Abstract
Distinct classes of protective immunity are guided by activation of STAT transcription factor (TF) family members in response to environmental cues. CD4+ regulatory T cells (Tregs) suppress excessive immune responses, and their deficiency results in a lethal, multi-organ autoimmune syndrome characterized by T helper 1 (Th1) and T helper 2 (Th2) CD4+ T cell-dominated lesions. Here we show that pathogenic Th17 responses in mice are also restrained by Tregs. This suppression was lost upon Treg-specific ablation of Stat3, a TF critical for Th17 differentiation, and resulted in the development of a fatal intestinal inflammation. These findings suggest that Tregs adapt to their environment by engaging distinct effector response-specific suppression modalities upon activation of STAT proteins that direct the corresponding class of the immune response.
The vertebrate immune system affords defense against different classes of pathogens by activation of a particular type of immune response. Intracellular pathogens induce protective Th1 responses, whereas parasitic helminthes induce Th2 cytokine production. In contrast, pathogenic yeast, fungi and extracellular bacteria elicit highly inflammatory Th17 responses associated with the production of interleukins (IL) -17, -22, -23, and granulocyte recruitment. Commitment of naïve T cells to these effector lineages is influenced by the cues derived from their microenvironment, particularly cytokines. Cytokine receptor signaling results in the activation of STAT family of transcription factors. Uncontrolled STAT activation can result in a variety of immune-mediated disease states. For instance, activation of Stat3 in response to the pro-inflammatory cytokine IL-6 in combination with transforming growth factor-β (TGF-β) leads to increased expression of orphan nuclear receptors RORγ and RORα, signature transcription factors for Th17 cells1,2,3,4. Deregulated Stat3-dependent Th17 responses have been implicated in pathogenesis of Inflammatory Bowel Disease (IBD), psoriasis, multiple sclerosis, and arthritis5.
Treg cells act ‘in-trans’ to suppress immune responses to self and to commensal microbiota, and to limit pathology associated with the immune responses to infection. The differentiation and maintenance of suppressive Tregs requires expression of the X-chromosome-encoded forkhead transcription factor Foxp36,7. In both humans and mice, mutations in Foxp3 result in a fatal immune disorder characterized by uncontrolled T cell proliferation and drastically elevated production of Th1 and Th2 cytokines, suggesting that Treg-elaborated suppression is involved in controlling these responses7. In contrast, Treg-mediated control of Th17 responses remains an unanswered question. Recent reports suggest that Th17 and peripherally induced Tregs represent competing fates of naïve T cell differentiation and the lineage choice is determined by relative amounts of IL-6 and TGF-β8,9,10. Therefore, Tregs might limit Th17 differentiation by “stealing” common precursors. Accordingly, a block in Th17 differentiation in IL-6 deficient mice correlates with an increase in Treg numbers11. We hypothesized that analogous to effector T cell differentiation, Tregs suppress a particular type of immune response by activating a distinct STAT family member in response to its cytokine microenvironment. We explored whether activation of Stat3 endows Tregs with the ability to suppress Th17 responses because Stat3 is a key factor in the initiation of Th17 differentiation.
Using co-immunoprecipitation and western blot analysis we found that in Tregs, Foxp3 was associated with the transcriptionally active, phosphorylated form of Stat3 (Fig. 1A). Stat3 association with Foxp3 was markedly diminished in cytokine-stimulated Tregs cultured in the presence of Stat3 dimerization or phosphorylation inhibitors (fig. S1). These data suggest that Stat3 association with Foxp3 is phosphorylation dependent. In contrast, Stat5, a related STAT family member activated down-stream of the IL-2 receptor, did not co-immunoprecipitate with Foxp3 (Fig. 1A).
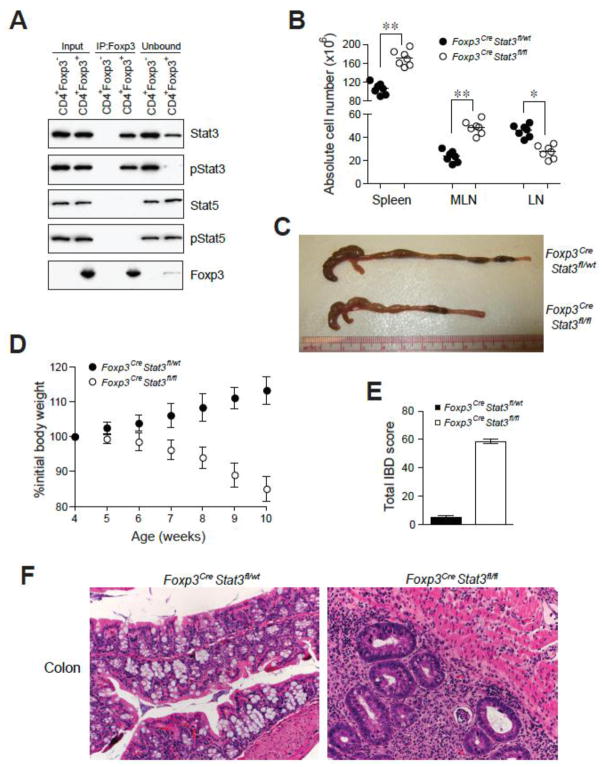
Fig. 1.
Phosphorylated Stat3 interacts with Foxp3 and its expression in Treg cells is required for suppression of fatal colitis. (A) Foxp3 immunoprecipitation from nuclear lysates of sorted CD4+Foxp3− and CD4+Foxp3+ cells followed by western blot analysis for the indicated proteins. The input lanes represent 3–5% of cell equivalents used for Foxp3 immunoprecipitation. (B) Spleen and lymph node cellularity. ** P<0.001; * P<0.01. (C) Examples of colon thickening. (D) Weight loss over time (n = 7). (E) IBD scores derived from histopathologic evaluation of colon and cecum from 8–9 week-old mice. Formalin fixed sections were H&E stained prior to examination (n = 4/group). (F) Representative H&E stained colon sections from 8–9 week old mice (original magnification, 20x).
To assess the role of Stat3 in Tregs in vivo, we induced deletion of a conditional Stat3 allele by crossing Stat3fl/fl mice to Foxp3Cre mice that express a yellow fluorescent protein (YFP)-Cre recombinase fusion protein under the control of the Foxp3 locus12. We determined that the deletion was efficient and specific to Tregs (fig. S2). Male Foxp3CreStat3fl/fl and female Foxp3Cre/CreStat3fl/fl were born at expected Mendelian ratios and initially appeared healthy. At six weeks of age, however, these mice displayed splenomegaly and a pronounced enlargement of the mesenteric lymph nodes (Fig. 1B). In contrast to Treg-deficient mice, we did not observe generalized lymphadenopathy characteristic of a systemic lymphoproliferative disorder. Instead, the size of lymph nodes, except for those draining the gastrointestinal tract, was reduced (Fig. 1B). Over time, Foxp3CreStat3fl/fl mice developed anemia, weight loss, rectal prolapse, and colon thickening and succumbed to the disease by 12–14 weeks of age (Fig. 1, C and D). These manifestations are hallmarks of IBD that was further confirmed by histological examination of the intestinal tissue, with the cecum and the colon of the diseased mice exhibiting massive lymphoid and neutrophilic infiltration (Fig. 1, E and F). In addition, hepatitis and liver lipidosis, which are thought to be secondary to colitis, were also observed (fig. S3). In contrast to the massive widespread tissue lesions observed in Foxp3− mice, in Foxp3CreStat3fl/fl mice pathology limited to the intestinal mucosa suggested that the absence of Stat3 only affects a particular subset of Treg functions (fig. S3).
As opposed to the systemic T cell expansion observed in Treg-ablated mice13, we observed reduced splenic and lymph node T cell frequencies in Foxp3CreStat3fl/fl mice, whereas the numbers of B cells, macrophages, and DCs were increased (Fig. 2A and fig. S4). Despite reduced or unchanged numbers of T cells in the lymph node and spleen, we observed a marked increase in activated CD44highCD62lowCD4+ T cells in these mice in comparison to control littermates (Fig. 2B). Increased Treg frequencies suggested, however, that the elevation in activated CD4+ T cell numbers was not due to reduced numbers of Stat3-deficient Tregs (Fig. 2C). Moreover, examination of surface phenotype of Tregs upon Stat3 ablation indicated the heightened activation of Tregs in these mice (fig. S5). Furthermore, we found Stat3-sufficient and -deficient Tregs expressed similar amounts of Foxp3, thus excluding altered Foxp3 expression as an explanation for the pathology in Foxp3CreStat3fl/fl mice (fig. S6).
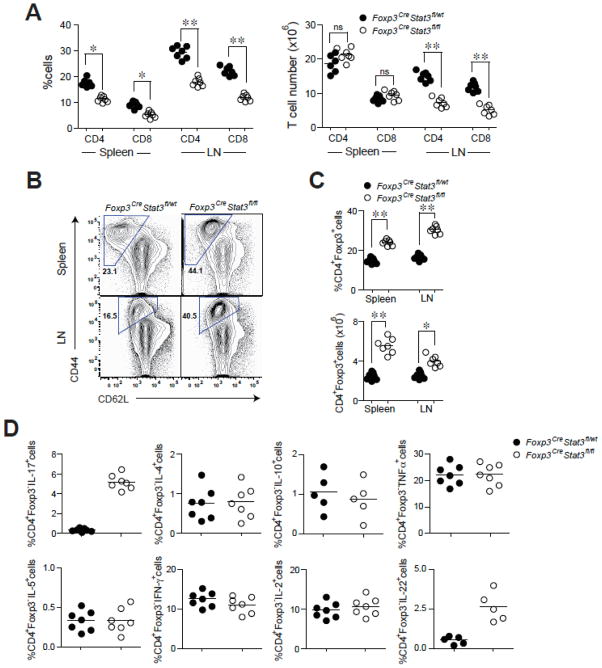
Fig. 2.
Stat3 ablation in Treg cells does not affect their numbers, yet leads to CD4+ T cell activation and selective increase in Th17 responses. (A) CD4+ and CD8+ T cell numbers in the spleen and lymph nodes. ** P<0.001; * P<0.01. (B) Flow cytometric analysis of CD44 and CD62L expression on CD4+ Foxp3− T cells in 6–8 wk-old mice. A representative of five independent experiments is shown. (C) Frequency and numbers of CD4+Foxp3+ T. ** P<0.001; * P<0.01. (D) Cytokine production by splenic CD4+Foxp3− T cells as determined by flow cytometric analysis. A representative of four independent experiments is shown.
Recent work suggests that pro-inflammatory Th17- and Th1-associated cytokines have a major impact on colitis development and progression14,15. Therefore, we assessed Th1-, Th17- and Th2- associated cytokine production in Foxp3CreStat3fl/fl mice. We observed increased production of Th17-associated cytokines, IL-17 and IL-22, by splenic CD4+ Foxp3− T cells in Foxp3CreStat3fl/fl mice (Fig. 2D and fig. S7). In contrast, Th1-and Th2-associated cytokine production was kept in check by Stat3-deficient Tregs as demonstrated by the comparable secretion of interferon-γ (IFN-γ), IL-4, and IL-5 in Foxp3CreStat3fl/fl and littermate control mice. IL-2, tumor necrosis factor-α (TNF-α), and IL-10 levels were also unaffected (Fig. 2D). Importantly, Stat3-deficient Foxp3+ Tregs did not produce any of these cytokines (fig. S8). These results demonstrate a selective dysregulation of Th17 responses upon Stat3 ablation in Tregs and identify Treg-dependent control of Th17 responses as an essential component of immune homeostasis.
We further analyzed Peyer’s patch (PP), intra-epithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) because the majority of Th17 cells in normal mice are induced in the gut associated lymphoid tissue (GALT) in response to intestinal flora16 and because we observed that the intestinal tract was the major site of inflammation in Foxp3CreStat3fl/fl mice. Whereas the numbers of LPLs and PP cells were diminished, we observed increased numbers of IELs, particularly CD8+ T cells and mononuclear cells, in agreement with the massive colonic infiltration revealed by the histopathological evaluation (Fig. 3A). Analysis of cytokine production by PP, LPL and IEL T cells revealed an increased frequency of IL-17-producing CD4+ Foxp3− T cells in Foxp3CreStat3fl/fl mice in comparison to controls, whereas IFN-γ production was reduced (Fig. 3B). Stat3 deficiency did not affect the frequencies of IL-2-, IL-4-, and IL-10-producing cells (fig. S9). We also observed elevated frequencies of IFN-γ-producing CD8+ T cells among IELs (Fig. 3B).
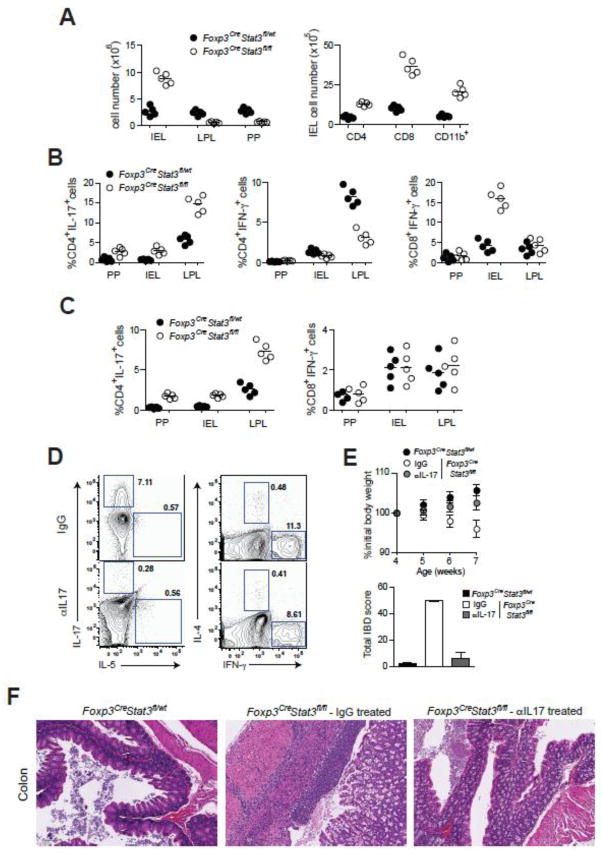
Fig. 3.
Increased IL-17 production triggers IBD development in Foxp3CreStat3fl/fl mice. (A) Total numbers of PP cells, IEL and LPL as well as CD4+, CD8+, CD11b+ cell numbers in the IEL population in 8 wk-old mice. (B) Frequencies of indicated cytokine secreting CD4+ and CD8+ T cells in PP, IEL and LPL populations in 7–8 week old mice. (C) Frequencies of indicated cytokine producing CD4+ and CD8+ T cells in PP, IEL and LPL in 3–4 wk-old mice. (D) Flow cytometric analysis of cytokine production by splenic CD4+Foxp3− T cells in Foxp3CreStat3fl/fl mice treated with isotype-matched IgG or IL-17 neutralizing antibody. A representative of three independent experiments is shown. (E) Weight loss and IBD scores and (F) representative H&E stained colon sections from 7–8 wk-old Foxp3CreStat3fl/fl mice treated with isotype-matched IgG control or neutralizing IL-17 antibody (original magnification, 10x).
We next sought to determine whether IL-17 produced by CD4+ T cells or IFN-γ produced by CD8+ IEL instigated the disease in Foxp3CreStat3fl/fl mice because both cytokines have been implicated IBD development14,15. We observed clinical symptoms of colitis in Foxp3CreStat3fl/fl mice at approximately 7–8 weeks of age whereas younger mice were disease-free (fig. S10). Assuming that the cytokine skewing should precede IBD development, we analyzed IL-17 and IFN-γ production in 3–4 week old mice. We observed significantly higher frequencies of IL-17-producing CD4+ Foxp3− T cells in the PP, LPL and IEL populations in Foxp3CreStat3fl/fl mice whereas the frequency of IFN-γ producing CD8+ and CD4+ T cells was similar in mutant and control mice (Fig. 3C). These results suggested that IL-17 responses act as its initial trigger. This was further supported by our finding that antibody-mediated IL-17 blockade in disease-free 3–4 week-old Foxp3CreStat3fl/fl resulted in the alleviation of colitis and a reduction in Th17 frequencies similar to those found in Foxp3CreStat3fl/wt controls (Fig. 3, D–F). In contrast, IFN-γ neutralization failed to prevent or delay colitis in Foxp3CreStat3fl/fl mice (fig. S11). Together, these data suggest that dysregulated Th17 responses drive colitis induction in Foxp3CreStat3fl/fl mice. Unrestricted Th17 responses are likely to facilitate the activation or recruitment of IFN-γ-producing CD8+ T cells at a later stage of disease progression. This phenomenon mirrors an initial requirement for a Th17 response to bring Th1 cells to infected or inflamed tissues17,18.
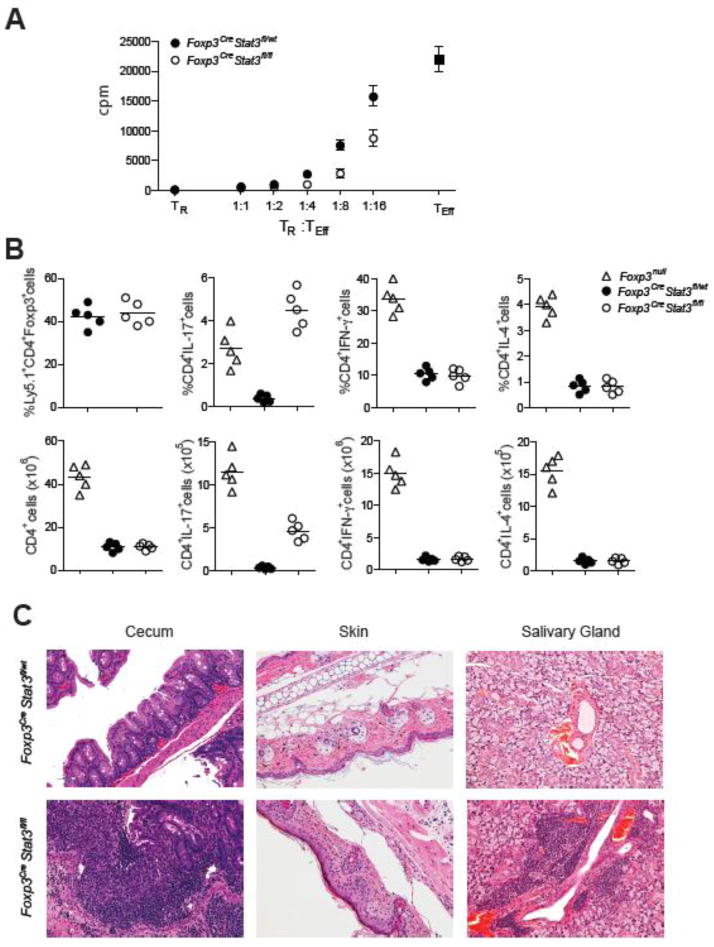
The selective dysregulation of Th17 responses and aggressive colitis observed in Foxp3CreStat3fl/fl mice suggested that only a distinct aspect of suppressor program was impaired in Stat3-deficient Tregs. Consistent with this assumption, their in vitro suppressor capacity was unaffected (Fig. 4A). To examine suppressor function of these cells in vivo, we co-transferred sorted Stat3-deficient or -sufficient Ly5.2+ Tregs and effector Ly5.1+ CD4 T cells from Foxp3− mice into Rag2−/−(Recombination activating gene 2) recipients. Prior to transfer, the effector CD4 T cell population was heavily Th1-skewed and contained ~40% of IFN-γ producing cells, but few IL-17-producing cells (fig. S12). Similar to unmanipulated Foxp3− mice, after four to six weeks, recipients of Foxp3− T cells developed a systemic multi-organ autoimmune syndrome, which was completely abrogated upon co-transfer of Stat3-sufficient Tregs. Recipient mice that only received Ly5.1+ Foxp3− CD4 T cells contained 3–5-fold higher frequencies and 15–20-fold higher numbers of IFN-γ and IL-4 producing cells as compared to recipient mice transferred with Ly5.1+ Foxp3− CD4 T cells mixed with either Stat3-deficient or -sufficient Tregs (Fig. 4B). In contrast, co-transfer of Stat3-deficient Tregs resulted in increased frequencies and absolute numbers of only IL-17-producing Ly5.1+ Foxp3− CD4 T cells (Fig. 4B). A similar trend was observed among LPLs in the recipient mice (fig. S13). These mice also developed a fulminant colitis similar to that seen in Foxp3CreStat3fl/fl mice as well as pronounced dermatitis and sialoadenitis, whereas other organs remained largely unaffected (Fig. 4C and fig. S13). In control, co-transfer of Stat3-sufficient Tregs prevented all autoimmune manifestations and tissue pathology. It is noteworthy that all three affected tissues are known targets of Th17-mediated inflammation. Thus, Stat3-deficient Tregs are selectively impaired in their ability to control Th17 responses in vivo and this impairment leads to fatal colitis.
Fig. 4.
Stat3-deficient Treg cells efficiently suppress in vitro T cell proliferation, but fail to suppress Th17 cell differentiation and colitis. (A) Stat3-sufficient and -deficient Treg cells from 5–6 wk-old mice suppress in vitro proliferative response of CD4+Foxp3− T cells (Teff). A representative of three independent experiments is shown. (B) Frequencies and numbers of splenic Treg cells, CD4+IL-17+Foxp3−, CD4+IFN-γ+Foxp3−, CD4+IL-4+Foxp3− T cells within the indicated donor-derived population 4 weeks after co-transfer of either Foxp3CreStat3fl/wt or Foxp3CreStat3fl/fl Treg cells with Foxp3− Teff cells into Rag2−/− recipients. (C) Representative H&E stained sections of cecum, skin, and salivary gland tissues collected from recipient mice 4 wk after adoptive T cell transfer (original magnification, 20x).
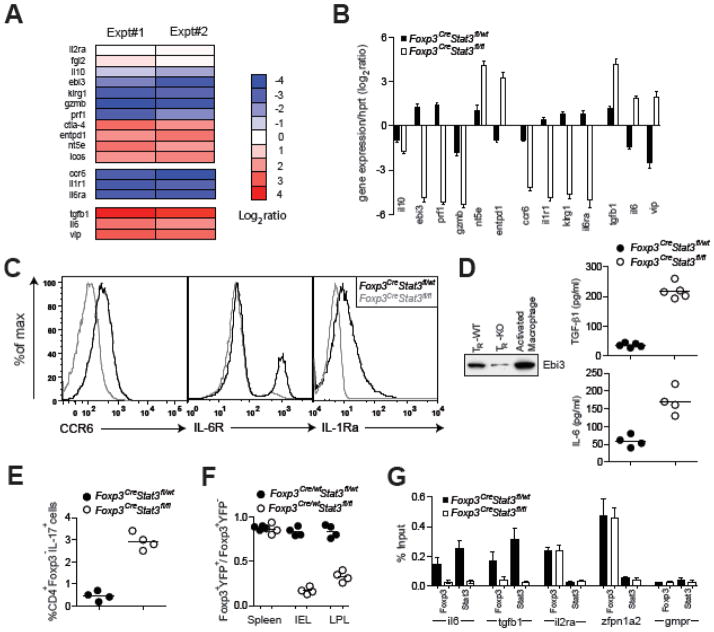
To gain insight into the potential mechanisms of Stat3-dependent regulation of Treg function we compared gene expression patterns in Stat3-deficient and -sufficient YFP+ Tregs isolated from healthy heterozygous Foxp3Cre/wtStat3fl/fl and Foxp3Cre/wtStat3fl/wt female mice. Cross-referencing of the resulting datasets with the previously identified set of Foxp3-dependent genes19 showed that 20% of these genes are also dependent upon Stat3 expression in Tregs (fig. S14). Within this group, we found decreased expression of Il10, Ebi3, Gzmb, and Prf1, genes implicated in Treg suppressor function (Fig. 5, A and B). In particular, Treg production of IL-10 and the Ebi3-containing cytokine, IL-35, is important for preventing colitis12,20. In contrast, expression of other genes encoding proteins related to Treg suppressor function was unaltered (Il2ra, Fgl2) or dramatically increased (Ctla4, Nt5e, Entpd1, Tgfb1).
Fig. 5.
Stat3-dependent gene expression in Treg cells. (A, B) Expression pattern of Stat3dependent genes potentially contributing to Treg suppressor function. The data represent average of two independent microarray experiments. qPCR analysis of relative expression of indicated genes in YFP-Cre+ Treg cells from indicated mice. The results represent mean and standard deviation of relative expression values for indicated genes over Hprt in two independent experiments using three replicates each. (C) Flow cytometric analysis of CCR6, IL-1R and IL-6R expression on Tregs from indicated mice. (D) ELISA and western blot analysis of amounts of TGF-β1, IL-6, and Ebi3 in supernatants of Stat3-sufficient and -deficient Tregs cultured in presence of IL-2. (E) Ratio of Foxp3+YFP+ to Foxp3+YFP− Tregs in spleen, IEL and LPL populations of indicated mice. (F) CD4+Foxp3− were stimulated with anti-CD3 in the presence of culture supernatants derived from Tregs isolated from indicated mice and the frequency of CD4+IL-17+ cells was assessed by flow cytometry. (G) qPCR analysis of Foxp3- and Stat3-bound chromatin isolated from wild-type Tregs using primer set corresponding to the promoter region of the indicated genes. The housekeeping gene Gmpr was used as a specificity control.
Substantially reduced expression of Ccr6 in Stat3-deficient Tregs (Fig. 5, A–C) may also contribute to failure of Stat3-deficient Tregs to suppress Th17-mediated inflammation. CCR6 is expressed by both Th17 and Tregs and plays an important role in migration of Th17 cells to inflammatory sites and in Treg-mediated suppression of thereof21,22. We therefore compared relative distribution of YFP-Cre+ Stat3-deficient and YFP-Cre− Stat3-sufficient Foxp3+ Tregs in the colon, secondary lymphoid organs and GALT in the absence of any inflammation in female heterozygous Foxp3Cre/wtStat3fl/fl mice. These cells were present at a ~1:1 ratio in spleen; however, we observed a diminished proportion of Stat3-deficient Tregs in the gut (Fig. 5E). These results are consistent with an idea that Stat3-deficient Treg cells are impaired in their ability to migrate to the gut tissue prior to establishment of colitis because Stat3-deficient and -sufficient Treg cell populations had similar proportions of cycling and apoptotic cells (fig. S15). In contrast to healthy Foxp3Cre/wtStat3fl/fl mice, increased numbers of Foxp3+ T cells were observed in the inflamed colon of Foxp3CreStat3fl/fl mice in agreement with a recent report that CCR6-deficient T cells enter CNS at late, but not at early stages of EAE progression18.
Additionally, low level of IL-1 receptor and IL-6 receptor expression in Stat3-deficient Tregs (Fig. 5C) can potentially reduce their ability to compete with effector T cells and other immune cell types for IL-1 and IL-6, cytokines known to enhance Th17 differentiation3,10,23. In this regard, IL-2R expressed on Tregs is capable of depriving effector T cells of IL-2, thereby, effectively limiting the immune response24. Consistent with this idea, Stat3-sufficient, but not Stat3-deficient Tregs were able to efficiently deplete IL-1 and IL-6 from the culture medium (fig. S17).
Unexpectedly, Stat3 deficiency in Tregs resulted in increased expression of Il6, Tgfb1, and Vip. Although Treg-produced TGF-β1 can mediate suppression25, TGF-β1 in combination with IL-6 also facilitates the generation of Th17 cells. Similarly, Vip-encoded vasoactive intestinal peptide (VIP) promotes Th17 differentiation26. Indeed, soluble factors produced by Stat3-deficient Tregs were able to facilitate differentiation of IL-17-producing T cells in vitro in the absence of exogenously supplied IL-6 and TGF-β1 (Fig. 5F). Increased expression of IL-6 and TGF-β1 in Stat3-deficient Tregs (Fig. 5, B and D) might amplify, but cannot fully account for the observed increase in IL-17 production in diseased Foxp3CreStat3fl/fl mice because heterozygous Foxp3Cre/wtStat3fl/fl female mice co-habited by Stat3-deficient and -sufficient Tregs do not exhibit augmented Th17 responses.
To test a possibility that Stat3 facilitates recruitment of Foxp3 to regulatory elements of Il6 and Tgfb1 genes, we employed chromatin immunprecipitation (ChIP) combined with quantitative PCR to examine Foxp3 binding to promoter regions of these genes in Stat3-sufficient and -deficient Treg cells. Indeed, we found that Foxp3 was bound to Il6 and Tgfb1 promoters in a Stat3-dependent manner. In contrast, Foxp3 binding to Zfpn1a2 (Helios) and Il2ra, well-known Foxp3-binding genes expressed in a Stat3-independent manner, was unaffected by the absence of Stat3 (Fig. 5G). These results suggest that Stat3 activation-dependent association with Foxp3 transcriptional complexes may result in modulation of Stat3-dependent gene expression in part through Stat3-dependent recruitment of Foxp3.
Thus, the activation of Stat3 in Tregs endows them with the ability to suppress Th17 responses plausibly through increased expression of a subset of suppressor molecules as well as cytokine and chemokine receptors, which may deprive immune effector cells of essential activation cues and facilitate the spatial proximity of Tregs and Th17 cells. Furthermore, Stat3 in Tregs limits the expression of soluble mediators of Th17 differentiation. We suggest that altered expression of a combination of genes, but not changes in any one of them can account for inability of Stat3-deficient Tregs to restrain Th17 responses.
Our findings support the idea that the same transcription factors integrate environmental cues that guide a particular immune response type and facilitate Treg cell ability to suppress the corresponding type of the immune response. Consistent with this notion, Irf4, an IRF transcription factor family member essential for Th2 differentiation, is required for their ability to suppress Th2 responses27. Furthermore, Treg expression of T-bet, a Th1-specific transcription factor, is required for Treg homeostasis under conditions of induced Th1 inflammation28. We propose that STAT-IRF axis of transcriptional regulation allows Tregs to adapt to a particular environment and ensures appropriate ‘class’-specific control of immune-mediated inflammation.
Supplementary Material
Acknowledgments
We thank K. Forbush, T. Chu, L. Karpik and A. Bravo for assistance with the mouse colony management, S. Akira for Stat3fl/fl mice and J. Renauld for anti-IL-22. This work was supported by grants AI-061816 and AI-034206 from the National Institutes of Health. A.C. is supported by the Cancer Research Institute and D.R. is supported by the Arthritis Foundation. A.Y.R. is an investigator with the Howard Hughes Medical Institute.
References and Notes
- 1.Mangan PR, et al. Nature. 2006;441:231. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 2.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. Immunity. 2006;24:179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, et al. Nat Immunol. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 4.Yang XO, et al. Immunity. 2008;28:29. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Korn T, Oukka M, Kuchroo VK. Nature. 2008;453:1051. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057. [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Nat Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov, et al. Cell. 2006;126:1121. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, et al. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, et al. Nature. 2008;453:236. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korn T, et al. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubtsov YP, et al. Immunity. 2008;28:546. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Rasmussen JP, Rudensky AY. Nat Immunol. 2007;8:191. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 14.Strober W, Fuss IJ, Blumberg RS. Ann Rev Immunol. 2002;20:495. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 15.Maloy KJ, Kullberg MC. Mucosal Immunol. 2008;1:339. doi: 10.1038/mi.2008.28. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov, et al. Cell Host Microbe. 2008;4:337. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khader SA, et al. Nat Immunol. 2007;8:369. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 18.Reboldi A, et al. Nat Immunol. 2009;10:514. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 19.Gavin MA, et al. Nature. 2007;445:771. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 20.Vignali DA, Collison LW, Workman CJ. Nat Rev Immunol. 2008;8:523. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinewietfeld M, et al. Blood. 2005;105:2877. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki T, et al. J Immunol. 2008;181:8391. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. J Exp Med. 2006;203:1685. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. Nat Immunol. 2007;8:1353. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 25.Li MO, Wan YY, Flavell RA. Immunity. 2007;26:579. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Yadav M, Rosenbaum J, Goetzl EJ. J Immunol. 2008;180:2772. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y, et al. Nature. 2009;458:351. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch MA, et al. Nat Immunol. 2009;10:595. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.