Abstract
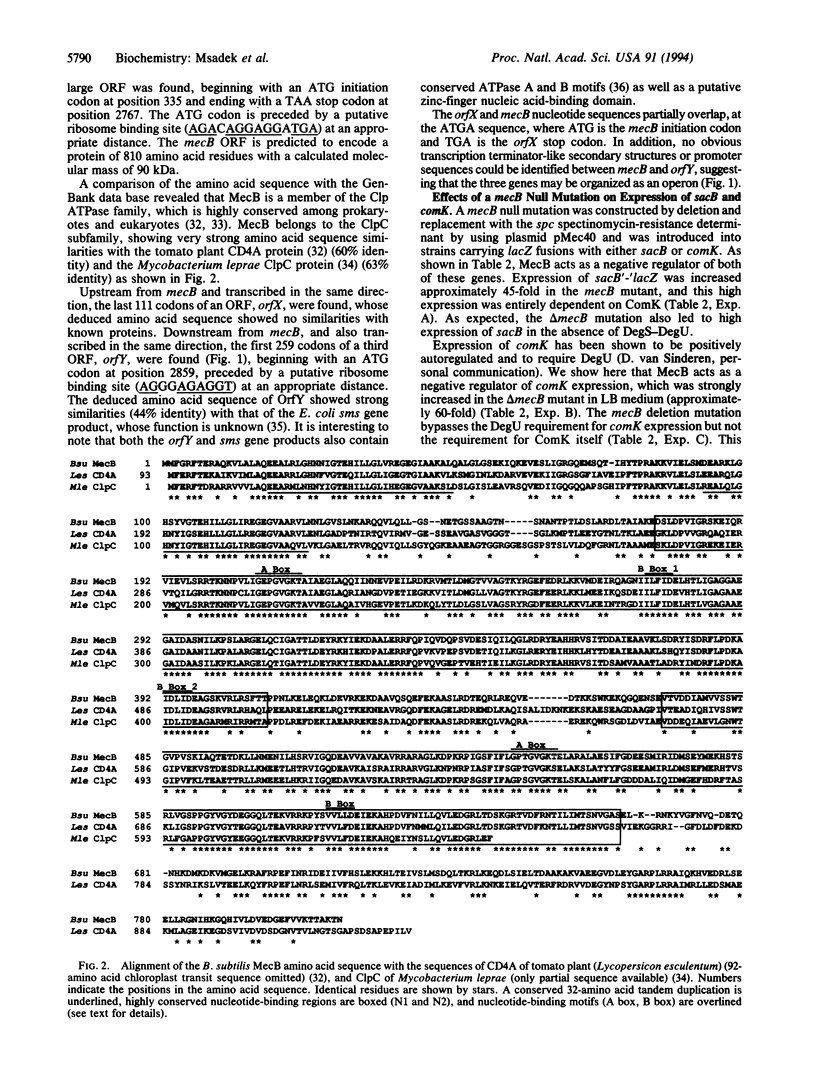
The Bacillus subtilis DegS-DegU histidine kinase-response regulator pair controls the expression of genes encoding degradative enzymes such as levansucrase (sacB) and of genes involved in genetic competence. The mecA and mecB mutations were previously isolated as allowing competence gene expression in complex media. We have shown that the mec mutations also lead to overexpression of sacB, bypassing the DegS-DegU requirement. This expression was shown to be entirely dependent upon ComK, a positive regulator of competence gene expression. The mecB gene was cloned and its nucleotide sequence was determined. The predicted MecB protein show very high similarity over its entire length with members of the ClpC family of ATPases (60% identity). MecB is essential for growth of B. subtilis at high temperature. MecB also acts as a negative regulator of ComK synthesis, thus preventing late competence gene expression. We suggest that under these conditions MecB may interact with MecA to sequester or otherwise inactivate ComK. In response to an unknown signal, active ComK would accumulate through a positive feedback loop, leading to expression of competence genes allowing DNA uptake.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Dubnau D. A. Cloning and characterization of a cluster of linked Bacillus subtilis late competence mutations. J Bacteriol. 1989 Oct;171(10):5376–5385. doi: 10.1128/jb.171.10.5376-5385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arantes O., Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene. 1991 Dec 1;108(1):115–119. doi: 10.1016/0378-1119(91)90495-w. [DOI] [PubMed] [Google Scholar]
- Calogero S., Gardan R., Glaser P., Schweizer J., Rapoport G., Debarbouille M. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J Bacteriol. 1994 Mar;176(5):1234–1241. doi: 10.1128/jb.176.5.1234-1241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl M. K., Msadek T., Kunst F., Rapoport G. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J Biol Chem. 1992 Jul 15;267(20):14509–14514. [PubMed] [Google Scholar]
- Dubnau D., Roggiani M. Growth medium-independent genetic competence mutants of Bacillus subtilis. J Bacteriol. 1990 Jul;172(7):4048–4055. doi: 10.1128/jb.172.7.4048-4055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel M., Alonso J. C. Expression of the recE gene during induction of the SOS response in Bacillus subtilis recombination-deficient strains. Mol Microbiol. 1989 Sep;3(9):1269–1276. doi: 10.1111/j.1365-2958.1989.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Glaser P., Kunst F., Arnaud M., Coudart M. P., Gonzales W., Hullo M. F., Ionescu M., Lubochinsky B., Marcelino L., Moszer I. Bacillus subtilis genome project: cloning and sequencing of the 97 kb region from 325 degrees to 333 degrees. Mol Microbiol. 1993 Oct;10(2):371–384. [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J., Yang M., Ferrari E. Localization of Bacillus subtilis sacU(Hy) mutations to two linked genes with similarities to the conserved procaryotic family of two-component signalling systems. J Bacteriol. 1988 Nov;170(11):5102–5109. doi: 10.1128/jb.170.11.5102-5109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. C., Huang X. F., Novel G., Novel M. Two genes present on a transposon-like structure in Lactococcus lactis are involved in a Clp-family proteolytic activity. Mol Microbiol. 1993 Mar;7(6):957–965. doi: 10.1111/j.1365-2958.1993.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa M., Wada C., Yoshioka S., Yura T. Expression of ClpB, an analog of the ATP-dependent protease regulatory subunit in Escherichia coli, is controlled by a heat shock sigma factor (sigma 32). J Bacteriol. 1991 Jul;173(14):4247–4253. doi: 10.1128/jb.173.14.4247-4253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Dubnau D. Regulation of competence-specific gene expression by Mec-mediated protein-protein interaction in Bacillus subtilis. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5793–5797. doi: 10.1073/pnas.91.13.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Siranosian K. J., Grossman A. D., Dubnau D. Sequence and properties of mecA, a negative regulator of genetic competence in Bacillus subtilis. Mol Microbiol. 1993 Jul;9(2):365–373. doi: 10.1111/j.1365-2958.1993.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Kunst F., Debarbouille M., Msadek T., Young M., Mauel C., Karamata D., Klier A., Rapoport G., Dedonder R. Deduced polypeptides encoded by the Bacillus subtilis sacU locus share homology with two-component sensor-regulator systems. J Bacteriol. 1988 Nov;170(11):5093–5101. doi: 10.1128/jb.170.11.5093-5101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Kunst F., Lepesant-Kejzlarová J., Dedonder R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol Gen Genet. 1972;118(2):135–160. doi: 10.1007/BF00267084. [DOI] [PubMed] [Google Scholar]
- Lewandoski M., Smith I. Use of a versatile lacZ vector to analyze the upstream region of the Bacillus subtilis spoOF gene. Plasmid. 1988 Sep;20(2):148–154. doi: 10.1016/0147-619x(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Msadek T., Kunst F., Henner D., Klier A., Rapoport G., Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990 Feb;172(2):824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msadek T., Kunst F., Klier A., Rapoport G. DegS-DegU and ComP-ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J Bacteriol. 1991 Apr;173(7):2366–2377. doi: 10.1128/jb.173.7.2366-2377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3") (9). Mol Gen Genet. 1985;200(1):33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- Nath I., Laal S. Nucleotide sequence and deduced amino acid sequence of Mycobacterium leprae gene showing homology to bacterial atp operon. Nucleic Acids Res. 1990 Aug 25;18(16):4935–4935. doi: 10.1093/nar/18.16.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald A. F., Berg D. E., Stauffer G. V. Mutational analysis of the Escherichia coli serB promoter region reveals transcriptional linkage to a downstream gene. Gene. 1992 Oct 12;120(1):1–9. doi: 10.1016/0378-1119(92)90002-7. [DOI] [PubMed] [Google Scholar]
- Pearce B. J., Yin Y. B., Masure H. R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993 Sep;9(5):1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Roggiani M., Hahn J., Dubnau D. Suppression of early competence mutations in Bacillus subtilis by mec mutations. J Bacteriol. 1990 Jul;172(7):4056–4063. doi: 10.1128/jb.172.7.4056-4063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner D. Z., LeDeaux J. R., Ireton K., Grossman A. D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991 Feb;173(4):1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Modulation of Bacillus subtilis levansucrase gene expression by sucrose and regulation of the steady-state mRNA level by sacU and sacQ genes. J Bacteriol. 1986 Oct;168(1):380–388. doi: 10.1128/jb.168.1.380-388.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Pedersen S., Ross B. M., Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J Bacteriol. 1991 Jul;173(14):4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Squires C. L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992 Feb;174(4):1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Völker U., Mach H., Schmid R., Hecker M. Stress proteins and cross-protection by heat shock and salt stress in Bacillus subtilis. J Gen Microbiol. 1992 Oct;138(10):2125–2135. doi: 10.1099/00221287-138-10-2125. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D., Galli G., Cosmina P., de Ferra F., Withoff S., Venema G., Grandi G. Characterization of the srfA locus of Bacillus subtilis: only the valine-activating domain of srfA is involved in the establishment of genetic competence. Mol Microbiol. 1993 May;8(5):833–841. doi: 10.1111/j.1365-2958.1993.tb01630.x. [DOI] [PubMed] [Google Scholar]
- van Sinderen D., Withoff S., Boels H., Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990 Dec;224(3):396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]
- van Sinderen D., ten Berge A., Hayema B. J., Hamoen L., Venema G. Molecular cloning and sequence of comK, a gene required for genetic competence in Bacillus subtilis. Mol Microbiol. 1994 Feb;11(4):695–703. doi: 10.1111/j.1365-2958.1994.tb00347.x. [DOI] [PubMed] [Google Scholar]