year-old female patient with a history of infertility associated with two consecutive miscarriages and endometriosis. Of the three patients positive for aPS/PT IgG, one had an astrocytoma and was also positive for LAC and aβ2GPI IgG. This patient had no clinical manifestations suggestive of APS; a follow-up may be of benefit to confirm the persistence of aPL and/or subsequent development of disease. The other two patients were a 64-year-old male with cervical spondylitis (aPS/PT IgG: 45 units) and a 49-year-old female with unproven diagnosis of retinal arterial occlusion (aPS/PT IgG: 40 units). Table 3 shows the summary of the demographic, clinical and laboratory attributes of the aPS/PT IgG- and/or IgM-positive patients in this cohort.
Table 3.
Clinical and laboratory characteristics of anti-phosphatidylserine/prothrombin complex (aPS/PT) antibody-positive patients
| Diagnosis | Patient ID | Sex/age (years) | APS clinical manifestations | aPS/PT (IgG/IgM) | Criteria aPL antibodies |
|---|---|---|---|---|---|
| Primary APS | HD-002 | Male/64 | AT | 122/0 | LAC+ |
| HD-021 | Female/22 | AT; Fetal demise | 115/69 | LAC+; aCL 17 GPL; 15 MPL | |
| HD-035 | Female/19 | VT | 158/210 | LAC+; aCL 88 GPL; aβ2GPI 44 SGU | |
| HD-075 | Female/60 | AT | 62/182 | LAC+ | |
| HD-138 | Female/21 | VT | 205/196 | LAC+ | |
| Secondary APS | HD-101 | Female/31 | 3 RPL | 59/64 | LAC+/− aCL 79 GPL aβ2GPI 116 SGU |
| SSc with Raynauds | HD-030 | Male/49* | None | 41/1 | None |
| AHA/SjS | HD-048 | Female/47* | None | 10/93 | aCL 16 MPL |
| Multiple sclerosis | HD-081 | Female/43 | None | 28/31 | None |
| Other (astrocytoma) | HD-012 | Male/41 | None | 47/1 | LAC+; aβ2GPI 25 SGU |
| ‘Other’ (‘probable’ APS) | HD-029 | Female/29 | RPL | 31/16 | LAC+/− |
| ‘Other’ (‘probable’ APS) | HD-041 | Female/37 | VT; AT | 8/79 | aCL 26 MPL |
| Other (unproven retinal arterial occlusion) | HD-062 | Female/49 | None | 40/9 | None |
| ‘Other’ (‘probable’ APS) | HD-070 | Female/20 | VT | 10/34 | aCL 20 MPL |
| ‘Other’ (‘probable’ APS) | HD-083 | Female/31 | 2 RPL | 124/209 | aCL 26 MPL |
| Other (cervical spondylitis) | HD-094 | Male/64** | None | 45/15 | LAC+/− |
The cut-off determination for the aPS/PT immunoglobulin (Ig)G and IgM assays were set at 30 units. Of the aPS/PT antibody-positive patients, 13 were of Caucasian, two Asian* and one non-Hispanic Latin American
descent. ‘Other’ (‘probable’ APS): if current clinical and/or laboratory criteria not fulfilled. + = positive; −/+ = equivocal. VT = venous thrombosis; AT = arterial thrombosis; RPL = recurrent pregnancy loss; SSc = systemic sclerosis; AHA = autoimmune haemolytic anaemia; SjS = Sjögren's syndrome; MPL = IgM aCL; GPL = IgG aCL; SMU = IgM beta2 glycoprotein I; SGU = IgG beta2 glycoprotein I; aPL = anti-phospholipid.
Fig 1.
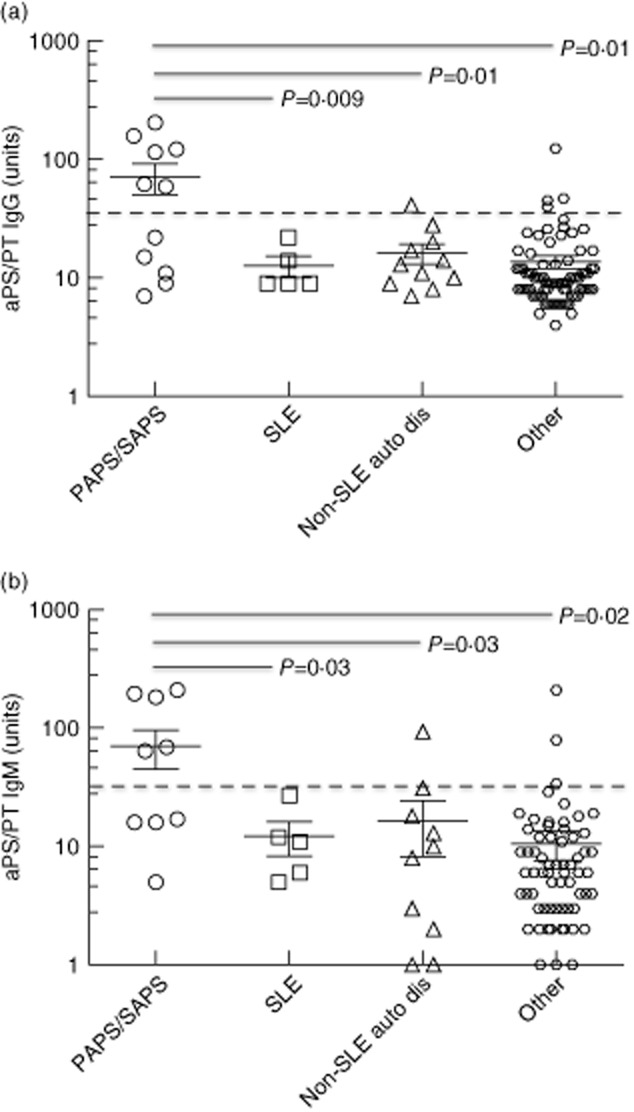
Scatterplot illustrating the mean levels for phosphatidylserine/prothrombin (PS/PT) immunoglobulin (Ig)G (a) and IgM (b) antibodies in the different patient groups. Each data point represents a single patient; horizontal lines and error bars show the mean ± standard error of the mean (s.e.m.) for the respective groups. Horizontal dotted line through each scatterplot indicates the cut-off of the test. PAPS/SAPS = patients with primary or secondary anti-phospholipid syndrome (APS). ‘Other’ refers to all patients with other clinical conditions as defined in Materials and methods.
Overall, the mean aPS/PT IgG or IgM levels in patients with primary APS or secondary APS were significantly higher than in any other patient groups (Fig. 1a,b). All patients with non-SLE autoimmune diseases, SLE or other diseases including those with probable APS were categorized in the ‘other’ group. The mean aPS/PT IgG antibody level was 71·4 U (range = 7–205 units) in the primary APS and secondary APS compared to 12·6 units (SLE only), 16·2 units (non-SLE autoimmune diseases) and 13·9 units (others). The trend was similar for the IgM isotype for all groups. Thus, the level of aPS/PT IgG or IgM was significantly higher in the primary and secondary APS than in all other groups, as indicated in Fig. 1. No significant differences in antibody levels were noted between the non-APS patient groups for both IgG and IgM isotypes.
Based on the presence or absence of primary APS or secondary APS in this cohort, the performance characteristics of the aPS/PT test were compared to those of the aPL test criteria (Table 4). Compared to the aCL and aβ2GPI immunoassays, the sensitivities of the aPS/PT IgG or IgM tests were higher, at 54·6 and 45·4.5%, respectively. The specificities were relatively lower, although still above 90%. Not surprisingly, the sensitivity and specificity of the LAC relative to the aPS/PT assays was superior. A receiver operating characteristic (ROC) AUC was constructed to evaluate the overall performance of aPS/PT immunoassays in relation to the criteria aPL assays. The aPS/PT IgG and IgM assays had AUC of 0·796 and 0·734, respectively. Compared to aCL and aβ2GPI assays, these values were closer in performance to the LAC test. The unadjusted OR for aPS/PT IgG was comparable to aCL IgG, but not aβ2GPI IgG: aPS/PT IgG 17·4 (95% CI = 4·1–73·9) versus aCL IgG 17·1 (95% CI = 2·5–117·5) versus aβ2GPI IgG: 34·5 (95% CI = 3·2–371·2). Overall, the aPS/PT IgM assay had better performance characteristics compared to the aCL or aβ2GPI IgM tests, but not LAC.
Table 4.
Diagnostic performance of specific aPL tests in the study cohort
| Test | % (95% CI) | Odds ratio (95% CI) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Area under curve | ||
| LAC | 81·8 (48·2–97·7) | 97·9 (92·5–99·7) | 0·898 (0·778–1·0) | 204·8 (25·7–1632·7) |
| aCL IgG | 27·3 (6·02–61·0) | 97·9 (92·5–99·7) | 0·626 (0·487–0·764) | 17·1 (2·5–117·5) |
| aCL IgM | 0·0 (0·0–32·1) | 96·8 (90·2–99·2) | 0·595 (0·417–0·773) | 1·1 (0·1–23·2)* |
| aβ2GPI IgG | 27·3 (6·02–61·0) | 98·9 (94·2–100·0) | 0·679 (0·459–0·899) | 34·5 (3·2–371·2) |
| aβ2GPI IgM | 0·0 (0·0–32·1) | 98·9 (94·2–100·0) | 0·653 (0·451–0·854) | 2·7 (0·1–69·8)* |
| aPS/PT IgG | 54·6 (23·4–83·3) | 93·6 (86·5–97·6) | 0·796 (0·624–0·968) | 17·4 (4·1–73·9) |
| aPS/PT IgM | 45·5 (16·8–76·6) | 94·6 (87·9–98·2) | 0·734 (0·516–0·951) | 14·7 (3·3–65·1) |
Logit correction since one of the values in the table was a zero. CI = confidence interval; aPL = anti-phospholipid; LAC = lupus anti-coagulant; aCL = anti-cardiolipin; GPI = glycoprotein I; aPS/PT = anti-phosphatidylserine/prothrombin complex; Ig = immunoglobulin.
In addition to the performance of the individual assays, the diagnostic characteristics of these tests in different combinations in a panel with and without LAC were examined (Table 5). Overall, no significant difference in outcome was observed if LAC and any two IgG-specific immunoassays were utilized. Exclusion of LAC testing significantly decreased the diagnostic performance for diagnosing APS. This was most evident when only aCL and aβ2GPI antibody tests were utilized. Without LAC testing, the best performance for diagnosis was observed with aPS/PT and aβ2GPI or aPS/PT and aCL. A combination of the three aPL (aCL, aPS/PT and aβ2GPI) did not improve diagnostic accuracy for disease. Lastly, the correlation between any two of the aPL antibody tests was also examined. Interestingly, LAC and aCL as well as LAC and aβ2GPI immunoassays did not have significant correlations. Significant correlations between any two aPL tests were observed between LAC and aPS/PT IgG/IgM (P = 0·002), aPS/PT IgG/IgM and aCL IgG/IgM (P = 0·002), aPS/PT IgG/IgM and aβ2GPI IgG/IgM (P = 0·03) and aCL IgG/IgM and aβ2GPI IgG/IgM (<0·0001).
Table 5.
Combined diagnostic performance of specific anti-phospholipid antibody tests with and without LAC for anti-phospholipid syndrome (APS)
| Test combinations | Isotype(s) | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| With LAC | aCL and aβ2GPI | IgG | 0·979 (0·988–0·999)** | 100 (71·5–100) | 95·7 (89·4–98·8) | 475·4 (23·1–9056·5) |
| IgM | 0·877 (0·755–0·999)** | 81·8 (48·2–97·7) | 93·6 (86·5–97·6) | 65·3 (11·4–372·2) | ||
| IgG/IgM | 0·957 (0·928–0·986)** | 100 (71·5–100) | 91·4 (83·8–96·2) | 231·4 (12·5–4280·4) | ||
| aPS/PT and aβ2GPI | IgG | 0·962 (0·935–0·989)** | 100 (71·5–100) | 92·5 (85·1–96·9) | 265·3 (14·2–4958·2) | |
| IgM | 0·906 (0·812–1·00)** | 90·9 (58·7–99·8) | 90·3 (82·4–95·5) | 93·3 (10·7–815·4) | ||
| IgG/IgM | 0·930 (0·895–0·966)** | 100 (71·5–100) | 86·0 (77·3–92·3) | 137·2 (7·6–2466·5) | ||
| aCL and aPS/PT | IgG | 0·962 (0·935–0·989)** | 100 (71·5–100) | 92·5 (85·1–96·9) | 265·3 (14·2–4958·2) | |
| IgM | 0·906 (0·812–1·00)** | 90·9 (58·7–99·8) | 90·3 (82·4–95·5) | 93·3 (10·7–815·4) | ||
| IgG/IgM | 0·925 (0·888–0·961)** | 100 (71·5–100) | 85·0 (76·0–91·5) | 126·1 (7·0–2260·4) | ||
| aCL, aPS/PT and aβ2GPI | IgG | 0·962 (0·935–0·989)** | 100 (71·5–100) | 92·5 (85·1–96·9) | 265·3 (14·2–4958·2) | |
| IgM | 0·906 (0·812–0·100)** | 90·9 (58·7–99·8) | 90·3 (82·4–95·5) | 93·3 (10·68–815·4) | ||
| IgG/IgM | 0·925 (0·888–0·961)** | 100 (71·5–100) | 85·0 (76·0–91·5) | 126·1 (7·0–2260·4) | ||
| Without LAC | aCL and aβ2GPI | IgG | 0·620 (0·481–0·759)* | 27·3 (6·0–61·0) | 96·8 (90·9–99·3) | 11·3 (1·9–65·1) |
| IgM | 0·521 (0·501–0·542) | 0·0 (0·0–32·1) | 95·7 (89·4–98·8) | 0·9 (0·04–17·1) | ||
| IgG/IgM | 0·599 (0·458–0·739)* | 27·3 (6·0–61·0) | 92·5 (85·1–96·9) | 4·6 (0·99–21·4) | ||
| aPS/PT and aβ2GPI | IgG | 0·786 (0·635–0·904)** | 63·6 (30·8–89·1) | 93·6 (86·5–97·6) | 25·4 (5·8–111·6) | |
| IgM | 0·690 (0·533–0·846)* | 45·5 (16·8–76·6) | 92·5 (85·1–96·9) | 10·2 (2·5–42·1) | ||
| IgG/IgM | 0·754 (0·601–0·907)** | 63·6 (30·8–89·1) | 81·7 (78·6–93·2) | 11·8 (3·0–46·5) | ||
| aCL and aPS/PT | IgG | 0·786 (0·635–0·937)** | 63·6 (30·8–89·1) | 93·6 (86·5–97·6) | 25·4 (5·8–111·6) | |
| IgM | 0·690 (0·533–0·846)* | 45·5 (16·8–76·6) | 92·5 (85·1–96·9) | 10·2 (2·5–42·1) | ||
| IgG/IgM | 0·748 (0·595–0·902)** | 63·6 (30·8–89·1) | 86·0 (77·3–92·3) | 10·8 (2·8–42·0) | ||
| aCL, aPS/PT and aβ2GPI | IgG | 0·786 (0·635–0·937)** | 63·6 (30·8–89·1) | 93·6 (86·5–97·6) | 25·4 (5·8–111·6) | |
| IgM | 0·690 (0·533–0·805)* | 45·5 (16·8–76·6) | 92·5 (85·1–96·9) | 10·2 (2·5–42·1) | ||
| IgG/IgM | 0·748 (0·595–0·902)** | 63·6 (30·8–89·1) | 86·0 (77·3–92·3) | 10·8 (2·8–42·0) | ||
P ≤ 0·002
P = 0·0001. AUC = area under the curve; CI = confidence interval; LAC = lupus anti-coagulant; Ig = immunoglobulin; aCL = anti-cardiolipin; aPS/PT = anti-phosphatidylserine/prothrombin complex; GPI = glycoprotein I.
Discussion
The absence of criteria aPL antibodies in patients with symptoms suggestive of APS has led to the phenomenon of ‘seronegative’ APS as well as the search for additional diagnostic tests. While this may be attributable to the poor performance characteristics for the available recommended tests for disease evaluation, there is evidence that antibodies directed against other proteins or protein-bound phospholipids may be useful in disease diagnosis and/or in predicting risk for certain clinical manifestation in APS. In addition, although the LAC assay is recognized as the most reliable and predictive marker for APS, it is fraught with analytical challenges at multiple levels, particularly interference from some anti-coagulant medications used in disease management 5–8. In this study, we focused on the prevalence, correlations and clinical significance of aPS/PT antibodies in a group of patients under evaluation for APS or APS-related clinical manifestations at the University of Utah Health Sciences Center. Our results demonstrate improved sensitivities for aPS/PT IgG and IgM relative to the aCL and aβ2GPI assays. In addition, aPS/PT antibodies were correlated highly with LAC results, suggesting that, in at least a subset of patients, this test will be of diagnostic and predictive relevance.
Because of the first observation, that prothrombin antibodies associate with significantly LAC 11, several studies have sought to define the diagnostic relevance of these antibodies in APS 12–15,17–19,21,23–29. However, conflicting results have been reported, due perhaps to the design of the assays, the populations investigated, as well as how the results have been interpreted 13–15,17,19,21–29. The main strength of this investigation is the prospective nature of the study and relative absence of selection bias. All specimens for evaluation were referred by clinicians from diverse specialities, albeit in an academic medical centre, and with requests for LAC and aCL or aβ2GPI IgG and/or IgM antibody tests indicating a high clinical suspicion for APS or related diseases. To date, except for a single study, to our knowledge this is the first study to examine the relevance of aPS/PT compared to other criteria aPL antibodies in a prospective manner 25. A major limitation of the study by Sanfelippo and colleagues 25 was the absence of full access to all 728 samples screened for antibodies. However, this study and others demonstrate higher sensitivities for aPS/PT IgG or IgM compared to aCL and aβ2GPI tests, as well as a significant correlation between LAC and aPT/PS antibodies 23–29.
In addition to investigating the prevalence and diagnostic performance of each of the aPL assays in the cohort, analyses of specific tests combinations with or without LAC for diagnosis of APS were performed. Overall, there was no significant difference between test combinations with LAC and any two aPL antibody tests. Moreover, using four tests (aβ2GPI, aCL, aPS/PT, LAC) did not improve the diagnostic performance for APS. The diagnostic performance for test combinations in our study differed somewhat from that found in a previous study in SLE 29. This may be explained by the study size and population characteristics, but it is likely that other factors may be involved. For test combinations not including LAC, only aβ2GPI IgG and aPS/PT IgG or aCL IgG and aPS/PT IgG panels had the highest AUCs (0·786; 95% CI = 0·635–0·904); these were significantly lower than any panel that included LAC testing.
Although our investigation is limited by the number of APS cases, the approach is unbiased and represents an important pathway for the possible adoption of aPS/PT IgG and IgM as a diagnostic test. The presence of aPS/PT antibodies in some patients with clinical manifestations suggestive of APS but with equivocal criteria aPL antibodies deserves consideration. Furthermore, the improved sensitivity of the aPS/PT IgG and its significant correlation between aPS/PT and LAC compared to the other criteria aPL tests suggests a potential additive value in APS evaluation, particularly in patients with inconclusive aPL antibody criteria results. While the lack of clinical follow-up in some aPS/PT-positive cases precludes a comprehensive understanding of the predictive value for thrombosis and/or specific pregnancy-related morbidity in this cohort, efforts to determine and validate the optimal aPL antibody combination and/or strategy in diagnostic algorithms for APS are warranted.
Acknowledgments
This work was supported by funds from the ARUP Institute for Clinical and Experimental Pathology, Salt Lake City, Utah. The kits for the detection of aPS/PT IgG and IgM antibody kits were provided free of charge for this investigation at Arup Laboratories by Inova Diagnostics (San Diego, CA, USA).
Disclosures
G. L. is an employee of Inova Diagnostics, which manufactures the aPS/PT IgG and IgM antibody kits described in this study. All other authors declare no competing financial interests.
References
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta 2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–2723. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. 2012;64:2311–2318. doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- Ortel TL. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. Am J Hematol. 2012;87(Suppl 1):75–81. doi: 10.1002/ajh.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreese KM. Antiphospholipid antibody testing and standardization. Int J Lab Hematol. 2014;36:352–363. doi: 10.1111/ijlh.12234. [DOI] [PubMed] [Google Scholar]
- Favaloro EJ. Variability and diagnostic utility of antiphospholipid antibodies including lupus anticoagulants. Int J Lab Hematol. 2013;35:269–274. doi: 10.1111/ijlh.12072. [DOI] [PubMed] [Google Scholar]
- Cervera R, Conti F, Doria A, Iaccarino L, Valesini G. Does seronegative antiphospholipid syndrome really exist? Autoimmun Rev. 2012;11:581–584. doi: 10.1016/j.autrev.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Nayfe R, Uthman I, Aoun J, Saad Aldin E, Merashli M, Khamashta MA. Seronegative antiphospholipid syndrome. Rheumatology (Oxf) 2013;52:1358–1367. doi: 10.1093/rheumatology/ket126. [DOI] [PubMed] [Google Scholar]
- Fleck RA, Rapaport SI, Rao LV. Anti-prothrombin antibodies and the lupus anticoagulant. Blood. 1988;72:512–519. [PubMed] [Google Scholar]
- Arvieux J, Darnige L, Caron C, Reber G, Bensa JC, Colomb MG. Development of an ELISA for autoantibodies to prothrombin showing their prevalence in patients with lupus anticoagulants. Thromb Haemost. 1995;74:1120–1125. [PubMed] [Google Scholar]
- Matsuda J, Saitoh N, Gotoh M, Kawasugi K, Gohchi K, Tsukamoto M. Phosphatidyl serine-dependent antiprothrombin antibody is exclusive to patients with lupus anticoagulant. Br J Rheumatol. 1996;35:589–591. doi: 10.1093/rheumatology/35.6.589. [DOI] [PubMed] [Google Scholar]
- Galli M, Beretta G, Daldossi M, Bevers EM, Barbui T. Different anticoagulant and immunological properties of anti-prothrombin antibodies in patients with antiphospholipid antibodies. Thromb Haemost. 1997;77:486–491. [PubMed] [Google Scholar]
- Atsumi T, Ieko M, Bertolaccini ML, et al. Association of autoantibodies against the phosphatidylserine–prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000;43:1982–1993. doi: 10.1002/1529-0131(200009)43:9<1982::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bertolaccini ML, Gomez S, Pareja JF, et al. Antiphospholipid antibody tests: spreading the net. Ann Rheum Dis. 2005;64:1639–1643. doi: 10.1136/ard.2005.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin N, Alessi MC, Dignat-George F, et al. Does the anti-prothrombin antibodies measurement provide additional information in patients with thrombosis? Immunobiology. 2007;212:557–565. doi: 10.1016/j.imbio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Jaskowski TD, Wilson AR, Hill HR, Branch WD, Tebo AE. Autoantibodies against phosphatidylserine, prothrombin and phosphatidylserine–prothrombin complex: identical or distinct diagnostic tools for antiphospholipid syndrome? Clin Chim Acta. 2009;410:19–24. doi: 10.1016/j.cca.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Bertolaccini ML, Sciascia S, Murru V, Garcia-Fernandez C, Sanna G, Khamashta MA. Prevalence of antibodies to prothrombin in solid phase (aPT) and to phosphatidylserine–prothrombin complex (aPS/PT) in patients with and without lupus anticoagulant. Thromb Haemost. 2013;109:207–213. doi: 10.1160/TH12-07-0527. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Sanaka T, Nishizawa A, Gotoh M, Gohchi K. Two antiprothrombin antibodies against prothrombin and prothrombin–phosphatidyl serine show partial but not total identity. Blood Coagul Fibrinolysis. 2002;13:697–702. doi: 10.1097/00001721-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Atsumi T, Ozaki Y, Koike T, Suzumori K. Phosphatidylserine-dependent antiprothrombin antibodies are not useful markers for high-risk women with recurrent miscarriages. Fertil Steril. 2004;82:1440–1442. doi: 10.1016/j.fertnstert.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Bertolaccini ML, Atsumi T, Koike T, Hughes GR, Khamashta MA. Antiprothrombin antibodies detected in two different assay systems. Prevalence and clinical significance in systemic lupus erythematosus. Thromb Haemost. 2005;93:289–297. doi: 10.1160/TH04-06-0382. [DOI] [PubMed] [Google Scholar]
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost. 2014;111:354–364. doi: 10.1160/TH13-06-0509. [DOI] [PubMed] [Google Scholar]
- Hoxha A, Ruffatti A, Tonello M, et al. Antiphosphatidylserine/prothrombin antibodies in primary antiphospholipid syndrome. Lupus. 2012;21:787–789. doi: 10.1177/0961203312441983. [DOI] [PubMed] [Google Scholar]
- Sanfelippo MJ, Joshi A, Schwartz S, Meister JA, Goldberg JW. Antibodies to phosphatidylserine/prothrombin complex in suspected antiphospholipid syndrome in the absence of antibodies to cardiolipin or beta-2-glycoprotein I. Lupus. 2013;22:1349–1352. doi: 10.1177/0961203313497120. [DOI] [PubMed] [Google Scholar]
- Pregnolato F, Chighizola CB, Encabo S, et al. Anti-phosphatidylserine/prothrombin antibodies: an additional diagnostic marker for APS? Immunol Res. 2013;56:432–438. doi: 10.1007/s12026-013-8421-z. [DOI] [PubMed] [Google Scholar]
- Žigon P, Čučnik S, Ambrožič A, et al. Detection of antiphosphatidylserine/prothrombin antibodies and their potential diagnostic value. Clin Dev Immunol. 2013;2013:724592. doi: 10.1155/2013/724592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žigon P, Ambrožič A, Čučnik S, Kveder T, Rozman B, Božič B. Modified phosphatidylserine-dependent antiprothrombin [corrected] ELISA enables identification of patients negative for other antiphospholipid antibodies and also detects low avidity antibodies. Clin Chem Lab Med. 2011;49:1011–1018. doi: 10.1515/CCLM.2011.162. [DOI] [PubMed] [Google Scholar]
- Sciascia S, Murru V, Sanna G, Roccatello D, Khamashta MA, Bertolaccini ML. Clinical accuracy for diagnosis of antiphospholipid syndrome in systemic lupus erythematosus: evaluation of 23 possible combinations of antiphospholipid antibody specificities. J Thromb Haemost. 2012;10:2512–2518. doi: 10.1111/jth.12014. [DOI] [PubMed] [Google Scholar]


