Abstract
Spondyloarthritis (SpA) is a group of immune mediated inflammatory diseases affecting joints, gut, skin and entheses. The inflammatory process involves activation of Toll-like receptor (TLR)-2 and TLR-4 and production of cytokines and chemokines such as monocyte chemoattractant protein 1 (CCL2/MCP-1). This proinflammatory chemokine recruits monocytes to sites of inflammation and is central in the development of several immune-mediated inflammatory diseases. Interleukin (IL)-19 is a member of the IL-10 family of cytokines. IL-19-deficient mice are more susceptible to innate-mediated colitis and develop more severe inflammation in response to injury. In this work, we studied inducers of IL-19 production and effect of IL-19 on the production of CCL2/MCP-1 and proinflammatory cytokines in peripheral blood mononuclear cells (PBMCs) from healthy controls (HCs) and in PBMCs and synovial fluid mononuclear cells (SFMCs) from SpA patients. Further, we measured IL-19 in plasma from HCs and in plasma and synovial fluid from SpA patients. Constitutive IL-19 expression was present in both PBMCs and SFMCs and the secretion of IL-19 was increased by TLR-2 and TLR-4 ligands. Neutralizing IL-19 in HC PBMCs and SpA SFMCs resulted in increased production of CCL-2/MCP-1. IL-19 concentrations were decreased in synovial fluid compared with plasma and associated inversely with disease activity in SpA. SpA SFMCs produced less IL-19 in response to LPS compared with HC PBMCs. These findings indicate that IL-19 production is diminished in SpA. Taken together, impaired IL-19 control of the innate immune system might be involved in the pathogenesis of SpA.
Keywords: IL-10, IL-19, innate immunity, Toll-like receptor, spondylitis
Introduction
Spondyloarthritis (SpA) is a group of immune-mediated inflammatory diseases affecting the joints and entheses. The disease group includes ankylosing spondylitis, psoriatic arthritis, arthritis associated with inflammatory bowel disease, reactive arthritis and undifferentiated arthritis 1. Proinflammatory cytokines and chemokines, including tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-17, IL-23 and chemokine (C-C motif) ligand 2/monocyte chemotactic protein 1 (CCL2/MCP-1), are important in the pathogenesis of SpA. This is highlighted by genetic associations, cytokine expression and effect of cytokine blockade in human SpA 2,3. For instance, CCL2/MCP-1 is essential for guiding monocytes to sites of inflammation. Growing evidence indicates that part of this proinflammatory profile results from abnormal innate immune responses, including activation of Toll-like receptors (TLRs) 4,5. First, TLR-2 and TLR-4 expression is increased in human SpA 6,7, and both receptors contribute to the pathogenesis of reactive arthritis 8,9. Secondly, endogenous TLR-2 and TLR-4 agonists are increased in the peripheral blood of SpA patients 10,11. Thirdly, presence of germ- and pathogen-derived TLR agonists is critical for the development of joint and gut inflammation in an animal model of SpA 12.
Interleukin (IL)-19 is a member of the IL-10 family of cytokines 13,14, and is produced primarily by epithelial cells and lipopolysaccharide (LPS)-stimulated monocytes 15,16. IL-19 shares the receptor complex of IL-20R1/IL-20R2 with IL-20 and IL-24 and the IL-20R1 subunit with IL-26 17–19. Expression of the IL-20R1 subunit was identified initially only in epithelial cells (e.g. skin, bone and gut), while the IL-20R2 subunit was also found in several peripheral blood leucocyte subsets 15,16. However, the IL-20R1 was induced in macrophages in the lung 18, and several studies have found effects of IL-19, IL-20, IL-24 and IL-26 on cells of the immune system indicating the presence of a functional receptor 20–26.
IL-19 has been associated with anti-inflammatory functions and suggested as a new therapeutic agent in inflammatory conditions 27. This is primarily because IL-19-deficient mice are more susceptible to dextran sodium sulphate-induced colitis 28 and develop more severe inflammation in response to injury 29. Also, single-nucleotide polymorphisms of the IL-19 gene are protective in psoriasis 30 and colitis 31, and altered methylation of the IL-19 gene is involved in Crohn's disease 32. Finally, IL-19 has anti-inflammatory functions in Crohn's disease 33,34, atherosclerotic disease 35,36 and cardiopulmonary bypass 37. In line with this, loss of IL-19 function has been suggested to contribute to the inflammatory mechanisms in these diseases. IL-19 has been studied in arthritis, but the mechanisms of action are not well described 38–41.
In this study we show increased IL-19 production by both TLR-2 and TLR-4 stimulation in mononuclear cell cultures, and the addition of IL-19 neutralizing antibodies resulted in increased production of CCL2/MCP-1. This supports that induction of IL-19 could be a regulatory mechanism in the innate immune system. IL-19 plasma concentrations associated inversely with disease activity and production of IL-19 was impaired in SpA. Taken together, an inability to increase IL-19 production and thereby to reduce inflammation might be involved in the pathogenesis of SpA.
Methods
Study subjects
A study population of 83 SpA patients with symptoms restricted to the axial skeleton (aSpA) was used for measuring the plasma concentration of IL-19. The patients all met the European Spondyloarthropathy Study Group (ESSG) criteria (Table 1) 42. As described previously 43, this study population was characterized with self-assessment scores and clinical scores comprising the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Bath Ankylosing Spondylitis Functional Index (BASFI), patient global visual analogue scale (VAS) score, patient pain VAS score, level of morning stiffness, the Bath Ankylosing Spondylitis Metrology Index (BASMI) and physician global assessment VAS score. The test results comprised C-reactive protein (CRP), human leucocyte antigen (HLA)-B27 status, lumbar radiography and magnetic resonance imaging (MRI) of the sacroiliac joint (SIJ) and lumbar spine. The MRI changes were graded using methods described previously 44,45. In summary, these methods differed from the Spondyloarthritis Research Consortium of Canada (SPARCC) methods primarily by using full three-dimensional assessment of the SIJ and the spine 46,47.
Table 1.
Clinical characteristics of the patients in each group used for measuring interleukin (IL)-19 concentrations in plasma and synovial fluid
| Characteristics | Axial SpA (n = 83) | Peripheral SpA (n = 16) | HC (n = 48) |
|---|---|---|---|
| Age (years, median) | 38 (30–43) | 49 (34–53) | 41 (32–53) |
| Gender (percentage female) | 58 | 75 | 54 |
| Diagnosis (percentage of patients) | |||
| Ankylosing spondylitis | 19 | – | – |
| Psoriatic arthritis | 11 | – | – |
| Enteropathic arthritis | 4 | – | – |
| Reactive arthritis | 15 | – | – |
| Undifferentiated SpA | 51 | – | – |
| HLA-B27 (percentage positive) | 61 | – | – |
| Disease duration (years, median) | 7·5 (5·0–11·0) | – | – |
| Treatment (number of patients) | |||
| MTX | 8 | – | – |
| Salazopyrin | 9 | – | – |
| Anti-TNF-α | 7 | – | – |
| Self-assessment scores | |||
| BASDAI (0–100, mean) | 32 (26–37) | – | – |
| BASFI (0–100, mean) | 20 (16–25) | – | – |
| Patient global (0–100, mean) | 32 (26–38) | – | – |
| Patient pain (0–100, mean) | 32 (26–38) | – | – |
| Level of morning stiffness (0–100, mean) | 36 (29–43) | – | – |
| Clinical scores | |||
| BASMI (0–100, median) | 0 (0–0) | – | – |
| Physician global (0–100, mean) | 16 (13–20) | – | – |
| Test results | |||
| CRP (mg/l, median) | 2·1 (1·3–3·9) | – | – |
| SIJ MRI activity (0–40, mean) | 7·5 (5·9–9·0) | – | – |
| SIJ MRI chronicity (0–48, mean) | 15 (12–18) | – | – |
| Spine MRI activity (0–81, median) | 1 (0–4) | – | – |
| Spine MRI chronicity (0–207, median) | 0 (0–4) | – | – |
Numbers in parentheses are 95% confidence interval for measures with a Gaussian distribution and 25–75% percentiles for measures with a non-Gaussian distribution. Anti-TNF-α = anti-tumour necrosis factor-alpha; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; BASMI = Bath Ankylosing Spondilitis Metrology Index; CRP = C-reactive protein; HC = healthy control; MRI = magnetic resonance imaging; MTX = methotrexate; SIJ = sacroiliac joint; SpA = spondyloarthritis; HLA = human leucocyte antigen; – = not available.
Another study population consisting of SpA patients with predominantly peripheral arthritis (pSpA) was included to be able to measure both plasma and synovial fluid concentrations of IL-19 (n = 16) and for culturing of peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) (n = 12) (Table 1). Patients with peripheral arthritis contacted the out-patient clinic because of a joint effusion. No disease activity or prognosis scores or test results were used for this study population.
Age- and gender-matched healthy controls (HCs) from the Donor Bank at Aarhus University Hospital were used to measure plasma concentrations of IL-19 (n = 43) and to culture PBMCs (n = 12) (Table 1).
Sample handling
All plasma samples were collected in ethylenediamine tetraacetic acid (EDTA) tubes and kept at −80°C until use. SFMCs and PBMCs were isolated by conventional Ficoll-Paque (GE Healthcare, Little Chalfont, UK) density-gradient centrifugation and cryopreserved at −135°C until time of analysis.
Ethics
Samples from the SpA study groups were collected at the out-patient clinic at Aarhus University Hospital. All samples were obtained after informed written consent according to the Declaration of Helsinki and approved by the Local Ethics Committee (project numbers 20050046, 20121329 and 20058432) and the Danish Data Protection Agency.
Stimulation of IL-19 production in PBMC and SFMC cultures
The stimulation of IL-19 secretion was studied with three different experimental set-ups. First, HC PBMCs were incubated with different recombinant human cytokines: TNF-α (Peprotech, Rocky Hill, NJ, USA) at 20 ng/ml, IL-1β (Peprotech) at 20 ng/ml, interferon (IFN)-γ (Peprotech) at 10 ng/ml and IL-23 (R&D Systems, Abingdon, UK) at 50 ng/ml. Secondly, HC PBMCs were incubated with different TLR agonists: the TLR-1/2 ligand Pam3CSK4 (InvivoGen, San Diego, CA, USA) at 1 μg/ml, the TLR-3 ligand polyinosinic:polycytidylic acid [poly(I:C)] (InvivoGen) at 25 μg/ml, the TLR-4 ligand LPS (Sigma Aldrich, St Louis, MO, USA) at 100 ng/ml and the TLR-9 ligand cytosine–phosphate–guanine (CpG) oligodeoxynucleotides (InvivoGen) at 2 μM. Thirdly, SpA PBMCs and SFMCs and HC PBMCs were incubated with LPS at a concentration of 100 ng/ml. In all experiments cells were incubated at a density of 2·0 × 106 cells/ml in RPMI medium supplemented with 10% fetal calf serum (FCS), penicillin, streptomycin and glutamine at 37°C and 5% CO2 for 48 h in a humidified incubator without changing the medium. Supernatants were harvested carefully after centrifugation of the culture plates at 300g for 5 min.
Further, the constitutive and induced IL-19 mRNA expression was examined. First, HC PBMCs and SpA SFMCs were incubated in culture medium to evaluate constitutive IL-19 mRNA expression. Then, HC PBMCs were stimulated for 6 h with the same concentrations of cytokines and TLR agonists as described above. The culture plates were centrifuged at 300g for 5 min and the supernatants were removed. Then, cells were lysed in the culture wells and transferred to filter tubes for mRNA isolation.
Inhibition of constitutive IL-19 in PBMC and SFMC cultures
The neutralization of endogenous IL-19 was studied with three different experimental set-ups. First, HC PBMCs were incubated with polyclonal goat anti-IL-19 antibody (AF1035, R&D Systems) at a concentration of 5 μg/ml or a negative control culture with goat immunoglobulin (Ig)G isotype (R&D Systems). Secondly, neutralization of IL-19 was tested after increasing the endogenous IL-19 secretion in HC PBMCs with LPS at a concentration of 1 ng/ml and then adding polyclonal goat anti-IL-19 antibody at 5 μg/ml. Thirdly, SpA SFMCs were incubated with polyclonal goat anti-IL-19 antibody at 5 μg/ml or goat IgG isotype. In all experiments cells were incubated at a density of 2·0 × 106 cells/ml in RPMI medium supplemented with 10% FCS, penicillin, streptomycin and glutamine at 37°C and 5% CO2 for 48 h in a humidified incubator without changing the medium. Supernatants were harvested after centrifugation at 300g for 5 min.
To validate the specificity of the IL-19 neutralization, SFMCs were also incubated with a mouse monoclonal anti-IL-19 antibody (MAB10351; R&D Systems) at a concentration of 5 μg/ml using a mouse IgG2b isotype (R&D Systems) as negative control. Furthermore, the polyclonal goat anti-IL-19 antibody was preincubated with polymyxin B (Sigma Aldrich) at a concentration of 100 μg/ml at 37°C for 2 h to eliminate the possible influence of endotoxin contamination. Supernatants were harvested after centrifugation of the culture plates at 300g for 5 min.
IL-19 enzyme-linked immunosorbant assay (ELISA)
The plasma concentration of IL-19 was quantified with an ELISA system, validated as described previously 48. Antibodies, recombinant human cytokines and streptavidin-horseradish peroxidase (HRP) for the IL-19 ELISA system were purchased from R&D Systems (DY1035). Nunc Maxisorp 96-well microplates were coated with 100 μl/well of coat antibody at a concentration of 2 μg/ml in phosphate-buffered saline (PBS) and incubated overnight at room temperature (RT). Each well was then washed four times with PBS/Tween. This washing step was repeated preceding all following steps. The wells were blocked with 300 μl 5% skimmed milk in PBS for 2 h at RT. Then, 100 μl of sample, positive controls and standards prepared in assay diluent were added in duplicate and plates were incubated overnight at 4°C. The assay diluent was prepared with protein-free PBS blocking buffer (ThermoScientific, Rockford, IL, USA) with 10 μg/ml mouse gamma globulin (Jackson ImmunoResearch, West Grove, PA, USA), 10 μg/ml bovine gamma globulin (Jackson ImmunoResearch), 10 μg/ml human immunoglobulin (Behring, King of Prussia, PA, USA) and 1 μg/ml mouse IgG2b (MAB004; R&D Systems). Samples were diluted 2:3. Next, 100 μl of biotinylated detection antibody was added to each well and plates were incubated for 1 h at RT. The detection antibody was used at a concentration of 0·5 μg/ml. Plates were then incubated with 100 μl/well of streptavidin–HRP for 15 min at RT. The signal was amplified with the ELAST ELISA Amplification System (PerkinElmer, Waltham, MA, USA) incubated with 100 μl/well of the biotinyl–tyramide solution for 15 min at RT and then for 30 min at RT with 100 μl/well streptavidin–HRP. Finally, the plates were incubated with 100 μl/well of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution at RT. Colour development was stopped with 50 μl/well of H2SO4. The optical density of each well was measured using a microplate reader set to 450 nm and wavelength correction set to 570 nm.
The concentration of IL-19 in cell culture supernatants was analysed as described for the plasma samples.
IL-19 quantitative real-time–polymerase chain reaction (qRT–PCR)
The amount of IL-19 mRNA in untreated and stimulated HC PBMCs and SpA SFMCs were analysed with qRT–PCR. The mRNA was isolated with the High Pure RNA Isolation kit following the manufacturer's instructions (Roche, Mannheim, Germany). Taqman Assays-On-Demand [6-carboxyfluorescein (FAM)-labelled minor groove binder (MGB)-probes] and an RNA-to-CT one-step kit (Applied Biosystems, Foster City, CA, USA) were used to quantitate IL-19 (Hs00604657_m1) and β-actin (hs01060665_g1) mRNA expression. IL-19 mRNA expression was normalized to β-actin expression.
CCL2/MCP-1, TNFα, IL-10, IL-17 and IL-22 ELISAs
Culture supernatants were analysed with commercially available ELISA kits for CCL2/MCP-1 (Biolegend, San Diego, CA, USA), TNF-α (Biolegend), IL-10 (Biolegend), IL-17 (eBioscience, San Diego, CA, USA) and IL-22 (eBioscience), following the manufacturer's instructions.
Statistics
Patient characteristics were expressed as the median with interquartile range (IQR) for parameters with a non-Gaussian distribution and with mean and 95% confidence intervals (95% CI) for parameters with a Gaussian distribution. IL-19 concentrations and mRNA expression ratios were log-transformed before analyses. Unpaired data were analysed using Student's t-test, while paired data were analysed using a paired t-test. The plasma IL-19 concentrations were correlated with disease activity parameters using Spearman's correlation for parameters with a non-Gaussian distribution and Pearson's correlation for parameters with a Gaussian distribution. Comparisons of cytokine ratios were made with the paired t-test or the repeated-measures one-way analysis of variance (anova) on log-transformed data. A two-tailed P-value below 0·05 was considered significant. Calculations and graphs were made with Stata (StataCorp LP, College Station, TX, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
IL-19 production is increased by both TLR-2 and TLR-4 stimulation in HC PBMCs
To identify stimulators of IL-19 production, we treated HC PBMC cultures with cytokines or agonists for pattern recognition receptors known to be involved in monocyte activation and measured IL-19 protein and mRNA. The secretion of IL-19 was increased by IL-1β (P = 0·0003), the TLR-1/2 ligand Pam3CSK4 (P = 0·0012) and the TLR-4 ligand LPS (P = 0·0008) (Fig. 1a,c). No induction was seen with TNF-α, IFN-γ, IL-23, the TLR-3 ligand poly(I:C) or the TLR-9 ligand CpG (Fig. 1a,c). IL-19 mRNA expression was increased significantly by IL-1β (P = 0·032) and LPS (P = 0·043) (Fig. 1b,d). Constitutive expression of IL-19 mRNA in untreated cells was found in both HC donors analysed with qRT–PCR. These findings suggest that IL-19 is expressed constitutively in HC PBMCs and greatly induced by ligands of TLR-2 and TLR-4.
Fig 1.
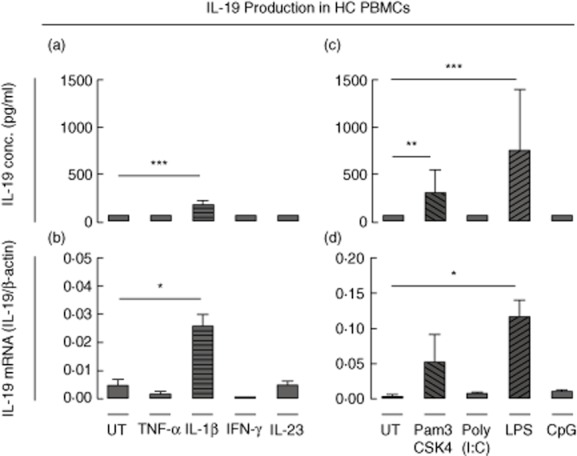
Stimulation of interleukin (IL)-19 production in healthy control (HC) peripheral blood mononuclear cell (PBMC) cultures. (a,b) IL-19 production in untreated (UT) HC PBMC cultures and in cultures stimulated with tumour necrosis factor (TNF)-α (20 ng/ml), IL-1β (20 ng/ml), interferon (IFN)-γ (10 ng/ml) and IL-23 (50 ng/ml). (a) IL-19 secretion (n = 6). (b) IL-19 mRNA expression (n = 2). Log-transformed data were analysed with the paired t-test. (c,d) IL-19 production from UT HC PBMC cultures and from cultures stimulated with the Toll-like receptor (TLR)-1/2 ligand Pam3CSK4 (1 μg/ml), the TLR-3 ligand poly(I:C) (25 μg/ml), the TLR-4 ligand lipopolysaccharide (LPS) (100 ng/ml) and the TLR-9 ligand cytosine–phosphate–guanosine (CpG) (2 μM). (c) IL-19 secretion (n = 6). (d) IL-19 mRNA expression (n = 2). Log-transformed data were analysed with the paired t-test. Boxes and bars indicate median and interquartile range (IQR). *P < 0·05; **P < 0·01; ***P < 0·001.
Inhibition of IL-19 increases CCL2/MCP-1 production in HC PBMCs
Further, to study the effect of IL-19 we neutralized constitutive IL-19 in HC PBMC cultures using a polyclonal goat anti-IL-19 antibody. Goat IgG isotype was used as a negative control. CCL2/MCP-1 was increased in supernatants from HC PBMCs incubated with neutralizing anti-IL-19 antibodies compared with supernatants from HC PBMCs incubated with isotype IgG (P = 0·021) (Fig. 2a). This set-up was also performed in HC PBMCs stimulated with LPS at 1 ng/ml to increase the endogenous IL-19 production. Neutralizing IL-19 in cultures stimulated with LPS at 1 ng/ml further increased the LPS-induced production of CCL2/MCP-1 (P = 0·0059) (Fig. 2b). This indicates that IL-19 has anti-inflammatory properties and that induction of IL-19 could be a regulatory mechanism in the innate immune system.
Fig 2.
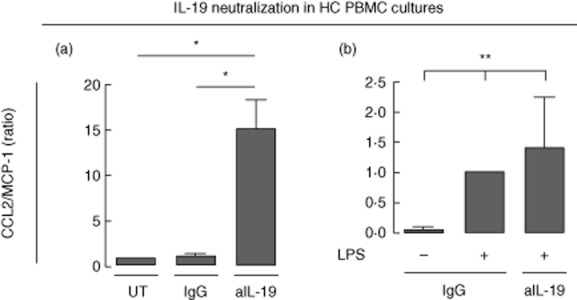
Effect of neutralizing interleukin (IL)-19 in healthy control (HC) peripheral blood mononuclear cell (PBMC) cultures. (a) Neutralization of IL-19 in HC PBMC with polyclonal goat anti-IL-19 antibody at a concentration of 5 μg/ml (n = 4). Goat immunoglobulin (Ig)G isotype served as a negative control. Ratios were calculated by normalizing to the chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-1) concentration in supernatants from untreated cultures. Log-transformed data were analysed with the paired t-test. (b) Neutralization of lipopolysaccharide (LPS)-induced IL-19 at 1 ng/ml in healthy control (HC) peripheral blood mononuclear cells (PBMCs) (n = 3). Goat IgG isotype served as a negative control. Ratios were calculated by normalizing to the CCL2/MCP-1 concentration in supernatants from cultures treated with LPS and isotype IgG. Log-transformed data were analysed with the repeated-measures one-way analysis of variance (anova). Boxes and bars indicate median and interquartile range (IQR). *P < 0·05; **P < 0·01.
Inhibition of IL-19 increases CCL2/MCP-1 and IL-10 production in SpA SFMCs
Next, we tested whether IL-19 also dampens immune reactions in SpA by determining the effect of neutralizing constitutive IL-19 in SpA SFMCs measuring a set of cytokines involved in the pathogenesis of SpA. With regard to the HC PBMCs, constitutive expression of IL-19 mRNA was also found in SFMCs from the two SpA donors tested with qRT–PCR. IL-19 neutralization resulted in increased secretion of CCL2/MCP-1 and IL-10 in SpA SFMC cultures (P = 0·0048 and P = 0·014, respectively) (Fig. 3). An increase was also seen in TNF-α, IL-17 and IL-22 production. However, this tendency was not significant for these cytokines. Removing potential endotoxin contamination of the anti-IL-19 polyclonal goat antibody with polymyxin B did not change the stimulatory effect of IL-19 inhibition (Supporting information, Fig. S1a). The stimulatory effect of IL-19 inhibition was seen using both the polyclonal goat antibody and a mouse monoclonal antibody (Supporting information, Fig. S1b). Our findings support that IL-19 regulates inflammation in SpA by inhibiting the production of cytokines and chemokines.
Fig 3.
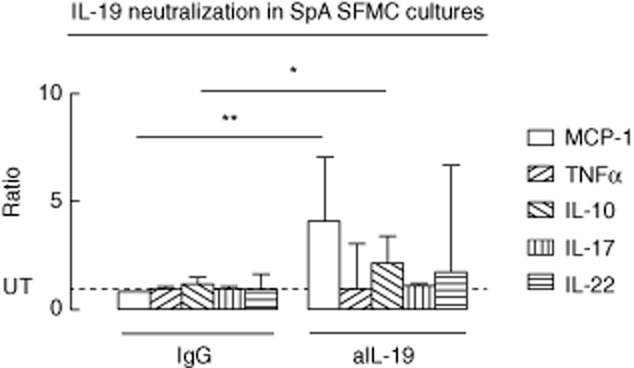
Effects of neutralizing endogenous interleukin (IL)-19 in spondyloarthrtis (SpA) synovial fluid mononuclear cell (SFMC) cultures. The effect of adding anti-IL-19 (5 μg/ml) on secretion of chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-)1 (n = 6), tumour necrosis factor (TNF)-α (n = 3), IL-10 (n = 3), IL-17 (n = 3) and IL-22 (n = 3) in SpA SFMC cultures. A polyclonal goat antibody was used as negative control in all experiments. Ratios were calculated by normalizing to the CCL2/MCP-1 concentration in supernatants from untreated cultures. Boxes and bars indicate median and interquartile range (IQR). Log-transformed data were analysed with the paired t-test. Truncated line indicates the level of untreated (UT) cultures. *P < 0·05; **P < 0·01.
IL-19 levels are decreased in synovial fluid compared with plasma in SpA
In order to determine whether IL-19 levels were altered in SpA patients we measured the levels of IL-19 in plasma from both aSpA and pSpA patients and HCs and in synovial fluid from pSpA patients. The levels of IL-19 in plasma from aSpA or pSpA patients were not significantly different compared with HCs (P = 0·20 and P = 0·62, respectively) (Fig. 4a). The IL-19 levels in synovial fluid from pSpA patients were decreased compared with the plasma levels in both pSpA and aSpA (P < 0·0001 and P = 0·0025) (Fig. 4a). The difference between pSpA synovial fluid IL-19 levels and HC plasma IL-19 levels did not reach statistical significance (P = 0·097) (Fig. 4a). No associations between gender or age and plasma IL-19 levels were observed for either SpA patients or HCs. Thus, despite increased expression of TLR-2 and TLR-4 and the presence of TLR-2 and TLR-4 agonists in SpA the plasma concentrations of IL-19 were not increased in SpA and synovial fluid levels were decreased compared with plasma levels.
Fig 4.
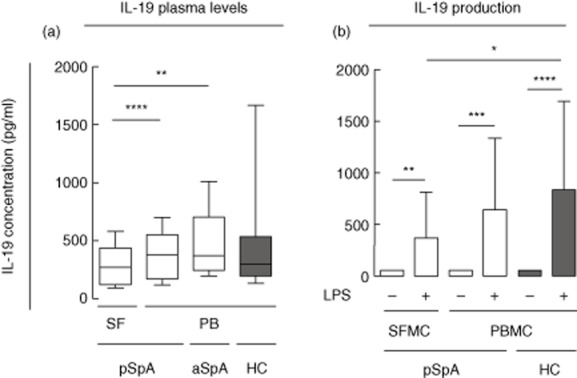
Interleukin (IL)-19 levels in synovial fluid from peripheral spondyloarthrtis (pSpA) patients and in plasma from axial SpA (aSpA) and pSpA patients and healthy controls (HCs) and IL-19 secretion from pSpA and healthy control (HC) mononuclear cell cultures. (a) The median value of IL-19 was 269 pg/ml [interquartile range (IQR) 123–439 pg/ml] in synovial fluid from pSpA patients, 380 pg/ml (IQR 174–557 pg/ml) in plasma from pSpA patients, 371 pg/ml (IQR 245–705 pg/ml) in plasma from aSpA patients and 294 (IQR 196–538 pg/ml) in plasma from HC. Lines and boxes indicate median and IQR and bars indicate 10th and 90th percentiles. Data were log-transformed and unpaired data were analysed with the t-test while paired data were analysed with the paired t-test. (b) Stimulation of pSpA SFMCs (n = 6) and peripheral blood mononuclear cells (PBMCs) (n = 6) and HC PBMCs (n = 12) with lipopolysaccharide (LPS) at a concentration of 100 ng/ml. Boxes and bars indicate median and IQR. Data were log-transformed and unpaired data were analysed with the t-test while paired data were analysed with the paired t-test. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Plasma levels of IL-19 associate inversely with disease activity in SpA
Possible associations between IL-19 and inflammatory activity in SpA were tested by correlating aSpA plasma IL-19 levels with disease activity parameters. These were BASDAI, BASFI, patient global VAS score, patient pain VAS score, morning stiffness, BASMI, physician global assessment score, CRP and sacroiliac joint and spine MRI activity and scores. IL-19 plasma concentrations were associated significantly inversely with the patient self-assessment scores BASDAI (r = −0·271, P = 0·034), patient pain (r = −0·246, P = 0·044) and morning stiffness (r = −0·265, P = 0·028) (Table 2). Inverse correlations were also observed for associations between IL-19 plasma concentrations and BASFI (r = −0·121, P = 0·32), patient global assessment score (r = −0·224, P = 0·067), physician global assessment score (r = −0·121, P = 0·3), CRP (rho = −0·046, P = 0·66) and sacroiliac joint MRI activity scores (r = −0·126, P = 0·26), but these did not reach statistical significance. To test whether IL-19 could be associated with new bone formation we studied the association between aSpA plasma IL-19 levels and sacroiliac joint and spine MRI chronicity scores. There was a positive association between IL-19 plasma concentrations and spine MRI chronicity score (rho = 0·246, P = 0·028). Taken together, we show that decreased IL-19 levels associate inversely with scores measuring disease activity in SpA; also, we show that decreased levels of IL-19 associate with increased inflammatory activity in SpA.
Table 2.
Associations between plasma concentrations of interleukin (IL)-19 in spondyloarthritis (SpA) patients and self-assessment disease activity scores
| BASDAI | BASFI | Patient pain | Patient global | Morning stiffness | ||
|---|---|---|---|---|---|---|
| IL-19 | r | −0·27 | −0·12 | −0·25 | −0·22 | −0·27 |
| P | 0·024 | 0·32 | 0·044 | 0·067 | 0·028 |
Numbers shown in bold type indicate significant correlations. Data were analysed with the Pearson correlation. BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index.
The production of IL-19 after TLR-4 stimulation is decreased in SpA SFMCs compared with HC PBMCs
To determine whether altered production of IL-19 could explain the decreased levels of IL-19 found in synovial fluid compared with plasma we measured the production of IL-19 from LPS-stimulated pSpA SFMCs and PBMCs and HC PBMCs. IL-19 secretion was increased by LPS stimulation at a concentration of 100 ng/ml in both pSpA SFMCs and PBMCs and HC PBMCs (P = 0·0072, P = 0·0002 and P < 0·0001, respectively) (Fig. 4b). The increase in IL-19 production after LPS stimulation was most pronounced in HC PBMC cultures, followed by pSpA PBMCs, and finally pSpA SFMCs (Fig. 4b). The difference in the IL-19 increase was significant when comparing pSpA SFMCs with HC PBMCs (P = 0·02). This supports that the decreased levels of IL-19 in synovial fluid could result from decreased production of IL-19 by SFMCs.
Discussion
The inflammatory process in SpA involves activation of TLRs and a proinflammatory cytokine profile with impaired regulatory mechanisms 2–5. Our findings show that both TLR-2 and TLR-4 stimulation can induce IL-19 production and indicate that endogenously produced IL-19 functions to dampen immune reactions. Plasma levels of IL-19 associated inversely with disease activity and IL-19 levels were decreased locally in the inflamed joint in SpA. Taken together, insufficient regulation of the innate immune system by IL-19 could be involved in the pathogenesis of SpA. This adds to the literature suggesting IL-19 as a therapeutic agent in inflammatory conditions 27.
Stimulation of IL-19 production through TLR-4 is well established 15,16. Also, IL-19 induction in response to TLR-2 stimulation has been proposed previously 33. Confirming these findings, both the TLR-1/2 agonist Pam3CSK4 and the TLR-4 agonist LPS increased IL-19 secretion from HC PBMCs. In contrast, the TLR-3 ligand poly(I:C) and the TLR-9 agonist CpG did not induce IL-19 secretion. IL-19 production was also increased by IL-1β, as described previously 38, but not by IFN-γ, TNF-α or IL-23. Taken together, IL-19 is produced primarily in response to bacterial components and ligands from host cells (TLR-2 and TLR-4) and not virus double-stranded RNA or DNA viruses (TLR-3 and TLR-9).
Neutralizing constitutive IL-19 resulted in increased production of CCL2/MCP-1. This was seen both when neutralizing constitutively produced IL-19 in untreated mononuclear cells and when neutralizing induced IL-19 in LPS-stimulated mononuclear cells. This is in line with previous studies, demonstrating greater responses to LPS in IL-19-deficient macrophages which could be normalized by adding recombinant human IL-19 28,34. Thus, our findings support the hypothesis that the induction of IL-19 functions to dampen excessive immune reactions. Such regulatory mechanisms have been suggested to prevent massive release of proinflammatory mediators 49. This is interesting in the context of autoinflammatory disease, because loss of inhibitory factors could then augment inflammation and disease activity. A previous study found that recombinant human IL-19 induces IL-10 production in PBMCs, which could potentially explain the regulatory effects of IL-19 21. However, in our study IL-10 was induced when neutralizing endogenously produced IL-19. The discrepancy could be a result of the different experimental approaches. However, there are several alternative explanations of the regulatory effects of IL-19; e.g. IL-19 has been suggested previously to skew the immune system towards a Th2 profile 20,25.
In this study of SpA two major findings suggest that impaired IL-19 production contributes to the uncontrolled regulation of immune reactions in these diseases.
First, the immune regulatory function of IL-19 found in HCs was also present in SpA patients. In this study the production of several proinflammatory cytokines was increased in SpA SFMC cultures when neutralizing constitutive IL-19. This is in accordance with several previous studies indicating the anti-inflammatory properties of IL-19 in inflammatory conditions 28–37,50. However, IL-19 has been suggested to have proinflammatory functions in arthritis. For instance, IL-19 inhibition attenuated arthritis in the collagen-induced arthritis model in rats, and high concentrations of recombinant human IL-19 induced the production of IL-6 and increased survival in fibroblast-like synovial cells 39,40. It can only be speculated that some of these discrepancies are explained by interspecies differences and differences between physiological and supraphysiological concentrations of IL-19 in cell cultures. However, IL-19 is up-regulated in the skin in psoriasis and in the gut in inflammatory bowel disease 51–53. Also, a recent comprehensive study of psoriasis showed the crucial role of IL-19 in IL-17-driven inflammation 54. In this way, IL-19 could act in different ways in different cell types and under different inflammatory conditions. This is in line with our finding that IL-19 actually associated positively with chronic MRI changes.
Secondly, concentrations of IL-19 were decreased in synovial fluid compared with plasma from SpA patients. The decreased synovial fluid IL-19 levels were, at least to some extent, explained by a decreased response to TLR-4 activation in SFMCs from SpA patients compared with PBMCs from HCs. This difference could reflect both differences in sensitivity to TLR stimulation and differences in the cell populations present in the cultures. However, our finding is in line with a study showing decreased IL-19 production from Crohn's disease PBMCs compared with HC PBMCs in response to LPS 33. The plasma concentrations of IL-19 were not decreased in SpA patients compared with HCs, as reported otherwise recently by others 41. However, the plasma concentrations were not increased, as seen with several other cytokines in SpA; e.g. IL-20 and IL-24 and were correlated inversely with disease activity scores 55. These findings are interesting, because TLR-2 and TLR-4 involvement in SpA and mouse models of these diseases is well established 6–9,12. Thus, the innate immune system is activated in SpA and this activation could be potentiated by the loss of IL-19 function.
No inflammatory disease controls were assessed, making the disease specificity of our findings in SpA uncertain. The impaired regulatory effect of IL-19 has been found in other diseases, such as vascular disease and inflammatory bowel disease, implying that these mechanisms are not SpA-specific 33,56. Thus, the mechanisms described here could be transferable to other immune-mediated inflammatory diseases.
Taken together, this study suggests that IL-19 restricts excessive proinflammatory immune reactions. This regulatory system seems to be impaired in SpA, potentially contributing to the activation of pathways in the innate immune system seen in these diseases.
Acknowledgments
T. W. K. helped to design the study, helped to collect the SFMC and PBMC samples, helped to carry out the experiments, analysed and interpreted the data and drafted the manuscript. T. A. and C. H. helped to plan and carry out the experiments. B. S. C. and A. G. J. helped to collect the patient samples and information. M. H. and B. D. helped to design the study and supervised the project. All authors helped to analyse and interpret the data, were involved in revising the manuscript and read and approved the final manuscript. We thank Karin Skovgaard Sørensen (Department of Biomedicine, Aarhus University) for excellent technical assistance concerning the ELISA systems. The Danish Rheumatism Association, the Danish Psoriasis Association, Aage Bangs Foundation and the Faculty of Health at Aarhus University funded this work.
Disclosure
The authors declare no conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Effect of neutralizing endogenous interleukin (IL)-19 in spondyloarthrtis (SpA) synovial fluid mononuclear cell (SFMC) cultures. (a) The effect of neutralizing IL-19 (5 μg/ml) on chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-1) secretion in SFMC cultures after removing potential endotoxin contamination of the anti-IL-19 polyclonal goat antibody (n = 3). (b) The effect of neutralizing IL-19 (5 μg/ml) on CCL2/MCP-1 secretion in SFMC cultures using either the polyclonal goat antibody or a mouse monoclonal antibody (n = 3).
References
- Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol. 2014;57:28–37. doi: 10.1016/j.molimm.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Romero-Sanchez C, Tsou H-K, Jan M-S, et al. Serum monocyte chemotactic protein-1 concentrations distinguish patients with ankylosing spondylitis from patients with mechanical low back pain. J Spinal Disord Tech. 2011;24:202–207. doi: 10.1097/BSD.0b013e3181e15cc8. [DOI] [PubMed] [Google Scholar]
- Ambarus C, Yeremenko N, Tak PP, Baeten D. Pathogenesis of spondyloarthritis. Curr Opin Rheumatol. 2012;24:351–358. doi: 10.1097/BOR.0b013e3283534df4. [DOI] [PubMed] [Google Scholar]
- Inman RD. Innate immunity of spondyloarthritis: the role of Toll-like receptors. In: Lopez-Larrea C, Diaz-Pefia R, editors. Advances in experimental medicine and biology. New York: Springer; 2009. pp. 300–309. [DOI] [PubMed] [Google Scholar]
- De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52:2146–2158. doi: 10.1002/art.21155. [DOI] [PubMed] [Google Scholar]
- Candia L, Marquez J, Hernandez C, Zea AH, Espinoza LR. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? J Rheumatol. 2007;34:374–379. [PubMed] [Google Scholar]
- Zhang X, Glogauer M, Zhu F, Kim T-H, Chiu B, Inman RD. Innate immunity and arthritis: neutrophil Rac and Toll-like receptor 4 expression define outcomes in infection-triggered arthritis. Arthritis Rheum. 2005;52:1297–1304. doi: 10.1002/art.20984. [DOI] [PubMed] [Google Scholar]
- Tsui FW, Xi N, Rohekar S, et al. Toll-like receptor 2 variants are associated with acute reactive arthritis. Arthritis Rheum. 2008;58:3436–3438. doi: 10.1002/art.23967. [DOI] [PubMed] [Google Scholar]
- Oktayoglu P, Em S, Tahtasiz M, et al. Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatol Int. 2013;33:1327–1331. doi: 10.1007/s00296-012-2578-y. [DOI] [PubMed] [Google Scholar]
- Duruöz MT, Turan Y, Cerrahoglu L, Isbilen B. Serum hyaluronic acid levels in patients with ankylosing spondylitis. Clin Rheumatol. 2008;27:621–626. doi: 10.1007/s10067-007-0757-0. [DOI] [PubMed] [Google Scholar]
- Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SR, Rösen-Wolff A, Tsokos GC, Hedrich CM. Biological properties and regulation of IL-10 related cytokines and their contribution to autoimmune disease and tissue injury. Clin Immunol. 2012;143:116–127. doi: 10.1016/j.clim.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Gallagher G, Dickensheets H, Eskdale J, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol. 2004;4:577–592. doi: 10.1016/j.intimp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Xu W, Brender T, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor–ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- Logsdon NJ, Deshpande A, Harris BD, Rajashankar KR, Walter MR. Structural basis for receptor sharing and activation by interleukin-20 receptor-2 (IL-20R2) binding cytokines. Proc Natl Acad Sci USA. 2012;109:12704–12709. doi: 10.1073/pnas.1117551109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S-C, Cheng Y-C, Wang Y-C, et al. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol. 2004;173:6712–6718. doi: 10.4049/jimmunol.173.11.6712. [DOI] [PubMed] [Google Scholar]
- Jordan WJ, Eskdale J, Boniotto M, et al. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol. 2005;35:1576–1582. doi: 10.1002/eji.200425317. [DOI] [PubMed] [Google Scholar]
- Oral HB, Kotenko SV, Yilmaz M, et al. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- Corvaisier M, Delneste Y, Jeanvoine H, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLOS Biol. 2012;10:e1001395. doi: 10.1371/journal.pbio.1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C, Park SH, Daley E, et al. Interleukin-19: a constituent of the regulome that controls antigen presenting cells in the lungs and airway responses to microbial products. PLOS ONE. 2011;6:e27629. doi: 10.1371/journal.pone.0027629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher G, Eskdale J, Jordan W, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Buzas K, Oppenheim JJ, Zack Howard OM. Myeloid cells migrate in response to IL-24. Cytokine. 2011;55:429–434. doi: 10.1016/j.cyto.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y-T, Nakajima H, Takeuchi T. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr Pharm Des. 2011;17:3776–3780. doi: 10.2174/138161211798357845. [DOI] [PubMed] [Google Scholar]
- Azuma Y-T, Matsuo Y, Kuwamura M, et al. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16:1017–1028. doi: 10.1002/ibd.21151. [DOI] [PubMed] [Google Scholar]
- Ellison S, Gabunia K, Richards JM, et al. IL-19 reduces ligation-mediated neointimal hyperplasia by reducing vascular smooth muscle cell activation. Am J Pathol. 2014;184:2134–2143. doi: 10.1016/j.ajpath.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõks S, Kingo K, Rätsep R, Karelson M, Silm H, Vasar E. Combined haplotype analysis of the interleukin-19 and -20 genes: relationship to plaque-type psoriasis. Genes Immun. 2004;5:662–667. doi: 10.1038/sj.gene.6364141. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Furusho JK, Alvarez-León E, Fragoso JM, Gozalishvilli A, Vallejo M, Vargas-Alarcón G. Protective role of interleukin-19 gene polymorphisms in patients with ulcerative colitis. Hum Immunol. 2011;72:1029–1032. doi: 10.1016/j.humimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Prendergast JG, Aldhous MC, et al. Genome-wide methylation profiling in Crohn's disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18:889–899. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- Cantó E, Garcia Planella E, Zamora-Atenza C, et al. Interleukin-19 impairment in active Crohn's disease patients. PLOS ONE. 2014;9:e93910. doi: 10.1371/journal.pone.0093910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma YT, Matsuo Y, Nakajima H, et al. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci. 2011;115:105–111. doi: 10.1254/jphs.10r02cr. [DOI] [PubMed] [Google Scholar]
- England RN, Autieri MV. Anti-inflammatory effects of interleukin-19 in vascular disease. Int J Inflam. 2012;2012:1–10. doi: 10.1155/2012/253583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am J Physiol Cell Physiol. 2013;305:C255–C265. doi: 10.1152/ajpcell.00069.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Cheng BC, Hsu CC, et al. Induced interleukin-19 contributes to cell-mediated immunosuppression in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2011;92:1252–1259. doi: 10.1016/j.athoracsur.2011.04.061. [DOI] [PubMed] [Google Scholar]
- Alanärä T, Karstila K, Moilanen T, Silvennoinen O, Isomäki P. Expression of IL-10 family cytokines in rheumatoid arthritis: elevated levels of IL-19 in the joints. Scand J Rheumatol. 2010;39:118–126. doi: 10.3109/03009740903170823. [DOI] [PubMed] [Google Scholar]
- Sakurai N, Kuroiwa T, Ikeuchi H, et al. Expression of IL-19 and its receptors in RA: potential role for synovial hyperplasia formation. Rheumatology (Oxf) 2008;47:815–820. doi: 10.1093/rheumatology/ken061. [DOI] [PubMed] [Google Scholar]
- Hsu Y-H, Hsieh P-P, Chang M-S. Interleukin-19 blockade attenuates collagen-induced arthritis in rats. Rheumatology (Oxf) 2012;51:434–442. doi: 10.1093/rheumatology/ker127. [DOI] [PubMed] [Google Scholar]
- Scrivo R, Conigliaro P, Riccieri V, et al. Distribution of IL-10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin Exp Immunol. 2014;179:300–308. doi: 10.1111/cei.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- Kragstrup TW, Jalilian B, Hvid M, et al. Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis. Arthritis Res Ther. 2014;16:R42. doi: 10.1186/ar4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KB, Jurik AG. Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res (Hoboken) 2010;62:11–18. doi: 10.1002/acr.20008. [DOI] [PubMed] [Google Scholar]
- Madsen KB, Jurik AG. MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol. 2010;65:6–14. doi: 10.1016/j.crad.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:703–709. doi: 10.1002/art.21445. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:502–509. doi: 10.1002/art.21337. [DOI] [PubMed] [Google Scholar]
- Kragstrup TW, Vorup-Jensen T, Deleuran B, Hvid M. A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springerplus. 2013;2:1–10. doi: 10.1186/2193-1801-2-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LAJ. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Valdez PA, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otkjaer K, Kragballe K, Funding AT, et al. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol. 2005;153:911–918. doi: 10.1111/j.1365-2133.2005.06800.x. [DOI] [PubMed] [Google Scholar]
- Romer J, Hasselager E, Norby PL, Steiniche T, Thorn J, Kragballe K. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine A or calcipotriol. J Invest Dermatol. 2003;121:1306–1311. doi: 10.1111/j.1523-1747.2003.12626.x. [DOI] [PubMed] [Google Scholar]
- Fonseca-Camarillo G, Furuzawa-Carballeda J, Granados J, Yamamoto-Furusho JK. Expression of interleukin (IL)-19 and IL-24 in inflammatory bowel disease patients: a cross-sectional study. Clin Exp Immunol. 2014;177:64–75. doi: 10.1111/cei.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte E, Kokolakis G, Witte K, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol. 2014;134:2757–2767. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- Kragstrup TW, Otkjaer K, Holm C, et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Cuneo AA, Herrick D, Autieri MV. IL-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol. 2010;49:647–654. doi: 10.1016/j.yjmcc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of neutralizing endogenous interleukin (IL)-19 in spondyloarthrtis (SpA) synovial fluid mononuclear cell (SFMC) cultures. (a) The effect of neutralizing IL-19 (5 μg/ml) on chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-1) secretion in SFMC cultures after removing potential endotoxin contamination of the anti-IL-19 polyclonal goat antibody (n = 3). (b) The effect of neutralizing IL-19 (5 μg/ml) on CCL2/MCP-1 secretion in SFMC cultures using either the polyclonal goat antibody or a mouse monoclonal antibody (n = 3).


