Abstract
Introduction
Rearrest occurs when a patient experiences cardiac arrest after successful resuscitation. The incidence and outcomes of rearrest following out-of-hospital cardiac arrest have been estimated in limited local studies. We sought provide a large-scale estimate of rearrest incidence and its effect on survival.
Methods
We obtained case data from emergency medical services-treated, out-of-hospital cardiac arrest from the Resuscitation Outcomes Consortium, a multi-site clinical research network with clinical centers in 11 regions in the US and Canada. The cohort comprised all cases captured between 2006 and 2008 at 10 of 11 regions with prehospital return of spontaneous circulation. We used three methods to ascertain rearrest via direct signal analysis, indirect signal analysis, and emergency department arrival vital status. Rearrest incidence was estimated as the proportion of cases with return of spontaneous circulation that experience rearrest. Regional rearrest incidence estimates were compared with the χ2-squared test. Multivariable logistic regression was used to assess the relationship between rearrest and survival to hospital discharge.
Results
Out of 18,937 emergency medical services-assessed cases captured between 2006 and 2008, 11,456 (60.5%) cases were treated by emergency medical services and 4396 (38.4%) had prehospital return of spontaneous circulation. Of these, rearrest ascertainment data was available in 3253 cases, with 568 (17.5%) experiencing rearrest. Rearrest differed by region (10.2% to 21.2%, p<0.001). Rearrest was inversely associated with survival (OR: 0.19, 95% CI: 0.14–0.26).
Conclusions
Rearrest was found to occur frequently after resuscitation and was inversely related to survival.
Keywords: Cardiac arrest, Resuscitation, Emergency Medicine, Electrocardiography
1. Introduction
Rearrest is one potential stumbling block in the pathway to survival for the out-of-hospital cardiac arrest (OHCA) patient. Rearrest occurs when a resuscitated patient experiences a subsequent cardiac arrest. Contextualized to OHCA, the term rearrest generally applies to the short term, covering the time period from first resuscitation to hospital admission. As a condition classification, rearrest captures cardiac arrest of all electrocardiogram (ECG) presentations, distinguishing itself from refibrillation, with an ECG presentation of ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT), by encompassing that condition as well as cardiac arrests presenting in pulseless electrical activity (PEA) and asystole, the classic flat-line ECG trace. Estimates of the incidence of rearrest among patients with return of spontaneous circulation (ROSC) as high as 79% when considering studies that only looked at refibrillation.1–6 However, most such studies used a defibrillation success criterion of “non-VF post-shock rhythm,” blurring the distinction between rearrest as a loss of pulses and rearrest as a transition between cardiac arrest ECG rhythms. In the few studies that have considered all-rhythm rearrest, rearrest incidence estimates ranged from 5% to 39%.7–10 Of critical interest, a number of studies in both categories have indicated that rearrest can be detrimental for patient survival, although the mechanism for this association remains unclear.3,6,9
While the ultimate goal in investigating rearrest would be to prevent it through an understanding of its causes, the first step in this process is to understand the epidemiology of rearrest and the general relationship of rearrest to survival. In the present study, we provide descriptive epidemiologic characterization of rearrest using data from multiple cities in North America.
2. Methods
The University of Pittsburgh Institutional Review Board approved this retrospective cross-sectional study. Case data were obtained from the Resuscitation Outcomes Consortium (ROC), a North American research network studying cardiac arrest and severe trauma in 11 US and Canadian cities, however all study analyses were conducted at the University of Pittsburgh. One of the primary aims of the ROC is to maintain an active surveillance program for OHCA, where cases are identified and captured at the emergency medical services (EMS) level with in-hospital follow up for outcomes assessment. For the purpose of this study, case data were obtained only for non-traumatic OHCA cases receiving some treatment from EMS. This eliminated cases that were presumed dead on arrival of EMS.
An additional inclusion criterion was any prehospital ROSC, a necessary precursor to rearrest. All case data were derived from OHCA events occurring in a fixed study period running from early January 2006 to December 2008, and came from 10 of the 11 ROC sites, with 1 site contributing no data during this time period. The study period corresponded to the post-ramp up phase of the ROC surveillance infrastructure and ended at the initiation of a ROC-wide clinical trial, which resulted in changes to resuscitation protocols at participating sites and a data embargo.
Data fields collected for each case by the ROC included a panel of variables capturing patient characteristics and outcomes, treatments, event order and timing, provider characteristics, and process measures. Data were abstracted by ROC research specialists from prehospital patient care reports, electronic defibrillator downloads, and hospital medical records and were made available to us as a single de-identified database. In addition, we were also given access to original electronic defibrillator downloads for cases when they were available.
Rearrest was ascertained in this study by three distinct methods. Three methods were used in order to maximize ascertainment in the absence of completed data fields, the breadth of which we describe in detail in the presentation of our results. In short, prior to our study, ascertainment of rearrest was not a priority of the ROC, so our retrospective investigation relied upon inference through available data. Fig. 1 provides a visual synopsis of the methodologies described below.
Fig. 1. Rearrest ascertainment methodology.
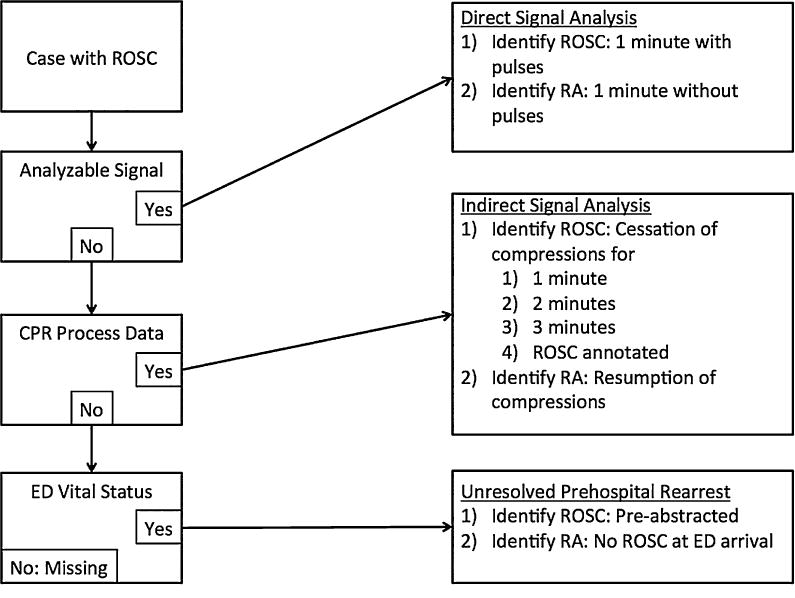
Abbreviations: ROSC, return of spontaneous circulation, ED Emergency Department, CPR cardiopulmonary resuscitation.
2.1. Rearrest Method 1
The first rearrest ascertainment method was the direct analysis of continuous defibrillator download data streams. While data stream content was manufacturer-dependent, defibrillator files available to us contained at a bare minimum continuous ECG and transthoracic impedance, a common signal modality used to detect chest compressions in resuscitation case review. Other signal modalities were often but not always present, and included end-tidal carbon dioxide, chest compression depth from sternal accelerometry, and chest compression force from a sternal strain gauge. When defibrillator downloads were available, we reviewed their content for evidence of rearrest, where signal-ascertained rearrest was defined as at least 1 min of the patient lacking a pulse post-ROSC, as evinced by obvious lethal arrhythmia in the ECG trace, chest compressions in any available chest compression reference data stream, or audible declaration of loss of pulses by paramedics in the audio data stream. In this method, ROSC was defined as 1 min of pulses, evinced by absence of chest compressions, non-lethal ECG rhythm, and, if present, audible paramedic confirmation of pulses.
2.2. Rearrest Method 2
The second rearrest ascertainment method involved the indirect analysis of defibrillator download data by way of previously extracted process metrics. In this way, even though a complete defibrillator download signal for a given case was not available, abstracted time-series data from the case could still be analyzed. During the 2006–2008 study period, ROC data abstractors had routinely tabulated cardiopulmonary resuscitation (CPR) process parameters throughout the first 20 available minutes of resuscitation. Process parameters were collected from electronic defibrillator downloads, which were analyzed but not retained, as minute-by-minute averages of chest compression rate and chest compression fraction, as well as raw chest compression count. Minute-by-minute process parameters were accompanied by qualifying variables indicating the reason for absence of parameters, if absent, at any of the 20 potential measurement time points. Included in this field was a value specifying that no parameters were recorded during a time point due to ROSC. Systematically analyzing the CPR process parameters over time with a custom MATLAB program (Mathworks R2011a, Nattick, MA), we first identified gaps in CPR. We could then infer to the presence of ROSC either by direct reference to the qualifying data field or through a time criterion. In the latter case, we separately considered periods of 1, 2, and 3 min without chest compressions as ROSC. Both methods were considered in separate analyses within this study. Rearrest could then be ascertained as the resumption of CPR following a ROSC-identified gap in the CPR process parameter timeline for a given case.
2.3. Rearrest Method 3
The third method was the assessment of a distinct, limited category of rearrest based on the presence or absence of pulses upon the patient's arrival at the hospital. Each case in our study cohort was identified as having ROSC prior to hospital arrival. Separately, ROC data abstractors identified patient vital status at emergency department (ED) arrival. It follows that any patient arriving at the ED without pulses had undergone rearrest at some point between achieving ROSC in the field and ED arrival.
2.4. Combined rearrest estimate
In order to produce an estimate of rearrest incorporating the maximum number of cases for which data were available, we created a conservative, composite rearrest variable incorporating data from each of the 3 methods. Rearrest status for each case was taken from one of the 3 methods, using the prioritization scheme illustrated in Fig. 1. In this way, direct signal analysis received the highest priority, while ED vital status received lowest. To produce the composite estimate, we erred on the sides of under-estimation and accuracy by only using Method 2 with annotated ROSC for the final overall analyses.
2.5. Analyses
Total rearrest incidence over the study period was estimated as the proportion of cases with rearrest out of all cases with available rearrest ascertainment data fields. Sub-estimates of incidence were also estimated for each method independently. Incidence estimates and other proportions were compared with the χ2 test. Logistic regression was used to assess the relationship between rearrest and the outcome of survival to hospital discharge. Multivariable regression models were constructed for both analyses and included age, sex, witness status, presenting cardiac arrest rhythm, and anonymized consortium site. In both multivariable models, the site coded ‘J’ was selected automatically by our statistical software as the reference site. All statistical analyses were conducted with Stata 12 (StataCorp, College Station, TX) with an alpha of 0.05.
3. Results
A total of 18,937 cases of EMS-assessed OHCA were detected by the ROC during the study period spanning 2006–2008. Of these, 11,456 (60.5%, 95% CI: 59.8–61.1) cases were treated by EMS, and a further subset of 4396 (38.4%, 95% CI: 37.5–39.3) cases were found to have a detectible prehospital ROSC event, forming the basis of our study cohort. Table 1 gives a summary of basic case characteristics for the overall study cohort.
Table 1.
Cohort characteristics by site.
| ROC Site | Age, years, mean (SD) | Male, % (95% CI) | Public location, % (95% CI) | VFVT presenting Rhx, %(95% CI) | EMS witnessed, %(95% CI) | Bystander resuscitation % (95% CI) | Survival % (95% CI) |
|---|---|---|---|---|---|---|---|
| A | 62.2 (16.6) | 63.9 (59.5–68.2) | 22.9 (19.1–26.7) | 42.9 (38.4–47.4) | 5.3 (3.3–7.3) | 60.9 (55.7–66.0) | 29.0 (24.8–33.2) |
| B | 62.3 (16.5) | 64.7 (61.4–68.0) | 25.3 (22.3–28.3) | 39.7 (36.2–43.1) | 14.7 (12.3–17.2) | 48.6 (45.2–52.1) | 35.4 (31.9–38.8) |
| C | 65.6 (16.2) | 62.4 (58.6–66.2) | 17.1 (14.2–20.1) | 40.3 (36.4–44.2) | 16.2 (13.3–19.0) | 31.0 (27.3–34.6) | 20.9 (17.6–24.1) |
| D | 60.4 (15.2) | 46.2 (30.3–62.0) | 15.8 (4.0–27.5) | 38.9 (22.7–55.0) | 12.8 (2.2–23.5) | 57.7 (38.3–77.1) | 12.9 (0.9–24.9) |
| E | 61.6 (19.5) | 65.9 (59.3–72.4) | 22.9 (17.2–28.7) | 43.6 (36.5–50.7) | 18.5 (13.2–23.9) | 39.1 (31.8–46.4) | 41.0 (34.1–47.9) |
| F | 63.9 (16.8) | 68.4 (65.5–71.4) | 25.2 (22.5–28.0) | 46.9 (43.7–50.1) | 12.2 (10.1–14.3) | 39.8 (36.7–42.9) | 25.1 (22.1–28.0) |
| G | 61.1 (19.2) | 50.4 (44.2–56.6) | 16.1 (11.6–20.7) | 24.9 (19.5–30.3) | 15.0 (10.6–19.4) | 26.0 (20.6–31.4) | 26.4 (20.8–32.0) |
| H | 64.9 (16.4) | 52.9 (45.8–59.9) | 16.6 (11.3–21.8) | 31.1 (24.5–37.7) | 8.8 (4.8–12.8) | 49.4 (42.1–56.8) | 29.7 (23.1–36.3) |
| I | 61.6 (16.4) | 61.0 (51.6–70.3) | 13.3 (6.8–19.9) | 43.2 (32.4–54.1) | 10.5 (4.6–16.4) | 47.2 (33.6–60.7) | 27.5 (18.2–36.7) |
| J | 67.0 (16.1) | 61.7 (58.2–65.2) | 18.7 (15.8–21.5) | 36.0 (32.5–39.5) | 15.8 (13.2–18.5) | 33.2 (29.8–36.7) | 25.0 (21.8–28.3) |
| Overall | 63.9 (16.9) | 63.0 (61.6–64.5) | 21.4 (20.1–22.6) | 40.1 (38.6–41.5) | 13.4 (12.3–14.4) | 40.6 (39.1–42.1) | 27.8 (26.4–29.2) |
Abbreviations: ROC, resuscitation outcomes consortium; VFVT, ventricular fibrillation/ventricular tachycardia; CI, confidence interval; SD, standard deviation; Rhx, rhythm.
Defibrillator download files (Method 1) were directly available to us for 370 (8.4%) cases from the study cohort, 20-min CPR process data (Method 2) were available for 1222 (27.8%) cases, and ROSC at ED arrival (Method 3) was available for 2913 (66.3%) cases. At least one rearrest ascertainment variable was available for a total of 3253 cases, or 74% of the study cohort, with 52.5% of cases having only one variable available, 14.5% having 2, and only 6.8% having all 3 variables available. Individual and combined rearrest incidence estimates are reported below and summarized in Table 2, along with odds rations for survival when each method of rearrest ascertainment represented rearrest status in separate multivariable logistic regression models.
Table 2.
Rearrest incidence by method summary.
| Rearrest ascertainment method | Analyzable Casesn | Rearrest rate % (95% CI) | Survival adjusteda OR (95% CI) |
|---|---|---|---|
| Method 1 – direct signal analysis | 294 | 38.4 (32.8–44.0) | 0.45 (0.22–0.90)# |
| Method 2.1 – CPR process (ROSC = 1 min) | 1222 | 16.1 (14.1–18.2) | 0.50 (0.32–0.79)# |
| Method 2.2 – CPR process (ROSC = 2 min) | 1222 | 6.5 (5.2–7.9) | 0.66 (0.34–1.28) |
| Method 2.3 – CPR process (ROSC = 3 min) | 1222 | 4.5 (3.3–5.7) | 0.61 (0.27–1.37) |
| Method 2.4 – CPR process (annotated ROSC) | 1222 | 2.3 (1.5–3.1) | 1.20 (0.46–3.12) |
| Method 3 – ED vital status | 2913 | 16.7 (15.3–18.0) | 0.14 (0.01–0.20)# |
| Combined rearrest with Method 2.1 | 3253 | 18.6 (17.3–20.0) | 0.20 (0.15–0.30)# |
| Combined rearrest with Method 2.2 | 3253 | 17.7 (16.4–19.1) | 0.20 (0.14–0.27)# |
| Combined rearrest with Method 2.3 | 3253 | 17.6 (16.3–18.9) | 0.19 (0.14–0.26)# |
| Combined rearrest with Method 2.4 | 3253 | 17.5 (16.2–18.8) | See Table 3 |
Abbreviations: CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; ED, Emergency Department; OR, odds ratio; CI, confidence interval.
Covariates: age, male, public location, VF/VT presenting rhythm, EMS witness status, ROC sites 1–10.
Statistically significant at 0.05 level.
3.1. Method 1 estimate
Of the 370 signals available to us, 294 (79.5%) were analyzable. Seventy-six signals either lacked a clear occurrence of ROSC, presented unusual ECG wherein lethal arrhythmia could not be ruled in or out, or lacked a CPR reference data stream by which to determine the cessation or resumption of chest compressions. Of those cases that were analyzable, rearrest was present in 113, yielding a rearrest incidence by direct signal analysis of 38.4%. Cases in this subset experienced, on average 1.6 (1.2) rearrest events (median: 1, IQR: 1–2) over a total of 4929 min of signal analyzed. The mean (SD) time to first rearrest was 6.6 (6.5) min from ROSC, with a median of 4.4 (1.8–9.3). Figs. 2 and 3 shows the ECG rhythm at time of rearrest for the first rearrest event in cases with analyzable ECG. The majority of first rearrest events presented with shockable rhythms.
Fig. 2. Rearrest incidence by presenting ECG rhythm.
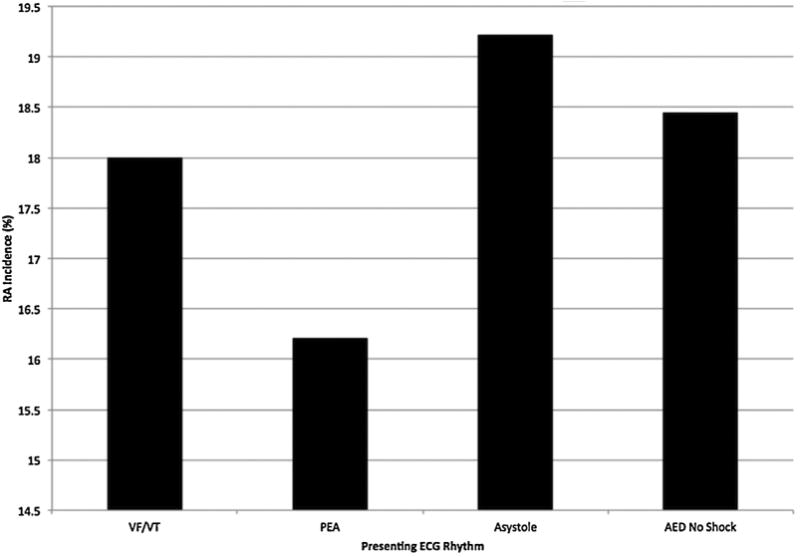
Abbreviations: VFVT, ventricular fibrillation/ventricular tachycardia, PEA, pulseless electrical activity, AED, automated external defibrillator.
Fig. 3. ECG Rhythm at first rearrest event.
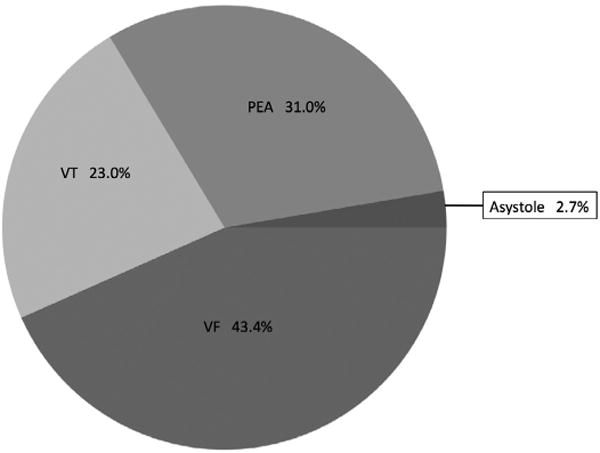
Abbreviations: VF, ventricular fibrillation, VT, ventricular tachycardia, PEA, pulseless electrical activity.
3.2. Method 2 estimate
Estimating rearrest incidence with an interpretation of 1 min of no CPR as ROSC resulted in a CPR process based incidence estimate of 16.1%. Estimating rearrest incidence conservatively for cases with available ROSC annotation data resulted in CPR process based incidence estimate of 2.3%. In these cases, bearing in mind that the data source imposed a 20-min ascertainment period limitation on our analyses, the average number of rearrest per case was 1.1 (0.4) (median: 1, range: 1–2) events over a total of 9133 min of CPR process data. The mean (SD) time to first rearrest was 3.5 (2.8) minutes from ROSC, with a median (IQR) of 3 (1–5)min.
3.3. Method 3 estimate
Of 2913 cases with a valid ROSC at ED arrival variable, 16.7% of cases qualified as rearrest. This method of classification of rearrest alone does not allow for the determination of number of rearrest events or relevant time intervals (e.g. when rearrest occurred).
3.4. Combined estimate and models
Allowing for any of the 3 methods of ascertaining rearrest and restricting Method 2 ascertainment to definite ROSC (Method 2.4), 568 cases (17.5%) out of 3253 cases with rearrest data experienced at least one rearrest event. Over the 10 sites, incidence of rearrest varied significantly from 10.2% to 21.2% (p = 0.01). Stratified by presenting cardiac arrest ECG rhythm, rates of rearrest did not differ significantly (p = 0.48); see Fig. 2. In a univariate model, rearrest was significantly predictive of survival to hospital discharge (OR: 0.25, 95% CI: 0.19–0.34). The effect remained in a multivariable model adjusting for age, sex, presenting ECG rhythm VF/VT, EMS witness status, bystander resuscitation, and public/private location of OHCA (OR: 0.19, 95% CI: 0.14–0.26). Results of the regression analysis are shown in Table 3.
Table 3.
Multiple logistic regression results.
| Predictor | OR | 95% CI | p |
|---|---|---|---|
| Rearrest (Method 2.4) | 0.19 | 0.14–0.26 | <0.001 |
| Age, y | 0.98 | 0.97–0.98 | <0.001 |
| Male | 1.16 | 0.94–1.42 | 0.17 |
| Public location | 1.99 | 1.61–2.46 | <0.001 |
| VF/VT presenting rhythm | 5.30 | 4.36–6.45 | <0.001 |
| EMS witnessed | 2.98 | 2.27–3.90 | <0.001 |
| Site A | 0.98 | 0.69–1.41 | 0.93 |
| Site B | 1.33 | 0.98–1.80 | 0.07 |
| Site C | 0.69 | 0.49–0.97 | 0.03 |
| Site D | 0.46 | 0.12–1.69 | 0.24 |
| Site E | 1.77 | 1.12–2.78 | 0.01 |
| Site F | 0.74 | 0.55–1.01 | 0.06 |
| Site G | 1.02 | 0.61–1.72 | 0.93 |
| Site H | 1.68 | 0.97–2.91 | 0.07 |
| Site I | 0.80 | 0.41–1.57 | 0.52 |
| Site J | Ref | Ref | Ref |
Abbreviations: VF/VT, ventricular fibrillation/ventricular tachycardia; EMS, emergency medical services; OR, odds ratio; CI, confidence interval.
In a sub-analysis of just those rearrest cases with analyzable ECG, cases with a shockable first rearrest rhythm had a survival rate of 33.3%, while cases with a non-shockable first rearrest rhythm had a survival rate of 3.9% (p = 0.003).
4. Discussion
Our principle finding is a general estimate of rearrest incidence. Overall, by way of our most conservative estimation scheme, we found that rearrest occurred in 17.5% of OHCA cases achieving ROSC. The selection process for our study cohort yielded an overall consortium rate of ROSC at 38.4%. Thus it follows that only 6.7% of EMS-treated OHCA patients experience rearrest. This figure is deceptively small, given that in the nearly 62% of patients who never regain pulses during resuscitation, rearrest is impossible and therefore clinically irrelevant. Still in comparison to previous single-site studies, our overall rearrest rate estimate is low. In single-city analyses, both our group working in Pittsburgh and Lerner et al. working in Milwaukee found local rearrest rates approaching 40%.8,9 However, it should be noted that our estimate of rearrest by Method 1 – 38.4% – was in very close agreement with these previous estimates. When we considered cases with presenting ECG rhythm of VF/VT alone, overall rearrest rate was not radically different from our all-rhythm estimate, but differed from estimates of refibrillation in previous studies.1–5 Compared to our previous single-site study, rates of VF rearrest were higher (43.3% vs 24.6%) and the overall proportion of non-shockable first rearrest rhythms was lower (33.7% vs 46.4%). Because little is known about the specific causes of rearrest, it is not possible to draw direct inference regarding the bases for these differences.
Also of significant interest, we found that rearrest was inversely predictive of survival. While this finding is intuitive and supports previous studies – though not explicitly reported, the OR for survival in the study by Lerner et al. was approximately 0.3 – the specific relationship between rearrest and survival is not clear. Patients with rearrest were significantly less likely than patients without rearrest to have a witnessed OHCA or a presenting ECG rhythm of VF/VT. This finding may provide further insight into the challenges of resuscitating initially non-shockable OHCA ECG rhythms. That non-shockable first rearrest rhythms were inversely correlated with survival is not surprising, given generally low survival rates for cases with non-shockable first rhythms at first EMS assessment.12 However there may be different mechanisms at work between initial presentation with a non-shockable rhythm and development of a non-shockable rhythm following ROSC.
We provide a broad geographic estimate of rearrest incidence. Rearrest rates differed significantly across the 10 participating consortium sites, evoking previously established diversity in OHCA incidence and survival rates across the same areas.11 We do not disclose site identity in this paper, out of agreement with the ROC, so we cannot explicitly assess the relationship between these past site-specific estimates and our current rearrest estimates. Furthermore, the scope of the present paper does not provide a basis for speculating to the causes of or risk factors for differential incidence of rearrest. Regional differences in underlying pathology, demographics, and EMS practices may all play a role in occurrence of rearrest. Whereas the aim of this study was to describe the incidence and outcomes of rearrest, future studies will examine its characteristics and causes.
Our study offers a new classification of rearrest, rearrest determined solely by ED vital status, which we call unresolved prehospital rearrest. While unresolved prehospital rearrest is the least detailed measure of rearrest available from our data set, it provides strong predictive power for negative outcomes after OHCA. The relationship between unresolved prehospital rearrest and poor outcomes could be useful with a full understanding of its determinants, characteristics, and consequences. Further characterization of this phenomenon is necessary.
We provide two estimates of mean time-to-rearrest in this study of 3.5 and 6.6 min by direct and indirect signal analysis. These times compare closely to our previous single-site estimate of 3.1 min.8 Time intervals between ROSC and rearrest of this magnitude provide a potential therapeutic window for preventing rearrest before it happens, with the caveat that such interventions are most likely to be necessary in the prehospital environment with all of the constraints and conditions that it carries.
Our study has several limitations related primarily to the retrospective data that formed the basis of our investigation. Due to several infrastructure issues outlined in our methods, we could not directly analyze the raw resuscitation signal for many of the cases in our cohort. This necessitated the adoption of alternative methods of rearrest ascertainment that indirectly inferred to the loss of pulses after ROSC. Both alternative methods are conservative, biasing ascertainment toward a non-event. Even so, all three methods – including Method 2.1 of indirect signal analysis – were independently predictive of survival in multivariable models, indicating some validity by effect equivalence. With respect to outcomes, we were unable to specifically investigate the in-hospital contributors to the survival or death of subjects in our cohort, including therapies, co-morbidities, secondary conditions, and indeed actual cause of death. Additionally, we did not have access to neurologic outcome data for our cohort, leaving open the possibility that rearrest, though inversely associated with survival, may or may not impact neurologic status at hospital discharge. Lastly, we derived our conclusions from data spanning a relatively narrow 2-year surveillance period in 2008 and 2009, leading to the possibility that both the temporal scope and the detachment from changes that have occurred since then may limit generalization of our findings. While it is certainly possible that critical changes the underlying pathology of OHCA and the effectiveness of treatments for this condition have occurred in the interim between the end of the data collection period and presentation of the present study, we believe that the findings of this study, while based on older data, may still provide useful insights, not the least being a basis for historical comparison for the outcome of rearrest.
5. Conclusion
In this geographically broad and inferentially conservative analysis, rearrest occurred on average in 1 of every 6 successfully resuscitated patients. Rearrest was inversely related to survival to hospital discharge.
Acknowledgments
Funding: This work was supported by a significant research grant from the National Heart, Lung and Blood Institute(contract 1R21HL104440-01) awarded to Dr. Menegazzi. All analyses were conducted at the University of Pittsburgh Department of Emergency Medicine.
Data acquisition assistance was provided by the Resuscitation Outcomes Consortium.
We would like to thank the Emergency Medical Services and Fire Services providers who serve the sites of the Resuscitation Outcomes Consortium for their participation in this study.
Footnotes
Spanish translated version of the abstract of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2014.10.011.
Conflict of interest statement: The authors have no conflicts to disclose.
References
- 1.Weaver WD, Cobb LA, Copass MK, Hallstrom AP. Ventricular defibrillation – a comparative trial using 175-J and 320-J shocks. N Engl J Med. 1982;307:1101–6. doi: 10.1056/NEJM198210283071801. [DOI] [PubMed] [Google Scholar]
- 2.Martens PR, Russell JK, Wolcke B, et al. Optimal response to cardiac arrest study: defibrillation waveform effects. Resuscitation. 2001;49:233–43. doi: 10.1016/s0300-9572(01)00321-5. [DOI] [PubMed] [Google Scholar]
- 3.van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation. 2003;59:181–8. doi: 10.1016/s0300-9572(03)00208-9. [DOI] [PubMed] [Google Scholar]
- 4.Hess EP, White RD. Recurrent ventricular fibrillation in out-of-hospital cardiac arrest after defibrillation by police and firefighters: implications for automated external defibrillator users. Crit Care Med. 2004;32:S436–9. doi: 10.1097/01.ccm.0000134258.72142.e5. [DOI] [PubMed] [Google Scholar]
- 5.Koster RW, Walker RG, Chapman FW. Recurrent ventricular fibrillation during advanced life support care of patients with prehospital cardiac arrest. Resuscitation. 2008;78:252–7. doi: 10.1016/j.resuscitation.2008.03.231. [DOI] [PubMed] [Google Scholar]
- 6.Berdowski J, ten Haaf M, Tijssen JG, et al. Time in recurrent ventricular fibrillation and survival after out-of-hospital cardiac arrest. Circulation. 2010;122:1101–8. doi: 10.1161/CIRCULATIONAHA.110.958173. [DOI] [PubMed] [Google Scholar]
- 7.Hartke A, Mumma BE, Rittenberger JC, Callaway CW, Guyette FX. Incidence of re-arrest and critical events during prolonged transport of post-cardiac arrest patients. Resuscitation. 2010;81:938–42. doi: 10.1016/j.resuscitation.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salcido DD, Stephenson AM, Condle JP, Callaway CW, Menegazzi JJ. Incidence of rearrest after return of spontaneous circulation in out-of-hospital cardiac arrest. Prehosp Emerg Care. 2010;14:413–8. doi: 10.3109/10903127.2010.497902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerner EB, O'Connell M, Pirrallo RG. Rearrest after prehospital resuscitation. Prehosp Emerg Care. 2011;15:50–4. doi: 10.3109/10903127.2010.519820. [DOI] [PubMed] [Google Scholar]
- 10.Chestnut JM, Kuklinski AA, Stephens SW, Wang HE. Cardiovascular collapse after return of spontaneous circulation in human out-of-hospital cardiopulmonary arrest. Emerg Med J. 2012;29:129–32. doi: 10.1136/emj.2010.108340. [DOI] [PubMed] [Google Scholar]
- 11.Nichol G, Thomas E, Callaway CW, et al. Resuscitation Outcomes Consortium Investigators. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally B, Robb R, Mehta M, et al. Centers for Disease Control and Prevention. Out-of-hospital cardiac arrest surveillance – Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005–December 31, 2010. MMWR Surveill Summ. 2011;60:1–19. PubMed PMID: 21796098. [PubMed] [Google Scholar]


