Abstract
Objective
Phase III trials have demonstrated a survival advantage for patients with optimally debulked epithelial ovarian cancer (EOC) who received intravenous (IV) and intraperitoneal (IP) chemotherapy compared to IV therapy alone. This was despite a significant proportion of patients in the IV/IP arms not completing all six planned cycles. Our objective was to evaluate the prognostic significance of the number of IV/IP cycles administered.
Methods/materials
Data were analyzed for all patients with stage III-IV EOC who underwent optimal primary cytoreduction followed by ≥1 cycle of IV/IP chemotherapy from 1/2005 to 7/2011 at our institution. A landmark analysis was performed to associate progression-free (PFS) and overall survival (OS) with the number of IV/IP cycles given.
Results
We identified 201 patients; 26 (13%) received 1-2 cycles of IV/IP chemotherapy, 41 (20%) received 3-4 cycles, and 134 (67%) received 5-6 cycles. Five-year PFS for patients who received 1-2, 3-4, and 5-6 cycles was 18%, 29%, and 17%, respectively. Five-year OS for patients who received 1-2, 3-4, and 5-6 cycles was 44%, 54%, and 57%, respectively. There was no significant difference in PFS (P=0.31) or OS (P=0.14) between the three groups. The most common reason for discontinuing IV/IP therapy was treatment-related toxicity (77%). Postoperative complications were the most common reason for not initiating IV/IP therapy (42%) in patients who subsequently transitioned to it.
Conclusions
We did not detect a significant survival difference between patients who received 1-2, 3-4, or 5-6 IV/IP chemotherapy cycles. Women may still derive a survival benefit if they receive <6 IV/IP cycles.
Keywords: Ovarian cancer, Intraperitoneal chemotherapy, Progression-free survival, Overall survival
Introduction
Ovarian cancer is the leading cause of gynecologic cancer-related mortality in the United States, with an estimated 14,270 deaths in 2014 [1]. The majority of new cases present with advanced-stage disease. Standard therapy for these patients consists of a combination of primary cytoreductive surgery and platinum and taxane-based chemotherapy [2]. Numerous studies have demonstrated a survival advantage for patients who undergo optimal cytoreductive surgery [3-5].
Because ovarian cancer typically remains confined to the peritoneal cavity for most of its natural history, the role of intraperitoneal (IP) chemotherapy in women with stage III optimally debulked (≤1cm residual) cancer has been evaluated in three randomized clinical trials [6-8]. The most recent one, Gynecologic Oncology Group (GOG) study-172, demonstrated a significant 16-month median overall survival (OS) advantage for patients who received intravenous (IV) paclitaxel plus IP cisplatin and paclitaxel compared to those who received IV paclitaxel and cisplatin chemotherapy [8]. This prompted the National Cancer Institute to issue a clinical announcement recommending that women with stage III ovarian cancer who undergo optimal cytoreduction be considered for IV/IP chemotherapy [9].
Despite this recommendation, the IV/IP regimen described in GOG-172 has not been widely accepted, owing primarily to its toxicity, the inconvenience of an inpatient regimen, and poor patient tolerance [10]. A modified outpatient regimen has been used at our institution since 2005, and is administered as follows: IV paclitaxel (135 mg/m2) over 3h on day 1, IP cisplatin (75 mg/m2) on day 2, and IP paclitaxel (60 mg/m2) on day 8, given every 21 days for 6 cycles. This regimen has been shown to have comparable survival outcomes to those reported in GOG-172, albeit with improved tolerability, a more favorable toxicity profile, and the convenience of outpatient administration [11].
It is important to note that in GOG-172, only 42% of patients in the IV/IP chemotherapy arm completed all six planned IV/IP cycles, mainly due to the high rate of treatment-related toxicity [8]. Despite this, the IV/IP arm was associated with increased survival over the IV only arm. It is unclear at this time if an increased number of IV/IP cycles leads to improved survival outcomes. The purpose of this study was to evaluate the prognostic significance of the number of IV/IP cycles administered. We also sought to evaluate the reasons for patients receiving <6 cycles of IV/IP chemotherapy.
Materials and Methods
After obtaining institutional review board approval, we identified all patients with International Federation of Gynecology and Obstetrics (FIGO) stage III-IV epithelial ovarian, fallopian tube, or peritoneal cancer who underwent optimal primary cytoreduction (≤1cm residual disease) followed by at least 1 cycle of platinum-based IV/IP chemotherapy from January 2005 to July 2011 at our institution. Patients who received IP treatment as consolidation were ineligible. Clinical data, chemotherapy details, and survival outcomes were retrospectively reviewed from the medical records. Data abstracted included: age, primary disease site, FIGO stage, histology, tumor grade, body mass index, albumin, CA-125, American Society of Anesthesiologists (ASA) score, residual disease at the end of cytoreductive surgery, and pre-chemotherapy Karnofsky performance status.
Details of the chemotherapy treatment were recorded and included: number of cycles administered, regimen used, start and end dates of treatment, and reasons for discontinuing or not initiating IV/IP chemotherapy (in patients who subsequently transitioned to it). Therapy was initiated for all patients with the intent to administer six cycles. Treatment modifications, therapy discontinuation, and need for dose reduction were decided at the discretion of the treating medical oncologist.
Patients were categorized into three groups depending on the number of IV/IP cycles administered: 1-2, 3-4, and 5-6 cycles. Categorical and continuous variables were compared using Fisher’s exact test and the Kruskal-Wallis test, respectively. The primary outcomes were progression-free survival (PFS) and OS. The date of progression was determined by computed tomography scan and/or CA-125 levels [12,13]. PFS was defined as the time interval from the date of surgery to the date of documented first recurrence or progression of disease. If there was no documented progression or death, PFS was calculated from the date of surgery to the date of last follow-up. OS was defined as the time interval from the date of surgery to the date of death or last follow-up. The date of surgery was used as the starting date to calculate PFS and OS for the entire cohort, and to assess univariate predictors of survival. However, due to the time-dependent nature of IV/IP chemotherapy, a landmark survival analysis was performed to associate survival with the number of IV/IP cycles administered. We used this method to calculate 5-year PFS, 5-year OS, and for multivariate survival analysis (in which the ‘number of IV/IP cycles’ covariate, which is time-dependent, was used). A landmark analysis is where the starting date for survival (landmark date) occurs after the independent variable of interest (number of IV/IP cycles administered) is known. Patients who develop disease progression or die prior to the landmark date are excluded from the analysis. Thus, for 5-year PFS, 5-year OS, and multivariate survival analysis, the landmark starting date for survival was defined as 7 months after the date of surgery, as that was the maximal duration needed for completion of IV/IP chemotherapy in our cohort. Four patients were excluded from the PFS analysis and one patient from the OS analysis as they progressed or died within 7 months after surgery, respectively.
The Kaplan–Meier method was used to estimate survival rates. The Cox proportional hazards model was used to estimate hazard ratios (HR) with respect to progression and death. Univariate analysis of continuous and categorical variables was performed using the Cox proportional hazards model and the log-rank test for significance, respectively. Multivariate analysis for survival was performed adjusting for variables with either statistical significance on univariate analysis or with clinical relevance. All statistical tests were two-sided, with P<0.05 considered significant. Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.15 (R development core team, 2013).
Results
Two hundred one women treated with at least one IV/IP cycle were identified over the study period. Twenty-six patients (13%) received 1-2 cycles of IV/IP chemotherapy, 41 (20%) received 3-4 cycles, and 134 (67%) received 5-6 cycles. Patient and tumor characteristics are shown in Table 1. One hundred ninety patients (95%) received cisplatin-based IV/IP chemotherapy, while 11 patients (5%) received carboplatin-based IV/IP therapy. Eighty-one percent of the patients in the cohort received our institution’s modified cisplatin-based IV/IP regimen [11]. Chemotherapy regimen details are shown in Table 2.
Table 1.
Patient and Tumor Characteristics (N = 201)
| Characteristic | 1-2 IV/IP cycles |
3-4 IV/IP cycles |
5-6 IV/IP cycles |
p |
|---|---|---|---|---|
| n = 26 | n = 41 | n = 134 | ||
|
| ||||
| Median age (range) | 59.5 yrs (38-74) |
56 yrs (36-83) |
58 yrs (23-79) |
0.94 |
|
| ||||
| Median BMI (range) | 24.5 kg/m2 (18.1 – 47.2) |
24.4 kg/m2 (19.9 – 54.6) |
25.6 kg/m2 (18.3 – 50.1) |
0.82 |
|
| ||||
| FIGO Stage | ||||
| IIIA | 2 (8%) | 1 (2%) | 6 (5%) | 0.51 |
| IIIB | 0 (0%) | 1 (2%) | 8 (6%) | |
| IIIC | 21 (81%) | 32 (78%) | 109 (81%) | |
| IV | 3 (11%) | 7 (17%) | 11 (8%) | |
|
| ||||
| Primary disease site | ||||
| Ovary | 17 (66%) | 30 (73%) | 96 (72%) | 0.48 |
| Fallopian tube | 5 (19%) | 5 (12%) | 27 (20%) | |
| Peritoneum | 4 (15%) | 6 (15%) | 11 (8%) | |
|
| ||||
| Tumor grade * | ||||
| 1/2 | 3 (11%) | 3 (7%) | 7 (5%) | 0.39 |
| 3 | 23 (89%) | 38 (93%) | 126 (95%) | |
|
| ||||
| Histology | ||||
| Serous | 26 (100%) | 39 (95%) | 124 (93%) | 0.44 |
| Clear cell/Endometrioid /Mixed/Other |
0 (0%) | 2 (5%) | 10 (7%) | |
|
| ||||
| Median preoperative albumin (range) † |
4.3 g/dL (3.5 – 4.7) |
4.1 g/dL (2.8 – 4.8) |
4.2 g/dL (2.6 – 4.8) |
0.35 |
|
| ||||
| Median preoperative CA-125 (range) ‡ |
400 U/mL (17 - 6459) |
325 U/mL (20 - 24,500) |
282 U/mL (4 - 8073) |
0.73 |
|
| ||||
| ASA class | ||||
| 1 | 0 (0%) | 1 (2%) | 8 (6%) | 0.84 |
| 2 | 17 (65%) | 27 (66%) | 81 (60%) | |
| 3 | 9 (35%) | 13 (32%) | 45 (34%) | |
|
| ||||
| Residual disease | ||||
| None | 14 (54%) | 24 (59%) | 85 (63%) | 0.59 |
| ≤1 cm | 12 (46%) | 17 (41%) | 49 (37%) | |
|
| ||||
| Pre-chemotherapy KPS § | ||||
| 70 | 3 (13%) | 2 (5%) | 3 (2%) | 0.17 |
| 80 | 8 (35%) | 10 (28%) | 44 (35%) | |
| 90/100 | 12 (52%) | 24 (67%) | 81 (63%) | |
|
| ||||
| Median total chemotherapy cycles (IV/IP + IV), (range) |
6 (5 – 8) |
6 (4 – 8) |
6 (5 – 8) |
0.25 |
|
| ||||
| Median number of days between surgery and first chemotherapy cycle (IV/IP or IV), (range) ∥ |
42 (21 – 70) |
37 (20 – 81) |
37 (20 – 90) |
0.51 |
Data missing for: one, patient.
Data missing for: four, patients.
Data missing for: seven, patients.
Data missing for: fourteen, patients.
Data missing for: one patient.
BMI, Body mass index; ASA, American Society of Anesthesiologists; KPS, Karnofsky performance status
Table 2.
Intraperitoneal Chemotherapy Regimens
| Chemotherapy Regimen | n (%) |
|---|---|
|
| |
| IP Cisplatin-based regimens | |
| Modified GOG 172 regimen: | 162 (81%) |
| IV Paclitaxel 135 mg/m2 over 3 h on day 1 | |
| IP Cisplatin 75 mg/m2 on day 2 | |
| IP Paclitaxel 60 mg/m2 on day 8 | |
| (Every 21 days for 6 cycles) | |
| IV Paclitaxel 135 mg/m2 over 3 h on day 1 | 28 (14%) |
| IP Cisplatin 75 mg/m2 on day 2 | |
| IP Paclitaxel 60 mg/m2 on day 8 | |
| IV Bevacizumab 15 mg/kg on day 1 beginning on cycle 2 | |
| (Every 21 days for 6 cycles) | |
| Followed by IV Bevacizumab 15 mg/kg for cycles 7 - 22 | |
| IP Carboplatin-based regimens | |
| IV Paclitaxel 135 mg/m2 over 3 h on day 1 | 6 (3%) |
| IP Carboplatin AUC = 6 on day 1 | |
| IP Paclitaxel 60 mg/m2 on day 8 | |
| (Every 21 days for 6 cycles) | |
| IV Paclitaxel 80 mg/m2 over 1 h on days 1, 8, and 15 | 5 (2%) |
| IP Carboplatin AUC = 6 on day 1, | |
| IV Bevacizumab 15 mg/kg on day 1 beginning on cycle 2 | |
| (Every 21 days for 6 cycles) | |
| Followed by IV Bevacizumab 15 mg/kg for cycles 7 - 22 | |
IP, Intraperitoneal; IV, Intravenous; AUC, Area under the curve
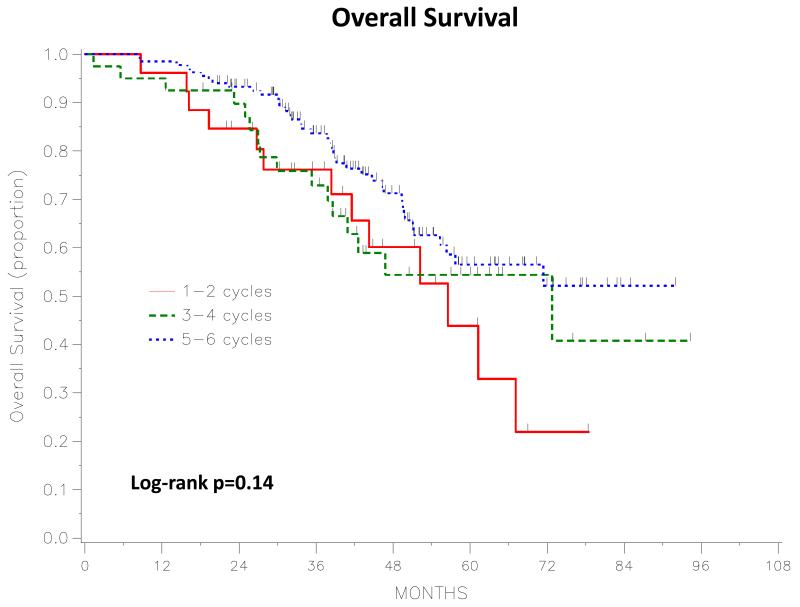
The median PFS and OS for the entire study population were 24 months (95%CI, 21-29) and 79 months (95%CI, 63-not estimable), respectively, with a median follow-up of 52 months (range, 20-102) for the 130 survivors. Figures 1 and 2 depict the PFS and OS curves based on the number of IV/IP cycles administered. Five-year PFS (starting date 7 months after surgery) was 17.9% (95%CI, 6-35.1) for patients who received 1-2 cycles, 29.1% (95%CI, 14-46.1) for those who received 3-4 cycles, and 16.9% (95%CI, 9.8-25.7) for those who received 5-6 cycles. There was no statistically significant difference in PFS between the three groups (log-rank p=0.31). Five-year OS (starting date 7 months after surgery) was 43.9% (95%CI, 19.6-65.9) for patients who received 1-2 cycles, 54.4% (95%CI, 35.1-70.1) for those who received 3-4 cycles, and 56.5% (95%CI, 44.5-66.9) for those who received 5-6 cycles. There was also no statistically significant difference in OS between the three groups (log-rank P=0.14).
Figure 1.
Progression-Free Survival: 1-2 vs 3-4 vs 5-6 Intravenous/Intraperitoneal cycles. Starting time is 7 months post-surgery.
Figure 2.
Overall Survival: 1-2 vs 3-4 vs 5-6 Intravenous/Intraperitoneal cycles. Starting time is 7 months post-surgery.
On univariate analysis, factors that were significantly associated with PFS were preoperative CA-125 (log transformation) (HR 1.13; 95%CI, 1.02-1.26; P=0.02) and residual disease (0mm vs 0.1-10mm: HR 0.57; 95%CI, 0.41-0.79; P<0.001). Factors that were significantly associated with OS were stage (III vs IV: HR 0.44; 95%CI, 0.21-0.89; p=0.02), histology (serous vs nonserous: HR 0.4; 95%CI, 0.17-0.92; P=0.03), and residual disease (0mm vs 0.1-10mm: HR 0.56; 95%CI, 0.35-0.9; P=0.02). On multivariate analysis (starting date 7 months after surgery), after adjusting for confounders, there remained no difference in PFS (3-4 vs 1-2 cycles: HR 0.62; 95%CI, 0.34-1.13; P=0.12; and 5-6 vs 1-2 cycles: HR 0.79; 95%CI, 0.48-1.29; P=0.34) between the three groups. Similarly, there remained no difference in OS (3-4 vs 1-2 cycles: HR 0.81; 95%CI, 0.39-1.69; P=0.57; and 5-6 vs 1-2 cycles: HR 0.56; 95%CI, 0.29-1.06; P=0.07) between the three groups. Multivariate models for PFS and OS are shown in Tables S1 and S2.
One hundred eleven patients (55% of our entire cohort) completed all 6 IV/IP cycles. Of the 90 women who received ≤6 cycles, 52 (58%) started with IV/IP chemotherapy and were subsequently switched to IV only (with the exception of 4 patients who did not receive further treatment), while 38 (42%) started with IV therapy and later transitioned to IV/IP treatment. Among those 52 patients who initiated IV/IP chemotherapy, IP treatment-related toxicity was responsible for stopping IV/IP therapy in 40 patients (77%), with poor performance status being the most common reason (n = 11). Three patients (6%) had their IV/IP therapy discontinued due to IP catheter-related complications, and the remaining 9 patients (17%) discontinued treatment due to other reasons, as outlined in Table 3. Among the 38 patients who started with IV chemotherapy, postoperative complications were the reason for not initiating IV/IP therapy with cycle 1 in 16 patients (42%), with wound infection being the most common complication (n=8). Other reasons for not initiating IV/IP therapy included poor performance status (n=6, 16%) and IP catheter-related complications (n=5, 13%) (Table 4).
Table 3.
Reasons for discontinuing IV/IP chemotherapy (N = 52)
| Reason | Number (%) |
|---|---|
|
| |
| IP treatment-related | |
| Poor performance status | 11 (21%) |
| Bowel obstruction | 7 (13%) |
| Renal/Metabolic | 7 (13%) |
| Gastrointestinal symptoms/Dehydration | 6 (11%) |
| Abdominal pain | 5 (10%) |
| Neuropathy | 4 (8%) |
|
| |
| IP catheter-related | |
| IP catheter blocked | 2 (4%) |
| IP catheter infection | 1 (2%) |
|
| |
| Other | |
| Progression of disease | 3 (6%) |
| Patient refusal | 2 (4%) |
| Fluid leak from vagina | 1 (2%) |
| Vesicovaginal fistula | 1 (2%) |
| Allergic drug reaction | 1 (2%) |
| N/A | 1 (2%) |
IP, Intraperitoneal; IV, Intravenous; N/A, Not available
Table 4.
Reasons for starting with IV chemotherapy in patients who subsequently transitioned to IV/IP chemotherapy (N = 38)
| Reason | Number (%) |
|---|---|
|
| |
| Postoperative complication | |
| Wound infection | 8 (21%) |
| Anastomotic leak | 3 (8%) |
| Pelvic hematoma | 1 (3%) |
| Bowel obstruction | 1 (3%) |
| Fluid leak from vagina | 1 (3%) |
| Vesicovaginal fistula | 1 (3%) |
| Myocardial infarction | 1 (3%) |
|
| |
| IP catheter-related | |
| IP catheter not placed during primary surgery | 3 (8%) |
| IP catheter blocked | 2 (5%) |
|
| |
| Other | |
| Poor performance status | 6 (16%) |
| Gastrointestinal symptoms | 2 (5%) |
| Ileostomy | 2 (5%) |
| Rapid ascites accumulation | 2 (5%) |
| Pleural effusion recurrence in a stage IV patient | 1 (3%) |
| Renal/Metabolic | 1 (3%) |
| Neuropathy | 1 (3%) |
| N/A | 2 (5%) |
IP, Intraperitoneal; IV, Intravenous; N/A, Not available
We performed a sensitivity analysis excluding patients who received carboplatin-based IP chemotherapy and/or bevacizumab treatment (n=39). Among the 162 patients who received the cisplatin-based modified IV/IP regimen, 22 (14%) received 1-2 cycles, 39 (24%) received 3-4 cycles, and 101 (62%) received 5-6 cycles. There was still no significant difference in PFS (p=0.29) and OS (p=0.15) between the three groups.
Discussion
In GOG-172, patients who received IV/IP chemotherapy had a 16-month OS advantage compared to those who exclusively received IV therapy, despite only 42% of women in the IV/IP arm completing all 6 planned cycles [8]. While the benefit of IV/IP chemotherapy may be greater if patients can complete all intended IV/IP therapy, it is also possible that less than 6 cycles are needed to achieve improved survival outcomes. In our study, we did not detect a significant PFS and OS difference between patients who received 1-2, 3-4, or 5-6 IV/IP chemotherapy cycles.
Only two studies have previously assessed the impact of the number of IV/IP cycles administered on survival outcomes [14-15]. In a retrospective analysis of 146 patients, Yen et al reported a survival advantage for patients who received ≥5 cycles of IV/IP chemotherapy compared to those who received ≤4 cycles [14]. The regimen in that study consisted of IV paclitaxel (175 mg/m2) over 3h, combined with IP cisplatin (100 mg/m2) or IP carboplatin (300 mg/m2). In an abstract presented at the 2013 Society of Gynecologic Oncology Annual Meeting, Tewari et al reported on the combined outcomes of patients from GOG-114 and GOG-172 who received IV/IP chemotherapy, and also indicated improved survival for patients who received ≥5 cycles [15]. The regimen used in GOG-114 consisted of 2 cycles of IV carboplatin (area under the curve 9) followed by 6 cycles of IV paclitaxel (135 mg/m2) and IP cisplatin (100 mg/m2) [7]. The regimen used in GOG-172 consisted of IV paclitaxel (135 mg/m2) over 24h on day 1, IP cisplatin (100 mg/m2) on day 2, and IP paclitaxel (60 mg/m2) on day 8, for 6 cycles [8].
Our study is different in several ways. The regimens used at our institution are different, with the majority of our patients receiving a modified GOG-172 cisplatin-based regimen [11]. We included patients who started with IV treatment and were subsequently transitioned to IV/IP therapy, which increases the applicability of our results. In addition, our study includes women with stage IV cancer, which we believe further increases the external validity of our analysis. Although the three GOG protocols assessing IP chemotherapy only evaluated patients with stage III disease [6-8], several institutions consider that the benefits of IV/IP chemotherapy should be extended to select women with optimally cytoreduced stage IV epithelial ovarian cancer. Indeed, recent publications reporting on IV/IP therapy have included these patients [16,17]. With regards to stage IV patients at our institution, IV/IP therapy is sometimes considered for those who are optimally debulked and who had resected pleural disease, supradiaphragmatic lymph nodes, isolated liver or cutaneous metastasis, or a malignant pleural effusion, with no remaining signs of extraperitoneal disease postoperatively. However, as there is no randomized evidence supporting the use of IV/IP in this population subset, we reanalyzed our data after excluding patients with stage IV cancer (n=21), with no change in our results (data not shown). Another strength lies in the fact that our study was conducted at a single center. All patients underwent primary cytoreductive surgery and received their chemotherapy at this institution, so there was little variation in surgical approach, supportive care, management of toxicity, or follow-up for these patients. Furthermore, there was uniformity in the baseline characteristics of all three groups. The majority had stage IIIC cancer that was high grade and of serous histology, with comparable preoperative prognostic factors.
In addition to the inherent biases of a retrospective analysis, our results are limited by the heterogeneity of the regimens used in our cohort. One hundred sixty-two patients (81%) received our institution’s modified cisplatin-based intraperitoneal regimen, which has been previously reported to have a median PFS of 29 months and OS of 67 months [11], comparable to the median PFS of 24 months and OS of 66 months seen in GOG-172 [8]. Of the remaining 39 patients, 28 (14%) received the same modified regimen with the addition of bevacizumab as part of a phase II trial conducted at our institution, which reported a median PFS of 28.6 months, also in the range of GOG-172 [18]. Six patients (3%) received a carboplatin-based IP regimen (due to factors such as baseline renal dysfunction or hearing loss), and 5 patients (2%) received a carboplatin-based IP regimen with dose-dense IV taxol and bevacizumab (as part of GOG-252). It is yet unknown if carboplatin-based IP regimens confer equivalent survival outcomes to cisplatin-based IP ones, and it is also unclear how bevacizumab affects OS in IP regimens, keeping in mind that the addition of bevacizumab to first-line adjuvant IV regimens has led to improved PFS but no difference in OS [19,20]. We addressed this heterogeneity by performing a sensitivity analysis and excluding all patients who received IP carboplatin and/or bevacizumab (n=39). When only analyzing the data of patients who received the modified cisplatin-based IP regimen (n=162), there was still no difference in survival between the three IV/IP groups. Another limitation is the sample size of patients who received 1-2 IV/IP cycles (n=26) and those who received 3-4 IV/IP cycles (n=41), which may not have been large enough to determine a statistically significant survival difference. We attempted to address this issue by combining those patients into a single group (n=67) and comparing them to those who received 5-6 cycles (n=134). There was no difference in survival between those groups (data not shown).
In our study, 121 women (55%) received all 6 planned IV/IP cycles. We evaluated the reasons for receiving <6 cycles separately for patients who had their treatment discontinued and for those who could not initiate it, as we considered these to be two distinct groups. The majority of those who had their therapy discontinued did so due to treatment-related complications/toxicity (77%), while postoperative complications were the most common reason for not initiating IV/IP therapy (42%) with cycle 1 of adjuvant chemotherapy. Among the 90 women who received <6 cycles, IP catheter-related factors were responsible in only 9% (n=8), in contrast to 34% of women in GOG-172 [10]. The IP catheter typically used at our institution is a Bardport silicone venous access device (Bard Access Systems, Salt Lake City, UT). The catheters used in GOG-172 included Tenckhoff or implanted ports with attached fenestrated catheters or ports attached to venous catheters, although it is unknown what proportion of each was used [10]. Black et al’s previous review of our institution’s experience with IP catheter use also cited a 10% rate of catheter-related complications as a reason for stopping IP therapy [21]. It is possible that the lower rate of IP catheter complications seen in our study reflects the type of IP catheters used at our center.
In another long-term follow-up of patients who received IV/IP therapy as part of GOG-114 and GOG-172, Landrum et al reported on prognostic factors associated with survival [22]. Predictors for PFS were stage, histology, and residual disease; while predictors for OS were age, histology, and residual disease. In our analysis, CA-125 and residual disease were significantly associated with PFS. Factors significantly associated with OS were stage, histology, and residual disease. While our results are different, residual disease is the common predictor for PFS and OS in both studies. This strongly underscores the fact that despite IV/IP chemotherapy leading to improved survival in this population, a maximal debulking effort is still essential prior to administering any chemotherapy regimen.
In conclusion, our results did not show a significant difference in survival outcomes between optimally debulked patients with ovarian cancer who received 1-2, 3-4, or 5-6 cycles of primary IV/IP chemotherapy. Patients who receive fewer than 6 cycles may still derive a survival benefit from regional, intraperitoneal treatment when compared to patients who receive IV therapy exclusively. Our data is hypothesis-generating, and despite not demonstrating a survival advantage associated with an increased number of IV/IP treatments, we believe that 6 IV/IP cycles should remain the goal of adjuvant treatment. Nevertheless, for those patients who are unable to initiate IV/IP chemotherapy due to postoperative complications or delayed recovery from surgery, there may still be a survival benefit by transitioning therapy to IV/IP once feasible. The impact of the number of cycles administered should be assessed further in future trials that evaluate IP chemotherapy regimens.
Supplementary Material
Acknowledgments
Funding: This study was supported by the Roy M. Speer Foundation, and NIH P30 CA008748 core grant.
Footnotes
Presented in part at the 45th Annual Meeting of the Society of Gynecologic Oncologists, Tampa, FL, March 22-25, 2014.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- [2].Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- [3].Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- [4].Chi DS, Franklin CC, Levine DA, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–4. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- [5].Winter WE, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- [6].Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- [7].Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecol. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- [8].Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- [9].NCI Clinical Announcement on Intraperitoneal Therapy for Ovarian Cancer [Accessed July 10 ,2014];Natl Cancer Inst. 2006 Jan 5; Available at: http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.
- [10].Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100:27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- [11].Barlin JN, Dao F, Bou Zgheib N, et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2012;125:621–4. doi: 10.1016/j.ygyno.2012.03.027. [DOI] [PubMed] [Google Scholar]
- [12].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [13].Rustin GJ, Marples M, Nelstrop AE, et al. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- [14].Yen MS, Twu NF, Lai CR, et al. Importance of delivered cycles and nomogram for intraperitoneal chemotherapy in ovarian cancer. Gynecol Oncol. 2009;114:415–9. doi: 10.1016/j.ygyno.2009.05.034. [DOI] [PubMed] [Google Scholar]
- [15].Tewari D, Java J, Salani R, et al. Long-term survival advantage of intraperitoneal chemotherapy treatment in advanced ovarian cancer: an analysis of a Gynecologic Oncology Group ancillary data study. 2013 SGO Annual Meeting. Gynecol Oncol. 2013;130:e4. Abstract 6. [Google Scholar]
- [16].Lesnock JL, Richard SD, Zorn KK, et al. Completion of intraperitoneal chemotherapy in advanced ovarian cancer and catheter-related complications. Gynecol Oncol. 2010;116:345–50. doi: 10.1016/j.ygyno.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [17].Teefey P, Bou Zgheib N, Apte SM, et al. Factors associated with improved toxicity and tolerability of intraperitoneal chemotherapy in advanced-stage epithelial ovarian cancers. Am J Obstet Gynecol. 2013;208:501.e1–7. doi: 10.1016/j.ajog.2013.03.012. [DOI] [PubMed] [Google Scholar]
- [18].Konner J, Grabon DM, Gerst SR, et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. 2011;29:4662–8. doi: 10.1200/JCO.2011.36.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- [20].Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- [21].Black D, Levine DA, Nicoll L, et al. Low risk of complications associated with the fenestrated peritoneal catheter used for intraperitoneal chemotherapy in ovarian cancer. Gynecol Oncol. 2008;109:39–42. doi: 10.1016/j.ygyno.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [22].Landrum LM, Java J, Mathews CA, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;130:12–8. doi: 10.1016/j.ygyno.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.