Abstract
Serine protease activity of Per a 10 from Periplaneta americana modulates dendritic cell (DC) functions by a mechanism(s) that remains unclear. In the present study, Per a 10 protease activity on CD40 expression and downstream signalling was evaluated in DCs. Monocyte-derived DCs from cockroach-allergic patients were treated with proteolytically active/heat-inactivated Per a 10. Stimulation with active Per a 10 demonstrated low CD40 expression on DCs surface (P < 0·05), while enhanced soluble CD40 level in the culture supernatant (P < 0·05) compared to the heat-inactivated Per a 10, suggesting cleavage of CD40. Per a 10 activity reduced the interleukin (IL)-12 and interferon (IFN)-γ secretion by DCs (P < 0·05) compared to heat-inactivated Per a 10, indicating that low CD40 expression is associated with low levels of IL-12 secretion. Active Per a 10 stimulation caused low nuclear factor-kappa B (NF-κB) activation in DCs compared to heat-inactivated Per a 10. Inhibition of the NF-κB pathway suppressed the CD40 expression and IL-12 secretion by DCs, further indicating that NF-κB is required for CD40 up-regulation. CD40 expression activated the tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6), thereby suggesting its involvement in NF-κB activation. Protease activity of Per a 10 induced p38 mitogen-activated protein kinase (MAPK) activation that showed no significant effect on CD40 expression by DCs. However, inhibiting p38 MAPK or NF-κB suppressed the secretion of IL-12, IFN-γ, IL-6 and TNF-α by DCs. Such DCs further reduced the secretion of IL-4, IL-6, IL-12 and TNF-α by CD4+ T cells. In conclusion, protease activity of Per a 10 reduces CD40 expression on DCs. CD40 down-regulation leads to low NF-κB levels, thereby modulating DC-mediated immune responses.
Keywords: allergen, CD40, dendritic cell, Per a 10, proteolytic activity
Introduction
Protease allergens from house dust mite, fungi, pollen grains, animal dander and cockroach cause barrier dysfunction, induce the production of proinflammatory cytokines in epithelial cells and keratinocytes, cleave various molecules and modulate the function of various cell types for the generation of T helper type 2 (Th2) responses 1–5. The primary immunological responses are initiated after antigen presentation by dendritic cells (DCs) that induce specific T cell stimulation 6. Proteases from Aspergillus species induce Th2 responses through limited maturation of DCs and reduced interleukin (IL)-12 production 7. The cysteine protease allergen Dermatophagoides pteronyssinus 1 suppresses IL-12 production by DCs, which favours a Th2 response 8. IL-12 secretion by DCs depends upon the ability of CD40 expressed by the DCs to receive CD40L-mediated signals from T cells 9,10. Signalling through CD40 in DCs leads to up-regulation of major histocompatibility complex (MHC) I and II, co-stimulatory molecules CD80/86 and proinflammatory cytokines such as IL-1α/β, IL-6 and IL-12, that participate in T cell function 11–15. Up-regulation of CD40 by DCs was observed after exposure to several skin sensitizers 9,10,16. NiSO4 induced CD40 expression and production of IL-6, IL-8 and IL-12p40 in human DCs 9. In contrast, stimulation with Der p 1 induced low CD40 expression on the DCs surface that suppressed IL-12 production 8.
Nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) family members are the two major pathways involved in DC maturation and activation. Studies also envisage the involvement of membrane raft-restricted recruitment of tumour necrosis factor (TNF)-receptor-associated factor(s) that further activate NF-κB and MAPK 9,10,16. In a previous study, serine protease activity of Per a 10 from Periplaneta americana augmented allergen-induced airway inflammation, characterized by enhanced airway hyperresponsiveness and increased cellular infiltration of lungs in a mouse model 17. The proteolyically inactive Per a 10, however, elicited a significantly low immune-inflammatory response. Immunotherapy studies with proteolytically inactive Per a 10 demonstrated a significant suppression of systemic airway inflammation and enhanced IL-10 secretion in mice compared to the proteolytically active Per a 10 18. Further, it was demonstrated that the protease activity of Per a 10 modulates DC functions by CD86 up-regulation and low IL-12 secretion 19. However, the signal transduction event(s) in DCs leading to activation after CD40 engagement and consequent cytokine secretion is poorly understood. In light of the studies showing that signalling through CD40 is essential for up-regulation of IL-12, the present study was aimed to elucidate the role of protease activity of Per a 10 on CD40 expression by DCs and the signal transduction component(s) involved in such CD40-mediated DC modulation.
Materials and methods
Per a 10 preparation
Per a 10 was purified from freeze-dried whole-body P. americana extract by affinity chromatography 20. The purity of isolated protein was checked using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteolytic activity tested on gelatin zymogram under non-reducing conditions 19. Per a 10 was heated at 95°C for 60 min. Enzymatic inhibition by heat treatment was confirmed by both azocollagen assay and gelatin zymogram 19.
Study subjects
Allergic patients diagnosed for asthma and/or rhinitis were recruited for the study. Patients of either sex were screened by clinical history, skin prick test (SPT) and specific immunoglobulin (Ig)E level against cockroach allergy. Spirometry was performed using a portable spirometer (Adage Medical Systems, Via Del Maggiolino, Rome, Italy) and interpreted using population reference standards. Patients showing positive SPT and high serum IgE to P. americana and/or Per a 10 were selected for the present study (Table 1). This work was approved by the Human Ethics Committee of the Vallabhbhai Patel Chest Institute, Delhi (approval number VPCI/DirOff/IEC/2012/1296) and written consent was obtained from the subjects for participation in the study.
Table 1.
Patients' demography and specific immunoglobulin (Ig)E OD values
| Demographic variables (n = 14) | |
|---|---|
| Male (%) | 9 (64·3%) |
| Female (%) | 5 (35·7%) |
| Age group (years) | 19–41 |
| *FVC % predicted, mean | 72 ± 6 |
| †FEV1 % predicted, mean | 74·5 ± 3·2 |
| FEV1/FVC % predicted, mean | 95·8 ± 2·7 |
| Intradermal test report (wheal size) | |
| Histamine | +++ |
| Periplaneta americana | +++ |
| Specific IgE ‡OD values | |
| P. americana | 0·52–0·81 (control IgE = 0·14 ± 0·02) |
| Per a 10 | 0·43–0·67 (control IgE = 0·09 ± 0·01) |
FVC = forced vital capacity;
FEV1 = forced expiratory volume in 1 s
OD = optical density.
Generation of DCs
Monocytes were purified from peripheral blood of cockroach-allergic patients by the method described previously 19. Briefly, peripheral blood mononuclear cells were separated from heparinized blood by density gradient centrifugation followed by monocyte separation by positive selection using anti-CD14 microbeads and the magnetic-activated cell sorting (MACS) system (Miltenyi Biotech, Bergisch Gladbach, Germany). Approximately 2 × 105 monocytes were cultured in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco, Invitrogen, Grand Island, NY, USA), 10 mM HEPES buffer, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma, St Louis, MO, USA) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (50 ng/ml) plus rhIL-4 (25 ng/ml) at 37°C in 5% CO2 incubator to obtain immature DCs. The immature DCs so obtained were washed and resuspended in serum-free medium for allergen stimulation.
Chemical treatment of DCs and allergen stimulation
Monocyte-derived DCs were pretreated with four signalling cascade inhibitors; namely, SB203580 (20 μM for 30 min; a p38 MAPK inhibitor, SP600125 (20 μM for 30 min); a c-Jun N-terminal kinase (JNK) inhibitor, U0126 (20 μM for 30 min); an extracellular-signal-regulated kinase (ERK) inhibitor or Bay 11–7085 (3 μM for 1 h), and a NF-κB inhibitor separately 10. Monocyte-derived DCs pretreated or not with the pharmacological inhibitor were then stimulated with 1 μg/ml of proteolytically active or heat-inactivated Per a 10, respectively. Cells were incubated for 48 h at 37°C in a 5% CO2 incubator. Cells treated with the inhibitor alone were taken as internal control. As dimethylsulphoxide (DMSO) was used to dissolve the inhibitors, a DMSO-treated cell was also taken as control. For CD40 blocking experiments, the DCs were pretreated with anti-human CD40 blocking antibody (10 μg/ml) for 2 h and stimulated with the respective allergens.
Staining for cell surface markers
After 48 h of maturation, DCs were harvested and washed with phosphate-buffered saline (PBS) containing 0·1% (w/v) sodium azide plus 1% (v/v) heat-inactivated fetal bovine serum. Cells were stained with monoclonal antibodies (mAbs) for mouse anti-human CD14-phycoerythrin, CD40-fluorescein isothiocyanate, CD83-phycoerythrin-cyanin 5 (BD Biosciences, San Jose, CA, USA) or irrelevant isotype controls for 30 min at 4°C in the dark. Cells were acquired on an LSR II flow cytometer and analysed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
After an adequate stimulation time with proteolytically active or heat-inactivated Per a 10, DCs were harvested and washed with ice-cold PBS. Cell lysates were prepared by resuspending the cell pellet in lysis buffer [20 mM Tris (pH 7·4), 137 mM NaCl, 2 mM ethylenediamine tetraacetic acid (EDTA) (pH 7·4), 1% Triton-X, 25 mM β-glycerophosphate, 1 mM Na3VO4, 2 mM sodium pyrophosphate, 10% glycerol, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 μg/ml aprotinin, 10 μg/ml leupeptin and 10 μg/ml pepstatin] 10. The homogenates were centrifuged at 17 600 g for 20 min at 4°C. An equal amount of denatured protein (50 μg) was loaded onto 12% SDS-PAGE gel and transferred onto nitrocellulose membrane 10. Membranes were incubated with antibodies directed against phosphorylated forms of p38 MAPK (Thr 180/Tyr 182), ERK1/2 or JNK 1/2 (Thr183/Tyr185). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
T cells stimulation assay
Autologous CD4+ T cells were isolated from peripheral blood of allergic patients using the MACS system, as described previously 19. DCs pretreated or not with signalling inhibitors and stimulated with active or heat-inactivated Per a 10 were co-cultured with autologous CD4+ T cells in triplicate at a ratio of 1:10 for 5 days at 37°C in a CO2 incubator. Culture supernatants were collected and analysed for cytokine measurement.
Enzyme-linked immunosorbent assay
The concentrations of IL-1α, IL-6, IL-12 (p70), interferon (IFN)-γ, TNF-α (BD Pharmingen, San Diego, CA, USA) and sCD40 (eBioscience, San Diego, CA, USA) were detected in undiluted culture supernatant of DCs and DC–T cell co-cultures using paired antibodies following the manufacturer's protocol. IL-4, IL-5 and IL-13 were also detected in DC–T cell culture supernatants. The detection limit for IL-1α, IL-4, IL-6, IL-12 (p70), IFN-γ, TNF-α and sCD40 used was 1·1, 7·8, 4·7, 7·8, 4·7, 7·8 and 6·92 pg/ml, respectively.
Statistical analysis
Data were analysed by GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined by one-way analysis of variance (anova) followed by Dunnett's multiple comparison tests. Data are expressed as mean ± standard deviation (s.d.). A P-value < 0·05 was considered significant.
Results
Per a 10 activity conditions DCs with reduced CD40 expression and low IL-12 secretion
Monocyte-derived DCs stimulated with proteolytically active or heat-inactivated Per a 10 were evaluated for CD40 expression by flow cytometry. Active Per a 10-stimulated DCs demonstrated low surface CD40 expression compared to the heat-inactivated Per a 10-stimulated DCs (Fig. 1a,b). The level of soluble CD40 was significantly higher (P < 0·05) in active Per a 10-stimulated DC cultures than the heat-inactivated Per a 10 (Fig. 1c). The IL-12 level was significantly lower (P < 0·05) in active Per a 10-stimulated DCs culture supernatant than in the heat-inactivated Per a 10-stimulated DCs (Fig. 2a). Further, to confirm that CD40 is required for IL-12 secretion, CD40 blocking experiments were performed. Blocking the surface CD40 demonstrated a significant reduction (P < 0·05) in IL-12 secretion by the DCs after stimulation with the heat-inactivated Per a 10 (Fig. 2b). This shows that CD40 participates in IL-12 secretion by the DCs in response to stimulation with Per a 10.
Fig 1.
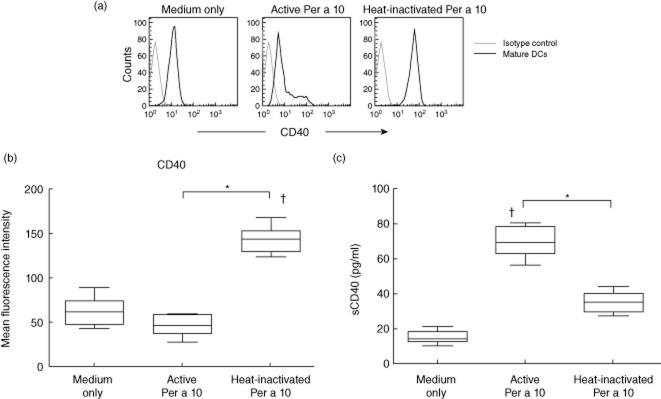
Effect of Per a 10 protease activity on CD40 expression by dendritic cells (DCs). (a) The histograms represent the CD40 expression by monocyte-derived DCs that were unstimulated (medium only), stimulated with proteolytically active or heat-inactivated Per a 10 and analysed by flow cytometry. (b) Mean fluorescence intensity (MFI) values of CD40 expression in unstimulated or stimulated DCs. The MFI value of samples is corrected with MFI of the isotype control. Data are representative of seven experiments. (c) Soluble CD40 level was estimated in culture supernatant of DCs stimulated with active or heat-inactivated Per a 10. Data (n = 7) are presented as mean ± standard deviation. *P < 0·05; †P < 0·05 in comparison to medium only.
Fig 2.
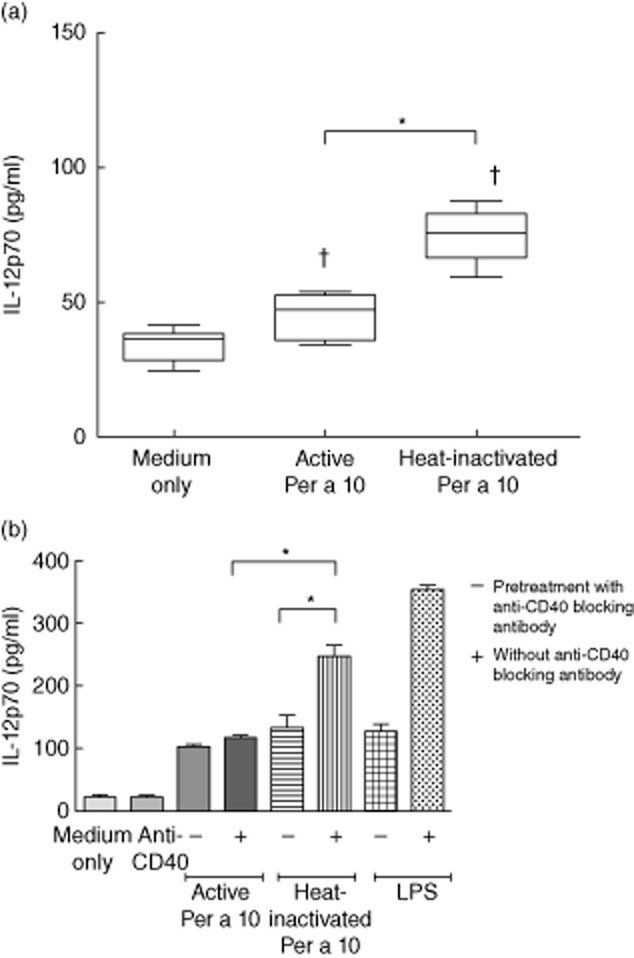
Interleukin (IL)-12 secretion in the culture-supernatant of dendritic cells (DCs). (a) DCs (n = 7) were stimulated with proteolytically active or heat-inactivated Per a 10 for 48 h and evaluated for IL-12 secretion by enzyme-linked immunosorbent assay (ELISA). (b) DCs (n = 3) were pretreated with CD40 blocking antibody followed by stimulation with proteolytically active or heat-inactivated Per a 10 and tested for IL-12 secretion. Lipopolysaccharide (LPS) was used as a positive control. Data are presented as mean ± standard deviation (s.d.). *P < 0·05; †P < 0·05 in comparison to medium only.
Protease activity of Per a 10 stimulates p38 MAPK that is not associated with CD40 expression by DCs
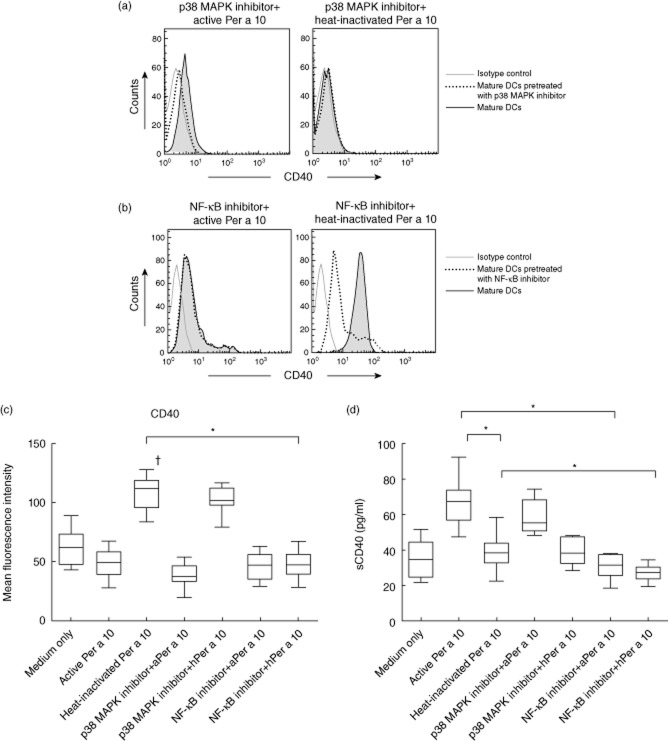
Up-regulation of CD40 by DCs is associated with the activation of several downstream signalling pathways. In the present study, protease activity of Per a 10 was evaluated for its role in activating the MAPK signalling pathway that might be associated with CD40 expression on DCs. Stimulation of DCs with proteolytically active Per a 10 activates p38 MAPK, as demonstrated by its phosphorylation in Western blot analysis (Fig. 3a), while heat-inactivated Per a 10 induced weak activation, suggesting the role of Per a 10 protease activity in activating the p38 MAPK pathway. However, inhibiting the p38 MAPK pathway depicted a similar level of CD40 expression on DCs compared to that expressed on the active or heat-inactivated Per a 10-stimulated DCs without prior inhibition (Fig. 4a,c). The sCD40 levels in the culture supernatant of DCs pretreated with p38 MAPK inhibitor also remained the same as those observed by active or heat-inactivated Per a 10-stimulated DCs (Fig. 4d). This suggests that although Per a 10 protease activity may be important for activating p38 MAPK, the activated pathway has no role in regulating CD40 expression by DCs. The role of the ERK and JNK pathways in CD40 expression by DCs was not observed after stimulation with either the active or heat-inactivated Per a 10 (data not shown).
Fig 3.
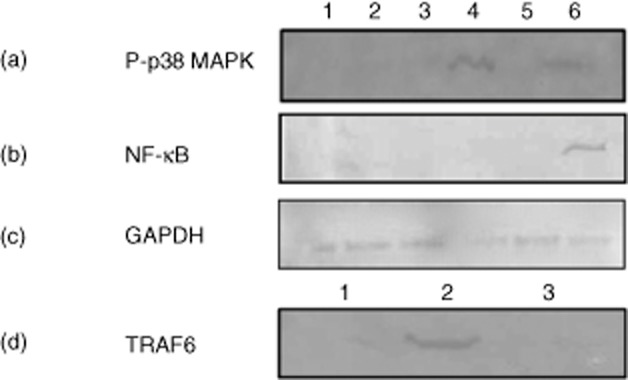
Activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) in Per a 10-stimulated dendritic cells (DCs). The DCs pretreated or not with inhibitor for (a) p38 MAPK or (b) NF-κB pathways and stimulated with active or heat-inactivated Per a 10 were analysed by Western blot. Well 1, immature DCs; 2, inhibitor alone; 3, inhibitor plus active Per a 10; 4, active Per a 10 alone; 5, inhibitor plus heat-inactivated Per a 10; and 6, heat-inactivated Per a 10 alone. (c) Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control and (d) expression of tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6) in Well 1, immature DCs; 2, heat-inactivated Per a 10; and 3, inhibitor plus heat-inactivated Per a 10.
Fig 4.
CD40 expression by dendritic cells (DCs) pretreated with (a) p38 mitogen-activated protein kinase (MAPK) inhibitor-SB203580 or (b) nuclear factor-kappa B (NF-κB) inhibitor Bay 11–7085 followed by stimulation with proteolytically active or heat-inactivated Per a 10. (c) Mean fluorescence intensity (MFI) values of CD40 expression of DCs pretreated with p38 MAPK or NF-κB inhibitor. Data are representative of 11 experiments performed. (d) Soluble CD40 levels in culture supernatant of DCs. ‘aPer a 10’ represents active Per a 10 and ‘hPer a 10’ represents heat-inactivated Per a 10. Data are representative of three experiments performed. *P < 0·05; †P < 0·05 in comparison to medium only.
Per a 10 activity decreases NF-κB expression, thereby reducing CD40 expression by DCs
Mature DCs showed intense NF-κB expression after stimulation with heat-inactivated Per a 10 compared to the active Per a 10 (Fig. 3b). Further, evaluating the role of NF-κB in CD40 expression showed that DCs pretreated with NF-κB inhibitor (Bay 11–7085) followed by stimulation with heat-inactivated Per a 10 reduced CD40 expression significantly compared to that expressed by the DCs stimulated with the heat-inactivated Per a 10 alone (Fig. 4b,c). Further, sCD40 levels were lower in DCs pretreated with NF-κB inhibitor followed by heat-inactivated Per a 10 stimulation compared to those stimulated with heat-inactivated Per a 10 alone (Fig. 4d). Also, the increased CD40 expression in heat-inactivated Per a 10-stimulated DCs was accompanied by elevated levels of TNF receptor-associated factor 6 (TRAF6) expression (Fig. 3d), which appears to be involved in NF-κB activation.
Per a 10-stimulated DCs secrete reduced levels of IL-12 and IFN-γ after inhibition of the NF-κB pathway
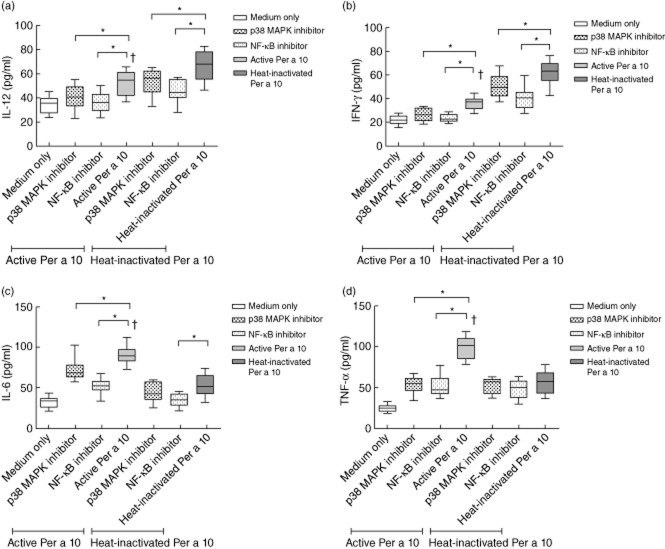
CD40 up-regulation leads to increased IL-12 secretion. Here, the role of NF-κB or p38 MAPK in regulating the secretion of IL-12 by Per a 10-stimulated DCs was analysed. DCs stimulated with proteolytically active Per a 10 secreted significantly (P < 0·05) lower levels of IL-12 (Fig. 5a) and IFN-γ (Fig. 5b) than heat-inactivated Per a 10. Pretreatment with NF-κB inhibitor and stimulation with active or heat-inactivated Per a 10 reduced the IL-12 and IFN-γ secretion compared to that secreted by the active or heat-inactivated Per a 10-stimulated DCs, respectively (P < 0·05). Pretreatment with p38 MAPK inhibitor and stimulation with active or heat-inactivated Per a 10 also showed a decline in IL-12 and IFN-γ secretion compared to treatment with the active or heat-inactivated Per a 10 directly (Fig. 5a,b). This indicates that both NF-κB and p38 MAPK participate in regulating the secretion of proinflammatory cytokines IL-12 and IFN-γ by Per a 10-stimulated DCs.
Fig 5.
Cytokine levels (a) interleukin (IL)-12, (b) interferon (IFN)-γ, (c) IL-6 and (d) tumour necrosis factor (TNF)-α in culture supernatant of mature dendritic cells (DCs) pulsed with proteolytically active or heat-inactivated Per a 10. DCs were pretreated with p38 mitogen-activated protein kinase (MAPK) inhibitor-SB203580 or nuclear factor-kappa B (NF-κB) inhibitor Bay 11–7085 and then stimulated with proteolytically active or heat-inactivated Per a 10. DCs treated with inhibitors alone were taken as internal control. Data (n = 11) are presented as mean ± standard deviation (s.d.). *P < 0·05; †P < 0·05 in comparison to medium only and heat-inactivated Per a 10 group.
Per a 10-induced IL-6 and TNF-α secretion is dependent upon p38 MAPK and NF-κB pathways
IL-6 is known to promote the proliferation of effector Th2 cells mediated by DCs. CD40 expression and the secretion of IL-6 and IL-12p40 has been shown previously to be regulated via the NF-κB pathway 8,9. In the present study, the IL-6 level was significantly high in culture supernatant of DCs stimulated with proteolytically active compared to heat-inactivated Per a 10 (P < 0·05) (Fig. 5c). The level of IL-6 was reduced significantly on pretreatment of DCs with p38 MAPK or NF-κB inhibitor compared to the cells treated directly with active Per a 10.
Secretion of TNF-α also declined by the DCs pretreated with p38 MAPK or NF-κB inhibitor, followed by stimulation with active Per a 10 (P < 0·05), compared to when the cells were treated directly with proteolytically active Per a 10 (Fig. 5d). However, no such differences were observed for the heat-inactivated Per a 10 group.
Th cell priming by Per a 10-stimulated DCs is dependent partly upon p38 MAPK and NF-κB
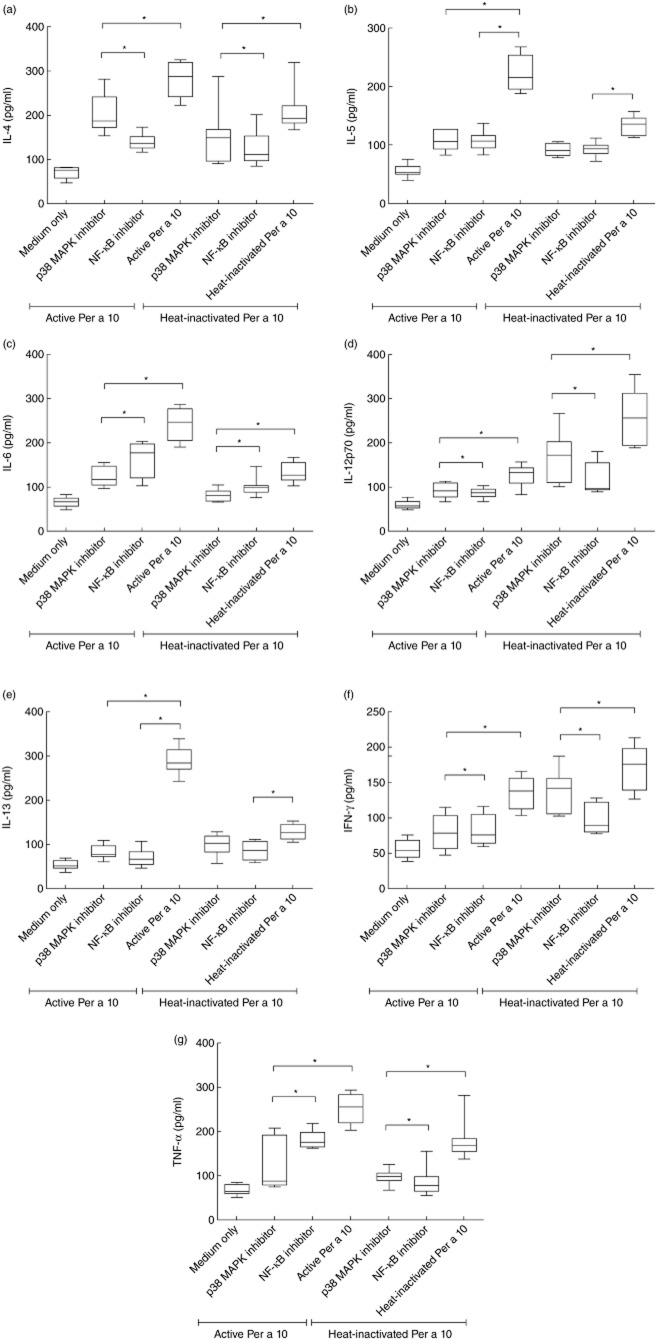
DCs treated with proteolytically active Per a 10 induced significantly high IL-4 (P < 0·05), IL-5 (P < 0·05), IL-6 (P < 0·05), IL-13 (P < 0·05) and TNF-α (P < 0·05) by Th cells compared to the Th cells that were primed by heat-inactivated Per a 10-treated DCs (Fig. 6). The levels of IL-4, IL-5, IL-6, IL-13 and TNF-α secreted by Th cells showed a reduction when DCs were pretreated with p38 MAPK or NF-κB inhibitor (Fig. 6a–c,e,g). Conversely, IL-12 and IFN-γ levels were significantly lower (P < 0·05) by Th cells primed by active Per a 10-treated DCs than those by heat-inactivated Per a 10-treated DCs (Fig. 6d,f). The IL-12 level secreted by Th cells was reduced further when the DCs were exposed to p38 MAPK (P < 0·05) or NF-κB inhibitor (P < 0·05) followed by stimulation with proteolytically active Per a 10 (Fig. 6d).
Fig 6.
Cytokine levels (a) interleukin (IL)-4, (b) IL-5, (c) IL-6, (d) IL-12p70, (e) IL-13, (f) interferon (IFN)-γ and (g) tumour necrosis factor (TNF)-α in culture supernatants of dendritic cell (DC)–T cell co-cultures. Autologous CD4+ T cells were co-cultured with proteolytically active or heat-inactivated Per a 10 [pretreated or not with signalling inhibitor (p38 MAPK inhibitor-SB203580 or nuclear factor-kappa B (NF-κB) inhibitor Bay 11–7085]-stimulated DCs. Data (n = 11) are presented as mean ± standard deviation (s.d.). *P < 0·05.
Discussion
Cockroaches are an important source of allergens among indoor pests. Exposure to cockroaches increases the risk of allergic sensitization that may lead to the development of asthma. Protease-rich P. americana extract has previously shown significant airway inflammation and increased cellular infiltration of lungs in mice. The protease activity of Per a 10 allergen from P. americana induced systemic IgE production and a Th2 response in a murine model of airway inflammation 17. Another study revealed that proteolytic activity of Per a 10 biases DC function by CD86 up-regulation and low IL-12 secretion 19. The low IL-12 secretion was suggested to be associated with CD40 cleavage by proteolytic activity of Per a 10, rendering the DCs less responsive to stimulation via CD40 signalling 19. The Per a 10-stimulated DCs further primed naive CD4+ T cells towards a type 2 response. The cysteine protease allergen Der p 1 demonstrated CD40 cleavage on the DC surface that deprives the DCs of receiving co-stimulatory signals from T cells, leading to significant reduction in IL-12 production 8. Despite several studies showing the role of CD40 in stimulating DCs for efficient specific T cell stimulation 9–15, signalling through CD40 in DCs remains poorly documented. Also, it is imperative to understand if allergen protease activity plays any role in CD40-mediated signalling in DCs. Therefore, the present study was undertaken to elucidate the role of Per a 10 protease activity in CD40-mediated signalling in DCs and the downstream components involved.
CD40-induced activation of cytokine gene expression in DCs is an important process in the initiation of primary immune response. In the present study, stimulation with proteolytically active Per a 10 induced a significantly low CD40 expression on the DC surface compared to that by heat-inactivated Per a 10. Active Per a 10-stimulated DCs also secreted low levels of IL-12 in cultures compared to heat-inactivated Per a 10 stimulation. Studies suggest that signalling through CD40 is required for efficient IL-12 production 8,11–13. Thus, the low level of IL-12 observed in the present study can be correlated with low CD40 expression by DCs which, in turn, might be due to the protease activity of Per a 10. In addition, the level of soluble CD40 was higher in active Per a 10-stimulated DC cultures than that in the heat-inactivated Per a 10. This indicates that the low CD40 expression on the DC surface is due to cleavage by proteolytic activity of Per a 10, and hence more sCD40 released into the culture supernatant. Further, blocking the surface CD40 showed a significant decline in IL-12 secretion after stimulation with heat-inactivated Per a 10, demonstrating the role of CD40 in IL-12 secretion by DCs.
CD40 engagement in DCs leads to the recruitment of TRAFs to its cytoplasmic domain that mediates the activation of NF-κB and the MAPK family 9,10,16,21. Earlier studies have indicated the role of p38 MAPK activation in CD40-induced IL-12 synthesis by DCs 22, while others suggest the involvement of the NF-κB pathway in CD40 expression and production of IL-6, IL-12p40 and IL-8 by DCs 9,10. In the present study, proteolytically active Per a 10 demonstrated the phosphorylation of p38 MAPK; however, inhibiting the p38 MAPK pathway prior to active Per a 10-stimulation showed no significant differences in CD40 expression by DCs, suggesting that CD40 expression in response to Per a 10 stimulation is independent of p38 MAPK. In contrast to this, NF-κB activation was observed by heat-inactivated Per a 10-stimulated DCs than that by active Per a 10. Inhibiting the NF-κB prior to heat-inactivated Per a 10-stimulation showed a significant reduction in CD40 expression on DCs. Thus, it appears that, first, CD40 expression by DCs is required for efficient NF-κB activation and secondly, NF-κB activation is important for sustained expression of CD40 on the surface of DCs. In addition, CD40 expression demonstrated the activation of TRAF6, suggesting that CD40-mediated NF-κB activation might be a TRAF6-mediated pathway. NF-κB activation is a widely documented consequence of CD40 signalling that leads to changes in gene expression 23. TRAFs have been suggested as mediators of CD40-induced NF-κB activation, although there is controversy regarding the involvement of specific TRAFs in such a response(s). TRAF2 was observed to be the critical mediator of anti-CD40-stimulated NF-κB activity and IL-6 gene transcription in fetal-skin dendritic cells (FSDC) and DC2·4 23. In another study, CD40 engagement induced the recruitment of TRAF2 and 3 that led to IL-1α and IL-1β mRNA expression via a MEK/ERK pathway in DCs 21.
In the present study, NF-κB inhibition led to reduced secretion of IL-12 and IFN-γ by DCs, providing more evidence that CD40-mediated NF-κB activation regulates the secretion of IL-12 and IFN-γ. Inhibiting p38 MAPK or NF-κB reduced the level of IL-6 and TNF-α by DCs, suggesting that secretion of IL-6 and TNF-α is dependent upon both pathways and that receptor molecules, apart from CD40 on the DCs surface, might also participate in regulating their secretion. Furthermore, Th cell priming ability of Per a 10-stimulated DCs was dependent upon both p38 MAPK and NF-κB, as demonstrated by the reduction of IL-4, IL-6, TNF-α and IL-12 after inhibiting either of the pathways.
Taken together, Fig. 7 illustrates that protease activity of Per a 10 induces CD40 cleavage on the DCs surface that leads to reduced IL-12 secretion. Active Per a 10 induces p38 MAPK phosphorylation while showing low NF-κB activation compared to heat-inactivated Per a 10. NF-κB activation is crucial for CD40 expression by DCs, while both p38 MAPK and NF-κB regulates the secretion of IL-6, IL-12, IFN-γ and TNF-α.
Fig 7.
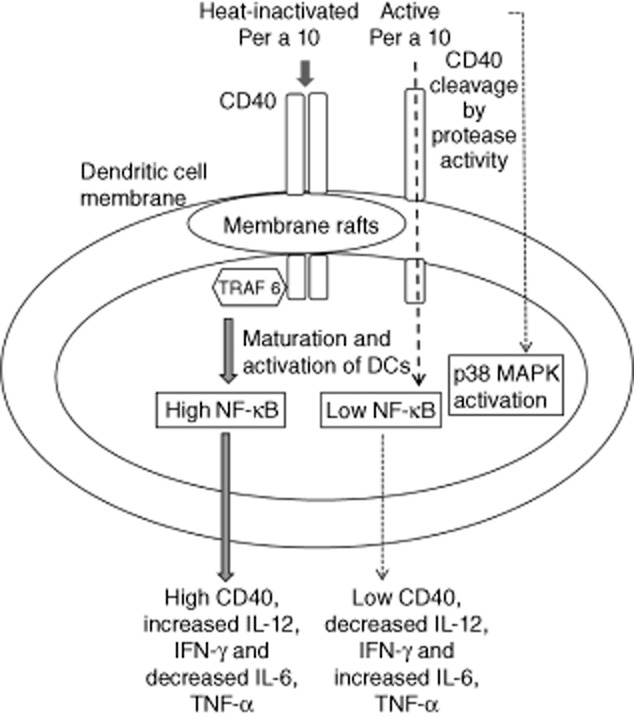
Diagrammatic representation of CD40-mediated signalling in human dendritic cells (DCs) in response to Per a 10 stimulation.
In conclusion, protease activity of Per a 10 is important in modulating CD40 expression on the DC surface. Engagement of CD40 on the DC surface leads to TRAF6 recruitment that induces NF-κB activation and secretion of proinflammatory cytokines, such as IL-12. Heat-inactivated Per a 10 could be explored further for immunomodulatory functions.
Acknowledgments
The authors thank Dr Ulaganathan Mabalirajan (CSIR – Institute of Genomics and Integrative Biology, Delhi, India) for help in blood collection. One of the authors (C. G.) is a Senior Research Fellow from the Indian Council of Medical Research, Delhi.
Disclosure
Authors have no financial conflicts of interest.
Author contributions
C. G. and N. A. designed the study and conducted the experiments. S. N. G. provided the study subjects and performed the SPT of patients. N. K. helped in performing the flow cytometry experiments and B. S. D. helped in interpreting the data. The paper was written by C. G. and N. A. and examined critically by N. K., B. S. D. and S. N. G.
References
- Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7:363–367. doi: 10.1007/s11882-007-0055-6. [DOI] [PubMed] [Google Scholar]
- Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190:1897–1902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami AM, Robins A, Gough L, Sewell HF, Shakib F. Human T cell subset commitment determined by the intrinsic property of antigen: the pro proteolytic activity of the major mite allergen Der p 1 conditions T cells to produce more IL-4 and less IFN-γ. Eur J Immunol. 2001;31:1211–1216. doi: 10.1002/1521-4141(200104)31:4<1211::aid-immu1211>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Segal DM. Controlling the Toll road to dendritic cell polarization. J Leukoc Biol. 2004;75:721–730. doi: 10.1189/jlb.1003482. [DOI] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi SE, Martin RE, Ito T, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy. 2002;32:1468–1475. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- Ade N, Antonios D, Kerdine-Romer S, Boisleve F, Rousset F, Pallardy M. NF-kB plays a major role in the maturation of human dendritic cells induced by NiSO4 but not by DNCB. Toxicol Sci. 2007;99:488–501. doi: 10.1093/toxsci/kfm178. [DOI] [PubMed] [Google Scholar]
- Antonios D, Rousseau P, Larangé A, Kerdine-Römer S, Pallardy M. Mechanisms of IL-12 synthesis by human dendritic cells treated with the chemical sensitizer NiSO4. J Immunol. 2010;185:89–98. doi: 10.4049/jimmunol.0901992. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso JF, Lapointe R, Hwu P. CD40 ligand and lipopolysaccharide enchance the in vitro generation of melanoma-reactive T-cells. J Immunol Methods. 2002;259:181–190. doi: 10.1016/s0022-1759(01)00513-0. [DOI] [PubMed] [Google Scholar]
- Tuettenberg A, Fondel S, Steinbrink K, Enk AH, Jonuleit H. CD40 signalling induces IL-10-producing, tolerogenic dendritic cells. Exp Dermatol. 2010;19:44–53. doi: 10.1111/j.1600-0625.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-α, and contact sensitizers. J Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- Sudha VT, Arora N, Singh BP. Serine protease activity of Per a 10 augments allergen-induced airway inflammation in a mouse model. Eur J Clin Invest. 2009;39:507–516. doi: 10.1111/j.1365-2362.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Mehta AK, Arora N, Gaur SN, Singh BP. Proteolytically inactive Per a 10 allergen of Periplaneta americana modulates Th2 response and enhances IL-10 in mouse model. J Clin Immunol. 2010;30:426–434. doi: 10.1007/s10875-009-9362-0. [DOI] [PubMed] [Google Scholar]
- Goel C, Govindaraj D, Singh BP, Farooque A, Kalra N, Arora N. Serine protease Per a 10 from Periplaneta americana bias dendritic cells towards type 2 by upregulating CD86 and low IL-12 secretions. Clin Exp Allergy. 2012;42:412–422. doi: 10.1111/j.1365-2222.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. 2008;63:768–776. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- Vidalain PO, Azocar O, Servet-Delprat C, Rabourdin-Combe C, Gerlier D, Manié S. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 2000;19:3304–3313. doi: 10.1093/emboj/19.13.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Shu GL, Magaletti D, et al. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol. 1999;163:5786–5795. [PubMed] [Google Scholar]
- Mann J, Oakley F, Johnson PW, Mann DA. CD40 induces interleukin-6 gene transcription in dendritic cells: regulation by TRAF2, AP-1, NF-kappa B, AND CBF1. J Biol Chem. 2002;277:17125–17138. doi: 10.1074/jbc.M109250200. [DOI] [PubMed] [Google Scholar]