Abstract
Lysophosphatidic acid (LPA) is a lipid mediator that modulates a wide variety of cellular functions. Elevated LPA signaling has been reported in patients with colorectal cancer or inflammatory bowel diseases, and the tumorigenic role of LPA has been demonstrated in experimental models of colon cancer. However, emerging evidence indicates the importance of LPA signaling in epithelial wound healing and regulation of intestinal electrolyte transport. Here, we briefly review current knowledge of the biological roles of LPA signalling in the intestinal tract.
Keywords: lysophosphatidic acid, colon cancer, inflammation, intestine
Introduction
The surface of the intestinal tract is lined with a layer of simple columnar epithelial cells. The surface of the small intestine is structurally divided into villus and crypt. The villus increases the surface area for absorption that is carried out by fully differentiated enterocytes. The crypt harbors stem cells and progenitors cells that regenerate the entire population of the intestinal epithelium every three to five days. In addition to carrying out digestion of food and absorption of nutrients, intestinal epithelial cells (IECs) form the first line of defense by separating the body from the lumen of the gut. The hostile luminal microenvironment damages the epithelial barrier that compromises the mucosal innate immunity that can lead to the pathologic conditions, such as inflammatory bowel diseases (IBD), infectious enterocolitis, and colorectal cancer (CRC) [1].
The integrity of intestinal epithelial cells is modulated by several factors that present within the intestinal lumen or the underling submucosa. These include growth factors (transforming growth factor-β, epidermal growth factor, platelet-derived growth factor, and vascular endothelial growth factor), regulatory peptides (trefoil and glucagon-like peptide-2), and non-peptide regulators (polyamine, adenine nucleotide, and glutamine) [1]. Damage to the intestinal surface and the breakdown of cell membrane lipid complexes lead to generation of eicosanoids, such as prostaglandins, thromoboxane, and leukotrienes, which are closely linked to pro-inflammatory responses, bacterial translocation, vasoconstriction, and cell survival [2]. LPA is a pleiotropic lipid molecule with potent effects on cell growth, motility, and inflammatory responses. Studies links LPA to inflammation and cancer, but emerging evidence indicates the roles of LPA in regulation of physiological functions in the gut.
LPA receptor expression in the intestine
During cell injuries and inflammation, LPA is produced by the activated platelets, fibroblasts and even by the injured epithelial cells. The majority of extracellular LPA is thought to be generated by at least two pathways. First involves hydrolysis of the fatty acid moiety from the membrane derived phosphatidic acid (PA) by phospholipase A1 (PLA1) and phospholipase A2 (PLA2). Another pathway requires the removal of choline moiety from lysophosphatidylcholine (LPC) by lysophospholipase D known as autotaxin (ATX) [3]. In addition to the cellular generation, LPA is present in significant amounts in several types of foodstuffs, including egg, soybean, and cabbage leaves [4]. Interestingly, egg yolk predominantly contains saturated LPA, whereas unsaturated LPA is the dominant LPA species in egg white [4]. Although the amounts of LPA in most of foodstuffs are not known, a recent study has shown the presence of PA in vegetables, such as cabbage leaves and Japanese radish leaves [5]. In an earlier study, the same group has shown that LPA is formed during mastication in the mouth by conversion of PA to LPA by PLA2 [6]. Because the gastrointestinal tract is the primary site of digestion, biological effects of food-borne LPA carry a great significance. It was shown that unsaturated fat-rich Western diet elevates unsaturated LPA levels without altering saturated LPA in the mouse small intestine that lacks low density lipid receptor [7]. This study potentially links Western diet to systemic inflammation and dyslipidemia via increased levels of unsaturated LPA.
LPA mediates its effects through a family of G protein-coupled receptors, LPA1–6 [8]. Multiple LPA receptors are expressed in the intestinal tract. The most abundant LPA receptors in mouse ileal and colonic epithelial cells are LPA1 and LPA5 based on quantitative RT-PCR [9]. The expression levels of LPA2, LPA3, and LPA4 are relatively low in mouse IECs. Similarly, normal intestinal epithelial cell lines such as rat IEC-6, YAMC (young adult mouse colonic epithelium), and MSIE (mouse small intestinal epithelium) cells express LPA1 at the highest level although expression of other LPA receptors varies depending on the cell lines [10]. Many of the colon cancer cell lines express elevated levels of LPA2, a trend often observed in other cancer cells [11]. LPA5 mRNA expression is abundant in freshly isolated IECs from mouse, but most of the cultured epithelial cells of intestine origin, including YAMC, MSIE, Caco-2, and HCT116, either lack LPA5 or express at a low level [9, 10].
The intestinal tract plays a critical role in immune system homeostasis. It was reported that LPA2 is expressed in human CD4+ T cells and CD19+ B cells, but not in CD8+ T cells [12]. A recent study showed that LPA5 is highly expressed in the intraepithelial lymphocytes of mouse intestine, with the highest in CD8+ T cells [13]. In addition, LPA5 is abundantly expressed in human mast cells [14].
Role of LPA in CRC
CRC results from the accumulation of multiple independent genetic instabilities and activation of oncogenic pathways that transform epithelial cells to cancerous cells [15]. In addition, growth factors, angiogenic factors, and motility factors that are produced by the tumor cells or surrounding environment play a critical role in malignant transformation. A body of evidence supports that LPA such a factor that stimulates proliferation, survival, and migration of malignant cells. ATX was originally identified as a motility factor from the culture supernatant of human melanoma cells [16]. Up-regulation of ATX in malignancies including breast ovarian, thyroid and lung cancer correlates with invasiveness and metastatic potential of cancer cells [8]. Similarly, ATX is highly expressed in infiltrating cells in human CRC tumor tissue in the submucosal layer. ATX expression shows a positive correlation with tumor angiogenesis in the early stage of CRC [17]. However, whether LPA levels are elevated in CRC patients is not known. Nevertheless, extracellular ATX enances locomotion of Caco-2 and MDCK cells [18], and the pan-antagonist of ATX and LPA receptor BrP-LPA is shown to be effective in limiting liver metastasis of HCT116 cells [19].
Among the LPA receptors, LPA2 provides a considerable pathophysiological relevance to cancer progression. The first observation of aberrant expression of LPA receptors in cancer came from the study by Goetzl et al. [20] that showed increased LPA2 transcript expression in ovarian cancer cells. Shida et al. [21] have shown elevated expression of LPA2 and concurrent decrease in LPA1 expression in CRC patients. The altered expression of LPA2 is a common occurrence in several colon cancer cell lines [11].
Much work has underscored the positive effect of LPA on cancer cell proliferation and migration. LPA promotes proliferation and migration of human colon cancer cells, including HCT116, LS174T, SW480, and LoVo, via LPA2 or LPA3 [22, 23]. Hepatic metastasis of colon cancer 26 cells by LPA is dependent on macrophage migration inhibitory factor (MIF) [24]. LPA also stimulates migration of SGC-7901 gastric cancer cells that predominantly express LPA2 [25]. Reduced expression of LPA1 in CRC patients and colon cancer cell lines [11, 21] appears to suggest that LPA1 has an anti-cancer role, but a line of evidence contradicts this. LPA1 stimulates colony scattering and migration of DLD1 cells [26, 27]. LPA also stimulates migration of LPA1-expressing NUGC-3 and MKN1 human gastric cancer cells in a Boyden chamber, but not in LPA2-expressing MKN28 and MKN74 cells [28]. Induction of vascular endothelial growth factor and IL-8 by LPA via LPA1-dependent pathways further suggests the potential contribution of LPA1 to cancer cell metastasis [11, 21]. However, no study has been performed to determine the role of LPA1 in tumor initiation and progression in vivo. Little is known about the role of LPA5. It was shown recently that LPA5 has an anti-migratory effect, inhibiting invasion of B16 melanoma cells across a Matrigel layer [29].
Colon cancer cell proliferation is achieved in part by activation of β-catenin, a co-activator of the TCF/LEF transcription factor in the Wnt pathway [22]. LPA activates β-catenin by inhibition of glycogen synthase kinase 3β [22]. Krüppel-like factor 5 (KLF5) is a transcription factor highly expressed in proliferation cells in the intestinal tract [30]. We have shown that KLF5 expression in colon cancer cells is induced by LPA via PKCδ- and MAPK-dependent pathways [23]. Silencing of either β-catenin or KLF5 blocked LPA-induced proliferation of HCT116, LS174T, and SW480 colon cancer cells, indicating their importance in cancer cell growth [22, 23]. It is not known whether β-catenin and KLF5 cooperatively or independently regulate cell proliferation, but KLF5 can physically interact with β-catenin in COS-1 cells to enhance the nuclear localization and transcriptional activity of β-catenin [31].
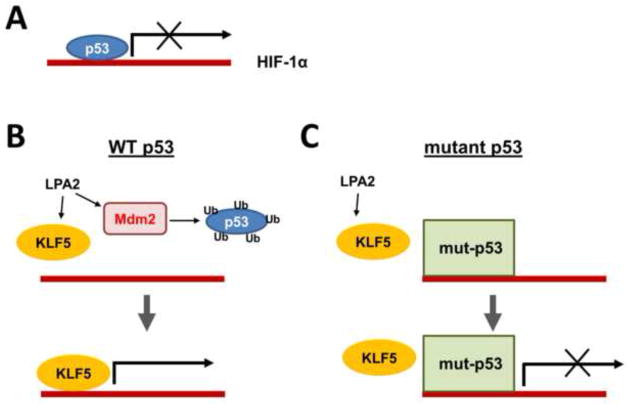
It has been shown that LPA suppresses p53 transcription in A549 lung carcinoma cells and protects the cells from actinomycin D-induced apoptosis [32]. Similarly, LPA induces the p53-specific ubiquitin ligase Mdm2, which suppresses p53 expression in colon cancer cells [33]. The transcription factor hypoxia-inducible factor 1 (HIF-1) is a pivotal regulator of cellular adaptation to hypoxia. In cancer cells, various growth factors, activated oncogenes, or loss-of-function mutations of tumor suppressor genes can induce HIF-1α expression under non-hypoxic conditions. We showed recently that the suppression of p53 is necessary, but not sufficient, for the induction of HIF-1α by LPA under non-hypoxic conditions. In addition to p53, HIF-1α expression requires KLF5, both of which bind to the Hif1α promoter. Hence, KLF5 transcriptionally regulates HIF-1α by displacing p53 from the hif1α promoter (Figure 1). However, mutant p53 is resistant to LPA-induced degradation and hence HIF-1α induction by LPA is limited to colon cancer cells harboring wild-type p53 [33]. Given that mutation in the p53 gene occurs at a late stage of colon tumor development, this finding implies that LPA may potentiate colon tumor progression by enhancing β-catenin activation while repressing the tumor suppressor function of p53.
Figure 1. Regulation of HIF-1α by LPA.
A. Under basal conditions, p53 functions as a negative regulator of Hif1α promoter. B. LPA stimulates Mdm2, which ubiquitinates and degrades p53. At the same time, LPA induces expression of KLF5 that transactivates Hif1α promoter and transcribes HIF1α mRNA. C. However, mutant p53 is resistant to LPA-mediated degradation of p53. Although KLF5 is induced by LPA in cells harboring mutant p53, KLF5 is unable to displace mutant p53 [33].
There are reports documenting the role of LPA in protection of IECs from radiation and chemotherapy-induced apoptosis. LPA prevents mitochondrial dependent apoptosis of IECs by inhibition of caspase-3, upregulation of Bcl-2 expression, and inhibition of apoptotic Bax and Bad [34, 35]. In addition, oral administration of a metabolically stable LPA analog protects enterocytes from γ-irradiation-induced apoptosis [34]. This protection is LPA2 dependent since LPA does not protect LPA2-null (Lpar2−/−) mice.
LPA2 contains a unique carboxyl terminal sequence that preferentially binds to Class I PDZ domains. Thus far, Na+/H+ exchanger regulatory factor 2 (NHERF2), membrane-associated guanylate kinase with inverted orientation-3 (MAGI-3), leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), and PDZ-RhoGEF are known to interact with LPA2 [8]. Cellular signaling and effects of LPA2 are modulated through the interaction with these PDZ proteins. NHERF2 facilitates activation of phospholipase C-β3 (PLC-β3) that activates COX-2, NF-κB, and JNK and promotes migration of colon cancer cells. On the other hand, the competitive binding of the LPA2 carboxyl terminus with MAGI-3 shows an anti-tumor effect by decreasing colon cancer cell invasion through the Matrigel layer [36].
The importance of LPA and LPA2 in CRC has been demonstrated in the rodent models of Apcmin and colitis-induced colon cancer [37, 38]. Oral administration of LPA for one month increased tumor incidence in Apcmin mice [38]. Similarly, weekly intraperitoneal injections of LPA for 30 weeks in the azoxymethane-induced rat model of adenocarcinoma significantly enhanced the development of pleural metastasis [39]. The loss of LPA2 (Lpar2−/−) in mice shows decreased tumor numbers and growth when exposed to a combination of azoxymethane and dextran sodium sulfate (DSS) or in the Apcmin background. In parallel, the expression of cyclooxygenase-2 (COX-2), HIF-1α, KLF5, MIF, and monocyte chemoattractant protein 1 is decreased in Lpar2−/− mice [37, 38].
Role of LPA in intestinal mucosa repair
When an epithelial surface is damaged, epithelial cells adjacent to the wound migrate to the denuded area closing the wound and reestablishing the epithelial barrier. A line of evidence shows that LPA stimulates migration of IECs. Migration of IEC-6 cells by LPA is pertussis toxin dependent, indicating the presence of a Gαi-couple receptor [40, 41]. On contrary, a recent study showed that LPA stimulates migration and proliferation of YAMC and MSIE cells by LPA1-and Gαq-dependent mechanisms [10]. Migrating cells undergo striking transition in cell shape that is orchestrated by the RhoA family of GTPase, actin cytoskeletal reorganization, and focal adhesion kinase (FAK). LPA rapidly induces reorganization of the actin cytoskeleton that forms lamellipodial protrusions in the leading edge [10, 41]. FAK plays a crucial role in LPA-induced assembly of focal adhesions and migration of IECs [41, 42]. LPA also shows chemotactic activity and regulates matrix metalloproteases that contribute to cell migration and wound healing [18]. We showed recently that Gαq-coupled LPA1 activates PLC-β1 and PLC-β2 in YAMC cells [10]. This study showed that PLC-β1 and PLC-β2 are required for proliferation and migration of YAMC cells, respectively. Gαq translocates to the nucleus where it interacts with PLC-β1 to stimulate cell cycle programing. PLC-β2, on the other hand, activates Rac1 at the plasma membrane contributing to cell migration. Although RhoA is often involved in cell migration, LPA1 decreases RhoA activity in YAMC cells, suggesting that RhoA may have an inhibitory effect. A question remains how migration of normal IECs and malignant cells by LPA differs at the cellular and molecular levels. While the mechanistic difference remains unclear, it is important to recognize that cancer cells and normal cells share many characteristics, such activation PLC-β, RhoGTPase, and FAK. One fundamental difference between migration of normal IECs and cancer cell is that normal wound healing is self-limiting, but cancer cells co-opt and dysregulate normal physiological processes to facilitate growth, migration, invasion and angiogenesis. More research is needed to clarify the complexity of LPA-mediated effects.
LPA1-null (Lpar1−/−) mice exhibit a cranofacial deformity and defective bone development [8]. The intestinal tract of Lpar1−/− mice does not display a gross change. However, a close examination revealed alterations that correlate with the role of LPA1 in vitro. The numbers of proliferating cells along the intestinal tract and the rate of migration of proliferating cells towards the villus in the small intestine or the crypt surface in the colon are significantly decreased in Lpar1−/− mice [10]. In contrast, no difference in proliferation or migration of IECs was observed in Lpar2−/− mice. Oral administration of LPA to wild-type but not Lpar1−/− mice results in increased proliferation and migration of IECs towards the luminal surface of the intestine. As dividing cells are expected to push the existing cells upwards, it is difficult to conclude whether the aberrant IEC migration in Lpar1−/− mice is entirely due to the defective migration or secondary to altered cell division.
The effect of LPA in wound healing in vivo was first demonstrated by Sturm et al. [40]. The authors showed that the extent of injury, assessed by weight loss and macroscopic mucosal damage, in the trinitrobenzene model of colitis was markedly decreased by topical LPA treatment. The protective potential of LPA was further highlighted by the observation that intragastric administration of soybean lecithin-derived LPA or LPA-rich Chinese medicine antyusan protected rats from stress-induced gastric ulcer [43]. The direct role of LPA1 was demonstrated by an in vivo wound healing assay where a mucosal wound was created by an endoscope in the mouse rectum [10]. Oral application of LPA enhanced the closure of the mucosal wound only in wild type mice but not in Lpar1−/− mice, demonstrating the role of LPA1 in epithelial restitution in vivo. In addition, the recovery from DSS-induced colitis was delayed by Ki16425, an antagonist for LPA1 and LPA3 [10, 44]. Our unpublished results indicate that Lpar1−/− mice are more susceptible to DSS-induced colitis, consistent with the protective effects of LPA1 described above. On the other hand, Lpar2−/− mice appear resistance to DSS.
The ATX-LPA axis is up-regulated in several inflammatory diseases, including multiple sclerosis, arthritis, fibrosis, and obesity. Increased ATX expression has been reported in liver fibrosis and chronic hepatitis [8]. Translocation of lymphocytes into the intestinal mucosal layer is of a central importance in the pathogenesis of IBD. It has been shown that ATX promotes the entry of T cells into secondary lymphoid organs [45]. A recent study has shown that the expression level of ATX is elevated in human patients with IBD [46]. LPA induces pro-inflammatory mediators, such as COX-2 and IL-8 [11, 47]. Administration of an ATX inhibitor, bithionol, decreased lymphocyte migration to the intestine and ameliorated inflammation caused by DSS in mice [46]. However, although DSS-induced colitis has some features of human IBD, it is primarily a model for acute inflammation and epithelial damage. The effects of ATX inhibition in chronic inflammation of the gut remain to be determined.
Regulation of electrolyte transport by LPA
Worldwide, diarrhea claims several million lives annually, mostly those of infants. Although its incidence is much lower in the more affluent nations, diarrhea remains one of the two most common visits to pediatric emergency rooms and is also common among the institutionalized elderly. The human intestine absorbed 8–9 L of electrolyte-rich fluid per day. The absorption of water results principally from the osmotic gradient created across the epithelium by absorption of electrolytes and nutrients. In the villus of IECs, Na+ absorption by the Na+/H+ exchanger type 3 (NHE3 or SLC9A3) is coupled with Cl− absorption by an anion exchanger to complete electroneutral absorption of NaCl [48]. In the crypt compartment, Cl− secretion by the cystic fibrosis transmembrane conductance regulator (CFTR) predominates [49]. It was shown that LPA2 attenuates cholera toxin-induced Cl− secretion in mouse intestine by inhibiting CFTR [50]. LPA2 and CFTR mRNA expression is relatively greater in the crypt region and LPA fails to regulate Cl− secretion in Lpar2−/− mice, confirming the importance of LPA2 in this regulation [51].
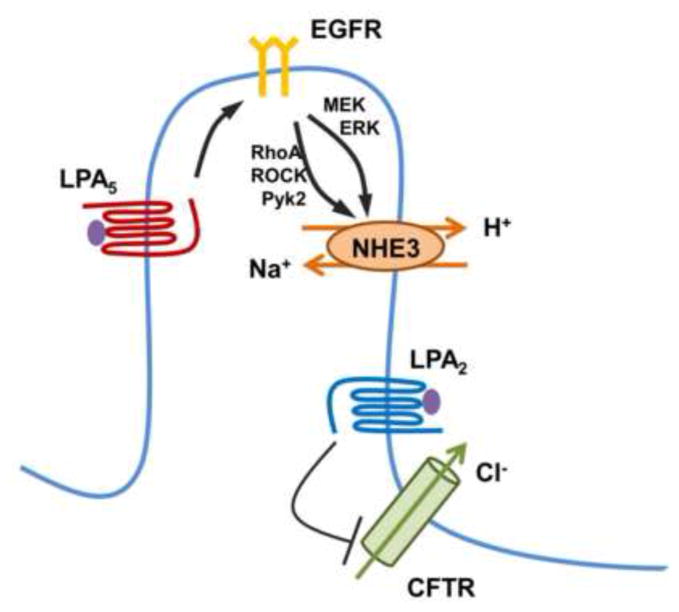
In the intestinal villus cells, LPA facilitates Na+ absorption by activation of NHE3 [9]. Unlike CFTR regulation, the activation of NHE3 by LPA is LPA5-dependent [9]. Stimulation of NHE3 by LPA5 involves transactivation of EGFR expressed in the apical membrane of IECs [52]. EGFR simultaneously activates the RhoA-ROCK (Rho associated kinase)-Pky2 (proline-rich tyrosine kinase 2) cascade and the MEK-ERK pathway (Figure 2). The anti-secretory activity of LPA through targeting NHE3 and CFTR suggests that LPA-rich food, single- or dual agonists of LPA2/LPA5 can be considered to have the potential for therapeutic intervention of certain forms of diarrheal disease. In this regard, LPA2-specific agonists are already under development [53].
Figure 2. Regulation of intestinal transport by LPA.
LPA5 stimulates Na+ absorption by activation of NHE3 in the villus [9]. Regulation of NHE3 is mediated by transactivation of EGFR, which activates the RhoA-ROCK-Pyk2 and MEK-ERK pathways converging onto NHE3 [52]. In the crypt compartment, LPA2 decreases cAMP generation by cholera toxin, leading to inhibition of CFTR Cl− channel [50].
Summary
Our understanding of the role of LPA and its receptor in the intestinal tract is rapidly growing. Much work has ascribed inflammation and malignant transformation to the LPA signaling cascade. Recent development of receptor-specific antagonists and receptor-null mice has led to profound insights into the mechanisms by which LPA regulates the epithelial integrity and homeostasis. There are additional complexities of LPA and further works is warranted to evaluate the balance between the pathological and restorative effects of LPA in the intestinal tract.
Highlights.
This paper presents multiple effects of LPA in the intestinal tract.
LPA promotes colon cancer progression.
LPA induces epithelial wound healing
An antidiarrheal effect of LPA
Acknowledgments
The studies by the authors have been supported by the National Institutes of Health grant DK061418 and DK071597, and Senior Research Award by the Crohn’s and Colitis Foundation of America.
Abbreviations
- LPA
Lysophosphatidic acid
- IEC
intestinal epithelial cell
- IBD
inflammatory bowel disease
- CRC
colorectal cancer
- PA
phosphatidic acid
- PLA
phospholipase A
- ATX
autotaxin
- YAMC
young adult mouse colonic epithelium
- MSIE
mouse small intestinal epithelium
- HIF-1
hypoxia-inducible factor 1
- KLF5
Krüppel-like factor 5
- MIF
macrophage migration inhibitory factor
- MAGI-3
membrane-associated guanylate kinase with inverted orientation-3
- NHERF
Na+/H+ exchanger regulatory factor
- RhoGEF
Rho guanine nucleotide exchange factor
- PLC-β
phospholipase C-β
- DSS
dextran sodium sulfate
- FAK
focal adhesion kinase
- MMP
matrix metalloprotease
- COX-2
cyclooxygenase-2
- NHE3
Na+/H+ exchanger 3
- CFTR
cystic fibrosis transmembrane conductance regulator
Footnotes
Conflict of interest: the authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of Barrier Function in Injured Intestinal Mucosa. Physiol Rev. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 3.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Nakane S, Tokumura A, Waku K, Sugiura T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids. 2001;36:413–419. doi: 10.1007/s11745-001-0737-1. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Kassai A, Ohmoto M, Morito K, Kashiwada Y, Takaishi Y, Urikura M, Morishige J, Satouchi K, Tokumura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem. 2012;60:4156–4161. doi: 10.1021/jf300147y. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Horiuchi G, Matsuoka M, Hirano K, Tokumura A, Koike T, Satouchi K. Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci Biotechnol Biochem. 2009;73:1293–1300. doi: 10.1271/bbb.80813. [DOI] [PubMed] [Google Scholar]
- 7.Navab M, Hough G, Buga GM, Su F, Wagner AC, Meriwether D, Chattopadhyay A, Gao F, Grijalva V, Danciger JS, Van Lenten BJ, Org E, Lusis AJ, Pan C, Anantharamaiah GM, Farias-Eisner R, Smyth SS, Reddy ST, Fogelman AM. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013;54:3403–3418. doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology. 2010;138:649–658. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Leoni G, Neumann PA, Chun J, Nusrat A, Yun CC. Distinct Phospholipase C-β Isozymes Mediate Lysophosphatidic Acid Receptor 1 Effects on Intestinal Epithelial Homeostasis and Wound Closure. Mol Cell Biol. 2013;33:2016–2028. doi: 10.1128/MCB.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 12.Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol. 2000;164:4996–4999. doi: 10.4049/jimmunol.164.10.4996. [DOI] [PubMed] [Google Scholar]
- 13.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 14.Lundequist A, Boyce JA. LPA5 Is Abundantly Expressed by Human Mast Cells and Important for Lysophosphatidic Acid Induced MIP-1β Release. PLoS ONE. 2011;6:e18192. doi: 10.1371/journal.pone.0018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 16.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- 17.Kazama S, Kitayama J, Aoki J, Mori K, Nagawa H. Immunohistochemical detection of autotaxin (ATX)/lysophospholipase D (lysoPLD) in submucosal invasive colorectal cancer. Journal of gastrointestinal cancer. 2011;42:204–211. doi: 10.1007/s12029-010-9186-4. [DOI] [PubMed] [Google Scholar]
- 18.Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res. 2008;314:530–542. doi: 10.1016/j.yexcr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prestwich GD, Gajewiak J, Zhang H, Xu X, Yang G, Serban M. Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim Biophys Acta. 2008;1781:588–594. doi: 10.1016/j.bbalip.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetzl EJ, Dolezalova H, Kong Y, Hu YL, Jaffe RB, Kalli KR, Conover CA. Distinctive Expression and Functions of the Type 4 Endothelial Differentiation Gene-encoded G Protein-coupled Receptor for Lysophosphatidic Acid in Ovarian Cancer. Cancer Res. 1999;59:5370–5375. [PubMed] [Google Scholar]
- 21.Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, Kishi Y, Yamaguchi H, Sasaki S, Sako A, Konishi T, Arai H, Nagawa H. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004;84:1352–1362. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T, An S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway. Proc Natl Acad Sci USA. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Bialkowska A, Rusovici R, Chanchevalap S, Shim H, Katz JP, Yang VW, Yun CC. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Kruppel-like factor 5. J Biol Chem. 2007;282:15541–15549. doi: 10.1074/jbc.M700702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S. Macrophage Migration Inhibitory Factor Promotes Tumor Invasion and Metastasis via the Rho-Dependent Pathway. Clin Cancer Res. 2005;11:1050–1058. [PubMed] [Google Scholar]
- 25.Yang D, Yang W, Zhang Q, Hu Y, Bao L, Damirin A. Migration of gastric cancer cells in response to lysophosphatidic acid is mediated by LPA receptor 2. Oncology letters. 2013;5:1048–1052. doi: 10.3892/ol.2013.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 27.Shin KJ, Kim YL, Lee S, Kim DK, Ahn C, Chung J, Seong JY, Hwang JI. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J Cancer Res Clin Oncol. 2009;135:45–52. doi: 10.1007/s00432-008-0441-z. [DOI] [PubMed] [Google Scholar]
- 28.Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, Yamashita H, Mori K, Sako A, Konishi T, Watanabe T, Sakai T, Suzuki R, Ohta H, Takuwa Y, Nagawa H. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res. 2004;301:168–178. doi: 10.1016/j.yexcr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA Is a Chemorepellent for B16 Melanoma Cells: Action through the cAMP-Elevating LPA5 Receptor. PLoS ONE. 2011;6:e29260. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009;69:4125–4133. doi: 10.1158/0008-5472.CAN-08-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murph MM, Hurst-Kennedy J, Newton V, Brindley DN, Radhakrishna H. Lysophosphatidic Acid Decreases the Nuclear Localization and Cellular Abundance of the p53 Tumor Suppressor in A549 Lung Carcinoma Cells. Molecular cancer research: MCR. 2007;5:1201–1211. doi: 10.1158/1541-7786.MCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of Hypoxia-inducible Factor 1α (HIF-1α) by Lysophosphatidic Acid Is Dependent on Interplay between p53 and Krüppel-like Factor 5. J Biol Chem. 2013;288:25244–25253. doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng W, Tsukahara SER, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, Vanmiddlesworth L, Johnson LR, Parrill AL, Miller DD, Tigyi G. The Lysophosphatidic Acid Type 2 Receptor Is Required for Protection Against Radiation-Induced Intestinal Injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusovici R, Ghaleb A, Shim H, Yang VW, Yun CC. Lysophosphatidic acid prevents apoptosis of Caco-2 colon cancer cells via activation of mitogen-activated protein kinase and phosphorylation of Bad. Biochim Biophys Acta. 2007;1770:1194–1203. doi: 10.1016/j.bbagen.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology. 2011;140:923–934. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin S, Lee SJ, Shim H, Chun J, Yun CC. The Absence of LPA receptor 2 Reduces the Tumorigenesis by ApcMin Mutation in the Intestine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1128–G1138. doi: 10.1152/ajpgi.00321.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsuta M, Iishi H, Baba M, Uedo N, Ishihara R, Higashino K, Mukai M, Ishiguro S. Induction by lysophosphatidic acid of peritoneal and pleural metastases of intestinal cancers induced by azoxymethane in Wistar rats. Cancer Lett. 2005;219:137–145. doi: 10.1016/j.canlet.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU. Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology. 1999;117:368–377. doi: 10.1053/gast.1999.0029900368. [DOI] [PubMed] [Google Scholar]
- 41.Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic Acid Stimulates Intestinal Restitution via Cytoskeletal Activation and Remodeling. J Surg Res. 2000;92:23–28. doi: 10.1006/jsre.2000.5941. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Jacamo R, Zhukova E, Sinnett-Smith J, Rozengurt E. RNA interference reveals a differential role of FAK and Pyk2 in cell migration, leading edge formation and increase in focal adhesions induced by LPA in intestinal epithelial cells. J Cell Physiol. 2006;207:816–828. doi: 10.1002/jcp.20629. [DOI] [PubMed] [Google Scholar]
- 43.Adachi M, Horiuchi G, Ikematsu N, Tanaka T, Terao J, Satouchi K, Tokumura A. Intragastrically administered lysophosphatidic acids protect against gastric ulcer in rats under water-immersion restraint stress. Dig Dis Sci. 2011;56:2252–2261. doi: 10.1007/s10620-011-1595-0. [DOI] [PubMed] [Google Scholar]
- 44.Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a Subtype-Selective Antagonist for EDG-Family Lysophosphatidic Acid Receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 45.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hozumi H, Hokari R, Kurihara C, Narimatsu K, Sato H, Sato S, Ueda T, Higashiyama M, Okada Y, Watanabe C, Komoto S, Tomita K, Kawaguchi A, Nagao S, Miura S. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab Invest. 2013;93:508–519. doi: 10.1038/labinvest.2013.45. [DOI] [PubMed] [Google Scholar]
- 47.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–5643. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol. 2010;2010:238080. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest. 2009;119:540–550. doi: 10.1172/JCI35541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na(+)/H(+) exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1008–1016. doi: 10.1152/ajpcell.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiss GN, Fells JI, Gupte R, Lee S-C, Liu J, Nusser N, Lim KG, Ray RM, Lin F-T, Parrill AL, Sumegi B, Miller DD, Tigyi GJ. Virtual Screening for LPA2-Specific Agonists Identifies a Nonlipid Compound with Antiapoptotic Actions. Mol Pharmacol. 2012 doi: 10.1124/mol.112.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]