Abstract
Purpose
To evaluate the effect of a topical non-steroidal anti-inflammatory drug, nepafenac 0.1%, in eyes with non-central diabetic macular edema (DME).
Methods
Multi-center double-masked randomized trial. Individuals with good visual acuity and non-center involved DME were randomly assigned to nepafenac 0.1% (N = 61) or placebo (nepafenac vehicle, N = 64) three times a day for 12 months. The primary outcome was mean change in OCT retinal volume at 12 months.
Results
Mean baseline retinal volume was 7.8 mm3. At 12 months, in the nepafenac and placebo groups respectively, mean change in retinal volume was -0.03 mm3 and -0.02 mm3 (treatment group difference: -0.02, 95% CI: -0.27 to 0.23, P = 0.89). Central involved DME was present in 7 eyes (11%) and 9 eyes (14%) at the 12-month visit (P = 0.79), respectively. No differences in visual acuity outcomes were identified. One study participant developed a corneal melt after using nepafenac in the non-study eye, which had a history of severe dry eye. No additional safety concerns were evident.
Conclusion
In eyes with non-central DME and good visual acuity, topical nepafenac 0.1% three times daily for 1 year likely does not have a meaningful effect on OCT-measured retinal thickness.
Keywords: Diabetic Retinopathy Clinical Research Network, Nepafenac, Non-central DME, Topical NSAID
Introduction
Recent population-based studies in the U.S. and else where report diabetic macular edema (DME) as the most common cause of vision loss in patients with diabetes mellitus.1, 2 Prevalence data from the Centers for Disease Control in the U.S. population over age 40 suggest that many cases of DME do not involve the central macula.3 Data from the ETDRS and the Protein Kinase C β Inhibitor study indicate that 22% to 30% of these cases may have central-involved DME by 1 year.4, 5 A common management of non-central DME is careful observation until either the center of the macula becomes thickened, or until it is perceived that the central subfield of the macula is threatened.6 A relatively safe treatment that reduces the risk of eyes with non-central involved DME from developing center-involved DME might be beneficial.
Elevated inflammatory markers have been found in patients with diabetic retinopathy suggesting that inflammation may have a role in the pathogenesis of DME.7, 8 Reports from animal models have shown that topical non-steroidal anti-inflammatory drops (NSAIDs) have the capability of reaching the posterior segment of the eye.9 Nepafenac rapidly penetrates the cornea and is deaminated by intraocular hydrolases to form the active metabolite amfenac. Nepafenac and amfenac inhibit activity from both cyclooxygenase isoforms (COX-1 and COX-2) responsible for prostaglandin synthesis and are frequently used to treat post-surgical (Irvine-Gass) cystoid macular edema.10 There is some evidence that NSAIDs penetrate to the retina which might affect resolution of macular edema.11, 12 Nepafenac is approved for use in the United States and elsewhere for the treatment of post-operative pain and inflammation associated with cataract surgery. In addition, it recently has been approved by some regulatory agencies in Europe,13 but not by the U.S. Food and Drug Administration, for macular edema associated with cataract surgery in adult diabetes patients.
Given the prevalence of non-central involved DME and frequency of worsening to central-involved DME, this Diabetic Retinopathy Clinical Research Network (DRCR.net) protocol was designed to assess whether topical nepafenac 0.1% might prevent worsening of DME as manifested primarily by optical coherence tomography (OCT) retinal volume and secondarily by other OCT and visual acuity outcomes among eyes with non-central involved DME.
Methods
This phase II, multicenter, double-masked randomized clinical trial was conducted by the DRCR.net at 43 clinical sites throughout the United States. The protocol and Health Insurance Portability and Accountability Act-compliant informed consent forms were approved by the institutional review board for each participating site. Each participant gave written informed consent to participate in the study. The study protocol (named “A Phase II Evaluation of Topical NSAIDs in Eyes with Non Central Involved DME”) is available on the DRCR.net website (www.drcr.net) and registered at www.clinicaltrials.gov (NCT01331005). Key aspects of the study are summarized below.
Study Population
Eligible study participants were at least 18 years of age, with type 1 or type 2 diabetes mellitus and had at least one eye with DME that did not involve the center of the macula. Patients were excluded if they were receiving systemic corticosteroids or vascular endothelial growth factor (VEGF) inhibitors, were concurrently using systemic prescription NSAIDs, or had an auto-immune disease judged to increase the risk for corneal complications.
Study-eligible eyes had a best corrected visual acuity Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS) letter score ≥74 (approximate Snellen equivalent of 20/32 or better), with definite retinal thickening by clinical examination, due to DME within 3000 μm of, but not involving, the center of the macula. In addition, eligible eyes had at least two of eight non-central macular subfields with OCT thickness above a threshold value, or at least one non-central macular subfield with OCT thickness ≥15 μm above a threshold value in Zeiss Cirrus (Carl Zeiss Meditec, Inc., Dublin, CA) or Heidelberg Spectralis (Heidelberg, Carlsbad, CA) OCT. Threshold values were defined as the average machine-specific OCT thickness in normal eyes + 2 standard deviations (Figure 1 online). In addition, the central subfield thickness was required to be less than the gender-specific mean thickness from a normal cohort + 2 standard deviations. Exclusion criteria included history of focal/grid macular laser within the last 6 months or other treatment for DME within the prior 4 months, an anticipated need for DME treatment during the course of study, lipid in the center of the macula, a history of panretinal photocoagulation (PRP) within 4 months prior to enrollment or an anticipated need for PRP within 6 months following randomization, aphakia, history of vitrectomy, cataract surgery within 1 year prior to enrollment, and any other major ocular surgery within 4 months prior to enrollment.
Figure 1.
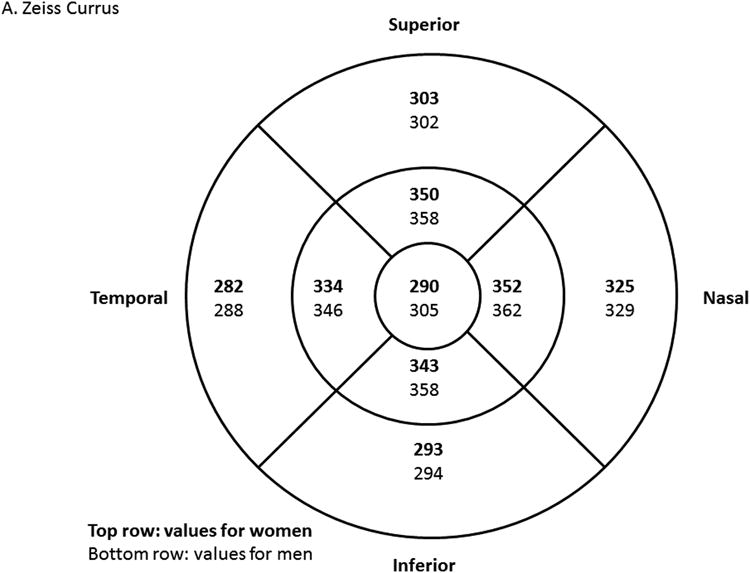
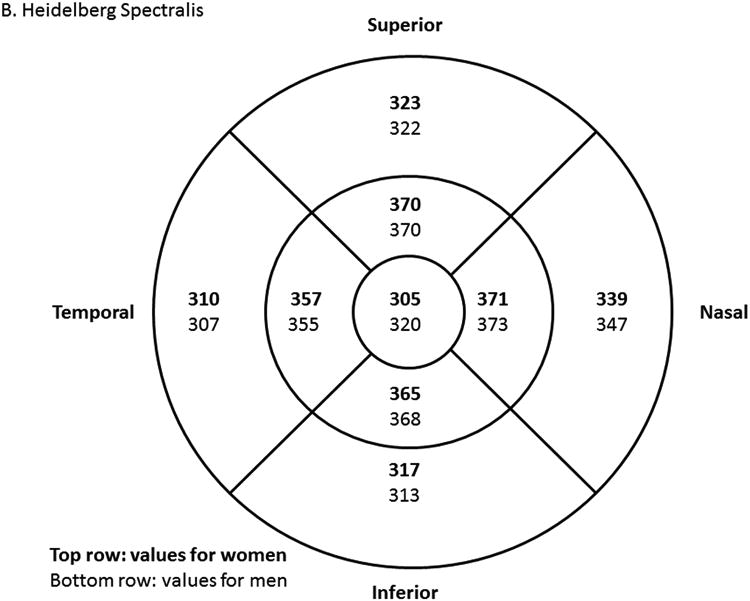
OCT threshold values to define eligibility in Zeiss Cirrus (Zeiss, Personal Communication, December 15, 2010) (A) and Heidelberg Spectralis14 (B) machines. Values in top row (bold font) are women-specific, and values in bottom row (regular font) are men-specific. Threshold is defined as the mean OCT thickness value from normal cohort+ 2 standard deviations (SD). For eligibility, study eye must have had central subfield thickness below the threshold, and meeting one of the following: 1) At least 2 non-central subfields with thickness values above threshold; or 2) At least 1 non-central subfield with thickness value more than 15 μm above threshold.
Study Design
Study participants were enrolled by investigators at participating DRCR.net clinical sites (Appendix 1). Only one eye of each participant was entered. If both eyes were eligible for the study, the study eye was chosen by the investigator. Enrollment procedures to screen for eligibility included blood pressure measurement; best corrected E-ETDRS visual acuity; ocular examination, including intraocular pressure assessment, slit lamp and fundus examination in each eye; and OCT scan of the study eye.
The study began with a 30- to 60-day run-in phase to assess compliance with daily drop placement, during which participants were required to apply one eye drop (artificial tears, Tears Naturale Forte® [Alcon Research Inc., Fort Worth, TX]) three times per day into the study eye. Participants were considered “compliant” if the weight of the artificial tear bottle at end of the run-in phase was 80% or more of the target level expected for the number of days the participant had been in the run-in phase.
Compliant participants at the end of run-in phase proceeded to the randomization phase. Before randomization, blood pressure measurement, best corrected E-ETDRS visual acuity, ocular examination, including intraocular pressure assessment, slit lamp and fundus examination in each eye, OCT scan and fundus photography of the study eye were performed to confirm that the participant still met the eligibility criteria. Eligible eyes were assigned randomly (1:1) to topical eye drops of nepafenac 0.1% (Alcon Research Inc., Fort Worth, TX) or placebo (consisting of the nepafenac vehicle). Randomization, completed on the study website, was stratified by site. Masking of investigators, other site personnel, and participants to treatment group assignment was achieved by using identical opaque study bottles for both groups. Protocol visits occurred at 4, 8 and 12 months after randomization with the primary outcome at the 12-month visit. At each protocol visit, information about adverse events was solicited, visual acuity was measured, an ocular examination performed, and an OCT of the study eye was obtained. Fundus photographs also were obtained at the 12-month visit. Visual acuity was measured following a standardized refraction using the E-ETDRS method by a masked study certified technician. Optical coherence tomography images were obtained by study-certified OCT technicians masked to treatment groups. Baseline and 12-month OCT images were graded by a central reading center (Duke Reading Center, Duke University, Durham, NC) as were retinal fundus photographs (Fundus Photograph Reading Center, University of Wisconsin-Madison, Madison, WI).
Participants were instructed to apply the study drug (masked nepafenac or placebo) to the study eye as one drop, 3 times per day with subsequent light closure of the eyelid for about 30 seconds. Participants who wore contact lens were asked to remove the lens prior to application of study drops. Prior to dispensing the study drug to the participant, the weight of each bottle was obtained using a calibrated scale. Participants were instructed to bring all bottles that were dispensed at the previous visit when they returned for the next visit.
Treatment for DME during the study was not allowed unless the central subfield retinal thickness increased to at least the gender-specific and OCT machine-specific threshold value (defined as the mean + 2 standard deviations of a normal cohort. [Zeiss, Personal Communication December 15, 2010]14) and there was at least a 10% increase in central subfield thickness from baseline. If the eye did not meet these criteria and the investigator believed it was in the study participant's best interest to receive treatment for DME, a discussion with the Protocol Chair was required. Before initiation of DME treatment, OCT scans and fundus photographs were obtained. Treatment with study drug was continued regardless of any DME treatment received.
Statistical Analysis
The primary efficacy analysis was a treatment group comparison of change in OCT volume from baseline to 12 months, adjusted for the baseline measurement (from randomization). Sample size was computed to be 120 eyes (60 per group) in order to have 90% power with type I error of 5% to detect a difference, assuming the true difference was 0.40 mm3 with a standard deviation of 0.60, allowing for 10% loss to follow up. The standard deviation of mean retinal volume change was based on unpublished DRCR.net data, in which the standard deviation for change in retinal volume from baseline to 1 year in 16 eyes with untreated non-center DME was 0.37 mm3 (95% confidence interval (CI): 0.28 to 0.56).
The primary analysis followed the intention-to-treat principle. Missing 12-month OCT and visual acuity measurements were imputed using the last observation carried forward. In eyes that received DME treatment during the study, the last OCT and visual acuity measurements prior to DME treatment were imputed for the 12-month visit value. Additional OCT measurements analyzed included the number of thickened subfields and retinal thickness change in the subfield with the maximum thickness at baseline. If randomization OCT values were not available or non-gradable, the enrollment visit OCT values were used as baseline (occurred in one eye in the nepafenac group). Optical coherence tomography values from spectral-domain machines were converted to time-domain equivalent values,15 since a formula for converting thickness values from one spectral-domain to another spectral-domain value is not currently available. The change in OCT retinal volume and thickness was calculated using the original machine values (i.e. without converting to time-domain equivalent values), since the same OCT machine was used within individuals at baseline and follow-up visits.
Analysis of covariance adjusting for baseline value of the dependent variable was used to compare the change from baseline of the specific parameter between the two treatment groups. Confounding was assessed by including variables imbalanced between treatment groups that were potentially associated with the outcome as covariates in a model. Comparison of the two treatment groups for categorical outcomes was conducted using Fisher's exact test. All reported P values were two-sided. SAS software, version 9.3 (SAS, Cary, NC), was used for all analyses.
Safety outcomes included corneal complications (including corneal edema, superficial keratitis, corneal erosion, corneal thinning, corneal ulceration and corneal melting), intraocular pressure changes, cataract formation and cataract surgery, ocular inflammation and/or infection, and local reactions or symptoms such as redness, burning, itching or watering.
Results
From June 2011 to November 2012, 169 participants were enrolled into the run-in phase of the study at 43 DRCR.net sites (Figure 2). Of these, 125 (74%) successfully completed the run-in phase and entered the randomized trial, with 61 assigned to the nepafenac group and 64 assigned to the placebo group. Median age was 60 years with 41% women and 66% White. Mean visual acuity letter score was 83 (approximate Snellen equivalent, 20/25). Mean time-domain equivalent retinal volume was 7.8 mm3 and mean time-domain equivalent central retinal subfield thickness was 223 μm. Participant and study eye characteristics according to treatment group are shown in Table 1.
Figure 2.
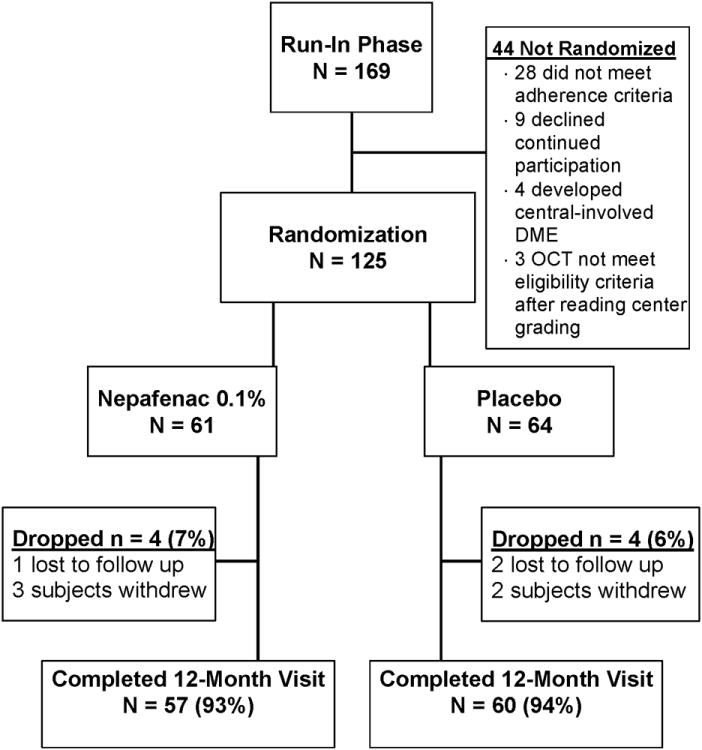
Summary of Study Recruitment.
Table 1. Baseline Characteristics of Participants in the Randomized Trial.
| Nepafenac | Placebo | ||
|---|---|---|---|
|
| |||
| N = 61 | N = 64 | ||
| Age, years | |||
| Median (25th, 75th percentile) | 60 (52, 68) | 59 (51, 66) | |
| ≥65, N (%) | 21 (34%) | 20 (31%) | |
|
| |||
| Gender, N (%) | |||
| Women | 23 (38%) | 28 (44%) | |
|
| |||
| Race/Ethnicity, N (%) | |||
| White | 44 (72%) | 38 (59%) | |
| African American | 7 (11%) | 13 (20%) | |
| Hispanic or Latino | 5 (8%) | 12 (19%) | |
| American Indian/Alaskan Native | 0 (0%) | 1 (2%) | |
| Native Hawaiian/Other Pacific Islander | 2 (3%) | 0 (0%) | |
| Asian | 2 (3%) | 0 (0%) | |
| More than one race | 1 (2%) | 0 (0%) | |
|
| |||
| Type of Diabetes, N (%) | |||
| Type I | 5 (8%) | 5 (8%) | |
| Type II | 53 (87%) | 56 (88%) | |
| Uncertain | 3 (5%) | 3 (5%) | |
|
| |||
| Duration of Diabetes, years | |||
| Mean±SD | 19±11 | 17±11 | |
| ≥10 years, N (%) | 51 (84%) | 44 (69%) | |
|
| |||
| HbA1c,%* | |||
| Median (25th, 75th percentile) | 8.1 (7.1, 8.7) | 7.9 (7.2, 10.0) | |
| <8% | 28 (47%) | 32 (54%) | |
| ≥8% | 32 (53%) | 27 (46%) | |
|
| |||
| Contact Lens Wear, % (N) | 2 (3%) | 3 (5%) | |
|
| |||
| E-ETDRS VA Letter Score, | |||
| Mean±SD | 82±6 | 83±7 | |
| Median (25th, 75thpercentile) | 82 (78, 86) | 84 (78, 89) | |
| Snellen Equivalent VA | |||
| Median (25th, 75thpercentile) | 20/25 (20/32, 20/20) | 20/20 (20/32, 20/16) | |
| History of DME treatment, N (%) | 30 (49%) | 28 (44%) | |
| History of Panretinal Photocoagulation, N (%) | 13 (21%) | 11 (17%) | |
| Level of Diabetic Retinopathy from Reading Center grading, N (%) | |||
| Microaneurysms only | 0 (0%) | 1 (2%) | |
| Mild to moderate NPDR | 28 (46%) | 25 (39%) | |
| Severe NPDR | 16 (26%) | 23 (36%) | |
| PDR or Prior PRP | 16 (26%) | 15 (23%) | |
| Cannot be graded | 1 (2%) | 0 (0%) | |
| Intraocular Pressure (mm Hg) | |||
| Mean±SD | 16±3 | 16±4 | |
| Lens Status, N (%) | |||
| Phakic | 40 (66%) | 53 (83%) | |
| PC IOL | 21 (34%) | 11 (17%) | |
| OCT Machine, N (%) | |||
| Zeiss Cirrus | 37 (61%) | 41 (64%) | |
| Heidelberg Spectralis | 24 (39%) | 23 (36%) | |
| OCT Central Subfield Thickness, μm† | |||
| Median (25th, 75thpercentile) | 228 (213, 242) | 215 (205, 236) | |
| Mean±SD | 227±29 | 218±25 | |
| OCT Retinal Volume, mm3 (Primary Outcome Variable)†† | |||
| Median (25th, 75thpercentile) | 7.8 (7.4, 8.2) | 7.7 (7.5, 8.0) | |
| Mean±SD | 7.9±0.6 | 7.7±0.4 | |
| Number of thickened non-central subfields, | |||
| Mean ±SD | 3±2 | 3±2 | |
SD = standard deviation; DR = diabetic retinopathy; NPDR = non-proliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; PRP = panretinal photocoagulation; OCT = optical coherence tomography; PC IOL = posterior chamber intraocular lens
Not available for 1 and 5 participants in the nepafenac and placebo groups respectively.
OCT CSF thickness conversion to Zeiss Stratus was applied as follows: -43.12 +1.01×Zeiss Cirrus; -72.76 + 1.03×Spectralis.
OCT retinal volume conversion to Zeiss Stratus was applied as follows: -1.21 + 1.02×((((CSF×(4/9)+inner superior subfield thickness × (8/9)+ inner temporal subfield thickness× (8/9)+ inner inferior subfield thickness× (8/9)+ inner nasal subfield thickness × (8/9)+ outer superior subfield thickness×3 + outer temporal subfield thickness ×3 + outer inferior subfield thickness ×3 + outer nasal subfield thickness ×3) ×3×3×3.14)/16)/1000); -2.05 + 1.06*Spectralis. One volume value in nepafenac group used enrollment visit value because randomization visit reading center-graded value was “nongradable”
Study Completion
The 12-month study visit was completed by 57 participants (93%) in the nepafenac group and 60 participants (94%) in the placebo group (Figure 2). There were no deaths among the 8 participants who did not complete the study.
Adherence with Study Drug
For participants who completed at least one protocol visit (58 [95%] in nepafenac and 61 [95%] in placebo), 57% and 62% of participants in the nepafenac and placebo groups, respectively, returned all bottles for compliance assessment during the study. Among the participants who did not return all bottles, the mean number of missing bottles was 3.0±2.6 and 3.8±4.0 bottles in the nepafenac and placebo groups, respectively, through the 12-month visit out of a mean total of approximately 19±3 bottles dispensed to each participant. Among participants with no missing bottles (33 in nepafenac, and 38 in placebo group), no participant had a cumulative final weight of the bottles that was less than 80% of the expected weight given the participant's duration in the study.
OCT Outcomes
The 12-month changes in retinal volume from baseline (adjusted for baseline value) was -0.03 mm3 (95% CI: -0.21 to 0.14) and 0.02 mm3 (95% CI: -0.19 to 0.16) in the nepafenac and placebo groups respectively. The 12-month treatment group difference was -0.02 mm3, 95% CI: -0.27 to 0.23, P = 0.89 (Table 2, Figure 3), and 0.004 mm3 [95% CI: -0.25 to 0.26] after adjusting for baseline lens status; results were similar when adjusting for duration of diabetes and HbA1c level. In the subgroup of eyes that returned all bottles dispensed (n = 33 for nepafenac and 38 for placebo), the 12-month mean change in retinal volume adjusted for baseline was −0.23 mm3 (95% CI: −0.43 to 0.03 mm3) in nepafenac group and −0.05 mm3 (95% CI: −0.24 to 0.14 mm3) in placebo group, with difference of −0.18 (95% CI: −0.46 to 0.10 mm3: P = 0.20). Treatment group differences among the other OCT outcomes (Table 2) could not be identified. Seven eyes (11%, 95% CI: 5% to 22%) in the nepafenac group and 9 eyes (14%, 95% CI: 7% to 25%) in the placebo group developed central-involved DME on OCT, defined as central subfield thickness at or more than the gender and OCT machine-specific average thickness from a normal cohort + 2 standard deviations with at least 10% increase from baseline (P = 0.79). In the nepafenac treated eyes, 6 of 40 (15%) phakic and 1 of 21 (5%) of the pseudophakic eyes developed central-involved DME versus 7 of 53 (13%) phakic and 2 of 11 (18%) pseudophakic eyes in the placebo group (P = 0.25 for interaction).
Table 2. OCT outcomes at 12-month visit (last observation carried forward).
| Nepafenac | Placebo | |
|---|---|---|
| N=61 | N=64 | |
| Retinal Volume (Primary Outcome)§ | ||
| At Baseline* | ||
| Median (25th, 75th Percentile) | 7.82 (7.36, 8.19) | 7.74 (7.45, 8.03) |
| Mean ±SD | 7.87±0.64 | 7.70± 0.43 |
| At 12-month Visit, mm3* | ||
| Median (25th, 75th Percentile) | 7.72 (7.28, 8.33) | 7.63 (7.18, 8.10) |
| Mean ±SD | 7.85±0.99 | 7.72±0.82 |
| Change from Baseline, mm3 | ||
| Median (25th, 75th Percentile) | -0.10 (-0.27, 0.10) | -0.10 (-0.30, 0.12) |
| Mean ±SD | -0.03±0.74 | -0.02±0.67 |
| Mean change from baseline adjusted for baseline value, estimate (95%CI), mm3 | -0.03 (-0.21 to 0.14) | -0.02 (-0.19 to 0.16) |
| Difference in mean change from baseline, adjusted for baseline value, estimate (95%CI) | -0.02 (-0.27 to 0.23) | |
| P value | 0.89 | |
| OCT Central Subfield¶ | ||
| At Baseline, μm* | ||
| Median (25th, 75th Percentile) | 228 (213, 242) | 215 (205, 236) |
| Mean ±SD | 227±29 | 218±25 |
| At 12-month Visit, μm* | ||
| Median (25th, 75th Percentile) | 230 (210, 244) | 219 (196, 243) |
| Mean ±SD | 236±55 | 226±45 |
| Change from Baseline, μm** | ||
| Median (25th, 75th Percentile) | 0 (-10, 9) | -1 (-11, 8) |
| Mean ±SD | 9±45 | 7±35 |
| Mean change from baseline adjusted for baseline value, estimate (95%CI), μm** | 9 (-1 to 19) | 7 (-3 to 17) |
| Difference in mean change from baseline, adjusted for baseline value, estimate (95%CI) | 2 (-13 to 16) | |
| P value | 0.81 | |
| ≥ 1 log OCT step increase (worsen), N (%)** | 4 (7%) | 5 (8%) |
| ≥ 1 log OCT step decrease (improve), N (%)** | 0 (0%) | 1 (2%) |
| Central-involved DME, N (%)**† | 7 (11%) | 9 (14%) |
| Change in number of thickened subfields, Median (Min, Max)** | 0 (-5, 4) | 0 (-3, 4) |
| 12-month change in thickness of the non-central subfield with maximum thickness at baseline, mean (95%CI), μm** | -1 (-10 to 8) | -3 (-15 to 8) |
Values are converted to time-domain equivalent values
Change values are not converted to time-domain equivalent values since they occurred within the same machine within subjects
Original 12-month values are not available in 6 and 9 eyes of nepafenac and placebo groups respectively because 12-month visit was not completed or non-gradable OCT value and were imputed from the last available measurement; values at or before DME treatment were carried forward in 5 and 3 eyes of nepafenac and placebo groups respectively.
Original 12-month values are not available in 4 eyes of each of nepafenac and placebo groups because 12-month visit was not completed or non-gradable OCT value and were imputed from the last available measurement; values at or before DME treatment were carried forward in 5 and 3 eyes of nepafenac and placebo groups respectively.
Define as CSF thickness at or more than the gender and OCT machine-specific average thickness from a normal cohort + 2 standard deviations, with at least 10% increase in thickness from baseline.
Figure 3.
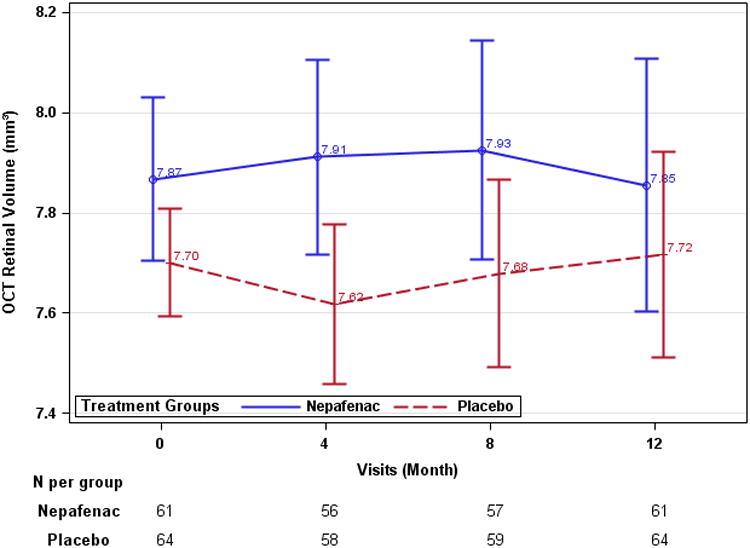
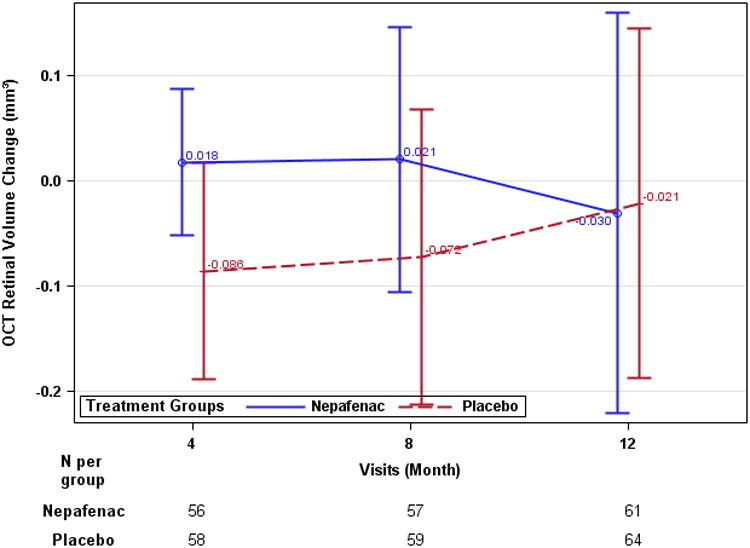
Optical Coherence Tomography retinal volume data (last observation carried forward). A: mean time-domain-equivalent retinal volume (mm3). B: mean retinal volume change from baseline (mm3). Error bars represent 95% confidence limit.
DME Treatment
Prior to the 12-month visit, 5 eyes (8%) in the nepafenac group and 3 (5%) in the placebo group received DME treatment (P = 0.48). Of eyes that received DME treatment, 3 in the nepafenac group, and 1 in placebo group did not meet the protocol criteria for DME treatment, but received treatment after discussions with the Protocol Chair. All other eyes that received DME treatment met the protocol-defined criteria for treatment. At the 12-month visit following the outcome assessments, an additional 5 eyes in each of the nepafenac and placebo groups, received DME treatment.
Visual Acuity
At the 12-month visit, the mean E-ETDRS letter scores were 82±7 (∼20/25 Snellen equivalent) and 83±8 (∼20/25 Snellen equivalent) in the nepafenac and placebo groups, respectively. The 12-month change in E-ETDRS letter scores from baseline, adjusted for baseline values were 0.09 and -0.15 in the nepafenac and placebo groups, respectively, for a treatment group difference of 0.2 letters (95% CI: -1.8 to 2.3 letter difference, P = 0.82). Three eyes (5%) in nepafenac and 2 eyes (3%) in placebo group lost ≥10 letters from baseline (Table 3).
Table 3. Visual Acuity at 12-month visit (Last Observation Carried Forward).
| Nepafenac | Placebo | |
|---|---|---|
| Visual Acuity¶ | N=61 | N=64 |
| At Baseline | ||
| Median (25th, 75th Percentile) | 82 (78, 86) | 84 (78, 89) |
| Mean ±SD | 82±6 | 83±7 |
| At 12-Month Visit | ||
| Median (25th, 75th Percentile) | 84 (78, 87) | 85 (78, 88) |
| Mean ±SD | 82±7 | 83±8 |
| Change from Baseline | ||
| Median (25th, 75th Percentile) | 0 (-3 to 3) | 0 (-3, 3) |
| Mean ±SD | 0.2±5.7 | -0.3±6.2 |
| Mean change in letter score from baseline adjusted for baseline value, estimate (95%CI) | 0.09 (-1.4 to 1.6) | -0.15 (-1.6 to 1.3) |
| Difference in mean change from baseline, adjusted for baseline value, estimate (95%CI) | 0.2 (-1.8 to 2.3) | |
| P value | 0.82 | |
| Change from Baseline, n (%) | ||
| ≥ 10 letters improve | 4 (7%) | 1 (2%) |
| 5-9 letters improve | 7 (11%) | 5 (8%) |
| Same ±4 letters | 40 (66%) | 46 (72%) |
| 5-9 letters worse | 7 (11%) | 10 (16%) |
| 14-10 letters worse | 2 (3%) | 1 (2%) |
| ≥ 15 letters worse | 1 (2%) | 1 (2%) |
Original 12-month values are not available in 4 eyes of each of nepafenac and placebo groups because 12-month visit was not completed and were imputed from the last available measurement; values at or before first DME treatment were carried forward in 5 and 3 eyes of nepafenac and placebo groups respectively.
Safety Outcomes
All reported ocular adverse events in the study eyes are listed in Table 4. One case of corneal melt occurred in the non-study eye of a participant who used the study drug (nepafenac) in that eye. The affected non-study eye had a pre-existing history of severe dry eye. Following treatment with a tarsorrhaphy and topical antibiotics, the melt resolved with a residual corneal scar. Other corneal complications reported included superficial keratitis in one eye in each of the treatment groups.
Table 4. All reported adverse events in study eyes.
| Ocular Adverse Events | Nepafenac N=61 | Placebo N=64 |
|---|---|---|
| N % | N % | |
| Blurred vision | 4 (7%) | 5 (8%) |
| Floaters | 3 (5%) | 4 (6%) |
| Eye itching | 0 (0%) | 3 (5%) |
| Proliferative diabetic retinopathy | 0 (0%) | 3 (5%) |
| Vision decreased | 3 (5%) | 3 (5%) |
| Burning eyes | 2 (3%) | 0 (0%) |
| Cataract | 2 (3%) | 2 (3%) |
| Conjunctivitis | 0 (0%) | 2 (3%) |
| Eye discharge | 2 (3%) | 0 (0%) |
| Eye pain | 0 (0%) | 2 (3%) |
| Visual acuity decreased | 2 (3%) | 1 (2%) |
| Watering eyes | 0 (0%) | 2 (3%) |
| Allergic conjunctivitis | 0 (0%) | 1 (2%) |
| Basal cell carcinoma | 0 (0%) | 1 (2%) |
| Blepharitis (eyelid irritation) | 1 (2%) | 1 (2%) |
| Cataract extraction | 0 (0%) | 1 (2%) |
| Chemical conjunctivitis | 0 (0%) | 1 (2%) |
| Corneal defect | 1 (2%) | 0 (0%) |
| Dot hemorrhages | 0 (0%) | 1 (2%) |
| Double vision | 1 (2%) | 0 (0%) |
| Eye ache | 1 (2%) | 1 (2%) |
| Eye irritation | 1 (2%) | 1 (2%) |
| Eye tearing | 1 (2%) | 0 (0%) |
| Eyelid disorder | 1 (2%) | 0 (0%) |
| Eyelid pain | 1 (2%) | 0 (0%) |
| Foreign body sensation in eyes | 0 (0%) | 1 (2%) |
| Glare | 1 (2%) | 1 (2%) |
| IVth nerve palsy | 1 (2%) | 0 (0%) |
| Iritis (anterior uveitis, iridocyclitis) | 0 (0%) | 1 (2%) |
| Itching | 1 (2%) | 0 (0%) |
| Mucus in eyes | 0 (0%) | 1 (2%) |
| Neovascularization | 0 (0%) | 1 (2%) |
| Nuclear sclerosis | 0 (0%) | 1 (2%) |
| Posterior vitreous detachment | 0 (0%) | 1 (2%) |
| Preretinal hemorrhage | 1 (2%) | 0 (0%) |
| Pseudoexfoliation of lens capsule | 0 (0%) | 1 (2%) |
| Ptosis | 1 (2%) | 1 (2%) |
| Sensitivity to light (photophobia) | 0 (0%) | 1 (2%) |
| Spots before eyes | 0 (0%) | 1 (2%) |
| Subconjunctival hemorrhage | 0 (0%) | 1 (2%) |
| Subconjunctival/conjunctival hemorrhage | 0 (0%) | 1 (2%) |
| Superficial punctate keratitis | 1 (2%) | 1 (2%) |
| Viral conjunctivitis | 0 (0%) | 1 (2%) |
| Visual field defect | 1 (2%) | 0 (0%) |
| Vitreous floater | 1 (2%) | 0 (0%) |
The mean IOP at 12 months was similar between the two treatment groups (16±3 and 15±3 mmHg in nepafenac and placebo groups, respectively). No eyes in either group had an increase in intraocular pressure of 10 mmHg or more nor an IOP of 30 mmHg or more. There were no differences in systemic adverse events between the two treatment groups that could not be attributed to chance.
Discussion
This placebo-controlled randomized trial of 125 eyes with non-center involved DME found no evidence of a beneficial effect of 12 months of topical nepafenac 0.1% on retinal thickening based on OCT or visual acuity outcomes. This is in contrast to recent reports indicating a beneficial effect of nepafenac 0.1% on macular edema in patients with diabetes, but presumably from a different mechanism, specifically macular edema in the setting of cataract surgery.16, 17 The results of this DRCR.net study also are not consistent with a recent report by Singh et al in which nepafenac compared with placebo showed a beneficial effect in preventing the development of macular edema in eyes of persons with diabetes who were undergoing cataract surgery.18 However, it is unknown whether the beneficial effect seen in that study was attributable to post-surgical macular edema or on DME. The effect of NSAIDs on DME remains controversial among the ophthalmic community,10 but the data from this DRCR.net study suggest that any beneficial effect of nepafanec on macular edema in patients with diabetes may be limited to a beneficial effect on macular edema associated with recent cataract surgery rather than DME.
Although this study was not powered to assess safety, nepafenac treatment appeared to be well-tolerated in the study eyes that were carefully screened for study enrollment and monitored for risks, with no difference in the reported adverse effects in study eyes between the two treatment groups. The one case of corneal melt in this study occurred in a non-study eye treated with the study-supplied nepafenac, which was not intended for the non-study eye, and was applied by the participant to the non-study eye that had a pre-existing history of severe dry eye. Nevertheless, to our knowledge, this study is the first to compare the effect of a topical NSAID with that of a placebo drop when given for 12 months within a protocol in which study participants were monitored carefully for adverse effects.
Optical coherence tomography measured retinal volume was selected as the primary outcome for this phase 2 study because it provides a global assessment of the macula accounting for both potential increase and decrease in retinal thickening throughout the macula. Based on prior studies using fundus photographs,4, 5 development of central DME among eyes with untreated non-central DME was projected to be 22% to 33%.5 Our study found an OCT-based progression rate of non-central to central-involved DME of 14% (95% CI: 7% to 25%) in the placebo group, without a meaningful reduction in the progression rate in the nepafenac group. Prior to this study, limited data were available among similar cohorts to assess expected changes in retinal volume over time. Ultimately the amount of increase in retinal volume that occurred in the placebo group over 12 months was small. Thus, there was little opportunity to show a benefit of nepafenac treatment in terms of an effect on increasing retinal volume. However, the mean baseline retinal volume of 7.8 mm3 is higher than the average retinal volume of 6.8 mm3 in persons with diabetes but without diabetic retinopathy, indicating a reduction in retinal volume from nepafenac was potentially possible.19
As with any topical eye drop treatment study, medication adherence by the participant can affect the outcome. However, this is inherent with this type of treatment and we do not believe that additional methods to try to enhance adherence would have altered the results because the conclusions were not altered when limiting analyses to the participants who returned all the dispensed bottles and, according to bottle weight, received at least 80% of the targeted study drug dosing.
The results of this study directly apply only to eyes with non-center involved DME and may not be similar in other presentations of DME. In particular, the results of this study do not address whether topical nepafenac may be beneficial in reducing macular edema following cataract surgery among individuals with diabetes. In addition, the lack of benefit found with topical nepafenac does not preclude the possibility of benefit from other delivery methods of non-steroidal anti-inflammatory drugs, such as intravitreous injection.20 Nevertheless, the results of this study in eyes with non-central DME and good visual acuity suggest that topical nepafenac 0.1% three times a day for 1 year does not have a meaningful effect on OCT-measured retinal thickness.
Acknowledgments
Financial Support: Supported through cooperative agreements from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY18817, EY023207
Appendix
Diabetic Retinopathy Clinical Research Network Clinical Sites that participated on this protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Study Investigator, (C) for Coordinator, (v) Visual Acuity Technician, and (P) for Photographer.
Lakeland, FL Florida Retina Consultants (13): Scott M. Friedman(I); Katrina L. Dawson(C); Damanda F. Fagan (C,V); Karen Sjoblom (C,V); Kimberly A. Williamson (C,V); Jessica Maldonado(C); Paige N. Walters(V); Steve Carlton(P); Allen McKinney(P) Boston, MA Joslin Diabetes Center (11): George S. Sharuk(I); Paolo S. Silva(I); Christopher Michael Andreoli(I); Lloyd Paul Aiello(I); Sabera T. Shah(I); Jennifer K. Sun(I); Margaret E. Stockman (C,V); Troy Kieser (C,V); Hanna Kwak(C); Elizabeth S. Weimann(V); Leila Bestourous(V); Rita K. Kirby(V); John Head(P); Kate A. Palitsch(P); Robert W. Cavicchi(P) Baltimore, MD Elman Retina Group, P.A. (9): Michael J. Elman(I); JoAnn Starr(C); Charlene K. Putzulo(C); Jennifer L. Belz(C); Dena Y. Salfer-Firestone(V); Pamela V. Singletary(V); Ashley Davis(V); Teresa Coffey(V); Daniel J. Ketner(P); Terri Cain(P); Peter Sotirakos(P) Paducah, KY Paducah Retinal Center (8): Carl W. Baker(I); Tracey M. Caldwell(C); Lynnette F. Lambert (C,V); Tracey R. Martin(V); Mary J. Palmer(V); Tana R. Williams(P); Samantha Kettler(P) Plantation, FL Fort Lauderdale Eye Institute (8): Stuart K. Burgess(I); Tirso M. Lara(I); Cindy V. Fernandez (C,V); Deborah Davis(V); Evelyn Quinchia(V); Karen Workman(P) Dubuque, IA Medical Associates Clinic, P.C. (6): Michael H. Scott(I); Philomina M. Ozpirincci(C); Shannon R. Walsh(C); Matthew Arensdorff(V); Marcia J. Moyle(P); Brenda L. Tebon(P) Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (6): Joseph T. Fan(I); Mukesh Bhogilal Suthar(I); Michael E. Rauser(I); Kara E. Halsey (C,V); Brandi J Perez (C,V); Rene G. Obispo(P); Jesse Knabb(P); Diana Povero(P) Glenview, IL North Shore University Health System (5): Manvi P. Maker(I); Veeral S. Sheth(I); Mira Shiloach(C); Courtney Kischuk (C,V); Qin Zhou (C,V); Nicole Pelkofer(P) Knoxville, TN Southeastern Retina Associates, P.C. (5): Joseph M. Googe(I); Kristina Oliver(C); Jennifer Beerbower(V); Kathy L. Schulz(V); Ann Arnold(V); Cecile Hunt(V); Nicole Grindall(V); Jerry K. Whetstone(P); Chris Morris(P); Sarah M. Oelrich(P) Oklahoma City, OK Dean A. McGee Eye Institute (5): Ronald M. Kingsley(I); Reshial D. Ellis (C,V); Sonny Icks (C,V); Brittany L Ross (C,V); Vanessa A. Bergman (C,V); Amanda M Butt(P); Russ Burris(P) Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (4): Justin C. Brown(I); Andrew N. Antoszyk(I); Ashley A. McClain (C,V); Jenna T. Herby (C,V); Merri F Walker (C,V); Sarah A. Ennis(V); Angella S. Karow(V); Swann J Bojaj(P); Michael D. McOwen(P); Lynn Watson(P); Loraine M. Clark(P); Donna R. Styles(P); Donna McClain(P); Susannah J Held(P); Uma M. Balasubramaniam(P); Lisa A. Jackson(P) Indianapolis, IN Raj K. Maturi, M.D., P.C. (3): Raj K. Maturi(I); Laura A. Bleau (C,V); Ashley M. Harless(C); Charlotte Harris(P); Thomas Steele(P); Kerri A Wellman(P); Abby Maple(P) Seattle, WA University of Washington Medical Center (3): James L. Kinyoun(I); Susan A. Rath (C,V); Brad C. Clifton(P); James D. Leslie(P) Syracuse, NY Retina-Vitreous Surgeons of Central New York, PC (3): G. Robert Hampton(I); Jamin S. Brown(I); Cindy J. Grinnell(C); Lynn M. Kwasniewski(V); Michelle L. Manley(V); Lynn A. Roderick(P); Nicole E. Robarge(P); Peter B. Hay(P); Teresa M. DeForge(P) Augusta, GA Southeast Retina Center, P.C. (2): Dennis M. Marcus(I); Geri L Floyd(C); Siobhan O. Ortiz(C); L. Allison Foster(V); Virginia Mims(V); Ken Ivey(P); Jared C. Gardner(P) Harinderjit Singh, MD (SI) Baltimore, MD Wilmer Eye Institute at Johns Hopkins (2): Sharon D. Solomon(I); Adrienne Williams Scott(I); Deborah Donohue (C,V); Mary Frey (C,V); Janis Graul(P); David Emmert(P); Charles Herring(P) Chicago, IL Northwestern Medical Faculty Foundation (2): Alice T. Lyon(I); Rukhsana G. Mirza(I); Lori Kaminski (C,V); Anna Liza M. Castro-Malek(C); Jeremy A. Chapman(C); Zuzanna Rozenbajgier(V); Evica Simjanoski(P) Lexington, KY Retina Associates of Kentucky (2): Thomas W. Stone(I); William J. Wood(I); Rick D. Isernhagen(I); John W. Kitchens(I); Diana M. Holcomb (C,V); Jenny L. Wolfe (C,V); Jeanne Van Arsdall(V); Michelle Buck(P); Edward A Slade(P) Milwaukee, WI Medical College of Wisconsin (2): Judy E. Kim(I); David V. Weinberg(I); Dennis P. Han(I); Krissa L. Packard(C); Vesper V. Williams(C); Judy Flanders(V); Vicki Barwick(V); Joseph R. Beringer(P); Dennis B. Backes(P); Kathy J. Selchert(P) OMAHA, NE University of Nebraska Medical Center, Department of Ophthalmology (2): Eyal Margalit(I); Thomas W. Hejkal(I); Donna G. Neely(C); Maria Blaiotta(V); Lori Hagensen(P) Palm Desert, CA Southern California Desert Retina Consultants, MC (2): Clement K. Chan(I); Steven G Lin(I); Kimberly S. alther(C); Tiana Gonzales(C); Lenise E. Myers(V); Kenneth M. Huff(P) Pittsburgh, PA Retina Vitreous Consultants (2): Karl R. Olsen(I); Pamela P. Rath(I); Judy C Liu(I); Lori A. Merlotti(C); Holly M. Mechling(C); Kellianne Marfisi(V); Veronica L. Bennett(V); Christina R. Fulwylie(V); Kimberly A. Yeckel(V); Amanda Fec(P); David Steinberg(P); Keith D McBroom(P) Portland, OR Retina Northwest, PC (2): Mark A. Peters(I); Colin Ma(I); Stephen Hobbs (C,V); Amanda C. Milliron (C,V); Christine Hoerner(P) West Des Moines, IA Wolfe Eye Clinic (2): Kyle J. Alliman(I); Jared S. Nielsen(I); Marianne Parker(C); Jennifer L. Coleman(V); Jamie Spillman(V); Marilyn A. Johnson(V); Jay Rostvold(P); Leigh S Schmidt(P); Bailey R. Bennett(P) Beachwood, OH Retina Associates of Cleveland, Inc. (1): Lawrence J. Singerman(I); Joseph M. Coney(I); Larraine Stone(C); Mary A Ilc(V); Vivian Tanner(V); John C. DuBois(P) Detroit, MI Henry Ford Health System, Dept of Ophthalmology and Eye Care Services (1): Paul Andrew Edwards(I); Janet Murphy (C,V); Julianne Hall (C,V); Tracy A. Troszak(P); Brian A. Rusinek(P); Steven F. Ogilvy(P); David Burley(P) Grand Rapids, MI Retina Specialists of Michigan (1): Thomas M. Aaberg(I); Scott J. Westhouse(I); Holly L. Vincent (C,V); Rebecca Malone(V); Kathy L. Karsten(P) Honolulu, HI Retina Associates of Hawaii, Inc. (1): John H. Drouilhet(I); Erica N. Lacaden(C); Deborah J. Nobler (C,V); Sarah G. Raybin(C) Joliet, IL Illinois Retina Associates (1): John S. Pollack(I); Barbara J. Ciscato(C); Belinda M. Kosinski(V); Daniel W. Muir(P) Kingsport, TN Southeastern Retina Associates, PC (1): Howard L. Cummings(I); Deanna Jo Long(C); Stacy Carpenter(V); Norma Mullins(P); Julie P. Maynard(P) Lincoln, NE Eye Surgical Associates (1): Daniel A. Chruscicki(I); Jody C. Hemberger(C); Suzanne M. Abele(C); Mary J. Judd(V); Gail Walker(V); Dana Meves(V); Sonya E. Gilliam(P); Mary Ann Kramer(P) Little Rock, AR Jones Eye Institute/University of Arkansas for Medical Sciences (1): Sami Hilmi Uwaydat(I); Deborah L. Troillett (C,V); Karin Aletter(P) McAllen, TX Valley Retina Institute (1): Victor Hugo Gonzalez(I); Deyla Anaya(C); Monica R. Cantu(V); Rachel Rodriguez(V); Hector Jasso(V); Karina Miranda(V); Santos Garza(P) Minneapolis, MN University of Minnesota (1): Dara Koozekanani(I); Wendy A. Elasky(C); Helen F. Roemhild(C); Sabrina M. Rolfer(V); Pamela K. Patterson(V); Pat Stanaitis Harvey(P); Mark J. Cohen(P) New Albany, IN John-Kenyon American Eye Institute (1): Howard S. Lazarus(I); Liana C. Davis(C); Debra Paige Bunch (C,V); Kelly Booth(V); Jay Moore(P); Margaret Trimble(P) Philadelphia, PA University of Pennsylvania Scheie Eye Institute (1): Alexander J. Brucker(I); Sheri Drossner (C,V); Beth Serpentine(P); Jim M. Berger(P); Laurel Weeney(P); Joan Dupont (C,V) Santa Barbara, CA California Retina Consultants (1): Dante J. Pieramici(I); Alessandro A. Castellarin(I); Jack Giust(C); Lisha Wan(C); Jerry Smith(V); Matthew Giust(P) Tampa, FL Retina Associates of Florida, P.A. (1): Ivan J. Suner(I); Marc C. Peden(I); Mark E. Hammer(I); Janet R. Traynom(C); Susan Ramsey(V); Anita Kim Malzahn(P) Trumbull, CT New England Retina Associates, PC (1): “Nauman A. Chaudhry(I); Gregory M. Haffner(I); Sumit P. Shah(I); Laura A. Fox (C,V); Alison Fontecchio(V); Marie Grace Laglivia(P); Emily Morse(P); Greg McNamara(P) Westlake Village, CA Retinal Consultants of Southern California Medical Group, Inc. (1): Kenneth R. Diddie(I); Deborah M. Cadwell(C); Louise Van Arsdale(V); Melissa L. Johnson(P); Joyce Galonsky(P)
DRCR.net Coordinating Center: Jaeb Center for Health Research, Tampa, FL (staff as of 05/23/14): Adam R. Glassman (Director and Principal Investigator), Roy W. Beck (Executive Director) Talat Almukhtar, Bambi J. Arnold-Bush, Brian B. Dale, Alyssa Baptista, Jasmine Conner, Sharon R. Constantine, Simone S. Dupre, Allison R. Ayala, Meagan L. Huggins, Paula A. Johnson, Brenda L. Loggins, Shannon L. McClellan, Michele Melia, Haijing Qin, Allam Sanchez, Eureca Scott, Cynthia R. Stockdale, Danielle Wales
Duke Reading Center: Durham, North Carolina: Beth Oakley, Brannon Balsley, John Choong, Cynthia A. Toth, MD, Cynthia Heydary, Garrett Thompson, Glenn J. Jaffe, MD, Katrina Winter, Kelly Shields, Russell Burns.
Fundus Photograph Reading Center: University of Wisconsin-Madison, Madison, WI (staff as of 5/23/14): Matthew D. Davis (Director Emeritus), Yijun Huang (Co-Director), Ronald P. Danis (Director and Principal Investigator), Amitha Domalpally (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Hugh Wabers (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Daniel Lawrence (Project Manager), James Allan (Data Management)
DRCR.net Operations Center: Johns Hopkins University School of Medicine, Baltimore, MD (staff as of 05/23/14): Neil M. Bressler (Past Network Chair and Principal Investigator), Connie Lawson, Peggy R. Orr, Beth Wellman.
DRCR.net Network Chair: Lee M. Jampol (2013-present)
DRCR.net Vice Chairs: Andrew Antoszyk (2013 – present), Carl W. Baker (2011-2013), Jennifer K. Sun (2012-present), John A. Wells (2013- present), Scott Friedman (2009-2012), Susan B. Bressler (2009-2011), Ingrid U. Scott (2009-2010).
National Eye Institute: Eleanor Schron (2009-current), Donald F. Everett (2003-2006, 2007-2009), Päivi H. Miskala (2006-2007)
Executive Committee: Lloyd Paul Aiello (2002-present; Chair 2002 – 2005), Andrew N. Antoszyk (2009; 2014 - present), Carl W. Baker (2009-present), Roy W. Beck (2002-present), Neil M. Bressler (2006-present; Chair 2006 - 2008), Susan B. Bressler (2009-Present), Ronald P. Danis (2004-present), Matthew D. Davis (2002-present), Michael J. Elman (2006-present; Chair 2009 and 2012), Frederick L. Ferris III (2002-present), Adam R. Glassman (2005-present), Jeffrey G. Gross (2012-present), Glenn J. Jaffe (2012-present), Lee M. Jampol (2012-present; Chair 2013-present), Raj K. Maturi (2009-2011, 2013- Present), Eleanor Schron (2009-present), Jennifer K. Sun (2009-present), John A. Wells (2012-present). Prior Members: Abdhish Bhavsar (2007-2008, 2010-2012), Alexander J. Brucker (2009-2011), Kakarla V. Chalam (2009-2011), Donald F. Everett (2002-2009), Joan Fish (2008 - 2009), Scott Friedman (2007 –2013), Joseph Googe, Jr. (2009-2011), Diana M. Holcomb (2011-2012), Andreas Lauer (2007-2008), Dennis M. Marcus (2011-2012), Ashley McClain (2013); Brandi J. Perez (2013), Ingrid U. Scott (2009-2010), JoAnn Starr (2009-2011).
Footnotes
Financial Disclosures: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript. Alcon provided the nepafenac and placebo (nepafenac vehicle) for the study. In addition, funds to DRCR.net to defray the study's clinical site costs, were provided by Alcon Inc.. As described in the Diabetic Retinopathy Clinical Research Network (DRCR.net) Industry Collaboration Guidelines (available at http://www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Writing Committee financial disclosures: Dr. Neil Bressler is principal investigator of grants at The Johns Hopkins University sponsored by the Bayer; Genentech, Inc, Novartis Pharma AG, Regeneron, and The Emmes Corporation through the Office of Research Administration of the Johns Hopkins University School of Medicine, and has a contract agreement from the American Medical Association to the Johns Hopkins University School of Medicine.
References
- 1.Bhagat N, Grigorian RA, Tutela A, et al. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo JF. Diabetic macular edema: Current management 2013. World J Diabetes. 2013;4:231–3. doi: 10.4239/wjd.v4.i6.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Diabetes Fact Sheet. Available at: http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 4.PKC-DMES Study Group. Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318–24. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 6.AAO. [Accessed: 27 February 2014];Preferred Practice Patterns: Diabetic retinopathy. Available at: http://one.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp--september-2008-4th-print.
- 7.Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–6. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 8.Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–9. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Kapin MA, Yanni JM, Brady MT, et al. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation. 2003;27:281–91. doi: 10.1023/a:1026024409826. [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–33. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Callanan D, Williams P. Topical nepafenac in the treatment of diabetic macular edema. Clinical Ophthalmology. 2008;2:689–92. doi: 10.2147/opth.s3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindstrom R, Kim T. Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac. Curr Med Res Opin. 2006;22:397–404. doi: 10.1185/030079906X89775. [DOI] [PubMed] [Google Scholar]
- 13.Nevanac. [Accessed: 27 February 2014];EPAR summary for the public. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000818/human_med_000928.jsp&mid=WC0b01ac058001d124.
- 14.Chalam KV, Bressler SB, Edwards AR, et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:8154–61. doi: 10.1167/iovs.12-10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetic Retinopathy Clincical Research Network. Reproducibility of spectral domain optical coherence tomography retinal thickness measurements and conversions to equivalent time domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.1698. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida DR, Khan Z, Xing L, et al. Prophylactic nepafenac and ketorolac versus placebo in preventing postoperative macular edema after uneventful phacoemulsification. J Cataract Refract Surg. 2012;38:1537–43. doi: 10.1016/j.jcrs.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Miyake K, Ota I, Miyake G, et al. Nepafenac 0.1% versus fluorometholone 0. 1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1581–8. doi: 10.1016/j.jcrs.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Alpern L, Jaffe GJ, et al. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clin Ophthalmol. 2012;6:1259–69. doi: 10.2147/OPTH.S31902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bressler NM, Edwards AR, Antoszyk AN, et al. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145:894–901. doi: 10.1016/j.ajo.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado RM, Vianna RN, Cardoso GP, et al. Intravitreal injection of commercially available ketorolac tromethamine in eyes with diabetic macular edema refractory to laser photocoagulation. Curr Eye Res. 2011;36:768–73. doi: 10.3109/02713683.2011.585734. [DOI] [PubMed] [Google Scholar]


