Abstract
Critically ill patients display a state of immunosuppression that has been attributed in part to decreased plasma arginine concentrations. However, we and other authors have failed to demonstrate a clinical benefit of L-arginine supplementation. We hypothesize that, in these critically ill patients, these low plasma arginine levels may be secondary to the presence of granulocytic myeloid-derived suppressor cells (gMDSC), which express arginase known to convert arginine into nitric oxide (NO) and citrulline. Indeed, in a series of 28 non-surgical critically ill patients, we showed a dramatic increase in gMDSC compared to healthy subjects (P = 0·0002). A significant inverse correlation was observed between arginine levels and gMDSC (P = 0·01). As expected, gMDSC expressed arginase preferentially in these patients. Patients with high gMDSC levels on admission to the medical intensive care unit (MICU) presented an increased risk of death at day 7 after admission (P = 0·02). In contrast, neither plasma arginine levels, monocytic MDSC levels nor neutrophil levels were associated with overall survival at day 7. No relationship was found between body mass index (BMI) or simplified acute physiology score (SAPS) score, sequential organ failure assessment (SOFA) score or gMDSC levels, eliminating a possible bias concerning the direct prognostic role of these cells. As gMDSC exert their immunosuppressive activity via multiple mechanisms [production of prostaglandin E2 (PGE2), interleukin (IL)-10, arginase, etc.], it may be more relevant to target these cells, rather than simply supplementing with L-arginine to improve immunosuppression and its clinical consequences observed in critically ill patients.
Keywords: arginine, biomarker, critically ill patients, granulocytic myeloid-derived suppressor cells (gMDSC), immunosuppression
Introduction
Myeloid-derived suppressor cells (MDSC) comprise a heterogeneous population of immature myeloid cells at different stages of differentiation 1. Although many subtypes of MDSC have been described in mice and humans, they can be classified into two major subsets: a monocytic MDSC population [CD14+human leucocyte antigen (HLA)-DRlow/−] and a granulocytic MDSC population (CD3, CD19, CD56, CD14)neg CD15+ HLA-DRneg). These cells do not express markers of the T, B and natural killer (NK) cell lineage and the granulocytic population is also devoid of markers of the CD14 monocyte lineage 2. MDSC are considered to be immunosuppressive cells, as they are able to inhibit many immune functions. Interestingly, in healthy subjects, cells with this phenotype are rare, non-immunosuppressive and differentiate rapidly into mature myeloid cells. In cancer patients, these cells are expanded and activated, resulting in acquisition of potent immunosuppressive activity and altered ability to differentiate 3,4.
MDSC production has also been reported in a number of pathological conditions other than cancer, including traumatic stress, burn injury and bacterial and parasitic infections 5–7.
Various non-exclusive mechanisms have been proposed to explain the immunosuppressive activity of these MDSC, including the expression or production of arginase I, reactive oxygen/nitrogen species [peroxynitrites, nitric oxide (NO)], transforming growth factor (TGF)-β, ADAM metallopeptidase domain 17 (ADAM17), galectin-9 and vascular endothelial growth factor (VEGF) 8. Among these various factors, extensive research has focused on the expression of arginase by MDSC and its subsequent activity on its substrate (i.e. arginine). Arginase transforms arginine into ornithine (and subsequently into aliphatic polyamines and urea), whereas NO synthase converts arginine into NO and L-citrulline. Depletion of arginine (i.e. arginine deficiency syndrome) by arginase I plays a major role in MDSC suppression independently of cell-to-cell contact 9. In the absence of arginine, T cells are arrested in G0–G1 of the cell cycle, leading to inhibition of their proliferation and functions 10. NO production suppresses T cell function by blocking phosphorylation or promoting nitration of signalling proteins, resulting in inhibition of interleukin (IL)-2 and interferon (IFN) responsiveness 11,12.
In humans, it has been shown that granulocytic MDSC express high levels of arginase I and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox1) 13–15, whereas the monocytic population of MDSC express inducible NO synthase (iNOS) (NOS2) together with sustained high NO production 16.
Various studies have reported decreased plasma arginine concentrations in critically ill patients secondary to sepsis or other causes 17–21. This arginine depletion has been associated with the immunosuppression state observed in these patients 22. Interestingly, our group recently reported a marked increase in ornithine synthesis in critically ill patients treated by L-arginine supplementation, while citrulline/NO synthesis was only minimally stimulated 21. These results suggest that, in these patients, exogenous arginine is metabolized via the arginase pathway, rather than the iNOS pathway. The aim of this ancillary study was to determine granulocytic MDSC in this group of patients, as these cells express arginase and low iNOS activity preferentially and could therefore contribute to arginine depletion, explaining the preponderance of arginase activity over the NOS pathway in these critically ill patients. The results showed a marked increase in granulocytic MDSC in these patients that was correlated inversely with plasma arginine concentrations and overall survival.
Patients and methods
Patients
Patients (n = 28) included in this study were part of a double-blind randomized clinical trial of L-arginine supplementation [200 mg/kg/day for 5 days from admission to the Medical Intensive Care Unit (MICU) versus standard enteral nutrition plus placebo]. The clinical results of this study have been published previously 21.
Inclusion criteria, recorded on the day of admission (day 1 of MICU stay), were as follows: age >18 years, medical patient (absence of recent surgery or trauma), initial aggression <5 days, mechanically ventilated with expected duration of mechanical ventilation >2 days, need for enteral nutrition, absence of previous immunosuppression and nasal NO <60 parts per billion (ppb). Exclusion criteria were as follows: severe sepsis, septic shock and pregnancy.
The study was approved by our institutional review board and the protocol was registered at ClinicalTrials.gov Identifier: NCT01038622. Patients were enrolled from 3 November 2009 to 1 October 2011. Patients were enrolled after obtaining next-of-kin written informed consent.
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Supporting information, Fig. S1 and the primary reasons for admission to the MICU are shown in Supporting information, Table S1.
Blood samples from 12 healthy subjects were also obtained from the Etablissement Français du Sang (EFS, Rungis, France) for the purposes of this ancillary study.
MDSC subpopulation determination
Granulocytic MDSC analyses were performed on whole blood after Ficoll-Paque density gradients to eliminate neutrophils. The following labelled anti-human monoclonal antibodies were used for staining: anti-lineage (LIN) fluorescein isothiocyanate (FITC) including anti-CD3 [clone A-dmDT390-bisFv(UCHT1)], -CD19 [clone CD19 antibody (HIB19)], -CD56 [clone N-CAM-16 monoclonal antibody (NCAM)16·2], -CD14 [clone CD14 antibody monoclonal (MoP9)] (Becton Dickinson, Pont de Claix, France), PE-labelled anti-CD33 (clone WM53) (Biolegend-Ozyme, Saint-Quentin-en-Yvelines, France) and allophycocyanin (APC)-labelled anti-HLA-DR (clone IMMU-357) (Beckman Coulter, Villepinte, France). At least 105 events were acquired for analysis. Briefly, following the initial forward-/side-scatter (FS/SSC) discrimination to eliminate cell debris and singlet selection (FSC-H versus FSC-A), the gate was set on LIN-negative cells; CD33+ cells were then gated and the percentage of HLA-DRneg/low cells was measured on these gated populations. The percentage of granulocytic MDSC was defined as the percentage of CD33+LINneg (including CD14neg cells) HLA-DRneg cells in total peripheral blood mononuclear cells (PBMC) (Supporting information, Fig. S2), as proposed by Fricke 23. As other groups have defined MDSC as the percentage of CD15+CD14negHLA-DRneg cells in total PBMC 24, we compared these two populations and demonstrated that they were correlated closely (Supporting information, Fig. S3). As granulocytic MDSC have been shown to be sensitive to cryopreservation/thawing 13, all analyses were performed on fresh samples.
For determination of CD14+HLA-DRneg/low cells, also called monocytic MDSC (mMDSC) 3, the analysis was also performed on whole blood after Ficoll-Paque density gradients. PBMC were stained with the following monoclonal antibodies: FITC-labelled anti-CD14 (BD-Bioscience) and APC-labelled anti-HLA-DR (Beckman Coulter) (Supporting information, Fig. S2b).
Isotype control antibodies were introduced in each experiment. Data acquisition was performed using a fluorescence activated cell sorter (FACS)Calibur flow cytometer (BD Biosciences) and were analysed using CellQuest (BD Biosciences), as described previously 25.
Intracellular staining for arginase detection
Cells were washed in flow cytometry fixation buffer (R&D Systems, Lille, France) and incubated at room temperature for 10 min. After washing, cells were then resuspended in flow cytometry permeabilization/wash buffer I (R&D Systems) including 5% human serum (PAA Laboratory, Velizy-Villacoublay, France). Anti-arginase 1 [human/mouse arginase 1 APC polyclonal sheep immunoglobulin (Ig)G] or isotype control (R&D Systems) were then incubated at 4°C for 45 min. After washing in 2 ml of flow cytometry permeabilization/wash buffer I, the final cell pellet was resuspended in 500 μl of flow cytometry staining buffer (R&D Systems).
Determination of L-arginine and nasal NO fraction
For arginine assay, blood was collected in heparin-containing tubes (17 IU/ml), which were centrifuged immediately (10 min, 2500 g, 4°C). The supernatant was stored at −80°C until analysis. Analysis was performed by ion-exchange chromatography, as described previously 26.
For nasal NO fraction, gas was sampled through a nasal prong introduced approximately 2 cm from the aperture of one nostril, as described previously 27.
Statistical analysis
For continuous variables, Student's t-test was used for normally distributed variables and the Wilcoxon test was used for non-normally distributed variables. The χ2 test was used for categorical variables.
Correlations between parameters were calculated using Spearman's rank correlations, when both variables were continuous. Logistic regressions were used to estimate associations between binary variables and continuous variables.
A P-value <0·05 was considered significant; sas statistical software (release 9·2; SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
Granulocytic MDSC (gMDSC) and monocytic MDSC (mMDSC) are increased in critically ill patients
gMDSC and mMDSC were measured in, respectively, 28 and 27 unselected, non-surgical critically ill patients without initial severe sepsis and admitted to the MICU. A dramatic increase of gMDSC was observed in critically ill patients on admission to the MICU compared to 12 healthy subjects, as the median percentage of gMDSC was 14% [interquartile range (IQR) = 6%; 23%] in critically ill patients versus 0·85% (IQR = 0·6%; 1·1%) in healthy subjects (Fig. 1) (P = 0·0002). Percentages of gMDSC ranging from 0·5 to 43% were observed in critically ill patients, while these percentages never exceeded 1·6% in healthy subjects (Fig. 1). Similarly, percentages of mMDSC were higher in critically ill patients (median: 4·18%; IQR = 1·46%; 12·38%), than in healthy subjects (median: 0·17%; IQR = 0·1%; 0·22%) (P = 0·0039).
Fig 1.
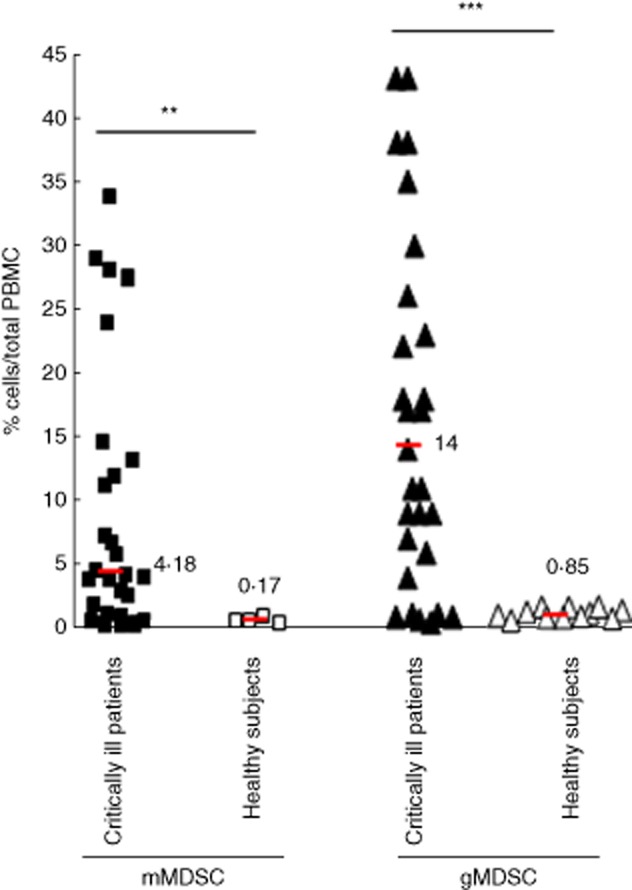
Granulocytic and monocytic myeloid-derived suppressor cells (MDSC) levels were increased in critically ill patients. Granulocytic MDSC [lineage (LIN)negCD33+human leucocyte antigen R-related (HLA-DR)neg] (n = 28) (▴, △) and monocytic MDSC (CD14+HLA-DRneg) (n = 27) (▪, □) were measured on day 1 [admission to the medical intensive care unit (MICU)] in critically ill patients (▴, ▪) without sepsis and in healthy subjects (△, □). Median is indicated by horizontal bars. *P < 0·01; **P < 0·01; ***P < 0·001.
We did not find any correlation between the age and gender of patients and the levels of gMDSC (Supporting information, Fig. S4).
gMDSC express high levels of arginase and are correlated inversely with L-arginine levels
To support the link between gMDSC and plasma L-arginine, arginase levels were measured in gMDSC and various myeloid cell subpopulations (mMDSC and HLA-DR+CD14+ cells). Very high levels of arginase were found in gMDSC, while very low levels were found in mMDSC and HLA-DR+ monocytes (Fig. 2). L-arginine levels were then correlated with granulocytic MDSC or mMDSC. As expected, plasma arginine levels were low in this group of patients [mean: 38 μmol/l (normal values: 60–80 μmol/l)]. A significant inverse correlation was observed between gMDSC levels and arginine levels (P = 0·0137) (Fig. 3a). In contrast, no correlation was observed between plasma arginine concentrations and mMDSC levels (P = 0·34) (Fig. 3b). These results support our hypothesis concerning a putative role of the arginase expressed by gMDSC on arginine depletion.
Fig 2.
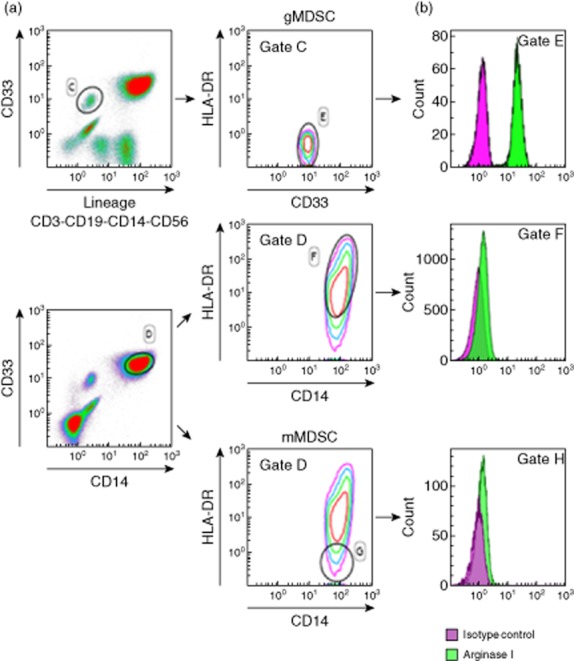
Granulocytic myeloid-derived suppressor cells (gMDSC) express high levels of arginase. Intracellular arginase (b) was measured by cytometry on gMDSC (a, top) and mMDSC and CD14+ human leucocyte antigen D-related (HLA-DR)+ monocytes (b, bottom), identified as described previously in Fig. 1 and Material and methods. Isotype controls were included for each antibody.
Fig 3.
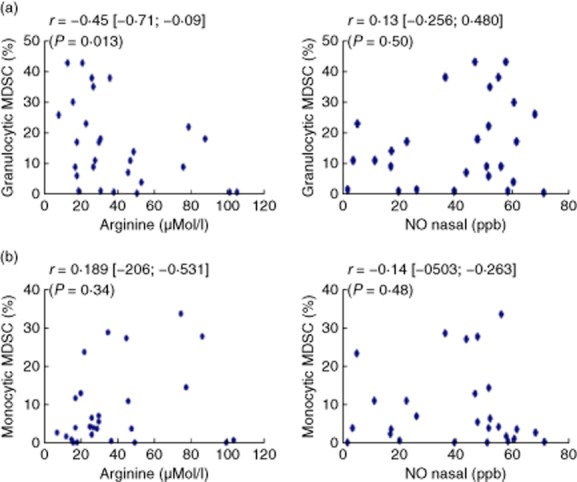
Correlation of myeloid-derived suppressor cells (MDSC) subpopulations with L-arginine and nitric oxide (NO). Granulocytic MDSC (a) and monocytic MDSC (b) measured in critically ill patients on day 1 were compared to plasma L-arginine (left) and nasal NO (right). Spearman's test was used to measure correlations between the two quantitative variables. P < 0·05 was considered statistically significant.
As these patients were part of a clinical trial of L-arginine supplementation versus placebo, we looked for a possible bias in gMDSC levels between the two initial groups of patients (L-arginine supplementation versus placebo groups) and assessed the influence of L-arginine supplementation on MDSC levels. No significant difference in gMDSC levels was observed between the two groups of patients on day 1 and L-arginine supplementation did not modify gMDSC levels compared to the placebo group (Supporting information, Table S2).
gMDSC are not known to express iNOS 28, and no correlation was demonstrated in our population of critically ill patients between nasal NO and gMDSC (Fig. 3a) or between NO and mMDSC, although these cells have been shown to express iNOS (Fig. 3b).
gMDSC levels correlate inversely with survival in critically ill patients
Patients with high gMDSC levels on admission to the MICU (day 1) presented an increased risk of death within the 7 days following inclusion in the protocol, regardless of the markers used to measure this risk, as the median of gMDSC, defined as CD15+CD14negHLA-DRneg cells, was higher (25%, IQR = 10·00; 36·00) in the 13 patients who died than in the 15 patients who were alive on day 7 (7%, IQR = 0·6; 18·00) (P = 0·02) (Table 1). A similar positive correlation was found when gMDSC were measured as LINnegCD33+HLA-DRneg cells (P = 0·049). In contrast, on day 1, neither plasma arginine levels nor mMDSC levels were associated with overall survival at day 7. As gMDSC are considered to be precursors of neutrophils, a parameter that is much easier to monitor than gMDSC, we also demonstrated that median neutrophil levels on day 1 were not correlated with mortality in this group of critically ill patients (Table 1).
Table 1.
Granulocytic myeloid-derived suppressor cells (MDSC) on day 1 correlates with survival in critically ill patients
| Death | P-value | |||
|---|---|---|---|---|
| Variables on day 1 | Yes | No | ||
| gMDSC† (%) | n | 13 | 15 | 0·0209 |
| Median (IQR) | 25 (10; 36) | 7 (0·6; 18) | ||
| gMDSC‡ (%) | n | 13 | 15 | 0·0499 |
| Median (IQR) | 18 (9; 35) | 9 (1; 18) | ||
| mMDSC (%) | n | 12 | 15 | |
| Median (IQR) | 4·72 (1·46; 12·38) | 4·18 (0·77; 14·47) | 0·932 | |
| Arginine μM/l | n | 13 | 17 | 0·2246 |
| Median (IQR) | 27 (17; 36) | 31 (23; 49) | ||
| Neutrophils (G/l) | n | 13 | 17 | 0·1266 |
| Median (IQR) | 12·5 (10·7; 17·9) | 11 (7·3; 12·55) | ||
Median levels of the various variables [granulocytic myeloid-derived suppressor cells (gMDSC), monocytic myeloid-derived suppressor cells (mMDSC), arginine and neutrophils] were compared between critically ill patients who were alive or dead on day 7; n = number of patients.
gMDSC defined as % CD15+CD14neghuman leucocyte antigen D-related (HLA-DR)neg/peripheral blood mononuclear cells (PBMC)
gMDSC defined as %lineage (LIN)negCD33+ HLA-DRneg/PBMC.
To eliminate a possible bias concerning the significance of these results, we then tested whether MDSC levels were correlated with conventional prognostic factors used to stratify these patients. No correlations were observed between body mass index (BMI) or simplified acute physiology score (SAPS) score or sequential organ failure assessment (SOFA) score and MDSC levels on inclusion of these patients (Table 2).
Table 2.
Correlation of MDSC levels on day 1 with conventional prognostic criteria in critically ill patients
| Prognostic criteria | Statistical test | n | Rho (95%CI) | P-value for H0: Rho = 0 | |
|---|---|---|---|---|---|
| gMDSC at day 1 | BMI | Spearman | 28 | −0·27 [−0·58; 0·11] | 0·15 |
| gMDSC at day 1 | SAPS score | Spearman | 28 | 0·28 [−0·1; 0·59] | 0·14 |
| gMDSC at day 1 | SOFA | Spearman | 28 | 0·24 [−0·14; 0·56] | 0·2 |
Granulocytic myeloid-derived suppressor cells (gMDSC) levels on day 1 [date of admission to the medical intensive care unit (MICU)] were correlated with the various known prognostic factors used to stratify these critically ill patients. Spearman's test was used for statistical analysis; n = number of patients. BMI = body mass index; CI = confidence interval; SAPS = simplified acute physiology score; SOFA = sequential organ failure assessment.
Discussion
In the present study, we show that critically ill patients display high levels of gMDSC compared to healthy subjects. In addition, gMDSC appear to be a new prognostic biomarker in critically ill patients, which is correlated inversely with plasma arginine levels and overall survival.
It has been demonstrated previously in cancer patients that increased arginase activity restricted to granulocytic MDSC results in significantly decreased plasma arginine levels 13–15,29. Depletion of these MDSC restored IFN-γ production and T cell proliferation in these cancer patients 30. We have not performed functional studies in the present work due to the limited volume of blood collected.
Arginase activity was also increased in the setting of sepsis and oxidative stress 31. Other mechanisms may explain the low arginine levels observed in critically ill patients, as arginine is derived from diet, protein breakdown and de-novo synthesis from citrulline by argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL), a step which occurs mainly in the kidney and liver 32. Gut enterocytes are an important source of citrulline production, which can be impaired by critical illness, leading to decreased availability of citrulline 33,34. Citrulline supplementation increases plasma citrulline and arginine in healthy subjects 35 and in various pathological states, including hypercatabolic situations 36. L-arginine depletion may therefore be multi-factorial, but our study strongly suggests a novel mechanism linked to high levels of gMDSC, which express high levels of arginase (Fig. 2) in this group of critically ill patients. Whiteside's group showed clearly that only HLA-DRnegLINnegCD15+ cells expressed high levels of arginase I, whereas monocytic MDSC, including CD14+HLADR−/low cells, tend to express iNOS 13. Other myeloid cell populations present in the blood, such as mMDSC and neutrophils, were not correlated with L-arginine levels (Fig. 3 and data not shown), supporting the relationship between gMDSC and plasma L-arginine levels 29.
Other myeloid cell populations, such as arginase-expressing ‘alternatively activated’ M2 macrophages, have been detected in subcutaneous and visceral adipose tissues from critically ill patients and may also participate in L-arginine depletion 37.
Various mechanisms may explain the increase in both gMDSC and mMDSC in these critically ill patients. Proinflammatory mediators, such as IL-1, IL-6, PGE2 and S100 proteins, known to be increased in critically ill patients 38, drive the expansion of MDSC. Nuclear factor-kappa B (NF-kB) signalling mediated by IL-1 is also required for MDSC expansion in some settings. Interestingly, increased NF-kB activation in myeloid cells has been reported in critically ill patients with poor prognosis 39. Induction of CXCR4 and its CXCL12 ligand by PGE2 also promotes recruitment of MDSC 40. Haematopoietic growth factors, such as stem cell factor, granulocyte–macrophage colony-stimulating factor (GM-CSF) and granulocyte-CSF (G-CSF), have also been involved in the expansion of MDSC 8. Few studies have distinguished clearly the specific mechanisms involved in gMDSC and mMDSC generation. Plasma IL-6 was correlated with gMDSC in cancer patients 41, and we found high IL-6 levels in our series of critically ill patients (data not shown). IL-6 signals via signal transducer and activator of transcription-3 (STAT-3), which up-regulates NADPH oxidase components in gMDSC 42. STAT-3 has also been shown to be essential for mobilization and tissue accumulation of MDSC 43. In contrast, STAT-1 is particularly important for mMDSC function via its effect on iNOS expression 44.
Expansion of myeloid progenitor cells and immature granulocytes, including promyelocytes, myelocytes and metamyelocytes, has also been described in a subgroup of critically ill patients with sepsis and appears to be helpful to discriminate infected and non-infected patients 45. However, these immature myeloid cells were not suitable as a prognostic marker for mortality, and their relationship with MDSC remains to be established 45. Preclinical studies in sepsis have reported a protective role of MDSC via the release of anti-microbial products and by reducing the magnitude of septic responses 43. In our study in non-surgical critically ill patients without severe sepsis, gMDSC were not associated with nosocomial infections during the first 7 days of the MICU stay, supporting the absence of a strict correlation between gMDSC and infection (data not shown).
High gMDSC levels were correlated inversely with mortality of critically ill patients during the first 7 days after admission to the MICU, while no association was observed between neutrophil levels, mMDSC (CD14+HLADRneg/low) levels and survival. Although the mMDSC cell population has been proposed as a surrogate marker of immune failure, it is now considered not to provide valuable information on the outcome of patients, as confirmed by the results of the present study 46.
Interestingly, a very recent study showed that in critically ill patients with sepsis, interphase neutrophils recovered after gradient density was increased. A direct relationship was demonstrated between ‘interphase neutrophils’ and T cell dysfunction. Because gMDSC are considered as atypical neutrophils (immature or activated) with low buoyant density (‘interphase neutrophils’), these results support our data and the link between gMDSC and immunosuppression 47.
To eliminate a bias related to a link between gMDSC and known clinical prognostic markers to stratify patients, we demonstrated an absence of correlation between gMDSC levels and BMI or the SAPS or the SOFA scores (Table 2).
This novel biomarker clearly needs to be evaluated in a larger series of patients, but it may provide additional information with respect to other biomarkers [procalcitonin (PCT), soluble triggering receptors expressed on myeloid (sTREM1), soluble urokinase-type plasminogen activator (suPAR), CD64 expressed by neutrophils] proposed to identify critically ill patients with sepsis 48.
Many studies have emphasized a link between critical illness and immunosuppression and the low L-arginine levels, often observed in these patients, were considered subsequently to be a possible mechanism to explain these patients' immune defects 49. However, several meta-analyses and our own study failed to demonstrate the clinical benefit of L-arginine supplementation 21,50. As the present study proposed that L-arginine depletion may be due partly to the high gMDSC levels present in these critically ill patients, novel therapeutic strategies designed to deplete these immunosuppressive cells could be more relevant than simple L-arginine supplementation as, in addition to arginase expression, other mechanisms (reactive oxygen species, PGE2, IL-10, etc.) also mediate the suppressive and deleterious effects of gMDSC, which could mask the beneficial effect of L-arginine supplementation alone. Interestingly, various compounds (Cox-2 inhibitors, synthetic triterpenoids, nitroaspirins, phosphodiesterase and STAT-3 inhibitors, etc.) that block multiple activities of MDSC have been developed recently 51. Further studies are required to determine whether they can be used to improve immunosuppression of critically ill patients by targeting these novel suppressive cells.
Conclusions
gMDSC are increased and correlate inversely with plasma arginine and overall survival in critically ill patients. As gMDSC exert their immunosuppressive activity via multiple mechanisms (production of PGE2, IL-10, arginase, etc.), it may be more relevant to target these cells rather than simply supplementing with L-arginine to improve immunosuppression in critically ill patients.
Acknowledgments
This work was supported by grants from Labex Immuno-oncology, Canceropole-région Ile-de-France, Agence Nationale de la Recherche (ANR), Site intégré de recherche intégré en cancérologie (SIRIC-Carpem), Institut National contre le Cancer (INCA) and Ligue Nationale contre le Cancer.
Disclosure
None.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Consort flow diagram.
Fig. S2. Gating strategy to measure granulocytic monocytic granulocytic myeloid-derived suppressor cells (MDSC) and monocytic MDSC. (a) To measure granulocytic MDSC, peripheral blood mononuclear cells (PBMC) were stained with anti-lineage (LIN) fluorescein isothiocyanate (FITC), including anti-CD3, -CD19, -CD56, -CD14 and phycoerythrin (PE)-labelled anti-CD33 and allophycocyanin (APC)-labelled anti-human leucocyte antigen D-related (HLA-DR). Following the initial forward/side-scatter (FSC/SSC) discrimination to eliminate cell debris and singlet selection (FSC-H versus FSC-A), the gate was set on LIN-negative cells, then gated on CD33+ cells, and finally the percentage of HLA-DRneg/low cells was measured on these gated populations to define the percentage of granulocytic MDSC in total PBMC. (b) To measure monocytic MDSC, cellular debris were first eliminated by a gating on FSC/SSC and then PBMC were stained with FITC-labelled CD14 and allophycocyanin (APC)-labelled HLA-DR. Isotype control antibodies were included in each experiment.
Fig. S3. Comparative analysis of granulocytic myeloid-derived suppressor cells (MDSC) defined by various combinations of markers. Granulocytic MDSC were defined as either lineage (LIN)neg (including CD14neg cells) HLA-DRneg CD33+ or CD15+ CD14neg HLA-DRneg in total peripheral blood mononuclear cells (PBMC). They were then plotted on a figure and correlations were searched between these two populations using Spearman's test.
Fig. S4. Absence of correlation between the age and the gender and granulocytic myeloid-derived suppressor cells (gMDSC) concentrations in critically ill patients. gMDSC were measured in the blood of critically ill patients and their levels compared with the age (a) and the gender (b) of patients. The correlations were searched using Spearman's test.
Table S1. No difference in the percentage of myeloid-derived suppressor cells (MDSC) before and after L-arginine administration in the two randomized groups of patients.
Table S2. Primary medical reasons for admission to the medical intensive care unit.
References
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartour E, Pere H, Maillere B, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–4302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- Mencacci A, Montagnoli C, Bacci A, et al. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunol. 2002;169:3180–3190. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmor T, Attias D, Polliack A. Myeloid-derived suppressor cells – their role in haemato-oncological malignancies and other cancers and possible implications for therapy. Br J Haematol. 2011;153:557–567. doi: 10.1111/j.1365-2141.2011.08678.x. [DOI] [PubMed] [Google Scholar]
- Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71:5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- Lu T, Gabrilovich DI. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin Cancer Res. 2012;18:4877–4882. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber PL, Thevenot P, Sierra R, et al. Subpopulations of myeloid-derived suppressor cells (MDSC) impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2013;134:2853–2864. doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond) 2009;117:23–30. doi: 10.1042/CS20080444. [DOI] [PubMed] [Google Scholar]
- Druml W, Heinzel G, Kleinberger G. Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73:908–913. doi: 10.1093/ajcn/73.5.908. [DOI] [PubMed] [Google Scholar]
- van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. Am J Clin Nutr. 2007;86:1438–1444. doi: 10.1093/ajcn/86.5.1438. [DOI] [PubMed] [Google Scholar]
- Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84:197–203. doi: 10.1093/ajcn/84.1.197. [DOI] [PubMed] [Google Scholar]
- Tadie JM, Cynober L, Peigne V, et al. Arginine administration to critically ill patients with a low nitric oxide fraction in the airways: a pilot study. Intensive Care Med. 2013;39:1663–1665. doi: 10.1007/s00134-013-2984-y. [DOI] [PubMed] [Google Scholar]
- Stephan F, Yang K, Tankovic J, et al. Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med. 2002;30:315–322. doi: 10.1097/00003246-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- Loi C, Zazzo JF, Delpierre E, et al. Increasing plasma glutamine in postoperative patients fed an arginine-rich immune-enhancing diet – a pharmacokinetic randomized controlled study. Crit Care Med. 2009;37:501–509. doi: 10.1097/CCM.0b013e3181958cba. [DOI] [PubMed] [Google Scholar]
- Tadie JM, Trinquart L, Janniere-Nartey C, et al. Prediction of nosocomial infection acquisition in ventilated patients by nasal nitric oxide: proof-of-concept study. Shock. 2010;34:217–221. doi: 10.1097/SHK.0b013e3181d67494. [DOI] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Bune AJ, Shergill JK, Cammack R, Cook HT. L-arginine depletion by arginase reduces nitric oxide production in endotoxic shock: an electron paramagnetic resonance study. FEBS Lett. 1995;366:127–130. doi: 10.1016/0014-5793(95)00495-u. [DOI] [PubMed] [Google Scholar]
- Curis E, Nicolis I, Moinard C, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- Cynober L. Citrulline: just a biomarker or a conditionally essential amino acid and a pharmaconutrient in critically ill patients? Crit Care. 2013;17:122. doi: 10.1186/cc12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piton G, Belon F, Cypriani B, et al. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41:2169–2176. doi: 10.1097/CCM.0b013e31828c26b5. [DOI] [PubMed] [Google Scholar]
- Rouge C, Des Robert C, Robins A, et al. Manipulation of citrulline availability in humans. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1061–1067. doi: 10.1152/ajpgi.00289.2007. [DOI] [PubMed] [Google Scholar]
- Cynober L. Potential interest of citrulline in critically ill patients. Reanimation. 2013;22:350–357. [Google Scholar]
- Langouche L, Marques MB, Ingels C, et al. Critical illness induces alternative activation of M2 macrophages in adipose tissue. Crit Care. 2011;15:R245. doi: 10.1186/cc10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling-regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28(Suppl. 4):N3–12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor kappa B activation in critically ill patients who die. Crit Care Med. 2000;28:1047–1051. doi: 10.1097/00003246-200004000-00022. [DOI] [PubMed] [Google Scholar]
- Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy-Bosse BL, Young GS, Bauer T, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE, Sackett SD, Dierssen U, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Nierhaus A, Klatte S, Linssen J, et al. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis – a prospective, observational study. BMC Immunol. 2013;14:8. doi: 10.1186/1471-2172-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intensive Care Med. 2003;29:1245–1252. doi: 10.1007/s00134-003-1686-2. [DOI] [PubMed] [Google Scholar]
- Darcy CJ, Minigo G, Piera KA, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S, Bene MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186:65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin N Am. 2008;55:647–668. doi: 10.1016/j.pcl.2008.02.009. , xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980–1990. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Consort flow diagram.
Fig. S2. Gating strategy to measure granulocytic monocytic granulocytic myeloid-derived suppressor cells (MDSC) and monocytic MDSC. (a) To measure granulocytic MDSC, peripheral blood mononuclear cells (PBMC) were stained with anti-lineage (LIN) fluorescein isothiocyanate (FITC), including anti-CD3, -CD19, -CD56, -CD14 and phycoerythrin (PE)-labelled anti-CD33 and allophycocyanin (APC)-labelled anti-human leucocyte antigen D-related (HLA-DR). Following the initial forward/side-scatter (FSC/SSC) discrimination to eliminate cell debris and singlet selection (FSC-H versus FSC-A), the gate was set on LIN-negative cells, then gated on CD33+ cells, and finally the percentage of HLA-DRneg/low cells was measured on these gated populations to define the percentage of granulocytic MDSC in total PBMC. (b) To measure monocytic MDSC, cellular debris were first eliminated by a gating on FSC/SSC and then PBMC were stained with FITC-labelled CD14 and allophycocyanin (APC)-labelled HLA-DR. Isotype control antibodies were included in each experiment.
Fig. S3. Comparative analysis of granulocytic myeloid-derived suppressor cells (MDSC) defined by various combinations of markers. Granulocytic MDSC were defined as either lineage (LIN)neg (including CD14neg cells) HLA-DRneg CD33+ or CD15+ CD14neg HLA-DRneg in total peripheral blood mononuclear cells (PBMC). They were then plotted on a figure and correlations were searched between these two populations using Spearman's test.
Fig. S4. Absence of correlation between the age and the gender and granulocytic myeloid-derived suppressor cells (gMDSC) concentrations in critically ill patients. gMDSC were measured in the blood of critically ill patients and their levels compared with the age (a) and the gender (b) of patients. The correlations were searched using Spearman's test.
Table S1. No difference in the percentage of myeloid-derived suppressor cells (MDSC) before and after L-arginine administration in the two randomized groups of patients.
Table S2. Primary medical reasons for admission to the medical intensive care unit.


