SUMMARY
Medulloblastoma is the most common malignant pediatric brain tumor. Current treatments including surgery, craniospinal radiation and high-dose chemotherapy have led to improvement in survival. However, the risk for recurrence as well as significant long-term neurocognitive and endocrine sequelae associated with current treatment modalities underscore the urgent need for novel tumor-specific, normal brain-sparing therapies. It has also provided the impetus for research focused on providing a better understanding of medulloblastoma biology. The expectation is that such studies will lead to the identification of new therapeutic targets and eventually to an increase in personalized treatment approaches.
KEYWORDS : immunotherapy for pediatric brain tumors, medulloblastoma, molecular classification, mouse models and preclinical studies, targeted agents for clinical studies, tumor epigenomics, tumor genomics
Practice points.
Medulloblastoma is a heterogeneous disease.
Molecular subgrouping and biology in conjunction with histopathology is increasingly driving prognostication.
Molecular mechanisms underlying metastatic disease remain to be fully understood.
Molecular subgrouping provides an opportunity for personalized medicine.
Combination chemotherapeutic approaches will be important to tackle treatment resistance.
Immunotherapy may be a novel tool for the treatment of pediatric brain tumors.
Approximately 400–500 new cases of medulloblastoma (MB) are recorded in the USA every year, primarily in children [1]. Current treatment includes surgery followed by radiation and chemotherapy [2,3]. Event-free survival and overall survival vary based on histology: (desmoplastic/nodular MB [DNMB] or MB with extensive nodularity [MBEN], classic MB [CMB] and large-cell/anaplastic [LCA]), extent of resection and presence of metastatic disease at diagnosis. Mortality rates have declined with 60–80% of patients surviving the disease [4,5]. Unfortunately, survivors have poor quality of life associated with disease and therapy-related side effects including long-term physical, endocrine, intellectual and cognitive impairment [6]. Furthermore, these children are at risk for recurrence and secondary malignancies [6]. Children younger than 3 years of age also tend to have worse outcomes [7]. Thus, there is an urgent need to re-evaluate and recalibrate clinical practice to limit damage to the developing brain and to improve survival. The research and clinical community have mined human tumor samples to help determine a path forward. As discussed below, studies focused on genetic and epigenetic analyses of patient tumors have shown that MB is not a single disease [8]. This is further complicated by intratumoral heterogeneity, leading to a growing recognition that in place of a uniform therapeutic approach for all MB patients, clinical decisions should take into consideration histopathology and clinical staging in conjunction with knowledge of tumor biology [8,9].
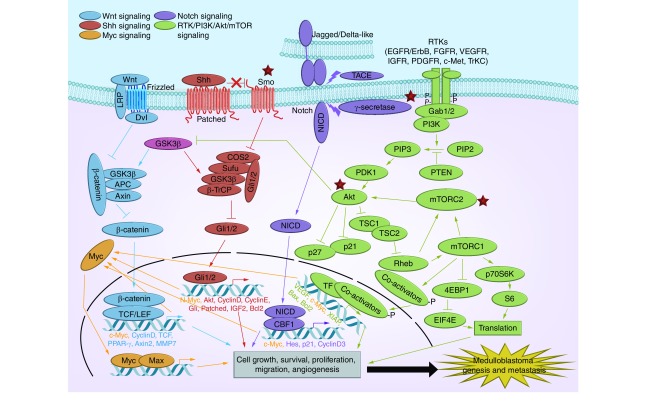
Here, we provide an overview of emerging data from high-throughput analyses of patient tumors, studies on signaling pathways with animal models and efforts to identify novel molecular targets for clinical application. We also discuss the state of newly initiated clinical trials to test molecularly targeted therapies and immunotherapy, and efforts to integrate conventional and novel treatment approaches (Figure 1).
Figure 1. . Overview of major signaling pathways and druggable nodels in medulloblastoma.
Targets of drugs under clinical investigation in children with pediatric solid/brain tumors are circled.
Cerebellar development & MB subtypes
Earliest studies of MB patients suggested a link between perturbations in cerebellar development and genesis of the disease. Familial inheritance accounts for a subset of MBs and is seen in patients with Gorlin's, Turcots and Li-Fraumeni syndrome [10]. Gorlin's syndrome associated with inactivating mutations in the PTCH-1, -2 and SUFU tumor suppressor genes, predisposes to MB development by deregulating the Sonic Hedgehog (Shh) developmental pathway [10]. Turcot's syndrome is characterized by inactivating mutations in the APC gene, and results in constitutive activation of the Wingless (Wnt) signaling pathway [10]. Finally, patients with Li–Fraumeni syndrome have germline mutations in the p53 tumor supressor gene, which predisposes them to various cancers including MB [10]. These observations have led to the generation of the first genetically engineered mice (GEM) models for Shh and the Wnt driven MBs [11–13]. The animal models in turn have been critical for the identification of the granule neural precursors (GNPs) and the rhombic lip precursors as the cells of origin of Shh and Wnt tumors respectively, the identification of downstream signaling cascade, and finally investigations on targeted therapy.
Sproadic MBs are driven by Shh pathway activation in approximately 20–25% of the cases, while Wnt pathway activation drives approximately 15% of these tumors. Amplifications in c-MYC and N-MYC occur in 5% of human MBs, while increased expression of gene or protein in the absence of amplification is common in 20–40% of tumors and is associated with poor prognosis [14].
MB genomics
In one of the first application of high-throughput methodologies to pediatric brain tumors, the Pomeroy group showed MBs and atypical teratoid rhabdoid tumors to be distinct disease entities [5]. The disconnect between histological subtyping and outcomes subsequently provided the impetus for international collaborative genome-based studies and efforts to reclassify MBs based on their molecular profile. These genetic and transcriptional profiling studies have led to the identification of four distinct molecular subtypes of MB: WNT/Wingless, SHH/Sonic Hedgehog, Group 3 and Group 4 [14–26]. Whereas WNT subgroup of tumors displayed predominantly CMB histology, SHH tumors included the DNMBs, CMBs and LCA subgroups. Group 3 and 4 tumors present as CMBs or highly aggressive LCAs [14–26]. The molecular classification of MBs in combination with histopathology has also allowed better prediction of likelihood of metastasis. Thus, patients with WNT tumors rarely have metastasis and respond well to therapy, whereas a subset of children with Shh-driven tumors as well as children with high-risk Group 3 and intermediate-risk Group 4 MBs have a significantly increased risk of developing disseminated disease [14–26].
Nevertheless, the same aggressive approach is used to treat all MB patients. SHH, Group 3 and Group 4 patients fail to benefit from the current treatment approaches [3]. Given the better outcomes seen in patients with Wnt-driven tumors, the merits and demerits of treatment de-escalation, specifically craniospinal radiation is being critically debated within the clinical community.
Time for paradigm shift in MB therapeutics?
MB genomics has not only significantly advanced our understanding of tumor biology, but also led to the molecular reclassification of these tumors and set the stage for recalibrating treatment based on specific needs of each patient. We summarize below the hallmarks of the various MB subgroups, preclinical investigations with mouse models and important clinical steps to help improve survival and quality of life.
WNT subgroup
Wnt tumors characterized by constitutive activation of Wnt signaling exhibit mutations in CTNNB1, AXIN1 and CTNNB1-associated chromatin re-modelers such as SMARCA4 and CREBBP and epigenetic silencing of genes encoding Wnt signaling antagonists, SFRP and DKK1 [23–29]. Mutations in p53 are seen in approximately 16% of WNT subgroup tumors [30].
GEM models have definitively shown that constitutive activation of Wnt-β-Catenin signaling in cells of the lower rhombic lip drives development of lesions with proliferating Zic1+ cells [13]. In agreement with data from patient samples, 15% of these mice suffer concurrent deletion of p53, resulting in tumors that recapitulate features of human WNT subtype of tumors [13]. These studies also identified genes that maintain this cell lineage (DDX3X), as well as mutated genes that initiate (CDH1) or cooperate (PIK3CA) in tumorigenesis [13].
Because patients with Wnt tumors have good prognosis and respond well to current standard of care, de-escalation of treatment has been prioritized for clinical evaluation so as to maintain optimum cure rates while aiming for a reduction in side effects [3].
SHH subtype
SHH-subtype MBs are distinguished by constitutive activation of Shh signaling due to loss-of-function mutations in PTCH1/2 and SUFU, gain of function mutations in SMO or GLI-1/2 amplification, and account for approximately 50% of Shh driven MBs [13–26]. P53 mutations are seen in a subset of patients with Shh-driven MBs, and portends poor prognosis [30]. These tumors are frequently of the DNMB or MBEN histological subtypes, although a few LCA variants are seen. Indeed, DNMB and MBEN histological subtypes are seen exclusively within the SHH subgroup of MBs. While the prognosis is generally good for patients with Shh-driven tumors, children that present with LCA tumors often have poor prognosis [13–26]. The mechanism(s) underlying this variability are not clear. Mutations in the gene encoding the telomerase reverse transcriptase were seen in approximately 83% of MBs obtained from adult patients, but had an interesting association with good prognosis [31].
GEM models carrying deletion of a copy of the PTCH1 gene or knock-in of commonly occurring SMO1 mutations in patients have unequivocally shown that Shh-driven MBs arise from cerebellar GNPs [11,12]. These animal models in conjunction with cell culture systems have unraveled the biology and regulatory network of Shh signaling, providing novel druggable nodes and the basis for numerous preclinical studies. For example, pharmacological inhibition of SMO blocks signal transduction and tumor cell proliferation [32–35]. However, even brief inhibition of Shh signaling in mouse models with the inhibitor HhAntag caused permanent defects in bone development in young mice, precluding further investigations [34]. MBs harboring mutations in PTCH1 are responsive to SMO inhibitors such as GDC-0449/vismodegib, whereas mutations (in SUFU) or amplification (of MYC-N) of downstream signaling molecules render tumor cells unresponsive to such agents [36,37]. Cholesterol and specific oxysterols are required for Shh pathway signaling, and pharmacological inhibition of their synthesis blocks signal transduction and tumor cell proliferation [38].
Receptor tyrosine kinases including IGF and HGF/c-Met signaling through PI3K are required for Shh-mediated tumorigenesis. PI3K inhibitors, AKT inhibitors, HGF-blocking antibodies alone or in combination with SHH ligand neutralizing antibodies, SMO antagonists and Gas and Survivin inhibitors have all elicited robust response in mouse models [39–45].
Although, the role of Notch signaling in MB genesis has been debated, a recent transcriptome analysis of pediatric MB samples showed that HES1 overexpression is directly related to shorter survival [46–48]. Although, these analyses were not conducted specifically in the context of Shh-driven tumors, the observations that Notch2 regulates GCP proliferation and that it plays a role in tumor development in SmoA1 mouse models suggest a role for Notch activation in Shh-driven tumors [47,49]. Interestingly, studies with a novel GEM model have shown Shh group of MBs to be generated by activation of Notch signaling in neural stem cells and or in glial cells [50]. If true, pharmacological inhibition of Notch signaling in tumor stem cells or in the tumor microenvironment could be applied for treatment of patients with Shh subgroup of MBs [51]. There is now evidence for negative regulation of Wnt signaling by SUFU, indicating cross-talk between Shh and Wnt signaling pathways as well [52]. These factors will impact the effectiveness of Shh inhibitors in patients and should be considered during trial design.
In one of the first studies to show therapeutic potential of targeting MB metabolism, Gershon and colleagues demonstrated PI3K signaling-dependent induction of aerobic glycolysis in tumors in Smo-M2 mice [53]. Loss of aerobic glycolysis blocked tumor growth and promoted long-term survival in tumor-bearing mice. Shh signaling has been linked to the regulation of the MB epigenome by promoting increased transcription and sustained activation of histone deacetylases (HDACs) leading to increased GNP proliferation [54]. Thus, HDAC inhibitors may have applications in the treatment of Shh-driven MBs.
The variable responsiveness of MBs to chemotherapy and radiation has been attributed to its heterogeneity and the presence of a population of cells called tumor-propagating cells [55]. These cells are often stem cell-like and are marked by the cell surface antigen CD15/SSEA-1. In PTCH mutant mice, CD15+ tumor-propagating cells have dysregulated expression of Aurora kinase and Polo-like kinases (PLK), proteins involved in control of G2-M transit [55]. This vulnerability could be targeted by pharmacological inhibition using the PLK antagonist BI2536, which also enhanced the sensitivity of tumor cells to conventional chemotherapy in vitro and in vivo [55,56].
PI3K/AKT activation is important in MB dissemination and radio-resistance in mice [57–60]. In preclinical studies, the drugs (PIK-75 and YM024) targeting the p110α catalytic subunit of PI3K suppressed MB growth [59]. In addition, YM024 and IC87114 (an inhibitor of the p110δ subunit of PI3K) impaired MB cell migration and invasion. The mTORC1 inhibitor RAPA (rapamycin) also suppressed proliferation and migration of MB cells, although the novel mTORC1/2 inhibitor-pp242 appeared to have greater efficacy in inhibiting these processes [59]. Targeting the AKT kinase PDK1 alone with OSU03012 or in combination with the RAPA analog CCI-779 also synergistically blocked AKT activation resulting in potent suppression of MB growth in vitro and in vivo [59]. Interestingly, an association between elevated expression of the chromatin remodeler, REST, and leptomeningeal disease development was shown in a subset of patients with Shh-driven tumors [61]. A similar observation was made by a separate study, although not necessarily in the context of constitutive Shh activation, which raises the possibility that REST may have a role in driving metastatic disease [62]. REST is associated with a number of druggable activities such as HDAC1/2, the histone methyl transferase-G9a and the histone lysine demethylase LSD1, and REST-high MBs are more sensitive to HDAC inhibitors compared to low-REST isogenic cells [61]. REST also represses the transcription of the anti-proliferative deubiquitylase USP37, and drugs that reactivate USP37 expression may have therapeutic applications [63].
Preclinical studies directed at understanding therapeutic resistance in Shh-driven MBs have identified mutations in SMO, GLI-2 amplification and activation of PI3K signaling as major contributors to drug resistance [36]. For example, resistance to the SMO inhibitor vismodegib was attributed to D473H point mutation in SMO [37]. However, resistance to the drug saridegib was independent of the D473H mutation and Gli2 amplification, and was attributed to induction of P-glycoprotein activity [35]. Resistance to the SMO antagonist NVP-LDE225 in vivo could be countered by inhibiting PI3K activity using either NVP-BKM120 (a PI3K inhibitor) or NVP-BEZ235 (a dual PI3K and mTOR inhibitor) and mitigated by PTEN loss, suggesting that PI3K activation constitutes a mechanism of drug resistance in Shh-driven MBs [64,65].
Group 3
These tumors account for 25% of all MBs and occur more commonly in males and young children, and hardly ever comprise adult patients [13–26]. They frequently encompass the LCA and CMB histologic subtypes, with 50% of the patients exhibiting metastasis at presentation [13–26]. Survival is the lowest in children in this group and is currently at a dismal 20% [13–26].
Recent data suggest that cerebellar GNPs may give rise to Group 3 tumors, although the drivers for this subgroup of tumors are likely to be distinct from constitutive Shh activation [66–68]. Mutations in p53 that are seen in Shh and Wnt tumor subgroups are absent in subgroup 3 tumors [30]. Gains in chromosome 1q, 7 and 17q and deletions of 10q,11, 16q and 17p are frequently detected, indicating a high level of genomic instability [66–68]. Elevated c-Myc expression often with focal amplification of the locus, PVT1-Myc fusion, elevated expression of OTX2, as well as an increased frequency of mutations in histone H3 lysine (K)-27 demethylases are hallmarks of Group 3 MBs [66–68]. OTX2 overexpression and knockdown is associated with up- and downregulated expression of several polycomb genes including EZH2, EED, SUZ12 and RBBP4 and genes encoding H3K27 demethylases: KDM6A, KDM6B, JARID2 and KDM7A [66,69]. A novel genetically engineered mouse model with constitutive OTX2 expression in the postnatal hind-brain showed accumulation of clusters of proliferative cells originating from neural progenitors of the rhombic lip (dorsal brain stem) and migrating GNPs in cerebellar white matter [70]. OTX2 knockdown in human MB cells increased survival of tumor-bearing mice, indicating that OTX2 is necessary for tumor maintenance [71]. Studies such as these not only provide insights into mechanisms by which chromatin remodeling is involved in tumor development, but also provide a new class of drug targets. For example, the OTX2 target-EZH2 can be pharmacologically manipulated by GS2816126, an agent under clinical investigation for adult patients with hematological malignancies [72].
A novel mouse xenograft model (HD-MB03) established from a patient tumor with molecular features Group 3 MBs including isochromosome 17q and MYC amplification revealed strong expression of a number of HDACs, including HDAC-2, -5, -8 and -9 [73]. Consistent with these findings, HD-MB03 cells displayed increased sensitivity to the HDAC inhibitors, Vorinostat and Panobinostat [73]. These inhibitors also conferred increased radiation sensitivity to HD-MB03 cells, providing support for the use of HDAC inhibitors for the treatment of patients with Group 3 MBs [73].
Molecules that contribute to leptomeningeal disease development in Group 3 tumors are understudied [74,75]. Myc is a prime candidate because of its known roles in regulating migration, invasion and angiogenesis, processes critical to tumor metastasis. Myc inhibition for cancer therapy has been investigated over the years with little success. Nevertheless, agents that target Myc such as S2T1–6OTD, a telomestatin derivative that can bind to the c-Myc promoter, as well as agents that can modulate Myc expression including all-trans-retinoic acid (ATRA), the quassinoid analog NBT-272, the anti-convulsant and HDAC inhibitor-valproic acid (VPA), the polyphenol resveratrol, have shown efficacy in vitro and in mice, and their further investigation in MYC-high MBs may be warranted [76,77]. The availability of three separate Myc-driven mouse models of LCA MB should further aid in such preclinical studies [78–81].
Immunotherapy is being increasingly viewed as a weapon for use in combination therapy or as an alternative to conventional treatments [82–85]. The ability of immune cells to traffic also increases their attractiveness for treatment of metastatic disease. However, MBs appear to be immunosuppressive in comparison to other pediatric brain tumors and have fewer infiltrating immune cells, which are dominated by immunosuppressive M2 macrophages, CD8+ and CD4+ T cells [86]. The elevated expression of the nonclassical MHC CD1d gene, which encodes a receptor for a class of cytotoxic T cells, was recently leveraged to show tumor regression in an Shh mouse model and could be an attractive option for other metastatic MB subgroups as well [87]. The application of T cells for MB treatment could however be hampered by the low expression of HLA-I in neural tumors. The use of chimeric antigen receptor (CAR)-T cells avoids this problem and the use of CAR-T cells specific for HER2 showed efficacy against MB in a murine model [88]. The requirement for tumor-associated antigens (TAAs) can also be circumvented by harnessing the power of components of the innate immune system, such as natural killer (NK) cells [89–91]. NK cells have been tested successfully in cell culture systems [89–91].
Because B-cell function appears to be unaffected in MB patients, antibodies specific for a few TAAs can be evaluated alone or in conjunction with radiation or chemotherapy [92]. SHH and HGF blocking antibodies have been studied for efficacy in murine xenograft models [41]. Finally, antibodies against immunosuppressive molecules or drugs such as HDAC inhibitors that can increase tumor immunogenicity and alter the sensitivity of MBs to immune cells appear attractive for MB treatment [93].
Group 4
Group 4 tumors occur more frequently in older children and accounts for 35% of all MBs. Tumors are frequently of CMB histology with a few instances of LCA [23–29]. Metastasis is observed in 33% of these cases at diagnosis [23–29]. Mutations in p53 have not been described [30]. These tumors exhibit a neuronal molecular signature and exhibit elevated expression of OTX2, N-Myc, FST and CDK6 [13–26]. Isochromosome 17q and deletion of 17p is a common occurrence [13–26]. Children with Group 4 tumors have an intermediate prognosis [13–26]. Group 4 tumors in adult patients have a particularly poor prognosis [13–26]. Although, these MBs are the most commonly occurring tumors, their biology is the least understood.
N-Myc expression is elevated in human Group 4 MBs and its overexpression driven by the hind-brain specific Glt-2 promoter in postnatal neural stem cells resulted in non-Shh dependent, disseminated tumors with classic and LCA histology. Metastatic disease development combined with elevated N-Myc and their non-Shh signature suggest that these tumors may resemble human subgroup 4 MBs [79].
Bmi-1 is a polycomb group repressor complex gene overexpressed across all MB subgroups but most significantly in Group 4 tumors and is associated with deregulation of cell adhesion molecules. In vitro assays identified Bmi-1 dependent perturbation of cell adhesion and motility through repression of bone morphogenetic proteins (BMPs) [94]. In vivo, Bmi-1 controlled invasion in a novel xenograft model of human MB [94]. Thus, BMP agonists may have potential application in the treatment of Group 4 MBs [94].
Tumor biology drives novel clinical trials
Despite considerable preclinical data for targeted therapy, only few agents have been investigated as single agents or in combination with standard of care drugs in pediatric clinical trials. The SMO inhibitor GDC-0449/vismodegib was recently evaluated in a Phase I clinical trial (NCT00822458) involving children with refractory or relapsed MB [95–98]. The drug was well tolerated with a recommended Phase II dose of 150 or 300 mg. Two dose-limiting toxicities were observed [98]. A partial response was seen in a participant with metastatic MB [98]. It is under active evaluation in combination with temozolomide in a Phase I/II study in children (NCT01878617) and adults (NCT01601184) with MB. NVP-LDE225 (sonidegib) is another SMO inhibitor under clinical investigation as monotherapy in pediatric and adult MB patients (NCT01125800) [99]. It was well tolerated and response was observed in a few patients [98]. A Phase I study of sonidegib in combination with buparlisib (PI3K inhibitor) in adults with advanced solid tumors is ongoing (NCT01576666). The AKT inhibitor MK-2206 was evaluated in a Phase I/II trial of pediatric patients with refractory solid tumors (NCT01231919); study results remain to be released. mTOR inhibitors have been scrutinized in a number of trials for pediatric solid tumors. In a Phase I study, the drug everolimusin was well tolerated with a maximum tolerated dose (MTD) of 5 mg/m2 [100]. Deforolimus, another mTOR inhibitor, was well tolerated in a Phase I trial of pediatric patients with advanced cancers, with one partial response several instances of stable disease [101]. The Phase I study of a third mTOR inhibitor, temsirolimus, revealed safety. However, an MTD was not obtained, and the drug failed to meet criteria for its use as a single agent [102]. Temsirolimus has also been paired with irinotecan and temozolomide (NCT01141244, COG-ADVL0918) in a completed Phase I study for young patients with relapsed or refractory solid tumors; study results have not been posted. The most recent mTOR inhibitor under evaluation in a Phase I trial is ridaforolimus, both alone (NCT01431534) and in combination with dalotuzumab (NCT01431547). Results of these studies have not been posted. A Phase I (NCT01670175) studying the combination of rapamycin (sirolimus), cyclophosphamide and topotecan, in pediatric and young adults with relapsed and refractory solid tumors is currently open. Sirolimus was previously studied in combination with vinblastine (NCT01135563); no results have been published to date.
The Notch pathway is known to be important for maintenance of tumor stem cells, a population believed to contribute to treatment resistance [103]. Notch inhibition by the agent MK-0752 was evaluated in a recently completed Phase I trial of pediatric patients with recurrent CNS tumors [103]. Though safety was demonstrated efficacy was modest, thus undermining its use in future trials [103].
HDAC inhibitors have been investigated in two separate Phase I trials in pediatric patients with relapsed/refractory CNS tumors. The HDAC inhibitor Vorinostat was well tolerated when combined with either temozolomide or Bortezomib (NCT01076530, NCT00994500) [104,105]. The combination of Vorinostat, Isotretinoin and chemotherapy is under investigation in young patients with embryonal tumor (NCT00867178).
Immunotherapy has been gaining ground as a therapeutic approach for CNS malignancies. The intrathecal infusion of lymphocyte-activated killer (LAK) cells from allogeneic donors in a cohort of six patients with disseminated MB showed some success with three patients displaying no disease or neurological toxicity following treatment [106]. One other case report also echoed this success, warranting further investigation of LAK cells for pediatric CNS tumors [107]. A novel clinical trial investigating the safety and feasibility of fourth ventricular infusion of ex vivo expanded and activated NK cells has recently received US FDA approval and is anticipated to begin accruing pediatric patients with recurrent/refractory tumors of the posterior fossa.
131I conjugated GD2 antibodies have been evaluated for the treatment of MB, although a major drawback has been neuropathy associated with the use of GD2 as a target [108]. The use of high-dose chemotherapy followed by autologous stem cell transplant currently being pursued in multiple clinical trials holds promise, and has been attributed to the ‘resetting’ of the immune system [109]. However, the high relapse rates underscore the need for new combinations to augment the host antitumor immune response.
Conclusion & future perspective
The above discussion has provided a panoramic view of the preclinical studies that have examined the feasibility of targeting MBs. Of these, a few novel agents targeting Shh signaling and PI3K pathway have been explored in Phase I clinical trials in children (Figure 1). At present, very few have been studied in Phase II/III trials. Trial designs should take into consideration inter- and intratumoral heterogeneity in MBs, and also leverage high-throughput genomics and epigenomics to arrive at a panel of biomarkers that will help predict patient response to therapeutics.
Footnotes
Financial & competing interests disclosure
Work in V Gopalakrishnan's laboratory is supported by grants from the NIH (R01NS079715 and R03NS077021), American Cancer Society (118165-RSG-09–273–01-DDC), Addis Faith, Noah's Light, Rally Vs Cancer, Hyundai Hope on Wheels and The Cure Starts Now Foundations. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;(Suppl. 4):IV1–IV63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Hoff K, Rutkowski S. Medulloblastoma. Curr. Treat. Options Neurol. 2012;14(4):416–426. doi: 10.1007/s11940-012-0183-8. [DOI] [PubMed] [Google Scholar]
- 3.Leary SE, Olson JM. The molecular classification of medulloblastoma: driving the next generation clinical trials. Curr. Opin. Pediatr. 2012;24(1):33–39. doi: 10.1097/MOP.0b013e32834ec106. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An exceptional overview of how biology is increasingly driving MB treatment.
- 4.Packer RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma. Curr. Neurol. Neurosci. Rep. 2007;7(2):130–132. [PubMed] [Google Scholar]
- 5.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 6.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume and neurological complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014;32(17):1760–1769. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- 7.Hudson TJ. Genome variation and personalized cancer medicine. J. Intern. Med. 2013;274(5):440–450. doi: 10.1111/joim.12097. [DOI] [PubMed] [Google Scholar]
- 8.Morrison LC, McClelland R, Aiken C, et al. Deconstruction of medulloblastoma cellular heterogeneity reveals differences between the most highly invasive and self-renewing phenotypes. Neoplasia. 2013;15(4):384–398. doi: 10.1593/neo.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat. Rev. Neurol. 2012;8(6):340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]; •• An excellent article reviewing the clinical impact of the new MB subgrouping.
- 10.Ohgaki H, Kim YH, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr. Opin. Neurol. 2010;23(6):583–591. doi: 10.1097/WCO.0b013e3283405b5f. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich LV, Millenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]; •• The first genetically engineered mouse model of Shh-driven MB.
- 12.Hatton BA, Villavicencio EH, Tsuchiya KD, et al. The Smo/Smo model: Hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68(6):1768–1776. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]; •• The first genetically engineered mouse model of aggressive Shh-driven MB in mice.
- 13.Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first genetically engineered mouse model of Wnt-driven MB in mice.
- 14.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J. Clin. Oncol. 2011;29(11):1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011;29(11):1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• High-throughput analyses describing molecular hallmarks of aggressive human MBs.
- 16.Pomeroy SL, Cho YJ. Molecular fingerprints of medulloblastoma and their application to clinical practice. Future Oncol. 2011;7(3):327–329. doi: 10.2217/fon.11.15. [DOI] [PubMed] [Google Scholar]
- 17.Schwalbe EC, Lindsey JC, Straughton D, et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin. Cancer Res. 2011;17(7):1883–1894. doi: 10.1158/1078-0432.CCR-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamayo P, Cho YJ, Tsherniak A, et al. Predicting relapse in patients with medulloblastoma by integrating evidence from clinical and genomic features. J. Clin. Oncol. 2011;29(11):1415–1423. doi: 10.1200/JCO.2010.28.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important study that describes the use of high-throughput analyses of human MBs to predict recurrence.
- 19.Parsons DW, Li M, Zhang X, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study to identify mutations in chromatin remodelers in human MBs.
- 20.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Consensus of a multi-institutional cooperative group regarding a new classification system for MBs.
- 21.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Consensus of a multi-institutional cooperative group regarding a new classification system for MBs.
- 22.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• New methodology applying NanoString technology for the sub typing of human MBs.
- 23.Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DT, Jager N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene. 2010;29(20):3017–3024. doi: 10.1038/onc.2010.32. [DOI] [PubMed] [Google Scholar]
- 28.Valdora F, Banelli B, Stigliani S, et al. Epigenetic silencing of DKK3 in medulloblastoma. Int. J. Mol. Sci. 2013;14(4):7492–7505. doi: 10.3390/ijms14047492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013;31(23):2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the incidence of p53 mutations in specific subgroups of human MBs.
- 31.Remke M, Ramaswamy V, Peacock J, et al. TERT mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013;126:917–929. doi: 10.1007/s00401-013-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat. Rev. Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Excellent overview of drugs that target Shh signaling.
- 33.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by Hedgehog pathway inhibition. Science. 2002;297(5586):1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]; •• First pre-clinical study to demonstrate the therapeutic value of inhibiting Shh signaling.
- 34.Kimura H, Ng JM, Curran T. Transient inhibition of Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. USA. 2012;109(20):7859–7864. doi: 10.1073/pnas.1114718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong H, Alicke B, West KA, et al. Pharmacokinetic-pharmacodynamic analysis of vismodegib in preclinical models of mutational and ligand-dependent Hedgehog pathway activation. Clin. Cancer Res. 2011;17(14):4682–4692. doi: 10.1158/1078-0432.CCR-11-0975. [DOI] [PubMed] [Google Scholar]
- 37.Kool M, Jones DT, Jäger N, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the use of high-throughput methodology to predict treatment response.
- 38.Corcoran RB, Scott MP. Oxysterols stimulate Sonic Hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. USA. 2006;103(22):8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corcoran RB, Bachar Raveh T, Barakat MT, Lee EY, Scott MP. Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched 1 heterozygous mice. Cancer Res. 2008;68(21):8788–8795. doi: 10.1158/0008-5472.CAN-08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kongkham PN, Onvani S, Smith CA, Rutka JT. Inhibition of the MET receptor tyrosine kinase as a novel therapeutic strategy in medulloblastoma. Transl. Oncol. 2010;3(6):336–343. doi: 10.1593/tlo.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coon V, Laukert T, Pedone CA, Laterra J, Kim KJ, Fults DW. Molecular therapy targeting Sonic Hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma. Mol. Cancer Ther. 2010;9(9):2627–2636. doi: 10.1158/1535-7163.MCT-10-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerreiro AS, Fattet S, Fischer B, et al. Targeting the PI3K p110alpha isoform inhibits medulloblastoma proliferation, chemoresistance, and migration. Clin. Cancer Res. 2008;14(21):6761–6769. doi: 10.1158/1078-0432.CCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 43.Castellino RC, Barwick BG, Schniederjan M, et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS ONE. 2010;5(5):e10849. doi: 10.1371/journal.pone.0010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brun SN, Markant SL, Esparza LA, et al. Survivin as a therapeutic target in Sonic Hedgehog-driven medulloblastoma. Oncogene. 2014 doi: 10.1038/onc.2014.304. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Zhang L, Chen Y, et al. The G protein a subunit Gas is a tumor suppressor in Sonic Hedgehog driven medulloblastoma. Nat. Med. 2014;20(9):1035–1042. doi: 10.1038/nm.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordeiro BM, Oliveira ID, Alves MT, et al. SHH, WNT and NOTCH pathways in medulloblastoma: when cancer stem cells maintain self-renewal and differentiation properties. Childs Nerv. Syst. 2014;30(7):1165–1172. doi: 10.1007/s00381-014-2403-x. [DOI] [PubMed] [Google Scholar]
- 47.Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of Sonic Hedgehog-induced medulloblastomas. Cancer Res. 2004;64(21):7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 48.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 49.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31(4):557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 50.Natarajan S, Li Y, Miller EE, et al. Notch-1 induced brain tumor models the Sonic Hedgehog subgroup of human medulloblastoma. Cancer Res. 2013;73(17):5381–5390. doi: 10.1158/0008-5472.CAN-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Implicates Notch signaling in MB genesis.
- 51.Brechbiel J, Miller-Moslin K, Adjei AA. Cross-talk between Hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat. Rev. 2014;40(6):750–759. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Baryawno N, Sveinbjornsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70(1):266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 53.Gershon TR, Crowther AJ, Tikunov A, et al. Hexokinase-2 mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1(1):2–26. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Lindsey S, Graves B, Yoo S, Olson JM, Langhans SA. The Sonic Hedgehog induced histone deacetylase activation is required for cerebellar granule precursor hyperplasia in medulloblastoma. PLoS ONE. 2013;8(8):e71455. doi: 10.1371/journal.pone.0071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markant SL, Esparza LA, Sun J, et al. Targeting Sonic Hedgehog-associated medulloblastoma through inhibition of Aurora and Polo-like kinases. Cancer Res. 2013;73(20):6310–6322. doi: 10.1158/0008-5472.CAN-12-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triscott J, Lee C, Foster C, et al. Personalizing the treatment of pediatric medulloblastoma: polo-like kinase 1 as a molecular target in high-risk children. Cancer Res. 2013;73(22):6734–6744. doi: 10.1158/0008-5472.CAN-12-4331. [DOI] [PubMed] [Google Scholar]
- 57.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3 pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo . Genes Dev. 2008;22(4):436–438. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mainwaring LA, Kenney AM. Divergent functions for eIF4E and S6 kinase by Sonic Hedgehog mitogenic signaling in the developing cerebellum. Oncogene. 2011;30(15):1784–1797. doi: 10.1038/onc.2010.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loh AHP, Brennan RC, Lang WH, Hickey RJ, Malkas LH, Sandoval JA. Dissecting the PI3K signaling axis in pediatric solid tumors: novel targets for clinical integration. Front. Oncol. 2013;3(93):1–10. doi: 10.3389/fonc.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Focuses on outcomes of several clinical trials targeting the PI3K signaling pathway in pediatric solid tumors including MBs.
- 60.Fernandez-L A, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated AKT activation. Oncogene. 2011;31(15):1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor P, Fangusaro J, Rajaram V, et al. REST is a novel prognostic factor and therapeutic target for medulloblastoma. Mol. Cancer Ther. 2012;11(8):1713–1723. doi: 10.1158/1535-7163.MCT-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu X, Northcott PA, Dubuc A, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482(7386):529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Implicates a chromatin remodeling complex in the development of metastatic MB.
- 63.Das CM, Taylor P, Gireud M, et al. The deubiquitylase USP37 links REST to the control of p27 stability and cell proliferation. Oncogene. 2013;32(13):1691–1701. doi: 10.1038/onc.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study to demonstrate the influence of proteasomal machinery in MB development.
- 64.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010;2(51):51–70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the identification of PI3K signaling as a critical mediator of therapeutic resistance in Shh MBs.
- 65.Metcalfe C, Alicke B, Crow A, et al. PTEN loss mitigates the response of medulloblastoma to Hedgehog pathway inhibition. Cancer Res. 2013;73(23):7034–7042. doi: 10.1158/0008-5472.CAN-13-1222. [DOI] [PubMed] [Google Scholar]
- 66.Ryan SL, Schwalbe EC, Cole M, et al. Myc family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol. 2012;123(4):501–513. doi: 10.1007/s00401-011-0923-y. [DOI] [PubMed] [Google Scholar]
- 67.Mumert M, Dubuc A, Wu X, et al. Functional genomics identifies drivers of medulloblastoma dissemination. Cancer Res. 2012;72(19):4944–4953. doi: 10.1158/0008-5472.CAN-12-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones DT, Northcott PA, Kool M, Pfister SM. The role of chromatin remodeling in medulloblastoma. Brain Pathol. 2013;23(2):193–199. doi: 10.1111/bpa.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bunt J, Hasselt NA, Zwijnenburg DA, Koster J, Veersteeg R, Kool M. Aberrant OTX2 sustains a bivalent-like state of OTX2-bound promoters in medulloblastoma by maintaining their H3K27me3 levels. Acta Neuropathol. 2013;125(3):385–394. doi: 10.1007/s00401-012-1069-2. [DOI] [PubMed] [Google Scholar]
- 70.Wortham M, Jin G, Sun JL, Bigner DD, He Y, Yan H. Aberrant Otx2 expression enhances migration and induces ectopic proliferation of hindbrain neuronal progenitors. PLoS ONE. 2012;7(4):e36211. doi: 10.1371/journal.pone.0036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adamson DC, Shi Q, Wortham M, et al. OTX2 is critical for the maintenance and progression of Shh-independent medulloblastomas. Cancer Res. 2010;70(1):181–191. doi: 10.1158/0008-5472.CAN-09-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCabe MT, Creasy CL. EZH2 as a potential target in cancer therapy. Epigenomics. 2014;6(3):341–351. doi: 10.2217/epi.14.23. [DOI] [PubMed] [Google Scholar]
- 73.Milde T, Lodrini M, Savelyeva L, et al. HD-MB03 is a novel group 3 medulloblastoma demonstrating sensitivity to histone deacetylase inhibitor treatment. J. Neuro-Oncol. 2012;110(3):335–348. doi: 10.1007/s11060-012-0978-1. [DOI] [PubMed] [Google Scholar]; • Xenograft model of human group 3 MBs.
- 74.Swartling FJ, Hede SM, Weiss WA. What underlies the diversity of brain tumors? Cancer Metastasis Rev. 2013;32(1–2):5–24. doi: 10.1007/s10555-012-9407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snurdel M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1075–1086. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ajeawung NF, Wang HY, Gould P, Kamnasaran D. Advances in molecular targets for the treatment of medulloblastomas. Clin. Invest. Med. 2012;35(5):E246. doi: 10.25011/cim.v35i5.18697. [DOI] [PubMed] [Google Scholar]
- 77.Bandopadhayay P, Bergthold G, Nguyen B, Schubert S, Gholamin S, Tang Y. BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin. Cancer Res. 2014;20(4):912–925. doi: 10.1158/1078-0432.CCR-13-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swartling FJ, Grimmer MR, Hackett CS, et al. Pleitropic role for MYCN in medulloblastoma. Genes Dev. 2010;24(10):1059–1072. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swartling FJ, Savov V, Persson AI, et al. Distinct neural stem cell populations give rise to disparate brain tumors in response to N-Myc. Cancer Cell. 2012;21(5):601–613. doi: 10.1016/j.ccr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of three GEM models of aggressive MB.
- 80.Pei Y, Moore CE, Wang J, et al. An animal model of MYC-driven medulloblastoma. Cancer Cell. 2012;21(2):155–167. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of three GEM models of aggressive MB.
- 81.Kawauchi D, Robinson G, Uziel T, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21(2):168–180. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of three GEM models of aggressive MB.
- 82.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]; •• Excellent review article highlighting immunotherapy for cancers.
- 83.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Excellent review highlighting immunotherapy for cancers.
- 84.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert Opin. Emerg. Drugs. 2012;17(2):181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonabend AM, Ogden AT, Maier LM, et al. Medulloblasoma: challenges for effective immunotherapy. J. Neuro-Oncol. 2012;108(1):1–10. doi: 10.1007/s11060-011-0776-1. [DOI] [PubMed] [Google Scholar]; • Important review, highlighting the challenges of applying immunotherapy for pediatric brain cancers.
- 86.Griesinger AM, Birks DK, Donson AM, et al. Characterization of distinct immunophenotypes across pediatric brain tumors. J. Immunol. 2013;191(9):4880–4888. doi: 10.4049/jimmunol.1301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu D, Song L, Brawley VS, et al. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clinical Immunol. 2013;149(1):55–64. doi: 10.1016/j.clim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmed N, Ratnayake M, Savoldo B, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67(12):5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 89.Castriconi R, Dondero A, Negri F, et al. Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur. J. Immunol. 2007;37(11):3190–3196. doi: 10.1002/eji.200737546. [DOI] [PubMed] [Google Scholar]
- 90.Fernandez L, Portugal R, Valentin J, et al. In vitro natural killer cell immunotherapy for medulloblastoma. Front. Oncol. 2013;3:94–101. doi: 10.3389/fonc.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weigering V, Eyrich M, Rutkowski S, Wolfi M, Schlegel PG, Winkler B. TH1 predominance is associated with improved survival in pediatric medulloblastoma patients. Cancer Immunol. Immunother. 2011;60(5):693–703. doi: 10.1007/s00262-011-0981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuan CT, Wikstrand CJ, McLendon RE, Zalutsky MR, Kumar U, Bigner DD. Detection of amino-terminal extracellular domain of somatostatin receptor 2 by specific monoclonal antibodies and quantification of receptor density in medulloblastoma. Hybridoma. 2009;28(6):389–403. doi: 10.1089/hyb.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguilera DG, Das CM, Sinnappah-Kang ND, et al. Reactivation of death receptor 4 (DR4) expression sensitizes medulloblastoma cell lines to TRAIL. J. Neuro-Oncol. 2009;93(3):303–318. doi: 10.1007/s11060-008-9788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merve A, Dubuc AM, Zhang X, et al. Polycomb gene BMI1 controls invasion of medulloblastoma cells and inhibits BMP-regulated cell adhesion. Acta Neuropathol. Commun. 2014;2:10–16. doi: 10.1186/2051-5960-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with Hedgehog inhibitor GDC-0449. N. Engl. J. Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Smaele E, Ferretti E, Gulino A. Vismodegib, a small-molecule inhibitor of the Hedgehog pathway for the treatment of advanced cancers. Curr. Opin. Investig. Drugs. 2010;11(6):707–718. [PubMed] [Google Scholar]
- 97.Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of Hedgehog pathway inhibitor GDC-0449 in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gajjar A, Stewart CF, Ellison DW, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin. Cancer Res. 2013;19(22):6305–6312. doi: 10.1158/1078-0432.CCR-13-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First pediatric trial to examine the safety of Shh inhibitors in children.
- 99.Rodon J, Tawbi HA, Thomas AL, et al. A Phase I, multicenter, open-label, first-in-human, dose–escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 2014;20(7):1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 100.Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J. Clin. Oncol. 2007;25:4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 101.Hartford CM, Desai AA, Janisch L, et al. A Phase I trial to determine the safety, tolerability and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin. Cancer Res. 2009;15:1428–1434. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spunt SL, Grupp SA, Vik TA, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J. Clin. Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fouladi M, Stewart CF, Olson J, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 2011;29(26):3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hummel TR, Wagner LM, Ahern CH, et al. A pediatric Phase I trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children's Oncology Group Phase I Consortium Study. J. Clin. Oncol. 2011;29(15 Suppl.):9579. doi: 10.1002/pbc.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muscal JA, Thompson PA, Horton TM, et al. A Phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children's Oncology Group Phase I consortium study (ADVL0916) Pediatr. Blood Cancer. 2013;60(3):390–395. doi: 10.1002/pbc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamoto Y, Shimizu K, Tamura K, et al. An adoptive immunotherapy of patients with medulloblastoma by lymphokine-activated killer cells (LAK) Acta Neurochirurgica. 1988;94(1–2):47–52. doi: 10.1007/BF01406615. [DOI] [PubMed] [Google Scholar]
- 107.Sankhla SK, Nadkarni JS, Bhagwati SN. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J. Neuro-Oncol. 1996;27(2):133–140. doi: 10.1007/BF00177476. [DOI] [PubMed] [Google Scholar]
- 108.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya [131]I-3F8. J. Clin. Oncol. 2007;25(34):5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 109.Petrosiute A, Auletta JJ, Lazarus HM. Achieving graft-versus-tumor effect in brain tumor patients: from autologous progenitor cell transplant to active immunotherapy. Immunotherapy. 2012;4(11):1139–1151. doi: 10.2217/imt.12.96. [DOI] [PubMed] [Google Scholar]