Abstract
Background
Cystic fibrosis (CF) patients exhibit a progressive decline in lung function accelerated by intermittent pulmonary exacerbations. There are urgent needs for clinically relevant biomarkers to aid in the diagnosis and management of a CF pulmonary exacerbation, in addition to providing insight into its pathophysiology. Club Cell Secretory Protein (CCSP) is produced by bronchial epithelial cells, known to have anti-inflammatory properties and may play a role in CF pulmonary exacerbations. Our objective was to measure sputum CCSP concentration during hospitalizations for CF pulmonary exacerbation and during quarterly outpatient clinic visits for two years. We explored the correlations between CCSP concentration, lung function and markers of inflammation and infection.
Methods
In this prospective, longitudinal cohort study, expectorated sputum, blood and lung function data were collected from 45 CF patients during 68 hospitalizations for pulmonary exacerbation and 193 clinic visits. Sputum CCSP concentration was measured and sputum and blood were assayed with a panel of inflammatory cytokines. We used a repeated measures model to compare log transformed sputum CCSP concentrations across multiple time points and to correlate those concentrations with related clinical variables.
Results
Our population had a mean age of 29 (16–58 years), and a median FEV1 % predicted of 60% (18–105%). Sputum CCSP concentration was significantly lower in the initial, interim and final exacerbation samples (p=0.0021, p=0.0005 and p=0.0274, respectively) compared to outpatient visits. Sputum CCSP concentration was negatively associated with sputum neutrophil elastase concentration (p=0.0373). Patients with Pseudomonas aeruginosa mucoid had a significantly lower sputum CCSP concentration (p=0.0129).
Conclusion
Sputum CCSP concentration is associated with CF pulmonary exacerbation.
Keywords: Cystic fibrosis, biomarker, sputum, inflammation, lung function, cytokines, infection
1. Introduction
CF lung disease begins silently in infancy and is characterized by infection, chronic inflammation, bronchiectasis, progressive lung function decline and intermittent pulmonary exacerbations (1). Pulmonary exacerbations are associated with an increase in pulmonary symptoms, decrease in lung function, loss of energy, weight loss and changes in physical exam findings (2). However, given the lack of a universally applicable definition and the knowledge that 25% of CF patients treated for an exacerbation do not recover to their baseline lung function, significant gaps in knowledge remain (3). There are urgent needs for clinically relevant biomarkers to aid in the diagnosis and management of a CF pulmonary exacerbation, in addition to providing insight into its pathophysiology.
Given that neutrophils and inflammatory cytokines are hallmarks of CF lung disease, biomarkers of airway inflammation have been an area of intense research focus in CF (4,5). However, anti-inflammatory mediators may also play a role in CF airway injury and may provide valuable insight into the mechanisms of lung disease progression (6). Club Cell Secretory Protein (CCSP, formerly known as Clara Cell Secretory Protein, CC10 or CC16) has anti-inflammatory and immunosuppressive properties and is primarily produced by non-ciliated Club Cells (formerly known as Clara Cells) in the conducting airways. Club cells secrete CCSP in very high concentrations in the epithelial lining fluid where it modulates the production and activity of phospholipase A2 (PLA2), interferon-γ and tumor necrosis factor-α. Its role in protecting the lung makes it an attractive candidate biomarker important to the defense against oxidative stress (7–9). CCSP is thought to be a biomarker of lung epithelial injury, with its airway concentration reflecting epithelial cell integrity and its plasma concentration assessing the permeability/leakiness of the lung epithelial barrier. Sputum CCSP was previously identified by proteomics analysis as a potential biomarker of CF lung disease warranting further study (10). Bronchoalveolar lavage fluid (BALF) concentration of CCSP was found to be lower in CF patients compared to those with chronic bronchitis and was inversely correlated with airway inflammation (6). These studies suggest CCSP may play an important role in CF; however, no information is known about CCSP concentration in CF patients during pulmonary exacerbation or longitudinally during times of clinical stability.
In this prospective, longitudinal cohort study, we collected expectorated sputum, blood and lung function data from patients with CF at multiple time points during hospitalization for a pulmonary exacerbation and during quarterly outpatient clinic visits for two years. We hypothesized that sputum CCSP concentration would be lower during a pulmonary exacerbation and would correlate with the severity of lung disease as measured by the degree of obstructive lung disease [forced expiratory volume in one second (FEV1)] and markers of inflammation and infection.
2. Materials and Methods
2.1 Study Population
Forty-five patients with a confirmed diagnosis of CF were identified on admission to the hospital for treatment of a pulmonary exacerbation and recruited into our study from 2008–2011 (11). For study purposes, a pulmonary exacerbation was defined as the need for hospitalization for intravenous (IV) antibiotics and airway clearance for an increase in pulmonary symptoms, and/or a 10% decrease in FEV1. Clinical stability was defined as no current use of IV antibiotics or lack of symptoms consistent with a pulmonary exacerbation. Patients were required to spontaneously expectorate sputum. The study protocol was approved by the University of Minnesota Institutional Review Board and informed consent and/or assent were obtained from each of the subjects and/or their parents or guardians.
2.2 Study Design
This was a single-center, prospective, two-year longitudinal cohort study of patients with CF during times of pulmonary exacerbation (hospitalization) and times of clinical stability (outpatient clinic visits). All hospitalized patients received standard-of-care therapies including airway clearance, nutritional support and IV antibiotics. Each subject provided an expectorated sputum sample and a blood sample, and underwent pulmonary function testing approximately within 24 hours of the initiation of IV antibiotics (initial sample), on day 3–4 (interim sample) and on day 5–7 (final sample). Subjects at the University of Minnesota are routinely discharged after <1 week in the hospital to finish therapy at home. Patients were not required to provide samples at all three time points to be included. Upon discharge, subjects were followed at quarterly outpatient clinic visits for two years. An expectorated sputum sample, blood sample and pulmonary function testing data were collected at each clinic visit. If a CF patient was on IV antibiotics or reported symptoms of a pulmonary exacerbation at an outpatient visit, samples collected were excluded from the analysis. Identical samples were collected if patients had subsequent hospitalizations for pulmonary exacerbation during the study period. Sputum was processed as previously described and frozen immediately after collection at −80°C prior to analysis (12).
2.3 Laboratory Assays
2.3.1 Sputum Sample Analysis
Proteases (neutrophil elastase and matrix metalloproteinase [MMP-9]) were measured in thawed specimens not treated with protease inhibitors. Free neutrophil elastase activity was quantified by a spectrophotometric assay based on the hydrolysis of the specific substrate MeO-suc-Ala-Ala-Pro-Ala-p-nitroanilide (Sigma Chemical Co, St. Louis, MO) and MMP-9 was measured by a commercially available ELISA kit (Quantikine; R&D Systems, Minneapolis, MN). The cytokines IL-23 and TGF-β1 were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN). IFN-γ, IL-1b, IL-2, IL-6, IL-8, MCP-1 and TNF-α, were analyzed as an eight-plex (EMD Millipore Corporation, Billerica, MA). Qualitative bacterial cultures were performed on an airway specimen obtained during each admission and for each clinic visit.
2.3.2 CCSP Assay
Sputum CCSP concentration was measured in the homogenized sputum supernatant treated with protease inhibitors using a human-specific competitive ELISA assay (APC Biotechnology Services, Inc. Rockville, MD). The LOD for this assay is 5ng/mL. Four parameter logistic curves were used that ranged from 5 to 500ng/mL. The intra-assay coefficient of variation (CV) was <12% and the inter-assay CV was <10%. Specimens were run in duplicate and a human serum control was used to ensure reproducibility.
2.3.3 Blood Sample Processing and Analysis
Plasma and serum were collected, aliquoted and stored at −80°C until analysis. Serum IL-8 and plasma TGF-β1 were measured using commercially available ELISA kits (Quantikine; R & D Systems, Minneapolis, MN). Plasma CCSP concentration was measured with the ELISA method noted above. An additional blood sample was sent for a complete blood count (CBC) and differential (Sysmex XE 2011 instrument) and circulating CRP (Vitros 5600, Immunoanalyzer System).
2.3.4 Pulmonary Function Testing
Pulmonary function tests were performed according to American Thoracic Society Guidelines (13). Functional indices measured included forced expiratory volume in one second (FEV1) and forced vital capacity (FVC).
2.4 Statistical Analysis
Descriptive statistics were calculated using medians and ranges where specified for continuous variables and percentages for categorical variables. Log transformation was used to normalize the distribution of all variables except FEV1 and FEV1 %predicted. Mixed effects models were used to investigate how sputum CCSP concentration changes over time during hospitalization and for comparison to clinic visits. Outpatient CCSP concentrations were not combined to generate a mean concentration for comparison; instead a mixed effects model was used to estimate the mean level for all visits. Mixed effects models were also used to assess the relationship between sputum CCSP concentration and lung function while controlling for the effect of visit type. These models had random effects for hospitalizations that were nested within random effects for subjects and treated the visit types as fixed effects. Restricted maximum likelihood was used to obtain parameter estimates and the Wald test was used to test for associations between variables in the models. To investigate associations among inflammatory markers we fit mixed effects models with all markers in the data set and then used stepwise, backward variable selection based on dropping the variable with the largest p-value at each step. Mixed effects models were used to test for an association between P. aeruginosa and CCSP concentration. The latter two analyses did not have random effects for exacerbations as the selection of samples subjected to these analyses did not allow for estimation of exacerbation specific random effects. To estimate the correlation between FEV1 and CCSP concentration a bivariate mixed effects model was fit to both variables (with exacerbation effects nested within subject specific effects). All calculations were conducted using R version 2.15.2 and mixed effects models were fit using the lme function in the nlme package.
3. Results
3.1 Patient Demographics
Demographic data from 45 patients, involving 68 hospitalizations for pulmonary exacerbation and 148 outpatient clinic visits are presented in Table 1. Sputum samples collected from 45 outpatient clinic visits were excluded given the subjects were either on IV antibiotics or reporting symptoms consistent with a pulmonary exacerbation. Four subjects were <18 years of age at the time of the first sample collection. Our population had moderate lung disease with a median FEV1 of 62% predicted, calculated using all outpatient clinic FEV1 data available. The distribution of genotypes in our study cohort reflects the overall distribution in the CF population. Seventeen subjects had more than one hospitalization during the study period.
Table 1.
Patient demographics. Values are presented as number (%) or median (range).
| Characteristics | Values |
|---|---|
| Subjects | 45 |
| Hospital Admissions | 68 |
| Clinic Visits | 148 |
| # Clinic Visits/subject | 4 (1–7) |
| Male Subjects | 19 (42%) |
| Age (years) | 29.1 (16.8 – 58.7) |
| FEV1 (L), initial | 2.1 (0.64 – 4.1L) |
| FEV1 (% predicted), initial | 55% (19 – 87%) |
| FEV1 (% predicted), clinic | 62% (22 – 105%) |
| F508del/F508del | 26 (58%) |
| F508del/other | 17 (38%) |
| other/other | 2 (4%) |
3.2 Sputum CCSP Concentration
We collected 156 expectorated sputum samples during 68 hospitalizations and 131 expectorated sputum samples during 148 outpatient clinic visits. Not every patient was able to expectorate at each time point. Pulmonary Exacerbation: During hospitalization, the initial untransformed median sputum CCSP concentration was 270.1ng/mL (range: 6.0–1504.0, n=66), the interim median concentration was 270.3ng/mL (range: 0.9–1698.0, n=59) and the final median concentration was 351.0ng/mL (range: 24.5–2331.0, n=31). Figure 1 illustrates the log-transformed sputum CCSP concentrations at each time point during hospitalization compared to the outpatient clinic visits. Upon admission to the hospital, sputum CCSP concentration was significantly lower in the initial (p=0.0021), interim (p=0.0005) and final (p=0.0274) sputum samples compared to the outpatient clinic visits. There was no significant increase in sputum CCSP concentration during hospitalization. Outpatient Clinic Visits: The median sputum CCSP concentration for all outpatient clinic visits was 421.6ng/mL (range: 18.5–5247.0, n=128). Although log-transformed sputum CCSP concentration declined over time during the two year follow-up period, it was not statistically significant for those patients with single or multiple exacerbations (p=0.1101). For those patients who had multiple exacerbations and a preceding clinic visit, CCSP concentration was unable to predict the time to a second exacerbation (p=0.8734, n=12). The reproducibility of sputum CCSP concentration during outpatient clinic visits was evaluated. The coefficient of variation was 0.1517 and the intra-class correlation was 0.4075. There was no significant relationship observed between sputum CCSP concentration, gender (p=0.5920) and age (p=0.2430). There was no significant correlation observed between sputum CCSP concentration and plasma CCSP concentration (Figure 2).
Figure 1.
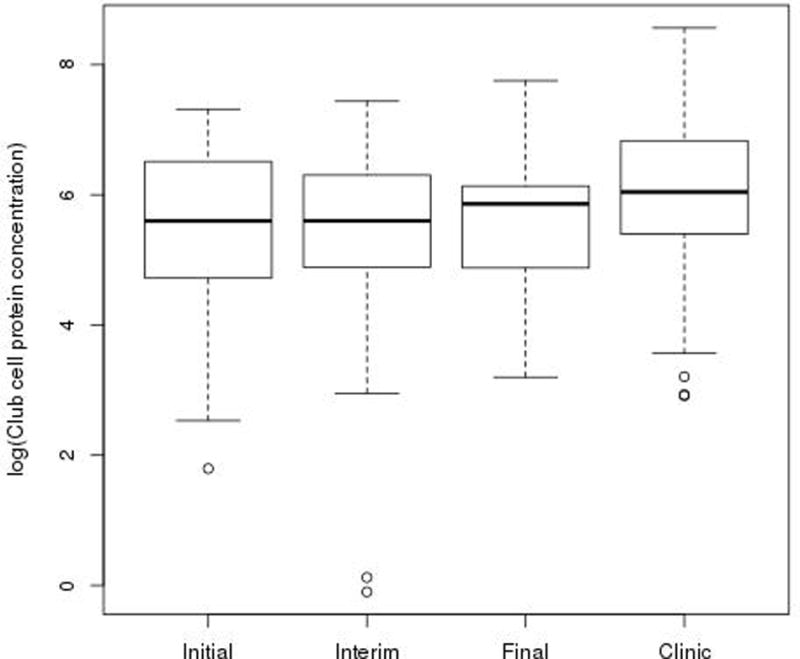
Sputum CCSP concentration was significantly lower in the initial exacerbation sample (p=0.0021), the interim exacerbation sample (p=0.0005) and the final exacerbation sample (p=0.0274) compared to baseline outpatient clinic visits. The long horizontal lines represent the median and limits of the interquartile range, the short ones represent the most extreme point that is within 1.5 IQR of the upper quartile.
Figure 2.
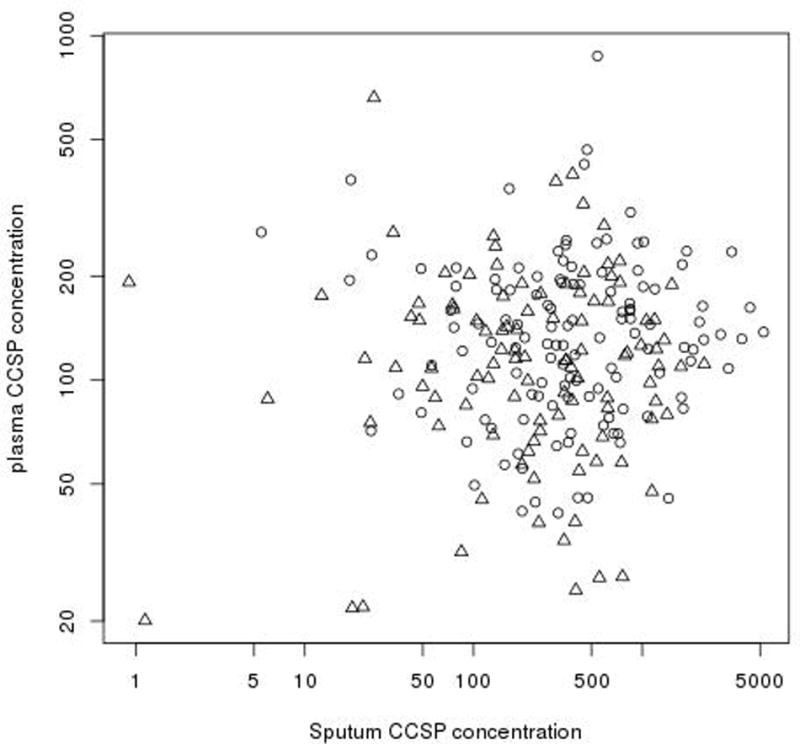
There is no significant correlation observed between sputum CCSP concentration and plasma CCSP concentration in our CF population. Δ = CF pulmonary exacerbation; ○ = outpatient clinic visits.
3.3 Relationships between Sputum CCSP Concentration, Lung Function and Blood and Sputum Inflammatory and Infectious Markers
3.3.1 Lung Function
Our patient population improved clinically during hospitalization for pulmonary exacerbation as demonstrated by a significantly higher FEV1 at the interim and final time points than upon admission to the hospital (Table 2, p<0.0001). Our patient population had a significantly lower FEV1 upon admission to the hospital compared to clinic visits (Table 2, p<0.0001). The cohort demonstrated a significant decline over the two year follow-up period in both absolute FEV1 (p=0.0470) and FEV1 %predicted (p=0.0279). The entire cohort had a decline in the FEV1 %predicted of 1.63% per year (95% CI, 0.18–3.08). When analyzed separately, those patients who had multiple exacerbations had a 3.18% decline per year (p=0.0021) while those patients with a single exacerbation did not have a significant change in their FEV1 %predicted. Although not statistically significant, a positive association was observed between sputum CCSP concentration and FEV1 % predicted (p=0.0866) and sputum CCSP concentration and FEV1 absolute (p=0.165) as illustrated in Figure 3.
Table 2.
The estimated differences between time points with standard errors and p-values for the differences. All data were first log transformed except FEV1 and 1 was added to the CRP values as one measurement was recorded as 0. All estimates are produced by a mixed effects model.
| Variables |
aInitial-bInterim Estimate and p-value |
Interim-cFinal Estimate and p-value |
Initial-Final Estimate and p-value |
Initial-dClinic Estimate and p-value |
||||
|---|---|---|---|---|---|---|---|---|
| Sputum club cell (log ng/mL) | 0.091 ± 0.155 | 0.557 | −0.142 ± 0.201 | 0.481 | −0.051 ± 0.196 | 0.795 | −0.529 ± 0.167 | 0.001 |
| FEV1 (absolute, L) | −0.222 ± 0.035 | <0.001 | −0.072 ± 0.043 | 0.094 | −0.294 ± 0.041 | <0.001 | −0.255 ± 0.034 | <0.001 |
| Sputum NE (log μg/mL) | 0.195 ± 0.293 | 0.505 | 0.149 ± 0.419 | 0.723 | 0.344 ± 0.410 | 0.402 | 0.111 ± 0.265 | 0.674 |
| Sputum IL-8 (log pg/mL) | 0.100 ± 0.154 | 0.515 | 0.463 ± 0.220 | 0.035 | 0.563 ± 0.215 | 0.009 | −0.146 ± 0.139 | 0.294 |
| Plasma IL-8 (log pg/mL) | 0.222 ± 0.174 | 0.204 | −0.315 ± 0.257 | 0.220 | −0.093 ± 0.253 | 0.713 | 0.561 ± 0.213 | 0.008 |
| Plasma CRP (log mg/L) | 0.327 ± 0.111 | 0.003 | 0.318 ± 0.144 | 0.027 | 0.646 ± 0.143 | <0.001 | 0.570 ± 0.111 | <0.001 |
Initial = initial pulmonary exacerbation sample during hospitalization
Interim = middle pulmonary exacerbation sample during hospitalization
Final = final exacerbation sample prior to discharge from the hospital
Clinic = sample collected during an outpatient clinic visit
Figure 3.
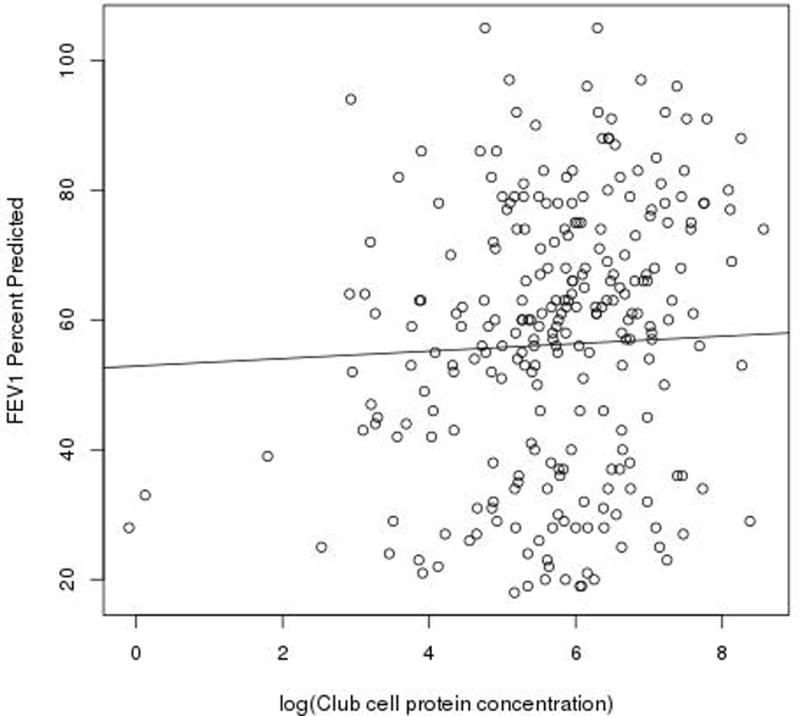
There is a trend towards a positive association between sputum CCSP concentration and inpatient and outpatient FEV1 percent predicted (p = 0.165). All available inpatient and outpatient CCSP and FEV1 data were used in this analysis.
3.3.2 Blood and Sputum Inflammatory Markers
Our patient population had a significant decrease in sputum IL-8 concentration (Table 2, p=0.0169) and plasma CRP (Table 2, p<0.0001) during hospitalization for a pulmonary exacerbation. We did not find a significant decrease in sputum NE or serum IL-8 concentration during hospitalization. Our patients had significantly higher plasma CRP concentrations upon admission compared to outpatient clinic visits (Table 2, p<0.0001). While serum IL-8 concentrations were higher upon admission, they were not significantly higher compared to outpatient clinic visits during the two year follow-up period (Table 2, p=0.3667). When investigating the relationships between sputum CCSP concentration and inflammatory markers, we found that sputum NE was negatively associated with sputum CCSP concentration (p=0.0373). While the remaining inflammatory markers (i.e. neutrophil elastase, MMP-9, IL-1β, IL-6, TNF-α and IL-8) were highly associated with one another, they were not significantly associated with sputum CCSP concentration. However, we did detect a significant positive association between CCSP concentration and MCP1 (p<0.0001) and IFN-γ (p=0.0318) and significant negative associations between CCSP and IL-2 (p=0.0004) and IL-23 (p=0.03575) in models that jointly considered the effect of all of these variables on CCSP given the effect of study visit.
3.3.3 Airway Infection
Those CF patients with any airway culture positive for Pseudomonas aeruginosa, mucoid had a significantly lower Club cell concentration than those who did not have a positive culture for P. aeruginosa, mucoid during the study period (p=0.0129).
4. Discussion
Our study is the first to measure sputum CCSP concentration longitudinally in CF patients. The study design provided the unique opportunity to compare the concentration of an anti-inflammatory protein during times of increased lung injury (pulmonary exacerbation) with times of baseline health (outpatient clinic visits). Although sputum CCSP concentration did not decline significantly over the two year study period, we found that it was significantly lower than outpatient clinic concentration during admission to the hospital for a pulmonary exacerbation. Sputum CCSP concentration was also inversely correlated with neutrophil elastase concentration and those patients infected with P. aeruginosa, mucoid had a lower sputum concentration. These are findings that offer insight into mechanisms of lung injury in CF lung disease as measured non-invasively in expectorated sputum.
CF lung disease is known to be a disease of the airways, characterized by intense neutrophil influx and a heightened pro-inflammatory cascade. The noted decrease in CCSP concentration in our population compared to outpatient clinic concentration may be due to a decreased production/secretion of the protein or to a reduction in the number of Club Cells in the conducting airways. Animal models of acute lung injury have reported decreased BALF CCSP concentrations with no corresponding decrease in the number of Club Cells, suggesting that a lower CCSP airway concentration reflects a down-regulation of CCSP production by mediators released into the airway during inflammation (14). Sputum CCSP concentration was inversely correlated with free NE activity in the sputum in our study population, lending support to this potential mechanism. Given that there was no correlation of plasma CCSP with our sputum CCSP concentration, the integrity of the alveolar-capillary membrane appears to be intact. A lower concentration of sputum CCSP was associated with the presence of P. aeruginosa, mucoid; however, it is not clear whether the decrease in protein concentration is a reflection of increased airway injury and/or whether a decrease in the protective effects of CCSP predisposes individuals to infection. CCSP-deficient mice have significantly higher morbidity and mortality than their wild-type counterparts to oxidative stress from a variety of insults in the conducting airways, concluding that this important protein may modulate lung inflammatory and immune responses to infection and injury (15–18). Unlike general markers of inflammation (i.e. cytokines), sputum CCSP concentration provides information regarding the direct effect of pulmonary exacerbation on the lung. With the mechanisms of CF pulmonary exacerbation still relatively undefined, our findings provide unique insight into this process.
Why is sputum CCSP concentration important in CF? There are a number of human lung diseases characterized by increased pulmonary inflammation in which a lower CCSP airway concentration is associated with increased morbidity and mortality. Cigarette smokers (19,20) and lung transplant patients with bronchiolitis obliterans (21) have lower CCSP concentration in the airway. There is a relationship between CCSP and lung function in COPD, with lower protein levels significantly associated with the faster rate of decline in FEV1 (22). Very low concentrations of CCSP have been found in the BALF and tracheal aspirate fluid (TAF) of ventilated premature infants and those with bronchopulmonary dysplasia (23–25). Data in preterm infants led to a small clinical trial of CCSP administered via the endotracheal tube to ventilated preterm infants with respiratory distress syndrome that resulted in a significant decrease in inflammation in the lungs (26). In addition we have recently reported that replacement therapy with CCSP can improve survival and reverse lung pathology in a murine model of obliterative bronchiolitis (27). There is the potential of utilizing exogenous CCSP as a therapeutic agent in CF, making the finding of a lower airway concentration of CCSP of vital importance for future study.
One of the main strengths of this cohort study is the two year longitudinal follow-up period and the comparison of sick samples (pulmonary exacerbation) with well samples (outpatient clinic visits). We demonstrated that CCSP can be reliably detected by an ELISA in expectorated CF sputum. With a coefficient of variation of 15% and an intra-class correlation of 0.41, we demonstrated reproducibility of multiple measurements, even across time points separated by three months. However, our study is not without limitation. First, we studied only patients with CF and did not measure CCSP concentration in healthy control sputum. Each patient served as their own control, which we felt was appropriate given our aim to investigate CCSP during a CF pulmonary exacerbation. We enrolled subjects when they were admitted to the hospital for treatment of a pulmonary exacerbation and did not measure sputum CCSP concentration prior to their admission. Our study patients were older with a median age of 29 years and had moderate lung disease with a median FEV1 (62% predicted), making its applicability to children limited. Finally, the length of stay in the hospital for our CF patients is rarely > 7 days, making sample collection at further time points challenging.
Our study provides the longitudinal data confirming that sputum CCSP concentration is indeed associated with CF pulmonary exacerbation, providing the foundation both for future study and for a potential clinical trial evaluating the efficacy of exogenously administered CCSP in CF lung disease.
Acknowledgments
This work was supported by the Cystic Fibrosis Foundation (LAGUNA08A0), the National Institutes of Health (CHRCDA K12 HD068322, UM CTSI UL1TR000114) and the Gold Family Fund.
Sources of Support: The Cystic Fibrosis Foundation (LAGUNA08A0), the National Institutes of Health (CHRCDA K12 HD068322 and the University of Minnesota CTSI UL1TR000114) and the Gold Family Fund.
Footnotes
Results of this work have been previously reported in abstract form at the American Thoracic Society International Conference 2013 (Philadelphia, PA) and at the North American Cystic Fibrosis Conference 2013 (Salt Lake City, UT).
References
- 1.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic Fibrosis Cystic Fibrosis in the Metabolic and Molecular Basis of Inherited Disease. 7. New York: McGraw-Hill; 2001. pp. 521–588. [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, AREST CF Investigators Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 5.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186(9):857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur Respir J. 2006;28(3):581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]
- 7.Levin SW, Butler JD, Schumacher UK, Wightman PD, Mukherjee AB. Uteroglobin inhibits phospholipase A2 activity. Life Sci. 1986;38(20):1813–1819. doi: 10.1016/0024-3205(86)90135-9. [DOI] [PubMed] [Google Scholar]
- 8.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal SL, Mukherjee AB. Human clara cell 10-kDa protein is the counterpart of rabbit uteroglobin. J Biol Chem. 1993;268(27):20343–20351. [PubMed] [Google Scholar]
- 9.Dierynck I, Bernard A, Roels H, De Ley M. The human clara cell protein: Biochemical and biological characterisation of a natural immunosuppressor. Mult Scler. 1996;1(6):385–387. doi: 10.1177/135245859600100621. [DOI] [PubMed] [Google Scholar]
- 10.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, Innes JA, Porteous DJ, Greening AP. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178(5):444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. cystic fibrosis foundation consensus panel. J Pediatr. 1998;132(4):589–595. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 12.Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 13.Lung function testing: Selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 14.Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A. Clara cell specific protein (CC16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. Am J Respir Crit Care Med. 2000;161(5):1624–1630. doi: 10.1164/ajrccm.161.5.9812157. [DOI] [PubMed] [Google Scholar]
- 15.Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol. 1997;17(2):147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- 16.Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol. 1998;275(2 Pt 1):L348–56. doi: 10.1152/ajplung.1998.275.2.L348. [DOI] [PubMed] [Google Scholar]
- 17.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275(5 Pt 1):L924–30. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 18.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171(2):1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 19.Bernard AM, Roels HA, Buchet JP, Lauwerys RR. Serum clara cell protein: An indicator of bronchial cell dysfunction caused by tobacco smoking. Environ Res. 1994;66(1):96–104. doi: 10.1006/enrs.1994.1047. [DOI] [PubMed] [Google Scholar]
- 20.Shijubo N, Itoh Y, Yamaguchi T, Shibuya Y, Morita Y, Hirasawa M, Okutani R, Kawai T, Abe S. Serum and BAL clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respir J. 1997;10(5):1108–1114. doi: 10.1183/09031936.97.10051108. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics. 2006;6(3):1001–1010. doi: 10.1002/pmic.200500105. [DOI] [PubMed] [Google Scholar]
- 22.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI, ECLIPSE Investigators Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 23.Dhanireddy R, Lim M, Mukherjee A. Uteroglobin-like protein levels in premature infants on long term ventilatory support. 1993;33(323A) [Google Scholar]
- 24.Bernard A, Roels H, Lauwerys R, Witters R, Gielens C, Soumillion A, Van Damme J, De Ley M. Human urinary protein 1: Evidence for identity with the clara cell protein and occurrence in respiratory tract and urogenital secretions. Clin Chim Acta. 1992;207(3):239–249. doi: 10.1016/0009-8981(92)90122-7. [DOI] [PubMed] [Google Scholar]
- 25.Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE. Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164(1):155–161. doi: 10.1164/ajrccm.164.1.2008022. [DOI] [PubMed] [Google Scholar]
- 26.Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, Shaffer T, Pilon AL, Davis JM. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res. 2005;58(1):15–21. doi: 10.1203/01.PDR.0000156371.89952.35. [DOI] [PubMed] [Google Scholar]
- 27.Wendt CH, Tram KV, Price AP, England KA, Stiehm A, Panoskaltsis-Mortari A. Club cell secretory protein improves survival in a murine obliterative bronchiolitis model. Am J Physiol Lung Cell Mol Physiol. 2013;305:L642–L650. doi: 10.1152/ajplung.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


