Abstract
The mechanism responsible for trafficking of monocyte-derived macrophages into kidney in the puromycin aminonucleoside model of nephrotic syndrome in rats (PAN-NS), and the significance of this infiltration, remain largely unknown. CXCL10, a chemokine secreted in many T helper type 1 (Th1) inflammatory diseases, exhibits important roles in trafficking of monocytes and activated T cells. We hypothesized that induction of circulating interferon (IFN)-γ and glomerular tumour necrosis factor (TNF)-α during PAN-NS would stimulate the release of CXCL10 by podocytes, leading to infiltration of activated immune cells and greater glomerular injury. We found that serum IFN-γ, glomerular Cxcl10 mRNA and intra- and peri-glomerular macrophage infiltration were induced strongly during the late acute phase of PAN-NS in Wistar rats, but not in nude (Foxn1rnu/rnu) rats lacking functional effector T lymphocytes. Wistar rats also developed significantly greater proteinuria than nude rats, which could be abolished by macrophage depletion. Stimulation of cultured podocytes with both IFN-γ and TNF-α markedly induced the expression of Cxcl10 mRNA and CXCL10 secretion. Together, these data support our hypothesis that increased circulating IFN-γ and glomerular TNF-α induce synergistically the production and secretion of CXCL10 by podocytes, attracting activated macrophages into kidney tissue. The study also suggests that IFN-γ, secreted from Th1 lymphocytes, may prime proinflammatory macrophages that consequently aggravate renal injury.
Keywords: chemokines, CXCL10, kidney injury, macrophages, nephrotic syndrome
Introduction
Nephrotic syndrome (NS) is a condition with diverse aetiologies and common symptoms that are largely a result of massive loss of serum proteins in the urine. The most common subtype of NS is minimal change nephrotic syndrome (MCNS), in which the glomerular injury leading to protein leakage is a consequence of effacement of the tertiary ‘foot’ processes of visceral epithelial cells (podocytes). The precise aetiology of MCNS is not known, although the primary hypothesis underlying the efficacy of anti-inflammatory treatments for MCNS is that cytokines, secreted by effector T cells, act on glomerular podocytes, resulting in altered permeability of the glomerular filtration barrier to plasma proteins.
A single injection of puromycin aminonucleoside (PAN) in rats is a model of human MCNS, comprising all its clinical features including massive proteinuria, oedema, hypoalbuminaemia, hypercholesterolaemia, responsiveness to glucocorticoid therapy and a absence of immune deposits in the glomeruli. The acute phase of PAN-NS lasts approximately 2 weeks, followed by a recovery phase with complete remission of proteinuria. However, proteinuria recurs within 8 weeks after PAN injection, and glomerular injury progresses leading to extensive glomerular sclerosis 1.
The renal insufficiency and massive proteinuria that occurs during the acute phase of PAN-NS is thought to be due to direct action of the drug on the glomerular podocyte 2, although glomerular and interstitial accumulation of activated macrophages occurs within a few days of PAN injection 3. Macrophages are heterogeneous phagocytic cell populations that can acquire differential inflammation-related phenotypes, proinflammatory (M1) and anti-inflammatory (M2), depending on the nature of the stimuli present in their microenvironment (e.g. cytokines). Interferon (IFN)-γ, secreted mainly by activated T helper type 1 (Th1) lymphocytes, natural killer (NK) and NK T cells, plays a crucial role in the development of M1 macrophages 4. Macrophage infiltration into glomeruli in PAN-NS coincides with acute renal insufficiency, and is greatest during the peak of proteinuria 5. The number and activation state of infiltrated macrophages has also been shown to correlate with the severity of renal damage and disease progression 6. These results suggest that the macrophage infiltration observed during the late acute phase of PAN-NS may have an important role in the glomerular injury induced by PAN, and may also contribute to glomerulosclerosis in the later, chronic phase of PAN-NS and in human glomerular disease.
The role of chemokines and their receptors in the precise co-ordination of inflammatory cell trafficking into damaged kidney tissue has been well established 7. Within glomerular tissue, chemokines and their receptors are expressed in resident as well as in infiltrating cells. Among them, IFN-γ-inducible protein of 10 kD (IP-10/CXCL10), a member of the α (C-X-C) subfamily and a known T cell and monocyte chemoattractant 8, has been reported to contribute to the severity of kidney diseases in several animal models of nephrosis 9. In cultured glomerular mesangial cells, CXCL10 was highly induced by stimulation with proinflammatory stimuli such as lipopolysaccharide (LPS), immune complexes, IFN-γ and tumour necrosis factor (TNF)-α 10. However, podocytes expressed the highest levels of CXCL10 in the glomerulus, and CXCL10 could modulate expression of podocyte slit diaphragm proteins 11. The precise role of podocyte CXCL10, as well as the mechanism of macrophage influx and their activation status, remains elusive in PAN-NS.
In this study, we present data supporting the hypothesis that a deficient T cell response is protective in PAN-NS in the latter stage of the acute phase of the disease. We confirmed that nude (Foxn1rnu/rnu) rats developed less proteinuria 12 days after induction of NS than immunocompetent control Wistar rats. The remarkable reduction of plasma IFN-γ, and the absence of infiltrating macrophages and glomerular CXCL10 production in nude rats, led us to propose a mechanistic model of glomerular injury. We hypothesized that significant glomerular injury occurs from release of IFN-γ by activated Th1 lymphocytes, leading to production of CXCL10 by glomerular podocytes, and the subsequent recruitment of monocyte-derived macrophages from the blood into the injured kidneys by CXCL10.
Materials and methods
Disease model
A single intravenous tail vein injection of 50 mg pf puromycin aminonucleoside (Sigma Aldrich, St Louis, MO, USA) per kg total body weight on day 0 was used to induce PAN-NS in male Wistar and athymic nude (Foxn1rnu/rnu) rats weighing ∼200 g (Harlan Laboratories, Indianapolis, IN, USA). Animals injected with saline vehicle served as controls. Macrophage depletion was by intraperitoneal injection of 5 mg clodronate liposome (CL) on days 4, 7 and 10 after PAN injection. Clodronate (Roche Diagnostics Corporation, Indianapolis, IN, USA) was encapsulated by Dr Nico van Rooijen 12. Proteinuria was measured in urine collected for 24-h periods by a modified Bradford assay 13. After euthanasia, kidneys were collected and cortex tissue isolated, embedded in optical cutting temperature compound (OCT) (Tissue-Tek, Torrance, CA, USA), or used for isolation of glomeruli by graded sieving 14. All animal procedures were conducted in compliance with the regulations of the Institutional Animal Care and Use Committee at Nationwide Children's Hospital.
Cell culture
The human podocyte cell line was the kind gift of Moin Saleem, and was cultivated and differentiated as described previously 15. Cells were treated for 6 h with 10 ng/ml IFN-γ and TNF-α (R&D Systems, Minneapolis, MN, USA) in fresh culture medium containing 1% fetal bovine serum. After medium change, the cells were cultured for an additional 24 h. Conditioned medium was removed, clarified by centrifugation and stored at −80°C.
The human monocytic cell line THP-1 [American Type Culture Collection, Manassas, VA, USA (ATCC no. TIB-202] was cultured in RPMI-1640 supplemented with 0·05 mM 2-mercaptoethanol, penicillin/streptomycin and 10% fetal bovine serum in a humidified 5% CO2 incubator at 37°C.
Cytokine measurements
Blood was collected from rats, centrifuged, and cytokine concentrations were measured in plasma using the Milliplex MAP Rat Cytokine/Chemokine assay (Millipore, Billerica, MA, USA). Results were read using the Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA). In a separate analysis, the quantity of IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (R&D Systems).
Immunofluorescence microscopy
Cryostat sections of 5-μm thickness were cut and fixed for 30 min in 2% paraformaldehyde in phosphate-buffered saline (PBS). After washing, sections were incubated in lysis/blocking buffer (5% normal donkey serum with 0·3% Triton X-100 in PBS) for 1 h and incubated with primary antibodies overnight at 4°C. Primary antibodies were directed against synaptopodin (#H-140; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD68 (ED1) and CD3 (both from AbD Serotec, Raleigh, NC, USA). Sections were washed in 0·05% Tween-20 in PBS, incubated with appropriate DyLight 594- and 488-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) for 1 h, washed and mounted [Prolong Anti-fade Gold with 4′,6-diamidino-2-phenylindole (DAPI); Invitrogen, San Diego, CA, USA]. Immunofluorescence microscopy was performed as described previously 16. Micrographs were adjusted for brightness and contrast using Adobe Photoshop (Adobe Systems, San Jose, CA, USA). Macrophages (ED1+) and T lymphocytes (CD3+) cells were counted in eight glomerular sections from each rat. Intra-glomerular cells were those within Bowman's capsule, while peri-glomerular cells were within ∼1 glomerular diameter.
Quantitative real-time–PCR (qRT–PCR)
Total RNA was extracted from glomeruli using the Trizol® reagent (Life Technologies, Grand Island, NY, USA), and reverse-transcribed using high-capacity cDNA reverse transcription (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed on the iQ5 (Bio-Rad,) using Absolute Blue™ SYBR® Green Supermix (Thermo Fischer, Waltham, MA, USA). cDNA transcribed from normal kidney cortex RNA (BioChain, Newark, CA, USA) was used to create standard curves. Melt curve analysis and agarose gel electrophoresis were performed to ensure a single major product of the proper molecular weight. After normalization to the housekeeping Rpl19 gene, the expression levels were calculated using the ΔΔCt method of Pfaffl 17. Primers (Table 1) were designed using PrimerBLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Table 1.
Primer sequences for quantitative real-time polymerase chain reaction (PCR)
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Cxcl1 | 5′-GCAAGAGCATGCACGTGGTCTCC-3′ | 5′-AGGACAGCTGGGGCCGATCTC-3′ |
| Ccl2 | 5′-GAGGCCAGCCCAGAAACCAGC-3′ | 5′-TGGGGCATTAACTGCATCTGGC-3′ |
| Ccl3 | 5′-AGGTCTCCACCGCTGCCCTT-3′ | 5′-CTTGGTCAGGAAAATGACACCCGGC-3′ |
| Ccl4 | 5′-AGCACCAATAGGCTCTGACCCTCC-3′ | 5′-TCGCTGGGGTCGGCACAGAT-3′ |
| Cxcl9 | 5′-CACTGTGGAGTTCGAGGAACCCT-3′ | 5′-TGTGCCTTGGCTGGTGCTGA-3′ |
| Cxcl10 | 5′-GAAGCACCATGAACCCAAGT-3′ | 5′-CAACATGCGGACAGGATAGA-3′ |
| Cxcl11 | 5′-GGCCACAACGGTTCCAGGCTT-3′ | 5′-GCTTGGATGTGGGGTCCAGGC-3′ |
| Cd169 | 5′-CCCCACCCGCTCCGTCATCT-3′ | 5′-TGGGGGCATGCTGCACTTGT-3′ |
| Nos2 | 5′-TGGAGGCCTTGTGTCAGCCCT-3′ | 5′-AGGCAGCAGGCACACGCAAT-3′ |
| Rpl19 | 5′-CCACAAACTGAAGGCAGAC-3′ | 5′-TCTTGGTCTCTTCCTCCTTG-3′ |
Migration assay
Monocytic (THP-1, 2·5 × 105/well) cells were suspended in conditioned medium from vehicle-treated MS13 cells and loaded into the upper compartments of chemotaxis chambers (5 μm pore Transwell; Costar, Lowell, MA, USA). Lower compartments were loaded with the same conditioned media containing 10, 50 and 250 ng/ml rhCXCL10 (rhCXCL10), 30 ng/ml monocyte chemoattractant protein-1 (MCP-1) (R&D Systems), 10 nM N-formylmethionyl-leucyl-phenylalanine (fMLP) (Sigma Aldrich) or with conditioned medium from MS13 cultures collected 1 day after treatment with IFN-γ, TNF-α or both. THP-1 cells were allowed to migrate for 180 min, and the number of cells that migrated into the lower chamber was measured by flow cytometry normalized to added fluorescein isothiocyanate (FITC)-labelled beads. For neutralization experiments, culture medium was pretreated for 30 min with 2 μg neutralizing antibody/ml.
Results
PAN nephrosis increased serum IFN-γ in Wistar but not nude rats
To assess signs of systemic inflammation in Wistar rats upon PAN-NS, we tested serum levels of an extensive panel of cytokines at 12 days after injection with PAN. Significantly greater concentrations of Th2 cytokines IL-4 (124 ± 53 versus 51 ± 6 pg/ml), IL-13 (244 ± 55 versus 126 ± 23), and especially the Th1 cytokine IFN-γ (292 ± 60 versus 17 ± 12), were found in serum from PAN-induced Wistar rats compared to vehicle-treated controls (Fig. 1a). No differences were observed in the plasma concentrations of IL-1β, IL-6, IL-17, IL-18, regulated upon activation normal T cell expressed and secreted (RANTES) or TNF-α (data not shown). Because of the significant increase in serum level of IFN-γ in the Wistar rat, we sought to evaluate the role of T cells in the production of this type-1 cytokine. Thus, in a separate experiment, we measured IFN-γ concentrations in serum from controls (sham-injected), Wistar and nude rats with PAN-NS (Fig. 1b). Twelve days after PAN injection, a significant increase in serum IFN-γ was found in blood from Wistar rats (437 ± 54 pg/ml), but not from nude rats (25 ± 2), compared with sham-injected Wistar controls (15 ± 4).
Fig 1.
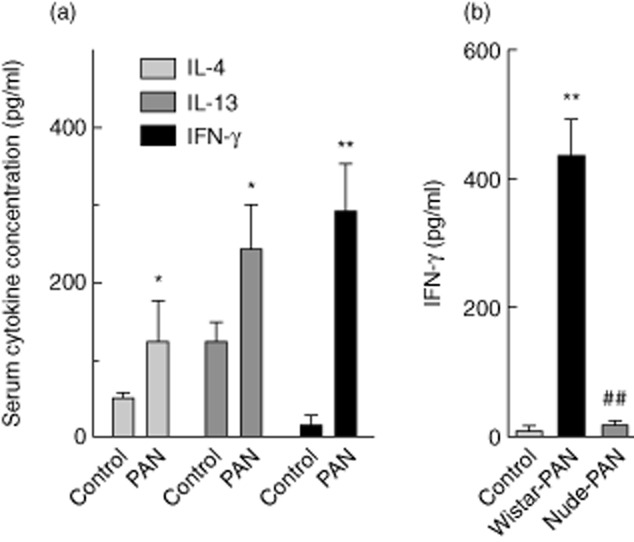
Serum interferon (IFN)-γ is induced at the peak of proteinuria induced by puromycin aminonucleoside (PAN) in Wistar but not nude (Foxn1rnu/rnu) rats. (a) Serum cytokine concentrations in blood samples taken from Wistar rats at 12 days after injection with vehicle alone (n = 4) or PAN (n = 8). (b) Serum IFN-γ concentrations in blood samples taken from Wistar or nude rats at 12 days after injection with vehicle alone (n = 4) or PAN (n = 8). Results are expressed as means ± standard error; *P < 0·05; **P < 0·01 compared with vehicle alone by unpaired, two-tailed t-test.
Proteinuria and podocyte injury after PAN-induced nephrosis
Proteinuria was measured at several time-points after PAN injection in both nude and Wistar rats, as well as in Wistar rats that had been depleted of macrophages after the initial development of proteinuria (Fig. 2a). Proteinuria at day 12 was significantly less in both nude (221 ± 62 mg/24 h) and macrophage-depleted Wistar rats (272 ± 104) than in Wistar rats (601 ± 56).
Fig 2.
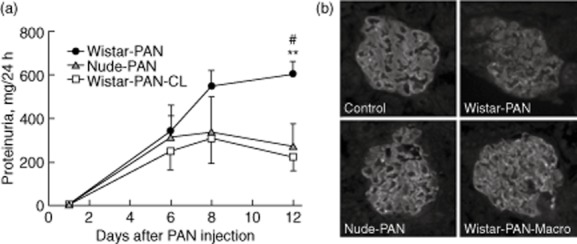
Proteinuria and podocyte injury in late stages of acute puromycin aminonucleoside (PAN) nephrosis are greater in Wistar rats than in nude rats, or in Wistar rats depleted of macrophages. (a) Plot of protein concentrations in 24-h urine samples taken at various times after PAN injection from Wistar (Wistar-PAN, n = 8) nude rats (nude-PAN, n = 4) and from Wistar rats whose macrophages were depleted by three intraperitoneal (i.p.) injections of clodronate liposomes at 4, 7 and 10 days after PAN injection (Wistar-PAN-CL, n = 4). Results are expressed as means ± standard error; **P < 0·01 compared with nude-PAN and #P < 0·05 compared with Wistar-PAN-CL by analysis of variance (anova) followed by Bonferroni's post-test. (b) Micrographs visualizing anti-synaptopodin antibody labelling of representative glomeruli in frozen sections from the same animals used in the experiment shown in (a), along with sham-injected control animals (control). Frozen sections were obtained from animals killked 12 days after PAN injection.
To assess injury to the glomerular podocyte in these models, synaptopodin was visualized in frozen sections obtained from rats euthanized on day 12 (Fig. 2b). As expected, anti-synaptopodin antibodies labelled capillary loops in glomeruli, a staining pattern characteristic of glomerular podocytes. In sections from control animals (sham-injected Wistar rats), podocyte labelling was strong and in the form of a continuous line outlining the capillary lumen. This pattern of staining was almost entirely disrupted in Wistar rats at day 12 of the PAN model. However, in both Wistar rats that had been depleted of macrophages and in nude rats there was much less disruption of the orderly capillary loop staining pattern by PAN, and the intensity of synaptopodin labelling was comparable to control animals. These results demonstrated that both macrophage depletion and the absence of mature T cells partially prevented the injury to glomerular podocytes that led to massive proteinuria in the PAN model of nephrotic syndrome.
Macrophage infiltration 12 days after PAN was greater in Wistar than nude rats
There was no significant intra-glomerular (IG) or the peri-glomerular (PG) infiltration of CD3+ T lymphocytes in nude and Wistar rats 12 days after injection with vehicle alone or PAN (data not shown). Few activated (ED1+) macrophages were observed in glomerular sections from Wistar or nude rats injected with vehicle alone (IG Wistar = 0·49 ± 0·12; IG nude = 0·40 ± 0·10; PG Wistar = 0·71 ± 0·11; PG nude = 0·64 ± 0·07 ED1+ cells/glomerulus, Fig. 3a,b) or from nude rats injected with PAN (IG = 0·40 ± 0·05; IG = 0·60 ± 0·10, Fig. 3a,b). In contrast, there was significant intra- and peri-glomerular influx of ED1+ cells in Wistar rats injected with PAN (IG = 6·1 ± 0·4; PG = 6·3 ± 0·7, Fig. 3a,b). We confirmed that injections of clodronate (CL), beginning just after initial development of proteinuria in Wistar rats on day 5, effectively prevented accumulation of peri- and intra-glomerular macrophages (IG = 0·96 ± 0·23; PG = 0·42 ± 0·36, Fig. 3b). As shown in Fig. 3c, the glomerular expression of genes encoding an activated macrophage marker (Cd169), and an enzyme characteristic of M1 macrophages (iNOS2), were also elevated in Wistar rats injected with PAN (Cd169 = 1·3 ± 0·17; Nos2 = 0·79 ± 0·18, fold change compared to normal kidney cortex) compared to vehicle-treated controls (Cd169 = 0·10 ± 0·03; Nos2 = 0·12 ± 0·06) and nude rats injected with PAN (Cd169 = 0·09 ± 0·02; Nos2 = 0·24 ± 0·11). In contrast, there were no differences between groups in the glomerular expression of Cd163, which encodes a marker for alternatively activated M2 macrophages.
Fig 3.
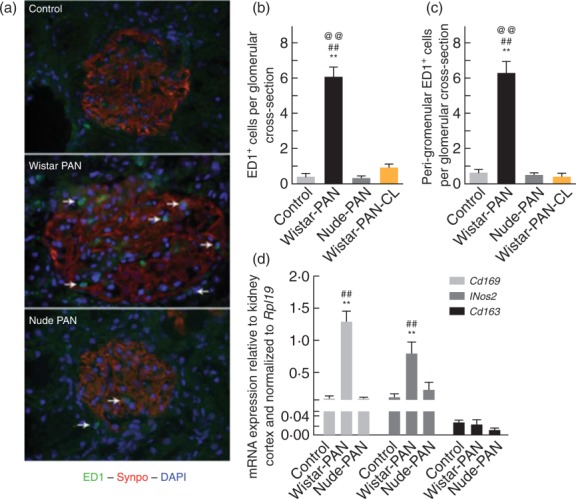
Activated (ED1+) macrophages accumulate in the glomerulus and peri-glomerular areas of Wistar rats [Wistar-puromycin aminonucleoside (PAN)], but not of nude rats (nude-PAN) or macrophage-depleted Wistar rats (Wistar-PAN-CL), during the late stage of acute PAN nephrosis. (a) Representative micrographs of glomeruli labelled with antibodies directed against ED1 (green), synaptopodin (red) and 4′,6-diamidino-2-phenylindole (DAPI) (blue) from Wistar rats injected with vehicle alone (control) or PAN (Wistar-PAN), or from nude rats injected with PAN (nude-PAN). The arrows show positions of glomerular and peri-glomerular activated (ED1+) macrophages. Results in studies of macrophage-depleted Wistar rats were similar to those shown for nude rats (not shown). Quantitative results of immunofluorescence analyses are shown in plots of intra- and peri-glomerular macrophage counts from these animals (b), including macrophage-depleted Wistar rats (Wistar-PAN-CL). Results are expressed as means ± standard error (s.e.), n = 4/group; **P < 0·01 compared with vehicle alone, ##P < 0·01 compared to nude-PAN, @@P < 0·01 compared to Wistar-PAN-CL. Shown in (d) are plots of the mRNA expression of markers of proinflammatory activated macrophages (Cd169, Nos2), and of alternatively activated macrophages (Cd163). Results are expressed as means ± s.e., n = 4/group; **P < 0·01 compared to vehicle alone and ##P < 0·01 compared to nude-PAN.
Podocyte Cxcl10 and Cxcl11 expression in vitro and in vivo
We hypothesized that the glomerular and peri-glomerular macrophage infiltration in Wistar rats during PAN-NS was a result of monocyte chemoattractants released from glomerular cells, most probably podocytes. To test this hypothesis, we measured glomerular expression of the chemokines Cxcl1, Cxcl9, Cxcl10, Cxcl11, Ccl2 (MCP-1), Ccl3 (MIP-1α) and Ccl4 (MIP-1β) (Fig. 4a). The most notable change was in the expression of members of C-X-C α chemokines, Cxcl10 and Cxcl11. Both were significantly greater in glomeruli from Wistar rats 12 days after PAN injection (Cxcl10 = 4·4 ± 1·1, Cxcl11 = 3·6 ± 0·53 fold versus normal kidney cortex) compared to controls injected with vehicle alone (Cxcl10 = 0·11 ± 0·03, Cxcl11 = 1·6 ± 0·37). Strikingly, the expression of Cxcl10 and Cxcl11 in PAN-treated nude rats was indistinguishable from expression in control, sham-injected animals (Cxcl10 = 0·18 ± 0·05, Cxcl11 = 0·24 ± 0·04). Although Ccl3 (MIP-1α) expression was modestly increased in the PAN-nude compared to the PAN-Wistar, this difference was not statistically significant when compared to control. No significant differences were observed in the expression levels of CXCL1, CXCL9, CCL2 or CCL4 in PAN-nude or PAN-Wistar compared to controls.
Fig 4.
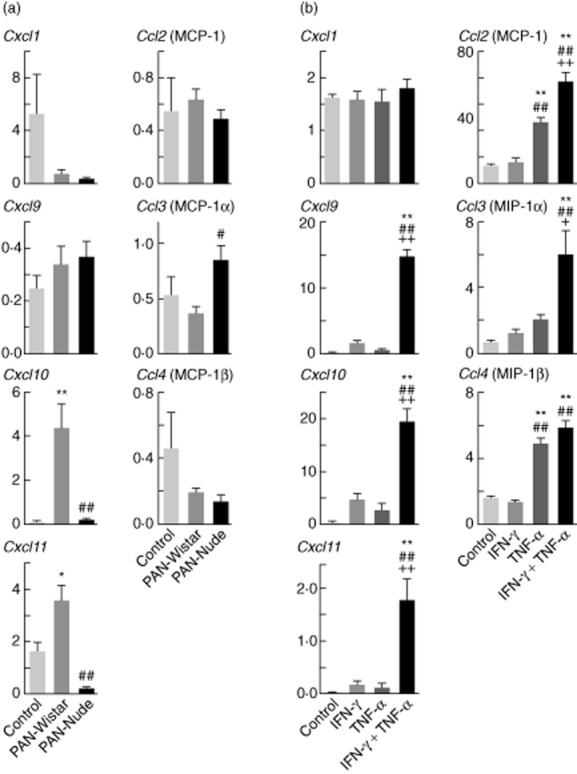
The expression of Cxcl10 and Cxcl11 is induced during late acute puromycin aminonucleoside (PAN) nephrosis in Wistar but not nude rats, and synergistically by interferon (IFN)-γ and tumour necrosis factor (TNF)-α in cultured human podocytes. (a) Plots of mRNA expression of various chemokines, relative to normal kidney cortex and normalized to Rpl19. The mRNA expression of genes encoding the C-X-C chemokines (Cxcl1, Cxcl9, Cxcl10, Cxcl11) as well as C-C chemokines (Ccl2, Ccl3, Ccl4) was evaluated in glomeruli isolated 12 days after Wistar or nude rats were injected with PAN (PAN-Wistar, PAN-nude), or with vehicle alone (control). Results are expressed as means ± standard error (s.e.), n = 4/group; *P < 0·05 compared with vehicle alone; **P < 0·01 or ##P < 0·01 compared with Wistar-PAN and vehicle alone. (b) Normalized expression of similar set of C-X-C and C-C chemokine genes as in (a). The mRNA expression was evaluated in cultured human podocytes after 4 h of treatment with IFN-γ, TNF-α or both cytokines together (each at 10 ng/ml). Results are expressed as means ± s.e., n = 6/group, **P < 0·01 compared with control, ##P < 0·01 compared with IFN-γ or ++P < 0·01 compared with TNF-α.
Several cell types in response to IFN-γ secrete the chemokines CXCL10 and CXCL11; therefore, to determine whether this cytokine induced the secretion of C-X-C or C-C chemokines, we stimulated human podocytes with IFN-γ and TNF-α (Fig. 4b) and measured the relative expression of a set of chemokines similar to those shown in Fig. 4a. As expected, the combination of IFN-γ plus TNF-α resulted in a potent type-1 immune stimulus that significantly increased the expression of not only the IFN-γ inducible chemokines CXCL9, CXCL10 and CXCL11, but also the main macrophage chemoattractants CCl2 (MCP-1), CCL3 (MIP-1α) and CCL4 (MIP-1α). These results support the hypothesis that podocytes act as initiators of macrophage recruitment in response to cytokine stimulation.
THP-1 monocytic cell chemotaxis is increased by CXCL10, but not CXCL11
For determining the specific effect of the chemoattractants released by podocytes in response to cytokine stimulation, we measured monocyte chemotaxis in response to supernatants from cultured podocytes treated with the type-1 immune-inducing stimuli IFN-γ and TNF-α. As only expression of Cxcl10 and Cxcl11 was induced in vivo in glomeruli during the course of PAN-NS in Wistar rats, we focused on these chemokines as potential chemoattractants for circulating monocytes. We first confirmed that recombinant human CXCL10 and CXCL11 could induce chemotaxis of a cultured monocyte cell line (THP-1), and found more than half-maximal stimulation at 5 ng/ml CXCL10/ml (Fig. 5a) compared to half-maximal stimulation with ∼35 ng recombinant human (rh)CXCL11/ml (Fig. 5b). The maximum stimulation of THP-1 chemotaxis by CXCL10 was comparable to that induced by chemoattractants known to induce THP-1 chemotaxis, MCP-1 18 (Fig. 5a), while maximal rhCXCL11 stimulation was only ∼25% of these values (Fig. 5b). Chemotaxis induced by both chemokines could be abrogated completely by blocking antibodies, but was not affected by blocking antibodies directed against the other chemokine (Fig. 5a,b). Chemotaxis of THP-1 cells was increased by conditioned medium from human podocyte cultures stimulated with IFN-γ (3·2 ± 0·04-fold greater than control) or TNF-α (3·5 ± 0·13, Fig. 5b). Medium from podocytes stimulated with both IFN-γ and TNF-α induced greater THP-1 chemotaxis (6·8 ± 0·54) than medium from cells stimulated with either cytokine alone. Chemotaxis induced by medium from podocytes treated with IFN-γ and TNF-α was blocked completely by preincubation with anti-CXCL10 blocking antibody (1·3 ± 0·08), but was not affected significantly by anti-CXCL11 blocking antibody (5·7 ± 0·49). To test the effect of type-2 cytokines on the production of IFN-γ inducible chemokines, we performed an additional set of experiments in which the prototypical type-1 stimuli, LPS + IFN-γ or TNF-α + IFN-γ, were added to human podocyte cultures. The conditioned media obtained from these cultures induced strong chemotactic responses in THP-1 cells (Fig. 5d). In contrast, the type-2 stimuli, IL-4 + IL-13, were unable to induce production of THP-1 chemoattractants by human podocytes. Interestingly, the treatment of human podocytes with the combination IL-4 + IL-13 only modestly reduced the ability of IFN-γ to induce secretion of THP-1 chemoattractants into the culture medium. These results demonstrated that IFN-γ, enhanced by TNF-α, induced secretion of a major monocyte chemoattractant by podocytes that blocking antibody experiments confirmed to be CXCL10.
Fig 5.
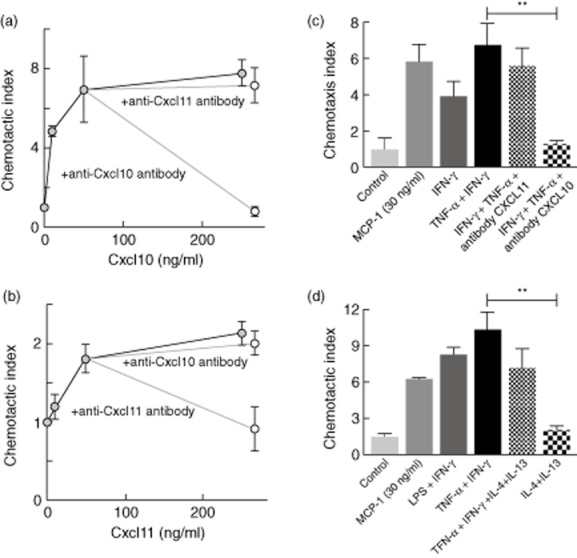
CXCL10 produced by cultured human podocytes in response to interferon (IFN)-γ and tumour necrosis factor (TNF)-α induces monocyte migration. The chemotactic index was calculated as the fold increase, compared to sham-treated controls, in the number of THP-1 monocytes migrating across a 5-μm-pore membrane in response to chemoattractants. (a) Migration after incubation over wells containing various concentrations of recombinant CXCL10, or with 250 ng of CXCL10/ml preincubated with neutralizing antibodies against CXCL10 or CXCL11. (b) Migration after incubation over wells containing various concentrations of recombinant CXCL11, or with 250 ng of CXCL11/ml preincubated with neutralizing antibodies against CXCL10 or CXCL11. (c) Migration after incubation over wells containing culture supernatants from human podocytes collected 24 h after a 6 h treatment with IFN-γ, or TNF-α + IFN-γ. (d) Migration after incubation over wells containing culture supernatants from human podocytes collected 24 h after a 6 h treatment with either type 1 stimulation [lipopolysaccharide (LPS) + IFN-γ, or TNF-α + IFN-γ] or type 1 + type 2 stimulation (TNF-α + IFN-γ + IL-4 + IL-13) or type 2 cytokines only (IL-4 + IL-13). Control supernatants are from untreated podocyte cultures. The chemoattractant CCL2 [monocyte chemoattractant protein-1 (MCP-1)] was used as positive control. Results are expressed as mean ± standard error, n = 4/group; **P < 0·01 compared to control.
Discussion
In the current study we found that nude rats, lacking mature T lymphocytes, developed less proteinuria in the late acute stage of PAN nephrosis than immune-competent Wistar rats. The greater proteinuria in Wistar rats was associated with increased serum concentration of IFN-γ, glomerular Cxcl10 and Cxcl11 mRNA expression and peri- and intra-glomerular infiltration of activated macrophages, none of which were observed in similarly treated nude rats. We found that podocytes responded to IFN-γ and TNF-α stimulation by release of CXCL10 capable of promoting monocyte chemotaxis. Importantly, we also showed that macrophage depletion after the initial development of proteinuria was sufficient to reduce the greater proteinuria in PAN-treated Wistar rats to the same level as in nude rats. Together, these results suggest that increased release of TNF-α from glomeruli 19, and IFN-γ by T lymphocytes, leads to production of CXCL10 by podocytes that attracts activated monocytes/macrophages to exacerbate glomerular injury.
Both innate and adaptive immune cells can produce IFN-γ. For instance, in response to stress or tissue damage, NK cells are important innate early secretors of this cytokine, which can prime macrophages to secrete proinflammatory cytokines as well as oxygen and nitrogen radicals to increase their injurious capacity 20. It has been reported that nude (Foxn1rnu/rnu) rats have more alloreactive NK cells in the blood and spleen than heterozygous Foxn1rnu/+ littermates 21. However, the production of IFN-γ by NK cells is transient and insufficient to maintain populations of activated macrophages, and antigen-specific Th1 helper cells are required for sustained production of IFN-γ 22. Thus, our observation that nude rats fail to maintain the high serum concentrations of IFN-γ found in Wistar rats during PAN-NS demonstrates that this is a consequence of the lack of a functional Th1 immune response. Previous work confirmed the role of IFN-γ in the development of glomerular injury, as crescentic glomerulonephritis in a mouse model was ameliorated by antibodies directed against IFN-γ and in IFN-γ-deficient mice 23. In addition, our conclusion that IFN-γ released by T lymphocytes is required for activated macrophage recruitment is directly supported by bone marrow transplant studies that demonstrated IFN-γ derived from both renal and bone marrow cells is required for renal recruitment of leucocytes in a murine model of crescentic glomerulonephritis 24.
Previous reports show that there is renal infiltration of immune cells during acute PAN nephrosis 25, and that infiltration of monocytes and activated T lymphocytes contributes to development of chronic renal damage 26,27. We observed infiltration of activated (ED1+) macrophages, but not CD3+ T lymphocytes, in Wistar rats at the peak of the proteinuria induced by PAN (12 days after injection), consistent with a previous study that found increased glomerular ED1+ macrophage infiltration in Sprague–Dawley rats at the same time after PAN injection 28. Infiltration of T lymphocytes into the glomerulus has also been observed in PAN-NS 29, but only at 5–7 days after PAN injection, consistent with our finding of no significant later infiltration of CD3+ T lymphocytes in any rats. The conclusion that early infiltration of T lymphocytes does not contribute to glomerular injury is supported by a study that depleted T lymphocytes by daily injections of OX19 antibody, and failed to detect any change in proteinuria or macrophage infiltration in PAN-NS in Lewis rats 30. However, in this study proteinuria was followed only until day 8 after PAN injection, too early to observe the decreased proteinuria we observed in nude rats at day 12. Serum levels of major T cell cytokines such as IFN-γ, IL-4 and IL-13, cytokines critical for the activation and/or suppression of macrophage function and chemotaxis into injured tissues, were also not measured in this study. In addition, IFN-γ is not only produced by T cells, but also by NK T cells 31,32. As the thymus is the primary site of NK T cell differentiation from double-positive thymocytes upon interaction with the thymic CD1d molecule 33, this could additionally explain the blunted IFN-γ response in our studies using nude rats. The depleted antibody used in Eddy et al.'s study were not able to deplete NK T cells in the periphery, as those cells do not express CD5 or CD8α, the target antigens for OX19 and OX8 antibodies, respectively 34. Therefore, this treatment is not sufficient to eliminate NK T cells in vivo 35. Overall, the fact that Eddy et al. still observed infiltrating macrophages in their studies after depletion of T cells could be explained by the effect of IFN-γ secreted from NK T cells. Moreover, these macrophages were observed in the tubulointerstitial compartment, which implied that the development of nephritis as a chronic consequence of initial nephrosis accompanied acute-phase PAN injury. In contrast, in our studies we examined intra- and extra-glomerular macrophages as a potential injurious factor. Indeed, we demonstrated that both secretion of the Th1 cytokine, IFN-γ, and the infiltration of activated macrophages were required for the greater glomerular injury apparent in Wistar rats in the late (12 days after injection) acute phase of PAN nephrosis. The injurious effect of infiltrating macrophages was also dependent on their state of activation, as the infiltrating macrophages we observed in Wistar rats expressed activation markers such as inducible nitric oxide synthase (iNOS) and sialoadhesin (CD169), but not CD163, a marker for alternatively activated M2 macrophages 36. This is consistent with studies that showed that activated but not resting macrophages could increase renal injury in murine adriamycin-induced nephrosis 6, and that macrophages isolated from normal glomeruli behave like uncommitted, undifferentiated macrophages, while those from nephritic glomeruli have the characteristics of IFN-γ-primed, TNF-α-activated macrophages 37. Thus, the increased IFN-γ and TNF-α during PAN-NS in Wistar rats is likely to be required for both the production of CXCL10, that induces monocyte infiltration (see below), and for the activation of macrophages to render them injurious.
The C-X-C motif chemokine, CXCL10, was isolated originally in a screen for genes induced by IFN-γ 38. Functionally, CXCL10 is a proinflammatory chemokine involved in orchestrating selective migration and adhesion of blood leucocytes during Th1-mediated responses at the sites of inflammation 39. However, a variety of studies demonstrated that CXCL10 is also a chemoattractant for circulating monocytes, leading to accumulation of macrophages in tissues 8,40. In addition, mice deficient in the CXCL10 receptor, CXCR3, have attenuated macrophage accumulation in various models of inflammation 41,42. Our finding that levels of serum IFN-γ are increased during PAN nephrosis, along with previous work showing induction of renal TNF-α in the same model 19, and synergistic induction of CXCL10 in response to IFN-γ and TNF-α 43, led us to hypothesize that our observed differences in proteinuria and macrophage infiltration between Wistar and nude rats was a consequence of differential podocyte CXCL10 induction. This hypothesis is supported by a study that found an association of greater infiltration of M1 macrophages with greater renal TNF-α and CXCL10 expression in a murine model of Alport nephropathy 44. The extra-renal origin of the activated macrophages is supported by a study that depleted resident kidney macrophages by irradiation without diminishing macrophage accumulation during PAN nephrosis 30. However, while CXCL10 expression was induced in the early phase of development of Thy1·1 glomerulonephritis, daily injections of an anti-CXCL10 blocking antibody was shown to aggravate proteinuria 11. Similarly, anti-CXCL10 blocking antibody also exacerbated proteinuria in the PAN model of nephrosis in rats 45. While the authors of these studies suggest that CXCL10 is necessary for maintenance of podocyte cell-cycle balance through action on p27Kip1 and cyclins A and E, they observed increased proteinuria due to CXCL10 blocking antibodies earlier in PAN-NS than our findings, and the use of blocking antibodies was associated with increased mesangiolysis and matrix deposition, but not a decrease in glomerular ED1+ cells. This suggests that circulating anti-CXCL10 antibodies target CXCL10 essential for mesangial cell function, but podocyte CXCL10 and consequent macrophage recruitment is largely unaffected by anti-CXCL10 antibodies due to their exclusion by the glomerular filtration barrier.
In conclusion, our work provides strong evidence that in the late acute phase of PAN-induced nephrosis, CXCL10 secreted from podocytes acts as a potent monocyte chemoattractant, directing their migration into the injured kidney to aggravate disease. Our macrophage depletion experiments demonstrated that macrophages are necessary for the increased proteinuria in late acute nephrosis in Wistar rats, and our studies in nude rats demonstrated that mature T lymphocytes are necessary for both glomerular induction of Cxcl10 and macrophage infiltration. These results are consistent with a role for T lymphocyte-derived IFN-γ in both the activation of macrophages and the stimulation of podocytes to produce CXCL10 to attract monocytes. Together, our findings imply that the podocyte response to circulating cytokines can lead to exacerbation of inflammatory responses, potentially leading to progressive glomerular disease and end-stage renal failure.
Acknowledgments
This work was supported by the National Institutes of Health grants, as follows: The National Institute for Diabetes, Digestive and Kidney Disorders (R01-DK07553 to R. F. R.) and The National Institute of Allergy and Infectious Diseases (R01-AI092117 to S. P.-S.). Partial support for M. P. through the scholar exchange programme was through a grant by the Serbian Ministry of Education and Science (175085). We thank Dr Nico van Rooijen at the Department of Cell Biology and Immunology in the Faculty of Medicine at Vrije Universitet, Amsterdam for the kind gift of encapsulated clodronate.
Disclosure
The authors have no conflicts of interest to declare.
References
- Anderson S, Diamond JR, Karnovsky MJ, Brenner BM. Mechanisms underlying transition from acute glomerular injury to late glomerular sclerosis in a rat model of nephrotic syndrome. J Clin Invest. 1988;82:1757–1768. doi: 10.1172/JCI113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer JR, Ratte J, Potter AH, Michael AF. Transfer of aminonucleoside nephrosis by renal transplantation. J Clin Invest. 1972;51:2777–2780. doi: 10.1172/JCI107099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner GF, Cotran RS, Unanue ER. Modulation of Ia and leukocyte common antigen expression in rat glomeruli during the course of glomerulonephritis and aminonucleoside nephrosis. Lab Invest. 1984;51:524–533. [PubMed] [Google Scholar]
- Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JR, Pesek I, Ruggieri S, Karnovsky MJ. Essential fatty acid deficiency during acute puromycin nephrosis ameliorates late renal injury. Am J Physiol. 1989;257:F798–807. doi: 10.1152/ajprenal.1989.257.5.F798. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cao Q, Zheng G, et al. By homing to the kidney, activated macrophages potently exacerbate renal injury. Am J Pathol. 2008;172:1491–1499. doi: 10.2353/ajpath.2008.070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerer S, Schlondorff D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol. 2007;27:260–274. doi: 10.1016/j.semnephrol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Luster AD, Leder P. Ip-10, a C-X-C-chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Chiarri M, Ortiz A, Gonzalez-Cuadrado S, et al. Interferon-inducible protein-10 is highly expressed in rats with experimental nephrosis. Am J Pathol. 1996;148:301–311. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Chiarri M, Hamilton TA, Egido J, Emancipator SN. Expression of Ip-10, a lipopolysaccharide- and interferon-gamma-inducible protein, in murine mesangial cells in culture. Am J Pathol. 1993;142:433–439. [PMC free article] [PubMed] [Google Scholar]
- Han GD, Koike H, Nakatsue T, et al. IFN-inducible protein-10 has a differential role in podocyte during Thy 1.1 glomerulonephritis. J Am Soc Nephrol. 2003;14:3111–3126. doi: 10.1097/01.asn.0000097371.64671.65. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Zor T, Selinger Z. Linearization of the bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Hoover RL, Karnovsky MJ. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978;14:21–30. doi: 10.1038/ki.1978.86. [DOI] [PubMed] [Google Scholar]
- Saleem MA, O'Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68:2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae CO, Anderson AO, Thompson HL, et al. Properties of monocyte chemotactic and activating factor (mcaf) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Chiarri M, Ortiz A, Lerma JL, et al. Involvement of tumor necrosis factor and platelet-activating factor in the pathogenesis of experimental nephrosis in rats. Lab Invest. 1994;70:449–459. [PubMed] [Google Scholar]
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- Reynolds CW, Timonen TT, Holden HT, Hansen CT, Herberman RB. Natural killer cell activity in the rat. Analysis of effector cell morphology and effects of interferon on natural killer cell function in the athymic (nude) rat. Eur J Immunol. 1982;12:577–582. doi: 10.1002/eji.1830120709. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–759. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Holdsworth SR, Kitching AR, Tipping PG. IFN-gamma production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol. 2002;168:4135–4141. doi: 10.4049/jimmunol.168.8.4135. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Michael AF. Acute tubulointerstitial nephritis associated with aminonucleoside nephrosis. Kidney Int. 1988;33:14–23. doi: 10.1038/ki.1988.3. [DOI] [PubMed] [Google Scholar]
- Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin Nephrol. 2007;27:250–259. doi: 10.1016/j.semnephrol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- Hattori T, Nagamatsu T, Ito M, Suzuki Y. The possible roles of hyperlipidemia and mononuclear cells in glomeruli in puromycin aminonucleoside nephrosis in rats. Jpn J Pharmacol. 1996;70:25–33. doi: 10.1254/jjp.70.25. [DOI] [PubMed] [Google Scholar]
- Ou ZL, Natori Y, Natori Y. Gene expression of CC chemokines in experimental acute tubulointerstitial nephritis. J Lab Clin Med. 1999;133:41–47. doi: 10.1053/lc.1999.v133.a94726. [DOI] [PubMed] [Google Scholar]
- Eddy AA, McCulloch L, Liu E, Adams J. A relationship between proteinuria and acute tubulointerstitial disease in rats with experimental nephrotic syndrome. Am J Pathol. 1991;138:1111–1123. [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Fox L, Wang X, Gumperz JE. Autoreactive natural killer t cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology. 2010;130:471–483. doi: 10.1111/j.1365-2567.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Rodenkirch L, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- Laloux V, Beaudoin L, Ronet C, Lehuen A. Phenotypic and functional differences between NKT cells colonizing splanchnic and peripheral lymph nodes. J Immunol. 2002;168:3251–3258. doi: 10.4049/jimmunol.168.7.3251. [DOI] [PubMed] [Google Scholar]
- van den Brink MR, Hunt LE, Hiserodt JC. In vivo treatment with monoclonal antibody 3.2.3 selectively eliminates natural killer cells in rats. J Exp Med. 1990;171:197–210. doi: 10.1084/jem.171.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Stewart K, Rees AJ. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am J Pathol. 2000;156:295–301. doi: 10.1016/S0002-9440(10)64730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD. Chemokines regulate lymphocyte homing to the intestinal mucosa. Gastroenterology. 2001;120:291–294. doi: 10.1053/gast.2001.21403. [DOI] [PubMed] [Google Scholar]
- Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol. 2002;169:2685–2693. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- Taub DD, Longo DL, Murphy WJ. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood. 1996;87:1423–1431. [PubMed] [Google Scholar]
- Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of mig expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med. 2001;194:1375–1384. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Liu E, Tager AM, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Clifford RL, Jindarat S, et al. TNFalpha and IFNgamma synergistically enhance transcriptional activation of CXCl10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010;285:29101–29110. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu M, Kulkarni OP, Radomska E, Miosge N, Gross O, Anders HJ. Bacterial CpG-DNA accelerates alport glomerulosclerosis by inducing an M1 macrophage phenotype and tumor necrosis factor-alpha-mediated podocyte loss. Kidney Int. 2011;79:189–198. doi: 10.1038/ki.2010.373. [DOI] [PubMed] [Google Scholar]
- Han GD, Suzuki K, Koike H, et al. IFN-inducible protein-10 plays a pivotal role in maintaining slit-diaphragm function by regulating podocyte cell-cycle balance. J Am Soc Nephrol. 2006;17:442–453. doi: 10.1681/ASN.2004090755. [DOI] [PubMed] [Google Scholar]


