Abstract
Fanconi anemia (FA) is an inherited DNA repair disorder associated with short stature and bone marrow failure usually requiring hematopoietic cell transplant (HCT). While low bone mineral density (BMD) has been reported in leukemia patients after HCT, little is known about BMD in FA children after HCT (FA HCT). This study's goals were to compare BMD in FA HCT to BMD in healthy controls, and in children who received HCT for hematologic malignancy (Cancer HCT), and to test for associations between BMD and risk factors for bone loss. This cross-sectional study included 20 FA HCT, 13 Cancer HCT, and 90 healthy controls, age-matched and <18 years old at evaluation. BMD Z-scores for total body (TBMD) and lumbar spine (LBMD) were measured by dual energy x-ray absorptiometry and adjusted for height-forage Z-score (HAZ). FA HCT had lower mean TBMDHAZ Z-score (by 0.8 SD) and higher fraction with Z-score ≤ −1 than healthy controls (42% vs. 11%). No LBMD deficits were detected. FA HCT and Cancer HCT groups did not differ significantly in TBMD or LBMD Z-scores. In FA HCT patients, lower BMI and lower percent fat were associated with lower BMD. This study highlights the importance of monitoring BMD to optimize bone health in FA patients.
Keywords: osteoporosis, DXA scan, bone mineral density, bone marrow transplantation, Fanconi anemia, children
INTRODUCTION
Fanconi anemia (FA) is an inherited DNA repair disorder associated with short stature, congenital anomalies, including limb and vertebral defects, and a high risk for bone marrow failure, leukemia, and other malignancy [1-4]. Hematopoietic cell transplantation (HCT) is the only potential curative therapy for these hematologic manifestations [5], but it also carries a risk of long-term consequences.
Low bone mineral density (BMD) has been documented in children and adults who received HCT for hematologic malignancies [6-9]. Risk factors include inadequate calcium or vitamin D intake, total body irradiation (TBI), conditioning regimens, age at HCT, sex, reduced lean body mass (LBM), graft-versus-host disease (GVHD), endocrinopathies such as hypogonadism and growth hormone deficiency. Although many of these risk factors are prevalent in FA patients [3, 4, 10, 11], bone health in the FA population is poorly understood. Only two published studies have addressed BMD in children with FA [9, 12], both using DXA. However, it remains unclear to what extent children with FA manifest BMD deficits after HCT, to what degree DXA results are impacted by short stature in this patient population, and which skeletal sites are most informative.
This study's primary goal was to evaluate BMD in FA children after HCT. Secondary goals were to determine if BMD of FA children is affected to a similar degree as BMD of children who received HCT for a malignancy, and to test for an association between BMD and risk factors for bone loss in FA. We hypothesized that 1) mean BMD Z-score is lower in FA HCT recipients than healthy controls; 2) FA HCT recipients have higher BMD Z-scores than Cancer HCT recipients as they have not been heavily treated with chemotherapy prior to the HCT preparative therapy, and 3) hypogonadism and vitamin D deficiency are associated with lower BMD Z-score in FA [13]. The current study addresses the existing gaps by including a control group of healthy subjects of a similar age, using both lumbar spine and total body BMD measures, and including FA patients who were all transplanted under the same protocol and thus had a uniform conditioning regimen.
MATERIALS AND METHODS
Participants
All FA patients who underwent an alternative donor HCT using the same regimen consisting of total body irradiation (TBI) 300 cGy, Cyclophosphamide 40 mg/kg, Fludarabine 140 mg/m2 and ATG 150 mg/kg, between 2006 and 2013, who survived at least one year after HCT were identified using the database of the University of Minnesota's Blood and Marrow Transplant Program. Forty patients were identified, aged 6.5-34.1 years.
Two comparison groups were selected for this cross-sectional study from previously published cohorts: normal healthy controls [14], and patients who received HCT for hematologic malignancy [6]. The original group of healthy controls included 208 siblings of cancer patients treated at the University of Minnesota and Children's Hospitals and Clinics of Minnesota, aged 9-18 years at evaluation [14]. The original group of patients who received HCT for hematologic malignancy included 151 patients, aged 10-50.5 years at evaluation, who were transplanted at the University of Minnesota or the Fred Hutchinson Cancer Research Center/Seattle Children's Hospital [6]. Because of significant age and hence Tanner stage differences between the groups (both of which are important in BMD assessment), to be able to compare results between the groups, the inclusion limits for age at evaluation (i.e., DXA scan) were progressively narrowed until the groups did not test significantly different for these two variables. The final study population included 20 FA children (called the FA HCT group), 90 normal healthy controls, and 13 children who received HCT for hematologic malignancy (called the Cancer HCT group). The study was approved by the Institutional Review Boards at the University of Minnesota Medical Center and the Fred Hutchinson Cancer Research Center/Seattle Children's Hospital.
Study procedures
All patients underwent a physical examination, including Tanner staging of pubertal development by trained pediatric providers, measurement of height, weight, body mass index (BMI), and a DXA scan (GE Healthcare Lunar Prodigy scanner; Madison, WI, USA) using enCORE software version 9.3 at the University of Minnesota and version 8.1 at Seattle Children's Hospital. DXA measures included Z-scores for total body BMD (TBMD) including head, and posterior anterior lumbar spine BMD (LBMD) at L1-L4 for FA patients, and at L2-L4 for healthy controls and cancer patients, with and without adjusting for height-for-age Z-score (HAZ) [15], as well as body composition (percent fat mass, lean body mass). The two GE Healthcare Lunar Prodigy DXA scanners were cross-calibrated using a custom-built phantom allowing calibration of bone, fat and lean tissue mass. Data from the two scanners were corrected for differences between them. Sex- and age-specific BMD Z-scores were calculated using enCORE reference data based on healthy, ambulatory subjects from the general population who were free from chronic diseases affecting bone and not taking bone-altering medications.
Laboratory testing included free thyroxine (free T4) by competitive immunoassay and total testosterone by liquid chromatography/tandem mass spectrometry. Chemiluminescent immunoassay was performed to measure 25-hydroxyvitamin D, estradiol, follicle stimulating hormone (FSH), and luteinizing hormone (LH). Hypothyroidism was defined by treatment with thyroid hormone replacement at the time of examination or free T4 < 0.7 ng/dL (9 pmol/L). Hypergonadotropic hypogonadism was defined by FSH > 40 IU/L in females >10 years or LH > 10 IU/L and testosterone below normal for Tanner stage in males >11 years [16].
Statistical analysis
Descriptive statistics are shown as frequencies and percentages, or as mean ± standard deviation (SD) for unadjusted analyses, or as adjusted average ± standard error (SE) for adjusted analyses. For unadjusted comparisons between FA HCT and Cancer HCT patients, P values are from two-sample t-test or Fisher's exact test. Otherwise all analyses had the form of multivariate linear or logistic regression, depending on the outcome, and all used generalized estimating equations (GEE) with robust standard errors to account for sibling relationships within the healthy control group. Analyses testing the association between BMD Z-score and risk factors in FA patients were adjusted for Tanner stage. Analyses comparing BMD Z-score between groups were adjusted for age at study, gender, and Tanner stage. Height-adjusted BMD Z-scores were calculated using a method described by Zemel et al. [15]. All analyses were done using the SAS system (v. 9.3; SAS Institute, Cary, NC). P values are two-sided and <0.05 is considered statistically significant except for analyses involving multiple comparisons, in which P value <0.0167 is considered statistically significant using a Bonferroni adjustment.
RESULTS
Characteristics of the study groups
Table 1 shows participant characteristics. By design, age and Tanner stage at DXA evaluation did not differ between the groups; all patients were 10-14 years old. At time of HCT, 19 FA patients had aplastic anemia and one patient had myelodysplastic syndrome. Complementation groups included FANCA (n=13), FANCC (n=4), FANCE (n=1), FANCG (n=1), and unknown (n=1). The Cancer HCT group had a higher percentage of males compared to healthy controls; six had acute myeloid leukemia, four acute lymphoblastic leukemia, one chronic myelogenous leukemia, one myelodysplastic syndrome, and one non-Hodgkin lymphoma. Compared to the FA HCT group, the Cancer HCT group was younger at HCT and had DXA scans later after HCT. The median time since HCT was 2.2 years for FA patients and 5.2 years for cancer patients. Only one FA patient received androgen treatment prior to HCT. Two FA patients had grade II-IV acute GVHD requiring systemic steroid therapy, one of whom also had chronic GVHD. The incidence of acute, but not chronic GVHD was higher in cancer patients than in FA patients.
Table 1.
Characteristics of the study groups.
| Characteristic | FA HCT [A] | Cancer HCT [B] | Healthy controls [C] | Pairwise P values |
||
|---|---|---|---|---|---|---|
| A vs C | A vs B | B vs C | ||||
| n | 20 | 13 | 90 | - | - | - |
| Male | 11 (55) | 12 (92) | 44 (49) | 0.61 | 0.04 | 0.0160 |
| Racea | ||||||
| Non-black | 19 (95) | 12 (92) | 90 (100) | 0.18 | >0.99 | 0.13 |
| Black | 1 (5) | 1 (8) | 0 (0) | |||
| Age at evaluation (yr) | 11.9 ± 1.4 | 12.2 ± 1.0 | 12.2 ± 1.1 | 0.20 | 0.77 | 0.23 |
| Range | 10.1-14.0 | 11.0-13.6 | 10.1-13.9 | |||
| Age at HCT (yr) | 9.0 ± 2.0 | 5.7 ± 2.8 | NA | NA | 0.0013 | NA |
| Range | 5.2-12.3 | 1.2-9.4 | ||||
| Years since HCT (yr) | 2.8 ± 1.6 | 6.5 ± 3.0 | NA | NA | 0.0010 | NA |
| Range | 1.0-6.1 | 2.7-11.3 | ||||
| Type of transplanta | ||||||
| Autologous | 0 (0) | 1 (8) | NA | NA | 0.39 | NA |
| Allogeneic | 20 (100) | 12 (92) | ||||
| Conditioning regimena | ||||||
| Chemotherapy only | 0 (0) | 0 (0) | NA | NA | 0.05 | NA |
| Chemo + TBI | 20 (100) | 10 (77) | ||||
| Chemo + TBI + CRT | 0 (0) | 3 (23) | ||||
| Acute GVHD | 3 (15) | 8 (62) | NA | NA | 0.0092 | NA |
| Chronic GVHD | 1 (5) | 5 (38) | NA | NA | 0.0248 | NA |
| Tanner stagea (mean) | 2.1 | 1.9 | 2.6 | 0.14 | 0.94 | 0.16 |
| I | 9 (50) | 6 (55) | 20 (22) | |||
| II-III | 5 (28) | 4 (36) | 46 (52) | |||
| IV-V | 4 (22) | 1 (9) | 23 (26) | |||
| Missing | 2 | 2 | 1 | |||
| Height Z-score | −2.3 ± 1.4 | −0.6 ± 0.4 | 0.4 ± 1.0 | <0.0001 | <0.0001 | <0.0001 |
| BMI Z-score | −1.2 ± 2.0 | 0.3 ± 1.0 | 0.5 ± 1.0 | 0.0006 | 0.002 | 0.93 |
| Percent fat mass | 26.4 ± 14.2 | 28.4 ± 9.6 | 25.5 ± 9.9 | 0.74 | 0.49 | 0.14 |
| Lean body mass | ||||||
| Unadjusted | 20.5 ± 5.4 | 28.0 ± 3.9 | 34.3 ± 7.2 | <0.0001 | <0.0001 | 0.98 |
| Adjustedb | 30.6 ± 1.4 | 30.9 ± 0.5 | 31.9 ± 0.4 | 0.40 | 0.80 | 0.003 |
| Hypothyroidism | 6 (30) | 7 (54) | 0 | <0.0001 | 0.28 | <0.0001 |
| Hypogonadismc | 3 (20) | 1 (8) | 0 | 0.003 | 0.61 | 0.13 |
| Testosterone (ng/dL) | 116 | 118 | 143 | 0.59 | 0.92 | 0.54 |
| Estradiol (pg/mL) | 53 | NA | 37 | 0.54 | NA | NA |
Data are presented as average ± SD, adjusted average ± SE for adjusted characteristics, or n (%).
P value refers to the difference, between groups, among the available categories.
Expressed in kg, adjusted for height (cm) and Tanner stage.
Hypergonadotropic hypogonadism for females >10 yr and males >11 yr. To account for multiple comparisons, P value <0.0167 is considered statistically significant using a Bonferroni adjustment (marked in bold). Cancer HCT, cancer patients who received HCT; FA HCT, FA patients who received HCT; NA, not applicable.
FA patients were the shortest of the three groups and had the lowest BMI Z-score, but their percent fat and lean body mass (LBM) were similar to the other groups after adjusting for height and Tanner stage. Consistent with previous reports, hypothyroidism was common among FA patients (30% in FA vs. 0% in healthy controls), although not as common as in cancer patients (54%). All patients with hypothyroidism were euthyroid (normal free T4 and TSH) on thyroid hormone replacement at the time of evaluation. A significant proportion of those FA patients who were in the pubertal age had hypergonadotropic hypogonadism (20% in FA vs. 0% in healthy controls). The mean levels of sex hormones (estradiol and testosterone), however, did not differ between the groups.
Bone mineral density in FA children vs. healthy controls
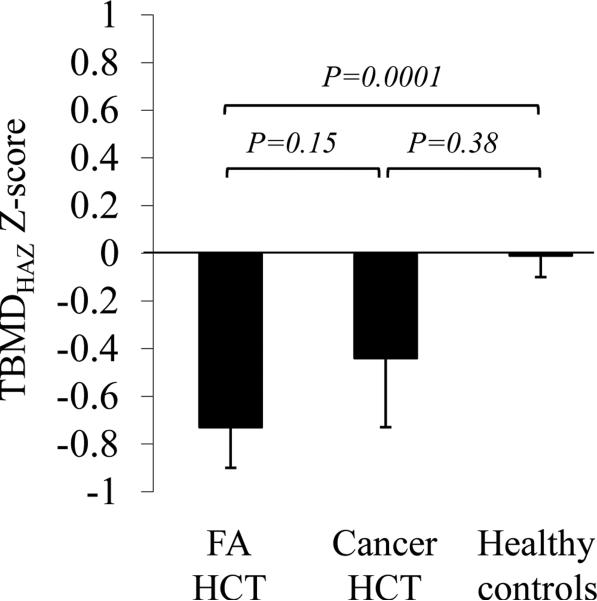
As shown in Table 2, FA patients had significantly lower TBMD Z-scores and LBMD Z-scores compared to healthy controls, and a higher proportion of FA patients had TBMD and LBMD Z-scores ≤ −1 compared to healthy controls. However, after adjusting for height-for-age Z-score (HAZ), only the differences in TBMD Z-scores remained significant. FA patients had mean TBMDHAZ Z-score -0.8 compared to 0.01 in healthy controls. Also, a higher proportion of FA patients had TBMDHAZ Z-score ≤ −1 compared to healthy controls (42% vs. 11%). Adjusting for age at study, gender, and Tanner stage did not change the statistical significance of any of these differences (Fig. 1). Six FA patients were on growth hormone treatment at the time of DXA evaluation. Their BMD measurements did not differ significantly from those of FA patients who were not receiving growth hormone (P = 0.11 for TBMDHAZ and P = 0.22 for LBMDHAZ).
Table 2.
DXA measures of bone mineral density.
| Characteristic | FA HCT [A] | Cancer HCT [B] | Healthy controls [C] | Pairwise P values |
||
|---|---|---|---|---|---|---|
| A vs C | A vs B | B vs C | ||||
| n | 20 | 13 | 90 | - | - | - |
|
Total body BMD Z-score | ||||||
| TBMDage Z-score (mean ± SD) | −1.3 (1.0) | −0.2 (0.7) | 0.7 (1.0) | <0.0001 | <0.0001 | 0.006 |
| TBMDHAZ Z-score (mean ± SD) | −0.8 (0.9) | −0.5 (0.8) | 0.01 (0.9) | 0.0004 | 0.16 | 0.11 |
| TBMDage Z-score ≤ −1, n (%)a | 13 (65) | 2 (15) | 3 (3) | <0.0001 | 0.0096 | 0.09 |
| TBMDHAZ Z-score ≤ −1, n (%)b | 8 (42) | 4 (31) | 10 (11) | 0.0032 | 0.24 | 0.02 |
|
Lumbar spine BMD Z-score | ||||||
| LBMDage Z-score (mean ± SD) | −0.9 (1.1) | −0.2 (0.9) | 0.2 (1.0) | 0.0003 | 0.04 | 0.62 |
| LBMDHAZ Z-score (mean ± SD) | 0.08 (0.9) | 0.04 (0.9) | 0.02 (1.0) | 0.77 | 0.91 | 0.94 |
| LBMDage Z-score ≤ −1, n (%) | 10 (50) | 3 (23) | 9 (10) | <0.0001 | 0.62 | 0.0032 |
| LBMDHAZ Z-score ≤ −1, n (%) | 1 (5) | 2 (15) | 11 (12) | 0.45 | 0.23 | 0.51 |
BMDage Z-score is based on chronological age and sex; BMDHAZ Z-score indicates adjustment for height-for-age Z-score (HAZ) using the approach of Zemel et al. JCEM 2010.
TBMD data were missing for 2 controls.
Height or TBMD data were missing for 2 controls and 1 FA patient.
cLBMD data were missing for 1 control.
dHeight or LBMD data were missing for 1 control and 1 FA patient. To account for multiple comparisons, P value <0.0167 is considered statistically significant using a Bonferroni adjustment (marked in bold).
Figure 1. Total body BMD Z-score adjusted for height-for-age Z-score, age at study, gender, and Tanner stage.
Average adjusted TBMDHAZ Z-score did not differ significantly between children with FA vs cancer after transplant. Cancer HCT group showed an intermediate adjusted average TBMDHAZ Z-score, between healthy controls and FA HCT group. Data are shown as adjusted average ± SE. P value is calculated from the analysis with adjustment of age at study, gender, and Tanner stage.
Bone mineral density in FA HCT group vs. Cancer HCT group
This study's secondary goal was to determine whether BMD is affected to a similar degree in children with FA and children who received HCT to treat cancer. BMD data are shown in Table 2. FA HCT and Cancer HCT groups did not differ significantly in HAZ-adjusted TBMD Z-scores, LBMD Z-scores, or the percentage of patients with HAZ-adjusted Z-scores ≤ −1. Cancer patients had intermediate values between the other two groups in terms of both TBMDHAZ Z-score (−0.8 in FA HCT vs. −0.5 in Cancer HCT vs. 0.01 in healthy controls), and prevalence of TBMDHAZ Z-score ≤ −1 (42% FA HCT vs. 31% Cancer HCT vs. 11% healthy controls). Adjusting for age at study, gender, and Tanner stage did not change the statistical significance of these findings (Fig. 1). The adjusted average TBMDHAZ Z-score was −0.7 in FA HCT (P = 0.0001 compared to healthy controls) vs. −0.4 in Cancer HCT (P = 0.15 compared to healthy controls) vs. 0.01 in healthy controls. Since FA patients and cancer patients differed in age at HCT and time since HCT, BMD analyses were additionally adjusted for these variables. Again, no significant differences between these two groups were found in TBMDHAZ or LBMDHAZ Z-scores.
Analysis of risk factors associated with lower BMD in FA patients
The following risk factors were evaluated to determine association with lower TBMDHAZ or LBMDHAZ Z-score in FA patients: age at HCT, sex, BMI Z-score, percent fat mass, lean body mass adjusted for height, vitamin D deficiency (defined as a 25-hydroxyvitamin D level <30 g/L), hypothyroidism, and hypergonadotropic hypogonadism. In FA patients, only lower BMI Z-score and lower percent fat mass were associated with lower TBMDHAZ Z-scores and lower LBMDHAZ Z-scores (Table 3). For example, 1 Z-score decrease in BMI was associated with 0.2 decrease in TBMDHAZ Z-score and 0.3 decrease in LBMDHAZ Z-score. For percent fat mass, 1 percent decrease was associated with 0.04 decrease in TBMDHAZ Z-score and 0.04 decrease in LBMDHAZ Z-score.
Table 3.
Factors significantly associated with lower BMDHAZ Z-scores in FA patients.
|
Total body BMDHAZ Z-score
| |||
| Risk factor | Comparison | Estimated change (SE) | P value |
| BMI Z-score | 1 Z-score decrease | −0.2 (0.094) | 0.0196 |
| Percent fat mass | 1 percent decrease | −0.04 (0.013) | 0.0068 |
|
Lumbar spine BMDHAZ Z-score | |||
| Risk factor | Comparison | Estimated change (SE) | P value |
| BMI Z-score | 1 Z-score decrease | −0.3 (0.082) | 0.0027 |
| Percent fat mass | 1 percent decrease | −0.04 (0.012) | 0.0005 |
This table includes only risk factors with P < 0.05. Other risk factors included age at HCT, sex, lean body mass adjusted for height, vitamin D deficiency (defined as vitamin D level <30 μg/L), hypothyroidism, and hypergonadotropic hypogonadism.
DISCUSSION
This study identified bone mineral deficits among FA patients previously treated with HCT. These deficits, averaging about 0.8 SD after adjusting for short stature, were observed specifically in total body BMD but not lumbar spine BMD. Different skeletal regions differ significantly in bone composition. Eighty percent of the whole skeleton is cortical bone, whereas the lumbar spine consists largely of trabecular (cancellous) bone (66%) [17]. Our study and others suggest predominant involvement of cortical bone in HCT recipients [6, 18]. Trabecular bone is more metabolically active than cortical bone and is likely to respond faster to factors that affect bone metabolism. In this study, FA patients were evaluated a median of 2.2 years (range 1-6) after HCT, so early changes in bone remodeling could have been missed. While differences in bone composition (i.e., cortical versus trabecular) may account for some of the observed differences in BMD measures depending on the skeletal site, a more direct measure is needed to distinguish cortical and trabecular bone, such as peripheral quantitative computed tomography (pQCT). Using pQCT, deficits in both cortical and trabecular bone were observed in children and young adults, mostly with leukemia, a median of 7 years after HCT [7]. Future studies are needed to address regional bone remodeling in FA patients before and after HCT.
In children as well as adults, DXA remains the most widely used modality for evaluating BMD due to its low radiation exposure, ease of use, and availability of robust reference data. It is recognized, however, that DXA tends to underestimate BMD in children with short stature. This is particularly relevant to children with FA, whose average height is < −2 SD [3]. Hence, methods have been developed to adjust for short stature [15, 19]. In this study, although adjustment for height increased BMD Z-scores in FA patients, their TBMD deficit remained substantial. The mean height-adjusted TBMD Z-score was −0.8 in FA patients compared to a mean of 0.01 in a normal population. A difference of 0.8 SD is likely to be clinically significant because fracture risk increases gradually along a gradient, not abruptly at a threshold. Although the incidence of fractures in the FA population is not known, in a general pediatric population the risk of fracture increases by an estimated 37% for a 0.5 Z-score reduction, based on logistic regression results in a study by Clark et al. [20]. Moreover, 42% of FA patients had height-adjusted TBMD Z-scores ≤ −1, which is remarkable given that these deficits are already present at a young age, at the time when reaching an optimal peak BMD is critical for long-term bone health.
The only other study reported in the literature that focused on BMD exclusively in FA patients was a cross-sectional analysis of LBMD (but not TBMD) in 37 FA patients (aged 4-23 years, 19 without HCT and 18 post-HCT) [12]. While about 50% of these FA children had LBMD Z-scores for chronological age below −1 SD, most patients had normal BMD when adjusted for height. Height-adjusted LBMD Z-scores for both transplanted and non-transplanted FA patients were within normal limits (average Z-scores of 0.4 and 0.8, respectively), leading to the conclusion that BMD is normal in children with FA. It was noted, however, that after adjusting for height, a higher proportion of FA patients who had previously undergone HCT had BMD < −1 SD compared to those without history of HCT (28% vs. 0%). Our study corroborates that LBMD does not appear to be affected in FA children, but also highlights the importance of measuring TBMD, which did show a deficit compared to healthy controls.
The pathogenesis of bone loss after HCT is multifactorial. We have previously shown that bone loss after pediatric HCT is due to a decrease in bone formation [9] and an increase in bone resorption [21]. FA patients were included in both studies. Both hypogonadism and vitamin D deficiency may contribute to the imbalance between bone formation and resorption. Although hypergonadotropic hypogonadism was prevalent among FA patients, it was not associated with lower HAZ-adjusted BMDHAZ, perhaps because the mean levels of sex hormones did not differ between the groups, suggesting only partial hypogonadism. Half of the FA patients had a 25-hydroxyvitamin D level <30 g/L even though one third of patients were already on vitamin D replacement at the time of evaluation. The recommended use of sunscreen to reduce the risk of skin cancer or exacerbation of cutaneous GVHD may contribute to a high prevalence of vitamin D deficiency in FA patients. Nevertheless, vitamin D deficiency was not associated with lower BMDHAZ in these patients. Exposure to TBI is also a risk factor for bone loss after HCT, presumably due to damaging effects of radiation on osteoblasts and the stromal microenvironment [7]. Although FA patients receive a lower dose of TBI and reduced chemotherapy doses relative to non-FA patients undergoing HCT, biologically, the effect is the same and fully myeloablative as FA patients have impaired DNA repair mechanisms. In this study, FA HCT patients were as severely if not more severely affected in terms of BMD outcome than Cancer HCT patients, who received significant amounts of chemotherapy for months or years prior to HCT.
Impaired bone metabolism may also be intrinsic to FA. The “intrinsic” hypothesis is supported by the observation that reduced BMD occurs in adults with FA without previous exposure to HCT [11], and by the presence of skeletal anomalies including radial-ray defects (thumb and radius abnormalities), brachydactyly, vertebral anomalies, and others [2], which indicate abnormal bone morphogenesis. A genetic component is likely to play an important role in FA patients as it is estimated that, in general, 60% to 80% of the variability in bone mass between individuals is determined by genetic factors [22]. Morphogenetic events during limb development are regulated by several developmental pathways, including fibroblast growth factor, sonic hedgehog, retinoid signaling, and wingless-type MMTV integration site family (Wnt) pathway [23, 24]. Among these, Wnt signaling emerges as an attractive candidate because it regulates not only skeletal embryonic development, but also postnatal bone homeostasis and hematopoiesis [25]. Wnt signaling regulates the function, differentiation, and survival of osteoblasts, osteoclasts, and chondrocytes within the growth plate. Defects in Wnt signaling lead to truncated limbs, chondrocyte differentiation defects, short stature, and osteoporosis [25-27]. One of the key mediators of Wnt signaling is β-catenin [27]. A recent study has shown that one of the proteins encoded by the FA-associated gene FANCL is directly involved in regulating posttranslational modification of β-catenin, enhancing its nuclear function [28]. FANCL-deficient progenitor cells exhibit reduced transcription of Wnt-responsive genes, resulting in impaired hematopoiesis. The interaction between FANCL and β-catenin may be facilitated by FANCA and FANCG [28]. It remains to be determined whether dysregulation of the Wnt/β-catenin or other developmental pathways can provide a unifying mechanism for a constellation of findings in FA patients, including short stature, limb and vertebral deformities, and abnormal bone homeostasis.
This study has some limitations. First, BMD evaluation was not performed before HCT, thus not allowing a separate estimate of HCT's effect. Second, the population was predominantly white, limiting the ability to generalize findings to other racial groups. Third, inclusion of the head in TBMD measures may have underestimated BMD deficits in FA patients. Fourth, data on fractures were not collected. Nevertheless, the inclusion of a large healthy control group strengthened the validity of the main observation that FA patients have reduced TBMD.
In summary, this study describes BMD deficits in post-HCT FA pediatric population. The study shows that 1) total body BMD, but not lumbar spine BMD, is reduced in FA patients compared to healthy controls even after adjusting for shortness, 2) FA HCT population and Cancer HCT population do not differ significantly in height-adjusted TBMD Z-scores or LBMD Z-scores, and 3) BMD deficit in FA after HCT cannot be explained by hypogonadism or vitamin D deficiency. The association between lower BMI and lower BMD underscores the importance of optimizing nutritional status in these patients. The current recommendation for children undergoing HCT is to perform a DXA scan before HCT, one year after HCT, and annually thereafter if BMD Z-score is < −1 [29]. This recommendation is also applicable to patients with FA. Following this regimented approach will allow better determination of the degree to which BMD deficits are intrinsic to FA patients, and the influence of HCT on BMD.
HIGHLIGHTS.
Children with FA after HCT have lower total body BMD Z-scores than healthy controls
Risk factors for lower BMD in FA include lower BMI and lower percent fat
It is important to monitor BMD to optimize bone health in FA patients
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (NIH): NCI grant RO1CA113930 to J.S., NCI grant RO1CA112530 to K.S.B, and the National Center for Research Resources (NCRR) Grants 1UL1RR033183 to the University of Minnesota Clinical and Translational Science Institute (CTSI). The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
AUTHOR's CONTRIBUTION
AP designed the research study, gathered the data, and wrote the paper, LEP participated in research design, KSB, JS, JEW, MLM and JLB contributed patient data, LZ and JSH performed statistical analyses, JSH, JLB, KSB, JEW, JS, LEP, and MLM critically read and revised the manuscript.
REFERENCES
- 1.Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, et al. X-linked inheritance of Fanconi anemia complementation group B. Nature genetics. 2004;36:1219–24. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach AD. Fanconi anemia and its diagnosis. Mutation research. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose SR, Myers KC, Rutter MM, Mueller R, Khoury JC, Mehta PA, et al. Endocrine phenotype of children and adults with Fanconi anemia. Pediatric blood & cancer. 2012;59:690–6. doi: 10.1002/pbc.24095. [DOI] [PubMed] [Google Scholar]
- 4.Wajnrajch MP, Gertner JM, Huma Z, Popovic J, Lin K, Verlander PC, et al. Evaluation of growth and hormonal status in patients referred to the International Fanconi Anemia Registry. Pediatrics. 2001;107:744–54. doi: 10.1542/peds.107.4.744. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how? British journal of haematology. 2010;149:14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]
- 6.Petryk A, Polgreen LE, Zhang L, Hodges JS, Dengel DR, Hoffmeister PA, et al. Bone mineral deficits in recipients of hematopoietic cell transplantation: the impact of young age at transplant. Bone marrow transplantation. 2014;49:258–63. doi: 10.1038/bmt.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:760–9. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauchmanova L, Colao A, Lombardi G, Rotoli B, Selleri C. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. The Journal of clinical endocrinology and metabolism. 2007;92:4536–45. doi: 10.1210/jc.2006-2870. [DOI] [PubMed] [Google Scholar]
- 9.Petryk A, Bergemann TL, Polga KM, Ulrich KJ, Raatz SK, Brown DM, et al. Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. The Journal of clinical endocrinology and metabolism. 2006;91:899–905. doi: 10.1210/jc.2005-1927. [DOI] [PubMed] [Google Scholar]
- 10.Forlenza GP, Polgreen LE, Miller BS, MacMillan ML, Wagner JE, Petryk A. Growth hormone treatment of patients with Fanconi anemia after hematopoietic cell transplantation. Pediatric blood & cancer. 2014;61:1142–3. doi: 10.1002/pbc.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. The Journal of clinical endocrinology and metabolism. 2007;92:2624–31. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 12.Rose SR, Rutter MM, Mueller R, Harris M, Hamon B, Bulluck AF, et al. Bone mineral density is normal in children with Fanconi anemia. Pediatric blood & cancer. 2011;57:1034–8. doi: 10.1002/pbc.22956. [DOI] [PubMed] [Google Scholar]
- 13.Weilbaecher KN. Mechanisms of osteoporosis after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2000;6:165–74. doi: 10.1016/s1083-8791(00)70039-5. [DOI] [PubMed] [Google Scholar]
- 14.Polgreen LE, Petryk A, Dietz AC, Sinaiko AR, Leisenring W, Goodman P, et al. Modifiable risk factors associated with bone deficits in childhood cancer survivors. BMC Pediatr. 2012;12:40. doi: 10.1186/1471-2431-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. The Journal of clinical endocrinology and metabolism. 2010;95:1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin's disease. Med Pediatr Oncol. 1996;27:74–8. doi: 10.1002/(SICI)1096-911X(199608)27:2<74::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Bonnick SL. Bone densitometry in clinical practice : application and interpretation. 3rd ed. Humana; New York, NY: 2010. [Google Scholar]
- 18.Buchs N, Helg C, Collao C, Chapuis B, Slosman D, Bonjour JP, et al. Allogeneic bone marrow transplantation is associated with a preferential femoral neck bone loss. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2001;12:880–6. doi: 10.1007/s001980170041. [DOI] [PubMed] [Google Scholar]
- 19.Fewtrell MS, Gordon I, Biassoni L, Cole TJ. Dual X-ray absorptiometry (DXA) of the lumbar spine in a clinical paediatric setting: does the method of size-adjustment matter? Bone. 2005;37:413–9. doi: 10.1016/j.bone.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:1489–95. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polgreen LE, Rudser K, Deyo M, Smith A, Baker KS, Petryk A. Changes in biomarkers of bone resorption over the first six months after pediatric hematopoietic cell transplantation. Pediatr Transplant. 2012;16:852–7. doi: 10.1111/j.1399-3046.2012.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 23.Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nature reviews Genetics. 2009;10:845–58. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama R, Kawakami H, Taketo MM, Evans SM, Wada N, Petryk A, et al. Distinct populations within Isl1 lineages contribute to appendicular and facial skeletogenesis through the beta-catenin pathway. Developmental biology. 2014;387:37–48. doi: 10.1016/j.ydbio.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 26.Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:327–37. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 28.Dao KH, Rotelli MD, Petersen CL, Kaech S, Nelson WD, Yates JE, et al. FANCL ubiquitinates beta-catenin and enhances its nuclear function. Blood. 2012;120:323–34. doi: 10.1182/blood-2011-11-388355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulsipher MA, Skinner R, McDonald GB, Hingorani S, Armenian SH, Cooke KR, et al. National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:334–47. doi: 10.1016/j.bbmt.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]