Abstract
The induction of mucosal tolerance has been demonstrated to be an effective therapeutic approach for the treatment of allergic diseases. Our previous study demonstrated that Peyer's patch B cells could convert naive T cells into regulatory T cells (so-called Treg-of-B(P) cells); however, it is important to characterize this particular subset of Treg-of-B cells for future applications. This study aimed to investigate the role of lymphocyte activating gene 3 (LAG3) in mediating the regulatory function of Treg-of-B(P) cells induced by mucosal follicular B (FOB) cells. Microarray analysis and real-time polymerase chain reaction (PCR) were used to assess the gene expression pattern of Treg-of-B(P) cells. To evaluate the role of LAG3, the in-vitro suppressive function and the alleviation of airway inflammation in a murine model of asthma was assessed. Our data indicated that FOB cells isolated from Peyer's patches had the ability to generate more suppressive Treg-of-B cells with LAG3 expression, compared with CD23loCD21lo B cells. LAG3 is not only a marker for Treg-of-B(P) cells, but also participate in the suppressive ability. Moreover, CCR4 and CCR6 could be detected on the LAG3+, not LAG3−, Treg-of-B(P) cells and would help cells homing to allergic lung. In the murine model of asthma, the adoptive transfer of LAG3+ Treg-of-B(P) cells was able to sufficiently suppress T helper type 2 (Th2) cytokine production, eosinophil infiltration and alleviate asthmatic symptoms. LAG3 was expressed in Treg-of-B(P) cells and was also involved in the function of Treg-of-B(P) cells. In the future, this particular subset of Treg-of-B cells might be used to alleviate allergic symptoms.
Keywords: airway hyperresponsiveness, mucosal tolerance
Introduction
Mucosal tolerance, which induces immunological tolerance to non-pathogenic antigens in the mucosa of the respiratory, gastrointestinal and urogenital tracts, has been used in humans for the treatment of allergic diseases for a century 1,2. In addition, both oral and nasal tolerance are used to treat several inflammatory diseases, including experimental autoimmune encephalomyelitis, arthritis and food allergies 3–5. It has been proposed that clonal deletion due to high-dose antigen exposure and regulatory T cell (Treg) production or anergy due to low-dose antigen exposure results in the induction of mucosal tolerance 6–9.
Organized lymphoid tissues are associated with each organ system and are thought to be the site of naive T cell priming and immune response initiation. Cervical lymph nodes (CLNs) and Peyer's patches are the major sites for tolerance induction 10. Previous studies have indicated that mucosal tolerance cannot be elicited in mice without CLNs or Peyer's patches 11,12. In addition to the microenvironment in lymph nodes, antigen-presenting cells play an important role in tolerance induction. Interleukin (IL)-10- and transforming growth factor (TGF)-β-producing dendritic cells (DCs) from the mesenteric lymph nodes (MLNs) of antigen-fed mice stimulate antigen-specific CD4+ T cells to produce IL-10 or TGF-β 13,14. Mucosal macrophages have been found to exert anti-inflammatory effects that inhibit T helper type 17 (Th17) cell differentiation 15. Recently, the function of B cells in tolerance has been noted. It has been reported that mucosal tolerance cannot be induced in B cell-deficient mice 3,16. The mucosal administration of antigen to B cell-deficient μMT mice resulted in a reduced number of forkhead box protein 3 (FoxP3)+ Treg cells and deficient Treg cell function 17. In addition, naive B cells can generate Treg cells without increasing FoxP3 expression 18. Our previous study demonstrated that mucosal B cells have a better ability to convert naive T cells into Treg cells, so-called Treg-of-B(P) cells 19. These Treg-of-B(P) cells, which produce more IL-10 and express cytotoxic T lymphocyte antigen 4 (CLTA-4), inducible co-stimulator (ICOS), OX40 (CD134), programmed death-1 (PD-1) and tumour necrosis factor (TNF)-RII, alleviate allergic airway inflammation.
Recently, lymphocyte activation gene 3 (LAG3) has been identified as a marker of Treg cells. LAG3 mRNA is expressed selectively by naturally occurring Treg (nTreg) cells and is not found in CD4+ CD25− T cells 20. In addition to modulating Treg cell function in vitro and in vivo, the ectopic expression of LAG3 confers a regulatory function to CD4+ T cells. A clinical study demonstrated that one particular Treg cell population, CD4+CD25hiFoxP3+LAG3+ cells, expanded preferentially in peripheral blood mononuclear cells (PBMCs) and tumour-infiltrating lymphocytes (TILs) in cancer patients and might contribute to tolerance at tumour sites 21. In Peyer's patches, CD4+LAG3+ T cells are enriched to approximately 8%, compared with 2% in the spleen, implying that LAG3 might participate in mucosal tolerance 22. In the present study, our data showed that LAG3 was expressed in Treg-of-B(P) cells and modulated the suppressive function of these cells. The number of LAG3+CD4+ T cells in the Peyer's patches increased after the oral administration of ovalbumin (OVA). We also demonstrated that follicular B (FOB) cells in Peyer's patches had a better ability to generate Treg-of-B(P) cells compared with CD23loCD21lo B cells. Finally, the results showed that sorted LAG3+FoxP3− Treg-of-B(P) cells could alleviate allergic airway inflammation and hypersensitivity.
Materials and methods
Animals
Male BALB/c mice, OVA-T cell receptor (TCR) transgenic (DO11·10) mice and FoxP3–green fluorescent protein (GFP) transgenic mice aged 6–8 weeks were obtained and maintained in the National Laboratory Animal Center. The DO11·10 mice had transgenic TCRs that recognize the 323–339 peptide fragments of OVA. DO11·10 mice were crossed with FoxP3–GFP mice to generate heterozygous OVA–TCR transgenic mice (F1 mice), in which GFP was detected along with FoxP3 expression. The Animal Research Committee of the college approved the animal study protocol.
Preparation of Treg-of-B cells
The protocol for Treg-of-B cell generation and the characteristics of these cells are as described previously 19. Naive CD4 T cells from spleens of DO11·10 × FoxP3–GFP F1 mice were enriched by negative isolation via immunomagnetic depletion (IMag; BD Pharmingen, San Diego, CA, USA) to purities of more than 90%. Separation of B cells from BALB/c mice resulted in 90–95% purity by B220 expression via immunomagnetic-positive selection (IMag; BD Pharmingen). Peyer's patches DCs are enriched to >90% purity by positive magnetic affinity cell sorter (MACS) selection using CD11c MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Different subsets of B cells are sorted by the expression of CD21 and CD23. First, B cells are isolated from Peyer's patches and spleen then stained with fluorescein isothiocyanate (FITC)-anti-CD21 and phycoerythrin (PE)-anti-CD23 (BD Pharmingen). These labelled cells were sorted on a fluorescence activated cell sorter (FACS)Aria (BD Biosciences, San Jose, CA, USA) through the service provided by the Cell Sorting Core Facility (the First Core Laboratory, National Taiwan University College of Medicine). Bone marrow-derived dendritic cells (BMDCs) were prepared as described previously 23. Briefly, bone marrow cells were cultured in RPMI-1640/5% fetal bovine serum (FBS) in the presence of recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (500 U/ml) and IL-4 (1000 U/ml) (PeproTech Inc., Rocky Hill, NJ, USA).
The protocol of Treg-of-B cells is as described previously 19. To assess the ability of Peyer's patch DCs and B cells to generate regulatory T cells, B cells and DCs were isolated from Peyer's patches and pulsed with OVA323–339 10 μg/ml and 1 μg/ml, respectively, in culture medium (RPMI-1640 supplemented with 5% FBS, 2·5 mM HEPES, 4 mM L-Gln and 100 U/ml penicillin, 100 μg/ml streptomycin and 0·25 μg/ml amphotericin) overnight, then co-cultured with CD4+CD25− T cells (B : T = 1:1, DCs : T = 1:10) for 3 days. BMDCs are harvested on day 8 and added with OVA323–339 1 μg/ml 4 h prior to culturing with naive T cells. As part of the LAG3 induction, T cells were cultured with anti-CD3 plus anti-CD28 1 μg/ml for 3 days then applied for LAG3 detection. B-cell-primed T cells (labelled as Treg-of-B) were applied to the following experiments.
Microarray analysis
RNA was extracted from nTreg, naive CD4 T and Treg-of-B(P) cells by the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA samples (0·2 μg for each) were amplified by low RNA Input Quick Amp Labeling kit (Agilent Technologies, Foster City, CA, USA) and labelled with cyanin 3–cytidine 5′-triphosphate (Cy3-CTP). All Cy3-labelled cRNAs were hybridized to Agilent Mouse G3 Whole Genome Oligo 8 × 60 K microarray. After washing and drying by nitrogen blowing, microarrays were scanned with an Agilent microarray scanner at 535 nm for Cy3. The scanned images were analysed by Feature Extraction version 10·5.1·1 software (Agilent Technologies) to quantify the signal and background intensity for each feature, and normalization by the 75 percentile method. Only the features with a signal-to-noise ratio of >2·6 were retrieved for further analysis.
Determination of the frequency of CD4+FoxP3−LAG3+ T cells in Peyer's patches and spleen
Mice were administered OVA 0·5 mg per day orally for 5 consecutive days and killed on days 0, 6 and 8. Cells were isolated from Peyer's patches and spleen then applied for FACS analysis.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from Treg-of-B cell using Trizol reagent (Invitrogen, Life Technologies, Paisley, UK) and then reverse-transcribed into cDNA using random hexamers [SMART reverse transcription–polymerase chain reaction (RT–PCR) kit; BD Biosciences Clontech, Palo Alto, CA, USA]. Gene expression of LAG3 was determined in triplicate by quantitative real-time PCR using SYBR Gene Expression Assays according to the manufacturer's protocol on an ABI 7500Fast (Applied Biosystems, Life Technologies, CA, USA). Amplification of the endogenous control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed in order to standardize the amount of sample cDNA added.
Determination of cytokine levels
Quantitative enzyme-linked immunosorbent assay (ELISA) was performed to assay IL-2, IL-4, IL-5, IL-10, eotaxin (R&D Systems, Abingdon, UK) and IFN-γ (BD OptEIA) using paired monoclonal antibodies (mAbs) specific for the corresponding cytokines.
Cultured supernatants of splenocytes, Treg-of-B cells and nTreg cells
In supernatants of splenocytes, cells isolated from spleen were cultured at 1 × 106 cells per well with OVA 5 μg/ml for 48 h. To determine the cytokine secreted by Treg-of-B cells, nTreg cells and naive T cells, 5 × 105 cells per well were stimulated with OVA323–339 1 μg/ml presented by irradiated splenocytes for 48 h. The supernatant was collected and stored at −20°C until further experiment.
Suppression function
To address the suppressive function of Treg-of-B cells, after 3 days of stimulation Treg-of-B cells were harvested and cultured with CD25−CD4+ T cells isolated from DO11·10 mice (as responder T cells) and irradiated splenocytes in the presence of OVA323–339 5 μg/ml for 96 h. Ten μg/ml neutralizing antibodies, including anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) (9H10; Biolegend, San Diego, CA, USA), anti-ICOS (7E, 17G9; Biolegend), anti-IL-10R (1B1·3a; BD Pharmingen) and anti-LAG3 (C9B7W; BD Pharmingen) antibodies, were added 1 h prior to adding responder T cells. Proliferative response was measured by addition of 1 μCi [3H]-thymidine incorporation into the culture for the last 16 h. Thymidine uptake was determined using a β-counter (Packard Instrument Co., Meriden, CT, USA) and expressed as counts per minute (cpm).
FACS analysis and cell sorting
For infiltrated eosinophil determination, isolated bronchoalveolar lavage fluid (BALF) cells were stained with fluorescence labelled mAbs. mAb against major histocompatibility complex (MHC)-II, CD3, B220 and CD11c were purchased from BD Pharmingen; mAb against CCR3 was purchased from R&D Systems). To prevent non-specific binding to Fc receptors, 2·4G2 blocking reagent (BD Pharmingen) was added. For cell surface marker staining, mAbs against CD4, CD194 (CCR4), CD196 (CCR6) and CD49b were purchased from Biolegend; mAbs against LAG3, latency associated peptide (LAP), CD25, CD44 were purchased from BD Pharmingen, mAbs against CD103 was purchased from eBioscience. For the detection of different B cell subpopulations, Peyer's patch B cells were stained with mAbs against B220, CD23 and CD21 (BD Pharmingen) 24. These labelled cells were sorted on a FACSAria (BD Biosciences) through the service provided by the Cell Sorting Core Facility (the First Core Laboratory, National Taiwan University College of Medicine).
Adoptive transfer of Treg-of-B cells for the alleviation of OVA-induced allergic airway inflammation
OVA-induced airway inflammation was established as described previously 19. Six to 8-week-old BALB/c mice were sensitized by intraperitoneal injections of 50 μg OVA emulsified in 4 mg of alum on day 0, and 25 μg OVA mixed with 4 mg of alum on days 14, 21 and 28. On days 42–44, mice were challenged with OVA 100 μg/mouse (in total volume 40 μl) by intranasal administration. On day 45, airway hyperresponsiveness was measured and mice were killed on day 46. FoxP3−LAG3+ Treg-of-B(P) cells (2 × 106 cells per mouse) were sorted (Supporting information, Fig. S1) and injected intravascularly into mice on day −1. Asthmatic control mice were injected in a similar manner with PBS. The naive group received challenge but without sensitization.
Measurement of OVA-specific antibodies
OVA-specific immunoglobulin (Ig)E, IgG1, IgG3, IgG2b and IgG2a titres in serum were determined by ELISA without pretreatment. Levels of antibodies were compared with standard serum and the concentration of all immunoglobulin subsets in standard serum was arbitrarily assigned one ELISA unit (EU): EU = (a sample − a blank)/(a positive − a blank).
Measurement of airway hyperresponsiveness
The airway responsiveness to aerosolized methacholine (MCh) (Sigma, St. Louis, MO, USA) was measured as described previously 25. The mice were placed into the main chamber (Buxco Electronics. Inc., Sharon, CT, USA) and challenged with aerosolized 0·9% normal saline, accompanied by increasing doses of MCh (6·25–50 mg/ml). The Penh [enhanced pause = pause × (peak expiratory box flow/peak inspiratory box flow)] values were determined. The Penh value was expressed as a relative increase ratio in response to PBS challenge.
BALF study
BALF was collected from each mouse, as described previously 19. Supernatant derived from the first lavage was stored at −20°C until measurement. Levels of cytokines were determined by ELISA and the cell pellet was resuspended to two subsequent lavages for cell counting and cell subset determination. Infiltrated cells were counted and classified as macrophages, lymphocytes, neutrophils or eosinophils with the expression of MHC-II, CD11c, B220, CD3 and CCR3 26 to analyse the inflammatory cell population in the BALF.
Cells were analysed on a FACSCalibur (BD Biosystems, Franklin Lakes, NJ, USA) using CellQuest (BD Immunocytometry Systems, San Jose, CA, USA). Lymphocytes were identified as forward-scatter (FSC)lo/side-scatter (SSC)lo and expressing CD3 or B220; B cells were distinguished from T cells by MHC-II expression in the (B220/CD3)+ gate. Granulocytes were recognized as non-autofluorescent highly granular (SSChi) cells, and within this gate eosinophils were defined as cells expressing the CCR3 27, intermediate levels of CD11c and very low to undetectable expression of MHC-II, B220 and CD3. Neutrophils had a similar scatter profile to eosinophils, but lacked CCR3 expression. DCs were identified as (CD3/B220)−, and expressing high levels of MHC-II and CD11c. Alveolar macrophages cells were identified as large autofluorescent cells.
Histopathological study
After lavage, the lungs were removed immediately, fixed in 10% buffered formalin, and embedded in paraffin. Sections (5 μm thick) were stained with haematoxylin and eosin (H&E) and examined by light microscopy for histological changes.
Statistical analysis
All statistical analyses were performed with Prism version 6·0 (GraphPad Software, San Diego, CA, USA) software. Dual comparisons were made with Student's t-test. Groups of three or more were analysed by analysis of variance (anova), with Dunnett's post-tests for experiments comparing treatments to controls. P-values < 0·05 were considered statistically significant.
Results
Treg-of-B(P) cells express LAG3
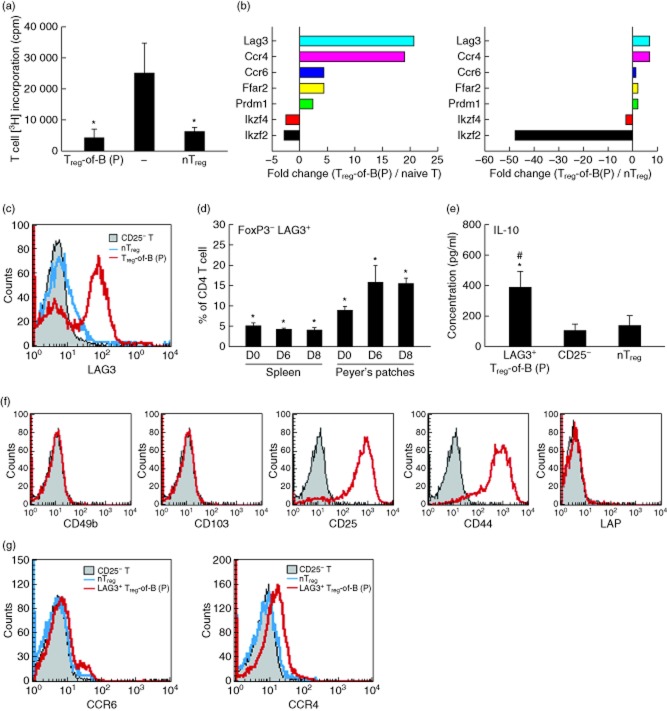
Similar to the previous study 19, CD4+FoxP3− Treg-of-B(P) (Treg-of-B(P) cells, which are generated by Peyer's patch B cells cultured with naive CD4+CD25− T cells in the presence of OVA323–339, suppressed the proliferation of responder T cells (Fig. 1a). In contrast to CD4+FoxP3+ nTreg cells, Treg-of-B(P) cells secreted higher levels of IL-10 (Fig. 1e). Thus, we hypothesized that certain molecules are involved in the regulatory function of Treg-of-B(P) cells. Microarray analysis was performed with single-colour mouse chips, and pairs of Treg-of-B(P) and naive T cells or Treg-of-B(P) and nTreg cells were compared. Previous studies have shown that the expression of both IKAROS family zinc finger (Ikzf)4 and Ikzf2, which are also known as Eos and Helios, respectively, and are highly expressed in nTreg cells 28,29, are decreased more than twofold in Treg-of-B(P) cells, compared with the naive T or nTreg groups (Fig. 1b). Compared with nTreg cells, Treg-of-B(P) cells expressed a higher level of LAG3, and this result was confirmed by immunofluorescence staining and real-time PCR (Fig. 1c and Supporting information, Fig. S2a). A similar result was also observed in B lymphocyte-induced maturation protein 1, Blimp1 (prdm1) expression (Supporting information, Fig. S2b). It has been reported that LAG3+CD4+ T cells are enriched in Peyer's patches to a greater degree than in the spleen 22, implying that LAG3+ T cells might be associated with oral tolerance. In the present study, our data showed that a greater number of FoxP3−LAG3+ T cells were present in Peyer's patches. In contrast to the number of FoxP3−LAG3+ CD4+ T cells in the spleen, the number of FoxP3−LAG3+CD4+ T cells in Peyer's patches increased after the administration of OVA 0·5 mg for 5 days, implying that naive T cells exposed to OVA presented by Peyer's patch antigen-presenting cells can become LAG3+ T cells (Fig. 1d).
Fig 1.
(a) The suppressive function of Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells]. Freshly isolated CD4+CD25+ naturally occurring regulatory T cells (nTreg) served as a positive control (nTreg), and CD4+CD25− T cells served as responder T cells (−). (b) The gene expression profile was evaluated by microarray analysis, and the results are shown as the fold change between Treg-of-B(P) cells and naive T cells (left) and between Treg-of-B(P) and nTreg cells (right). (c) Lymphocyte activation gene 3 (LAG3) expression was detected in Treg-of-B(P) cells by fluorescence staining. (d) The frequency of CD4+ forkhead box protein 3 (FoxP3)− LAG3+ T cells in Peyer's patches and spleen by mice fed with ovalbumin (OVA) was determined on days 0, 6 and 8 (right, labelled D0, D6 and D8, respectively). (e) Interleukin (IL)-10 production by LAG3+ Treg-of-B(P), CD4+CD25− naive T cells (CD25−) and nTreg cells. (f) Representative expression of CD49b, CD103, CD25, CD44 and latency-associated peptide (LAP) (red line) with isotype controls (grey-shaded) on Treg-of-B(P) cells. (g) Representative expression of CCR6 and CCR4 on LAG3+ Treg-of-B(P) cells, n = 5 per experiment. The same results were obtained in three other experiments. The results are expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05 compared with the responder T only group, Peyer's patches D0 group or CD25− group. #P < 0·05 compared with the nTreg group.
Because Treg-of-B(P) cells could secrete higher amounts of IL-10 and express LAG3, we further investigated the expression of CD25, CD44, CD49b and CD103, which were expressed on the Tr1-type cells and inducible regulatory T cells 30–32. FACS data showed that Treg-of-B(P) cells expressed higher levels of CD25 and CD44. In contrast, CD49b, CD103 and LAP were not detected (Fig. 1f). Chemokine receptors, which mediated cell migration, were also investigated. Microarray data showed that CCR4 and CCR6 are more highly expressed in Treg-of-B(P) cells. CCR6 regulates cell migration to lung and gut 33. CCR4 is critical for cell migrating to allergic lungs 34. Our data showed that Treg-of-B(P) cells, which gated on the LAG3+ population, display increased CCR4 and CCR6, compared with naive T cell and nTreg cell. In contrast, the LAG3− population did not express these receptors (Fig. 1g and Supporting information, Fig. S2c). This finding indicated that LAG3+ Treg-of-B(P) cells would be chemoattracted to inflammatory lung though these two receptors. Moreover, free fatty acid receptor 2 (FFAR2) is highly expressed in Treg-of-B(P) when compared to CD25− cells, and in LAG3− compared to LAG3+ cells (Supporting information, Fig. S2d).
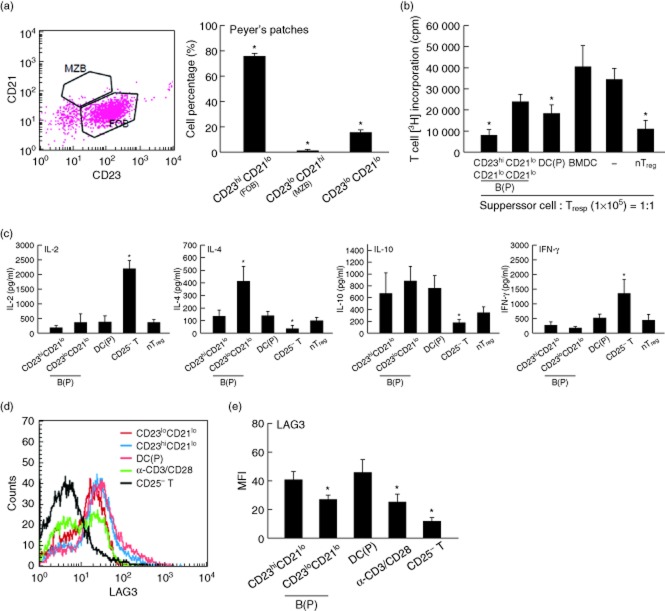
Treg-of-B(P) cells generated by follicular B cells in Peyer's patches have better suppressive function than by CD21lo CD23lo B cells
B cells play an important role in mucosal tolerance induction and maintain the microenvironment in the intestine 3. Different subsets of B cells express CD23 and CD21, including CD21loCD23hi follicular B (FOB) cells and CD21hiCD23lo marginal zone B (MZB) cells. In several models of infectious diseases, splenic MZB cells are the major source of IL-10 and play a protective role in mice with severe susceptibility to bacteria 24,35. In this study, we found that B cells in Peyer's patches, in contrast to B cells in the spleen, comprise FOB cells and CD23loCD21lo B cells, and FOB cells accounted for the largest cell population (Fig. 2a). To determine which B cell subset was able to generate Treg-of-B(P) cells, FOB and CD23loCD21lo B cells were sorted and cultured with naive T cells. The other two types of antigen-presenting cells, Peyer's patch dendritic cells (DCs) and bone marrow-derived dendritic cells (BMDCs), were used as controls 36. Figure 2b shows that compared with CD23loCD21lo B cells, FOB cells were better in inducing Treg cells. As expected, naive T cells activated by mature BMDCs did not suppress the proliferation of responder T cells. Peyer's patch DCs, which have been suggested to be able to capture oral antigens and induce the homing of Treg cells to the gut 37, also generated Treg cells, although FOB cells seemed to be more efficient. The higher level of IL-10 production could be detected in Treg cells generated by FOB and CD21loCD23lo Payer's patch B cells; however, increased IL-4 was shown in Treg cells induced by CD23loCD21lo B cells (Fig. 2c). The higher level of IL-4 might lead to less effective suppression 38,39. LAG3 expression is another candidate to give Treg-of-B(P) cells suppressive ability. Similar to T cells cultured with Peyer's patch DCs, Treg cells generated by Peyer's patch FOB cells had increased LAG3 on the cell surface, compared with T cells cultured with CD23loCD21lo B cells or activated by anti-CD3 and anti-CD28 antibodies (Fig. 2d). Based on the better suppressive ability of Treg cells, which are generated by Peyer's patch FOB cells and Peyer's patch DCs and could express LAG3 and IL-10, we speculated that IL-10 and LAG3 might be involved in the regulatory function of Treg-of-B(P) cells.
Fig 2.
Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells] generated by follicular B cells in Peyer's patches have better suppressive function. (a) Different B cell subsets that express CD23 and CD21, including follicular B (FOB) cells, marginal zone B (MZB) cells and CD23lo CD21lo cells, are found in Peyer's patches. FOB cells are the most prevalent cell population in Peyer's patch B cells. Treg-of-B cells generated by FOB cells had a better suppressive function (b). (c) After 48 h of restimulation, Treg-of-B(P) cells generated by FOB cells secreted lower interleukin (IL)-2, IL-4 and interferon (IFN)-γ levels and higher IL-10 levels, in contrast to the higher IL-4 levels secreted by CD23loCD21lo B cells generating Treg cells. (d) Lymphocyte activation gene 3 (LAG3) expression by T cells cultured with Peyer's patch FOB cells, Peyer's patch MZB cells, Peyer's patch DCs and anti-CD3 plus anti-CD28 antibodies, n = 4 per experiment. The same results were obtained in five other experiments. The results are expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05 compared with CD23hiCD21lo cells or responder T cells only (labelled as −) or naive CD4+CD25− T cells (labelled as CD25− T).
LAG3 and IL-10 participate in the suppressive function of Treg-of-B(P) cells
Because the level of LAG3 expression was elevated in Treg-of-B(P) cells, we determined whether LAG3 was required for the regulatory function of Treg-of-B(P) cells. First, CD4+FoxP3− naive T cells were isolated and cultured with Peyer's patch B cells. To exclude contaminating nTreg cells, FoxP3–GFP × DO11·10 F1 male mice were used. In these mice, nTreg cells express GFP and CD4+ T cells recognize the OVA peptide. After 3 days of co-culturing, CD4+LAG3+ Treg-of-B(P) cells were sorted and used in the suppressive function assay in the presence of anti-LAG3 antibodies. Due to the elevated expression of CTLA-4 and ICOS by Treg-of-B(P) cells, and the higher IL-10 production, we also determined whether CTLA-4, ICOS and IL-10 contribute to the suppressive function of these cells. The results indicated that in contrast to blocking CTLA-4 or ICOS, blocking LAG3 and IL-10R abrogated the suppressive function of Treg-of-B(P) cells (Fig. 3). This result suggests that LAG3 is not only a marker of Treg-of-B(P) cells, but is also involved in their function.
Fig 3.
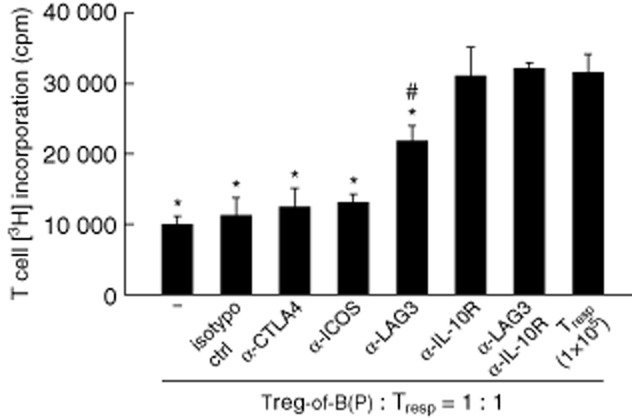
Lymphocyte activation gene 3 (LAG3) participates in the suppressive function of Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells]. Treg-of-B(P) cells were cultured with responder T cells, ovalbumin (OVA) peptide-pulsed irradiated antigen-presenting cells (APCs) and neutralizing antibodies. In the presence of the anti-LAG3 and anti-interleukin (IL)-10R antibody, the suppressive function of Treg-of-B(P) cells was abrogated, in contrast to the effect of blocking the cytotoxic T lymphocyte antigen 4 (CTLA-4) and inducible co-stimulatory (ICOS) signals, n = 3 per experiment. The same results were obtained in five other experiments. The results are expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05 compared with responder T cells only (labelled as Tresp). #P < 0·05 compared with Treg-of-B(P) cells (−).
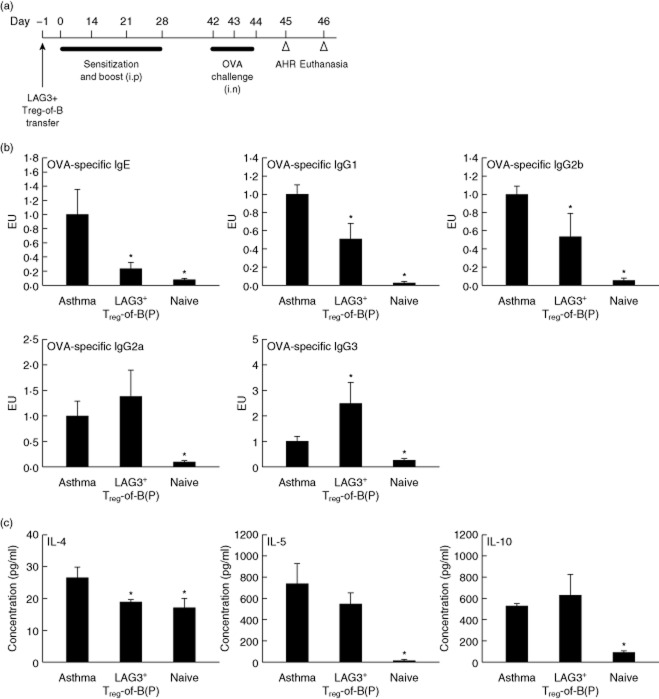
LAG3+ Treg-of-B(P) cells decrease OVA-specific IgE and Th2 cytokine production and alleviate asthmatic symptoms
An OVA-immunized murine model of asthma was used to determine the biological effect of LAG3+ Treg-of-B(P) cells in vivo. LAG3+ Treg-of-B(P) cells were sorted and transferred adoptively into mice on day −1. Mice were sensitized on day 0, boosted on days 14, 21 and 28 and challenged on days 42–44. One day later, airway hyperresponsiveness was evaluated, and the mice were killed on day 46 (Fig. 4a). Mice transferred with PBS and sensitized with OVA comprised the asthma group. The naive group was composed of mice transferred and sensitized with PBS. In the presence of LAG3+ Treg-of-B(P) cells, the mice had decreased OVA-specific IgE, IgG1 and IgG2b levels and increased OVA-specific IgG2a and IgG3 levels (Fig. 4b). Cytokine production by splenocytes stimulated with OVA was also determined by ELISA. In comparison with the asthmatic group, the LAG3+ Treg-of-B(P) cell-transferred group had decreased levels of IL-4 (Fig. 4c). This result suggests that LAG3+ Treg-of-B(P) cells have a regulatory effect on systemic Th2 responses, including antibody class-switch and cytokine production.
Fig 4.
Lymphocyte activation gene 3 (LAG3+) Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells] suppress ovalbumin (OVA)-specific immunoglobulin (Ig)E and T helper type 2 (Th2) cytokine production. (a) Sensitization protocol. (b) Serum was collected 1 day after aryl hydrocarbon receptor (AHR) detection (day 46) to measure the levels of OVA-specific IgE, IgG1, IgG2b, IgG2a and IgG3. The antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) and compared with the levels in standard serum. The Ig concentrations in the standard serum were set to arbitrarily one ELISA unit (EU), where EU = (a sample – a blank)/(a positive – a blank). (c) Splenocytes were collected 48 h after the last challenge and cultured with OVA for 48 h. The levels of interleukin (IL)-4, IL-5 and IL-10 in the splenocyte culture supernatants were determined by ELISA, n = 6 per group. The same results were obtained in two independent experiments. The results are expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05 compared with the asthma group.
The effect of LAG3+ Treg-of-B(P) cells on asthmatic symptoms was evaluated based on the severity of airway hyperresponsiveness, the extent of inflammatory cell infiltration in the lungs and pulmonary histology. When LAG3+ Treg-of-B(P) cells were present, OVA-sensitized mice had lower increases in the Penh value when stimulated with higher concentrations of methacholine, suggesting that these mice exhibited lower airway hyperresponsiveness (Fig. 5a, left). This lower reaction might be resulted from decreased eosinophil infiltration due to the diminished IL-5 and eotaxin production in the lungs (Fig. 5a–c). In addition, these results indicated that LAG3+ Treg-of-B(P) cells generated by Peyer's patch B cells were capable of alleviating allergic airway inflammation (Fig. 6).
Fig 5.
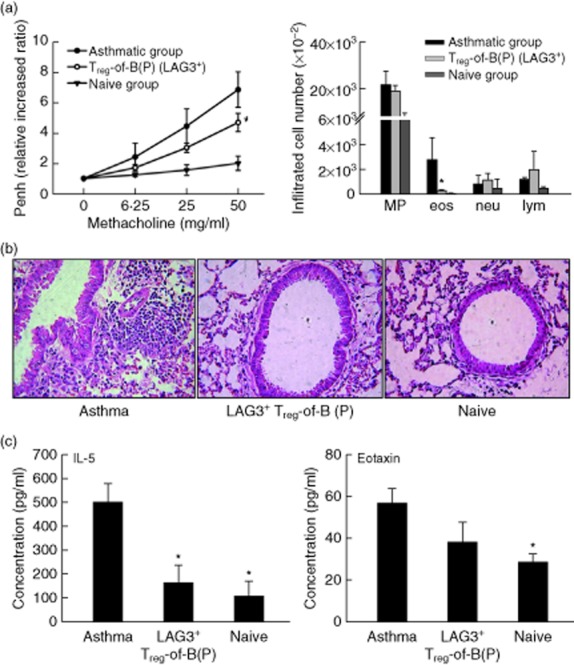
The effect of lymphocyte activation gene 3 (LAG3+) Treg-of-B cells on pulmonary inflammation. Mice were transferred adoptively with LAG3+ Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells]. Asthmatic mice were transferred with phosphate-buffered saline (PBS) and then immunized with ovalbumin (OVA). Naive mice were transferred adoptively and immunized with PBS. (a) The LAG3+ Treg-of-B(P) group had decreased airway hyperresponsiveness (left) and fewer infiltrated eosinophils in the bronchoalveolar lavage (BALF) (right). (b) Non-sensitized mice had normal pulmonary tissue sections. In sensitized and challenged mice, cells infiltrating the airway were observed; in contrast, the adoptive transfer of LAG3+ Treg-of-B cells decreased the level of cell infiltration. (c) The interleukin (IL)-5 and eotaxin levels were decreased in the adoptive transfer group. Sections stained with haematoxylin and eosin (H&E) (original magnification, c–e: ×200), n = 6 per group. The same results were obtained in two independent experiments. The results are expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05 compared with the asthma group.
Fig 6.
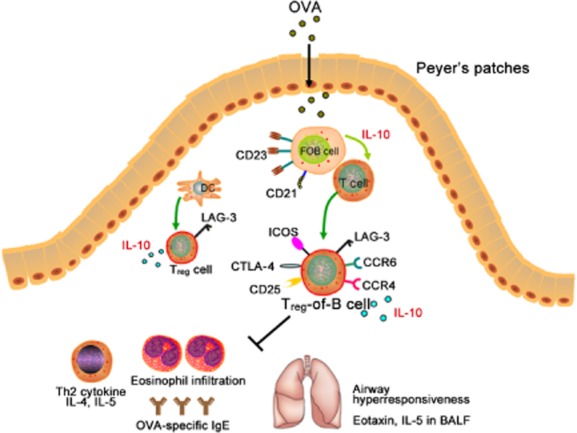
Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells] express lymphocyte activation gene 3 (LAG3) and have therapeutic effects, alleviating allergic airway hypersensitivity. In our study, we proposed a model of oral tolerance induction: when an antigen [ovalbumin (OVA)] enters the intestines, it is captured by B cells in Peyer's patches and presented to naive T cells. Through cell–cell contact and interleukin (IL)-10 production by B cells 19, naive T cells are converted into regulatory T cells (Treg-of-B(P) cells) that express cytotoxic T lymphocyte antigen 4 (CTLA-4), inducible co-stimulatory (ICOS), LAG3 and CCR6. LAG3 regulates the function of Treg-of-B(P) cells, and CCR4 and CCR6 helps Treg-of-B(P) cells to migrate to inflamed lungs and alleviate airway inflammation.
Discussion
Recently, the role of LAG3 in Treg cell function has been investigated more extensively. LAG3, which is a homologue of CD4, could bind to MHC-II molecules with a higher affinity 40,41 and result in the suppression of DC maturation 42. It has been demonstrated that LAG3 is expressed highly selectively in induced Treg cells and modulates the function of Treg cells both in vitro and in vivo 20. In this study, we found that naive CD4+ T cells stimulated by Peyer's patch B cells became Treg-of-B(P) cells and expressed higher LAG3 levels, which participated in the suppressive ability (Figs 1 and 3). It has been reported that, compared with the spleen, Peyer's patches are enriched in CD4+LAG3+ T cells (approximately 8%) 22. This T cell population is hypoproliferative and is able to inhibit the induction of colitis. Similar to the results of a previous study, higher numbers of LAG3+ T cells were observed in Peyer's patches than in the spleen in the present study. Furthermore, after the oral administration of OVA for 5 days, the proportion of LAG3+CD4+ T cells was increased in Peyer's patches (approximately 15%), although this phenomenon was not found in the spleen (Fig. 1d). These data implied that when antigens enter the intestines, they might be loaded on Peyer's patch B cells and presented to naive T cells. This would help naive T cells to become LAG3+FoxP3− regulatory T cells.
Several studies indicate that different subsets of inducible Treg cells participate in regulating immune responses. Tr1 cells, which co-express CD49b and LAG3, are shown to maintain immune tolerance in several diseases with higher IL-10 production 30. CD4+FoxP3−LAP+ Treg cells, which are induced by nasal tolerance, could suppress asthmatic lung inflammation 31. In the present study, our Treg-of-B(P) cells express LAG3, CD25 and CD44; however, CD49b, LAP and CD103 are not detectable. In addition, the amounts of TGF-β are undetectable in Payer's patch cells and Treg-of-B(P) cells cultured supernatants with OVA stimulation (data not shown). This implies that Treg-of-B(P) cells do not belong to these Treg cell subsets.
A previous study showed that the LAG3 gene is also expressed in nTreg cells; however, the protein expression is lower in nTreg cells 20, as shown in our data, and up-regulation of LAG3 expression requires contact by nTreg cells and antigens presented by APCs (Supporting information, Fig. S3). Our observations showed that, in contrast to naive T cells stimulated with anti-CD3 and anti-CD28, naive T cells cultured with Peyer's patch B cells express higher levels of LAG3 on the cell surface, suggesting that B cells might provide some molecules that are required for LAG3 expression. Another point to consider is that in the human system, Treg cells might suppress activated T cells through the binding of LAG3 to MHC-II molecules expressed by activated T cells and APCs 43. However, murine T cells do not express MHC-II after activation 44. Thus, it is unclear whether there are pathways other than the inhibition of DC maturation.
B cells are important in the induction of mucosal tolerance 3,16. Our previous study indicated that Peyer's patch B cells can generate Treg cells 19. In the present study, we further investigated the ability of different subsets of Peyer's patch B cells to induce the production of Treg cells. The major Peyer's patch B cell population is comprised of FOB cells (approximately 80%), and MZB cells account for fewer than 1% (Fig. 2a). The main function of FOB cells is to differentiate into antibody-secreting cells in response to thymus-dependent (TD) and thymus-independent (TI) antigens 45,46. In this study, we found that compared with CD23loCD21lo B cells, Peyer's patch FOB cells can be useful APCs to generate Treg-of-B(P) cells with higher LAG3 expression. In comparison with Peyer's patch B cells, the expression of LAG3 by different splenic B cells was also determined. B220+ splenic B cells are composed of MZB, FOB and CD23−CD21− B cells. Our data indicate that LAG3 expressed by T cells cultured with splenic MZB cells is higher, compared with splenic FOB and CD23− CD21− B cells (Supporting information, Fig. S4); however, the suppressive ability of these T cell cells remains to be determined. In addition to LAG3 mediating the suppressive function, IL-10 is involved in the suppressive ability of Treg-of-B(P) cells (Fig. 3). Furthermore, the levels of IL-10 are similar in cultures with Peyer's patch FOB cells and DCs, and IL-10 might participate not only in the suppressive function of Treg-of-B(P) cells but also in the suppression function of Treg generated by DCs.
We would like to emphasize the important role of B cells in mucosal tolerance. As found in another study, Peyer's patch DCs can generate Treg cells 36 (Fig. 2b); however, B cells are the major cell population in Peyer's patches and are capable of presenting antigens to naive T cells and generating Treg cells 19. T cells stimulated by Peyer's patch DCs express α4β7 and CCR9, which are required for the homing of T cells to the gut 47. We found that Peyer's patch B cells induce naive T cells express FFAR2, which is related to gut-homing 48; however, this receptor is detected in only LAG3− Treg-of-B(P) cells, not LAG3+ Treg-of-B(P) cells. In contrast, CCR4 and CCR6 are expressed in LAG3+ Treg-of-B(P) cells (Fig. 1g). CCL20 is the only ligand of CCR6 and is expressed by pulmonary epithelial cells and intestinal epithelial cells 33. The Th2 response, including elevated IL-4 and IL-13 levels, influences CCL20 expression. Given that CCR6-deficient mice suffer from lung and gut inflammation 49,50 and CCR4 is critical for T cell migration to allergic lung 34, we hypothesized that LAG3+ Treg-of-B(P) cells could be recruited to the airway through CCR4 and CCR6 expression and regulate allergic airway inflammation. Thus, it is suggested that Peyer's patch B cells, which generate Treg cells, might exert a better therapeutic effect on treating airway inflammation than Peyer's patch DCs.
To elucidate the effect of LAG3+ Treg-of-B(P) cells on airway allergic inflammatory disease, we transferred sorted LAG3+ Treg-of-B(P) cells into OVA-sensitized mice and determined the severity of the airway hyperresponsiveness and systemic Th2 responses. Our data suggested that LAG3+ Treg-of-B(P) cells have the ability to alleviate allergic airway inflammation (Figs 4 and 5). It is noted that Th2-type immunoglobulins (IgE, IgG1 and IgG2b) are decreased and Th1-type immunoglobulins (IgG2a and IgG3) 51 are increased in the adoptively transferred group. Therefore, we speculate that LAG3+ Treg-of-B(P) cells have the potential to balance the immune system. Our study has shown that most Treg-of-B(P) cells migrate to the mucosal lymph nodes (cervical lymph nodes and Peyer's patches) and the spleen after transfer into mice 19. The effects of Treg-of-B(P) cells on other inflammatory diseases remain to be investigated.
Taken together, we found that the oral administration of OVA could increase the number of LAG3+CD4+ T cells in Peyer's patches. Peyer's patch FOB cells convert naïive CD4+FoxP3−LAG3− T cells into FoxP3−LAG3+CCR4+ IL-10-producing T cells, which have a regulatory function. It would be necessary to define the molecules which are required for Treg-of-B(P) cell induction. Knowing the molecules could help us to generate Treg-of-B(P) cells more efficiently. Although additional experiments needed to be tested, our data indicated that Treg-of-B(P) cells, which are generated in vitro, have the potential to apply to allergic disease treatment.
Acknowledgments
This project was supported by a grant, NHRI-EX102-10253SI, from the National Health Research Institute, Republic of China.
Disclosure
The authors declare no conflicts of interest.
Author contributions
K. H. C. designed the study, performed all the experiments, analysed the data and drafted the manuscript. B. L. C. conceived the study and helped draft the manuscript. Both authors have read and approved the final manuscript.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. To examine the role of lymphocyte activation gene 3 (LAG3) in Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells], we purified CD4+ forkhead box protein 3 (FoxP3)− naive T cells and cultured then with Peyer's patch B cells, as described in Materials and methods. After 3 days, LAG3+CD4+ Treg-of-B(P) cells were sorted on a fluorescence activated cell sorter (FACS)Aria with the gating strategy to exclude the dead cells and doublets. The purity of CD4+ LAG3+ T cells was approximately 99%.
Fig. S2. Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells] could express lymphocyte activation gene 3 (LAG3), B lymphocyte-induced maturation protein 1 (prdm1), FFAR2 and prdm1. The expressions of LAG3 (a), prdm1 (b) and free fatty acid receptor 2 (FFAR2) (d) on Treg-of-B(P) cells are detected by real-time polymerase chain reaction (PCR). The expression of CCR4 and CCR6 are detected by flow cytometry and represented by mean fluorescence intensity (MFI) (c). The same results were obtained in three other experiments. The results are expressed as the mean ± standard error of the mean. *P < 0.05 compared with CD4+CD25− (labelled as CD25−) T cells. #P < 0.05 compared with naturally occurring regulatory T cells (nTreg) cells.
Fig. S3. Lymphocyte activation gene 3 (LAG3) expression by activated CD25− T cells, naturally occurring regulatory T cells (nTreg) cells. CD4+ CD25− T cells and nTreg cells were activated by antigen-presenting cells pulsed with ovalbumin (OVA)323–339 peptide for 24 h then applied for the detection of LAG3.
Fig. S4. Lymphocyte activation gene 3 (LAG3) expression by T cells cultured with splenic follicular B (FOB) cells, marginal zone B (MZB) and CD23−CD21− cells. Naive T cells were cultured with different B cell subsets isolated from spleen. After 3 days, cells were stained with fluorescence conjugated anti-LAG3 antibody.
References
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Samsom JN. Regulation of antigen-specific regulatory T-cell induction via nasal and oral mucosa. Crit Rev Immunol. 2004;24:157–177. doi: 10.1615/critrevimmunol.v24.i3.10. [DOI] [PubMed] [Google Scholar]
- Gonnella PA, Waldner HP, Weiner HL. B cell-deficient (mu MT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166:4456–4464. doi: 10.4049/jimmunol.166.7.4456. [DOI] [PubMed] [Google Scholar]
- Broere F, Wieten L, Klein Koerkamp EI, et al. Oral or nasal antigen induces regulatory T cells that suppress arthritis and proliferation of arthritogenic T cells in joint draining lymph nodes. J Immunol. 2008;181:899–906. doi: 10.4049/jimmunol.181.2.899. [DOI] [PubMed] [Google Scholar]
- Frossard CP, Hauser C, Eigenmann PA. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J Allergy Clin Immunol. 2004;114:377–382. doi: 10.1016/j.jaci.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Van Houten N, Blake SF. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J Immunol. 1996;157:1337–1341. [PubMed] [Google Scholar]
- Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- Ostroukhova M, Seguin-Devaux C, Oriss TB, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G, Samsom JN, Mebius RE. The importance of regional lymph nodes for mucosal tolerance. Immunol Rev. 2006;213:119–130. doi: 10.1111/j.1600-065X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- Wolvers DA, Coenen-de Roo CJ, Mebius RE, et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol. 1999;162:1994–1998. [PubMed] [Google Scholar]
- Song F, Wardrop RM, Gienapp IE, et al. The Peyer's patch is a critical immunoregulatory site for mucosal tolerance in experimental autoimmune encephalomylelitis (EAE) J Autoimmun. 2008;30:230–237. doi: 10.1016/j.jaut.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166:4843–4852. doi: 10.4049/jimmunol.166.8.4843. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002;14:659–667. doi: 10.1093/intimm/dxf032. [DOI] [PubMed] [Google Scholar]
- Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. J Immunol. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- Reichardt P, Dornbach B, Rong S, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- Chu KH, Chiang BL. Regulatory T cells induced by mucosal B cells alleviate allergic airway hypersensitivity. Am J Respir Cell Mol Biol. 2012;46:651–659. doi: 10.1165/rcmb.2011-0246OC. [DOI] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2011;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T, Fujio K, Shibuya M, et al. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci USA. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YH, Fu CL, Lo YC, Chiang BL. Adenovirus expressing Fas ligand gene decreases airway hyper-responsiveness and eosinophilia in a murine model of asthma. Gene Ther. 2004;11:1497–1505. doi: 10.1038/sj.gt.3302325. [DOI] [PubMed] [Google Scholar]
- Lee C-C, Kung JT. Marginal zone B cell is a major source of IL-10 in Listeria monocytogenes susceptibility. J Immunol. 2012;189:3319–3327. doi: 10.4049/jimmunol.1201247. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–121. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Grimaldi JC, Yu NX, Grunig G, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- Cai Q, Dierich A, Oulad-Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. J Immunol. 2009;183:2303–2311. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- Pan F, Yu H, Dang EV, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Duan W, So T, Mehta AK, Choi H, Croft M. Inducible CD4+LAP+Foxp3- regulatory T cells suppress allergic inflammation. J Immunol. 2011;187:6499–6507. doi: 10.4049/jimmunol.1101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurr M, Ladell K, Besneux M, et al. Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(−) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol. 2013;7:428–439. doi: 10.1038/mi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Carson WFt, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino L, da Fonseca DM, Takenaka MC, et al. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013;190:2614–2621. doi: 10.4049/jimmunol.1202354. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Min S-Y, Park K-S, Cho M-L, et al. Antigen-induced, tolerogenic CD11c+, CD11b+ dendritic cells are abundant in Peyer's patches during the induction of oral tolerance to type II collagen and suppress experimental collagen-induced arthritis. Arthritis Rheum. 2006;54:887–898. doi: 10.1002/art.21647. [DOI] [PubMed] [Google Scholar]
- Nagatani K, Sagawa K, Komagata Y, Yamamoto K. Peyer's patch dendritic cells capturing oral antigen interact with antigen-specific T cells and induce gut-homing CD4(+)CD25(+) regulatory T cells in Peyer's patches. Ann NY Acad Sci. 2004;1029:366–370. doi: 10.1196/annals.1309.020. [DOI] [PubMed] [Google Scholar]
- Or R, Renz H, Terada N, Gelfand EW. IL-4 and IL-2 promote human T-cell proliferation through symmetrical but independent pathways. Clin Immunol Immunopathol. 1992;64:210–217. doi: 10.1016/0090-1229(92)90202-y. [DOI] [PubMed] [Google Scholar]
- Lorre K, Van Damme J, Ceuppens JL. A bidirectional regulatory network involving IL 2 and IL 4 in the alternative CD2 pathway of T cell activation. Eur J Immunol. 1990;20:1569–1575. doi: 10.1002/eji.1830200724. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979;150:246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooten E, Klous P, Elsen P, Holling T. Lack of MHC-II expression in activated mouse T cells correlates with DNA methylation at the CIITA-PIII region. Immunogenetics. 2005;57:795–799. doi: 10.1007/s00251-005-0051-8. [DOI] [PubMed] [Google Scholar]
- O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariappa A, Mazo IB, Chase C, et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med. 2007;175:1165–1172. doi: 10.1164/rccm.200602-256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyestani TR, Woodward WD, Hillyer L. Serum levels of Th2-type immunoglobulins are increased in weanling mice subjected to acute wasting protein-energy malnutrition. Iran J Allergy Asthma Immunol. 2004;3:1–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. To examine the role of lymphocyte activation gene 3 (LAG3) in Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells], we purified CD4+ forkhead box protein 3 (FoxP3)− naive T cells and cultured then with Peyer's patch B cells, as described in Materials and methods. After 3 days, LAG3+CD4+ Treg-of-B(P) cells were sorted on a fluorescence activated cell sorter (FACS)Aria with the gating strategy to exclude the dead cells and doublets. The purity of CD4+ LAG3+ T cells was approximately 99%.
Fig. S2. Peyer's patch B cell-converted naive T cells into regulatory T cells [Treg-of-B(P) cells] could express lymphocyte activation gene 3 (LAG3), B lymphocyte-induced maturation protein 1 (prdm1), FFAR2 and prdm1. The expressions of LAG3 (a), prdm1 (b) and free fatty acid receptor 2 (FFAR2) (d) on Treg-of-B(P) cells are detected by real-time polymerase chain reaction (PCR). The expression of CCR4 and CCR6 are detected by flow cytometry and represented by mean fluorescence intensity (MFI) (c). The same results were obtained in three other experiments. The results are expressed as the mean ± standard error of the mean. *P < 0.05 compared with CD4+CD25− (labelled as CD25−) T cells. #P < 0.05 compared with naturally occurring regulatory T cells (nTreg) cells.
Fig. S3. Lymphocyte activation gene 3 (LAG3) expression by activated CD25− T cells, naturally occurring regulatory T cells (nTreg) cells. CD4+ CD25− T cells and nTreg cells were activated by antigen-presenting cells pulsed with ovalbumin (OVA)323–339 peptide for 24 h then applied for the detection of LAG3.
Fig. S4. Lymphocyte activation gene 3 (LAG3) expression by T cells cultured with splenic follicular B (FOB) cells, marginal zone B (MZB) and CD23−CD21− cells. Naive T cells were cultured with different B cell subsets isolated from spleen. After 3 days, cells were stained with fluorescence conjugated anti-LAG3 antibody.