Abstract
Expectations shape the way we experience the world. In this study, we used fMRI to investigate how positive and negative expectation can changes pain experiences in the same cohort of subjects. We first manipulated subjects’ treatment expectation of the effectiveness of three inert creams, with one cream labeled “Lidocaine” (positive expectancy), one labeled “Capsaicin” (negative expectancy) and one labeled “Neutral” by surreptitiously decreasing, increasing, or not changing respectively, the intensity of the noxious stimuli administered following cream application. We then used fMRI to investigate the signal changes associated with administration of identical pain stimuli before and after the treatment and control creams. Twenty-four healthy adults completed the study. Results showed expectancy significantly modulated subjective pain ratings. After controlling for changes in the neutral condition, the subjective pain rating changes evoked by positive and negative expectancy were significantly associated. fMRI results showed that the expectation of an increase in pain induced significant fMRI signal changes in the insula, orbitofrontal cortex, and periaqueductal gray, whereas the expectation of pain relief evoked significant fMRI signal changes in the striatum. No brain regions were identified as common to both “Capsaicin” and “Lidocaine” conditioning. There was also no significant association between the brain response to identical noxious stimuli in the pain matrix evoked by positive and negative expectancy. Our findings suggest that positive and negative expectancy engage different brain networks to modulate our pain experiences, but, overall, these distinct patterns of neural activation result in a correlated placebo and nocebo behavioral response.
Keywords: placebo, nocebo, expectancy, positive expectancy, negative expectancy, fMRI, pain
Introduction
Expectations shape the way we experience the world, for better or for worse (Tracey, 2010). Physicians and clinical investigators have found that positive expectancy of relief can enhance the therapeutic effect of treatment and negative expectancy can diminish it (Atlas and Wager, 2012; Atlas et al., 2012; Bingel et al., 2011; Carlino et al., 2014; Finniss and Benedetti, 2005; Finniss et al., 2010; Tracey, 2010). In the context of pain perception, positive expectations of treatment can elicit analgesia while negative expectation can elicit hyperalgesia. In a clinical setting, it has been demonstrated that either or both placebo (positive expectancy of pain relief) and nocebo effects (negative expectancy of increased pain) influence the effectiveness of medical treatment (Kam-Hansen et al., 2014; Pollo et al., 2001).
There is an increasing body of literature suggesting that placebo effects can enhance the therapeutic benefits of care through the context in which the treatment is administered (Brody and Miller, 2011; Cleophas, 1995; de la Fuente-fernandex et al., 2002; Di Blasi et al., 2001; Finniss et al., 2010; Kaptchuk, 1998; Price et al., 2008; Thomas, 1994). Similarly, there is evidence suggesting that negative expectations can contribute to a variety of side effects and adverse events in clinical trials and medical care (Amanzio et al., 2009; Barsky et al., 2002; Colloca and Finniss, 2012; Petersen et al., 2014). Investigators have explored the neurobiological mechanisms underlying placebo analgesia extensively over the past decades. Many have employed brain imaging technologies (Amanzio et al., 2013; Atlas and Wager, 2012; Benedetti, 2008; Benedetti et al., 2006; Buchel et al., 2014; Enck et al., 2008; Finniss and Benedetti, 2005; Finniss et al., 2010; Kong et al., 2007; Miller et al., 2009; Tracey, 2010; Zubieta and Stohler, 2009). Relatively fewer studies have focused on nocebo hyperalgesia (Benedetti et al., 2003; Colloca and Benedetti, 2007; Colloca and Finniss, 2012; Geuter and Buchel, 2013; Kong et al., 2008; Schmid et al., 2013; Scott et al., 2008).
In order to understand the mechanisms underlying the placebo and nocebo effects, it is important not only to understand them separately but also study the association between them. It is not yet clear whether any or all of the mechanisms that have been proposed to account for positive and negative modulation of pain perception are contributory, singly or in combination. Moreover, there is no clear consensus on whether bidirectional mechanisms contribute to placebo analgesia and nocebo hyperalgesia or whether they are completely separable cognitive constructs. To date, only a few studies have directly compared placebo and nocebo effects. Most of these studies have involved behavioral measures only (Benedetti et al., 2014; Benedetti et al., 2003; Colloca et al., 2010; Colloca et al., 2008). Based on the existing data, investigators have formed two main hypotheses regarding the relationship between placebo and nocebo effects (Petrovic, 2008; Scott et al., 2008). One postulates that placebo and nocebo are manifestations of the same type of brain network with different activation / deactivation changes or, using Petrovic’s term, ‘sides of the same coin’ (Petrovic, 2008). The other posits that placebo and nocebo are separate cognitive constructs grounded in different behavioral patterns and their associated brain networks (Benedetti et al., 2006; Kong et al., 2008).
In the present experiment, we first manipulated subjects’ treatment expectation of the effectiveness of three inert creams, with one cream labeled “Lidocaine” (positive expectancy), one labeled “Capsaicin” (negative expectancy), and one labeled “control” by surreptitiously decreasing, increasing or not changing, respectively, the noxious stimulus intensity after application. We then investigated the subjective pain rating and fMRI signal changes associated with administration of identical pain stimuli before and after the different “treatments.” Our study is unique in that it involved the use of a completely inert treatment, a moisturizing cream, to elicit both placebo and nocebo effects within each individual subject in the same session. This experimental design allowed us to investigate the association between the placebo and nocebo effects and directly compare the brain networks between these two important clinical phenomena in the absence of active medication.
Methods
The Institutional Review Board at Massachusetts General Hospital approved all study procedures. All enrolled subjects provided written informed consent before beginning any study procedures and we debriefed them at the end of the study. All subjects were offered the option to remove their data from the study if they had any concerns due to the inherent need for deception in the experimental paradigm. No subject reported any concern and all subjects allowed their data to be used.
Subjects
Healthy, right-handed, English-speaking subjects participated in the study. We excluded individuals who reported ongoing or past major medical, neurological, or psychiatric illnesses; pregnancy, breast feeding, menopause, and/or irregular menstrual cycles; a history of substance abuse or dependence; a history of impaired urinary elimination; use of psychotropic drugs within the past year; claustrophobia; head trauma; or any other contraindications to MRI.
Experimental Design
The study involved three sessions, each separated by 2–14 days: a training session, a conditioning session, and a scan session. In all sessions, we delivered calibrated heat pain stimuli to the right volar forearm of each subject using a Pathway Medoc (Contact Heat-Evoked Potential Stimulator, Medoc LTD Advanced Medical Systems, Rimat Yishai, Israel). All stimuli initiated at a baseline temperature of 32°C and subsequently increased to a given target temperature. Each stimulus lasted 12 seconds, including a ramp up from baseline (2.5 seconds) to the target temperature (7 seconds) and a ramp down to baseline (2.5 seconds).
Session 1
In the training session, we familiarized subjects with the heat pain stimuli and the Gracely Scales (0–20) (Gracely et al., 1978a; Gracely et al., 1978b) that they would use to rate their pain in order to determine the temperatures required to elicit heat pain for each subject and control for rating strategy and learning effects (Kong et al., 2008; Kong et al., 2006).
Specifically, we drew a 3×3 grid comprised of 2×2 cm regions on the right volar forearm of the subject (2 columns on the inner arm and a third column on the radial, lateral part of the arm). We then administered one or two ascending sequences consisting of stimuli that got progressively more painful over the course of the sequence followed by one or two sequences consisting of three mild [rated as 5–6 out of 20], three moderate [rated as 10–11 out of 20], and three strong [rated as 14–15 out of 20]) pain stimuli interspersed in random order. Finally we administered one or two sequences consisting of six identical moderate heat pain stimuli. Each sequence was administered to a separate region within the grid on the forearm.
Session 2
Session 2 was a behavioral conditioning session. This session involved an expectancy manipulation model employed in some of our laboratory’s previous studies (Kong et al., 2008; Kong et al., 2006; Kong et al., 2009a; Kong et al., 2009b). We informed all subjects that the aim of the study was to investigate the analgesic effect of Lidocaine cream and the hyperalgesic effect of Capsaicin cream on their experience of pain. We told subjects that we would apply three creams (Lidocaine, Capsaicin, and a neutral moisturizing cream) to different regions of their right volar forearm and test their response to heat pain stimuli both before and after the application of the creams (Figure 1).
Figure 1.
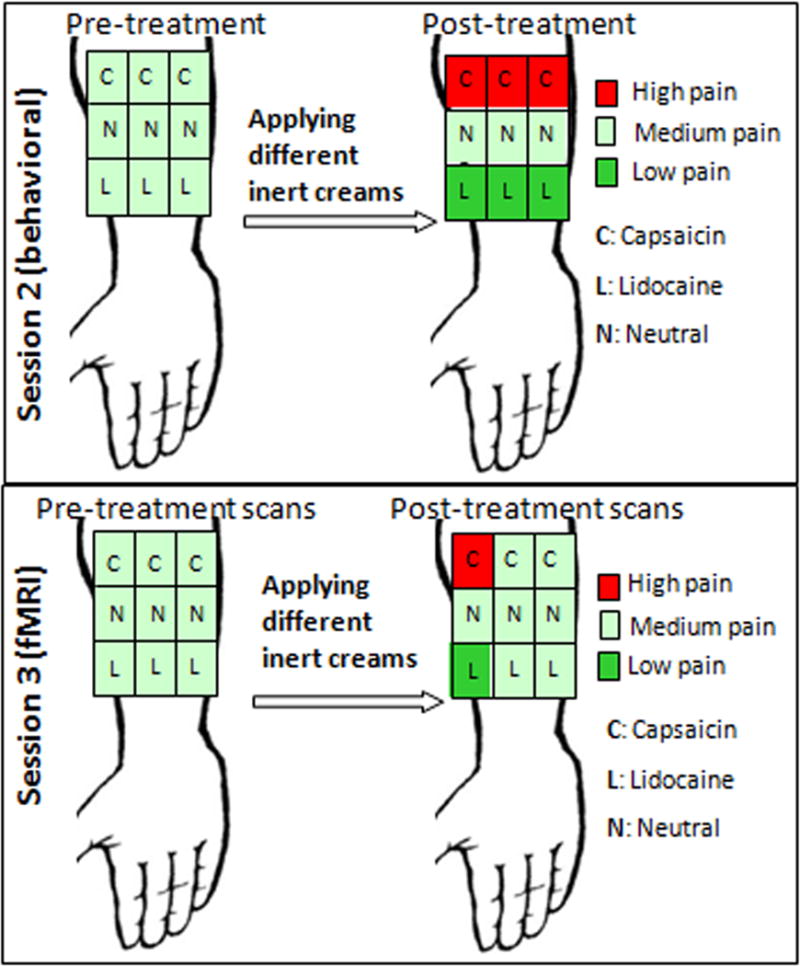
Experimental procedure. In session 2, 9 moderate heat pain sequences were applied on a 3×3 grid on the right inner arm. Then we administered one cream to each row on the grid and counterbalanced the order of cream application across subjects. Following the 20-minute waiting period, we surreptitiously lowered the heat pain intensity in the “Lidocaine” squares, raised the heat pain intensity in “Capsaicin” squares and used identical moderate stimuli in the neutral squares. In session 3, subjects were informed we would repeat the same procedure of session 2 in fMRI scanner. In realty, we only boosted surreptitiously changed the pain intensity at one “Lidocaine” and “Capsaicin” square. For rest of spots, identical moderate pain stimuli were applied.
In reality, we used three samples of one inert moisturizing cream, each dyed a different color. One sampling was dyed light blue and labeled “Lidocaine,” one was dyed pink and labeled “Capsaicin,” and one was left white and labeled “neutral.”
We drew a 3×3 grid identical to that of Session 1 on the inner arm of each subject and proceeded to administer 9 heat pain sequences (one sequence per square on the grid), each about 6 minutes in duration and each including 6 identical heat pain stimuli at the temperature that elicited a moderate (10–11 out of 20) rating as determined in the previous session. Then we applied one cream to each row (set of 3 adjacent squares) on the grid and counterbalanced the order of cream application across subjects. To balance the design, we started the administration of sequences of heat pain stimuli at the most lateral column and moved medially across all subjects. We told subjects that we would wait 15–20 minutes for the creams to take effect and to identify any allergic reactions they might have to the creams. We also read them a script stating that those who experience decreased pain from the Lidocaine and enhanced pain from the Capsaicin should continue and consistently respond that way over the course of the study.
Following the 20-minute waiting period after cream application, we conducted the experimental manipulation. In this conditioning paradigm, we informed subjects that they would be receiving 9 heat pain stimuli sequences comprised of stimuli at temperatures identical to those they had received prior to cream application. In reality, we surreptitiously lowered the heat to temperatures that elicited mild pain ratings in the “Lidocaine” squares, and raised the temperatures to elicit strong pain ratings in the “Capsaicin” squares. To reinforce these effects, identical moderate intensity stimuli were administered to the neutral squares (Eippert et al., 2009; Kong et al., 2006; Scott et al., 2008; Wagner et al., 2005). Only subjects who could distinguish between the pre- and post-treatment stimuli on the “Lidocaine” and “Capsaicin” regions, as indicated by average pain ratings, were permitted to continue with the study.
Before and after conditioning in Session 2, we measured expectancy with two scales by asking each subject to rate how much they expected Lidocaine to relieve their pain (0–10 scale, with 0 indicating no change and 10 indicating complete relief) and how much they expected Capsaicin to enhance their pain (0–10 scale, with 0 indicating no change and 10 indicating extreme pain sensitivity).
Session 3
The third session took place in the MRI scanner. We informed subjects that the proceedings of Session 3 would be identical to those of Session 2. In reality, Session 3 was designed to test the placebo and nocebo effects evoked by the expectancy manipulation in Session 2. Thus, the process of Session 3 was identical to that of Session 2, with the exception that it took place in the fMRI scanner and the temperature of the post-treatment heat pain was moderate on the final 6 regions demarcated on the volar forearm. First, we applied moderate heat pain stimuli (pre-treatment pain) to all nine regions. Then, we administered the creams while the subject remained in the scanner. As in Session 2, we waited 15–20 minutes and told the subjects that this time would allow for the creams to take effect. During this 15–20-minute wait period, we ran structural brain scans. Then we started heat pain stimuli application again. In order to re-boost the expectancy of the subjects, we surreptitiously altered the temperature of the heat pain stimuli (lowered to evoke mild pain for “Lidocaine” and raised to evoke strong pain for “Capsaicin” with no change for neutral) to the 3 boxes in the most lateral column of the 3×3 grid. Last, we administered identical moderate pain stimuli to the remaining 6 boxes in the center and medial rows of the grid on the forearm (post-treatment pain). The pre- and post-treatment changes in subjective pain ratings and fMRI signal changes evoked by the post-treatment identical moderate pain stimuli serve as the primary outcomes of this study (Figure 1).
During scanning, subjects were instructed to focus on a small black fixation cross in the center of a screen in front of them. The fixation cross turned red to cue the onset and duration of each heat pain stimulus (12 s) and then turned black during the variable inter-stimulus interval duration (4, 6, or 8 s). After administration of each stimulus, we displayed the Gracely Sensory Scale on the screen (8 s) and subjects used a button press device controlling a pointer to indicate their subjective ratings. We also measured expectancy with the scales used in Session 2 before treatment, immediately after the treatment boost, and after the scan was complete.
fMRI data acquisition and analysis
We performed brain imaging with a three-axis gradient head coil in a 3-Tesla Siemens TIM Trio MRI System equipped for echo-planar imaging. We acquired thirty axial slices (4 mm thick with 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain with 2000 ms repetition time, 40 ms echo time, 90° flip angle, and 3.13 × 3.13mm in-plane spatial resolution. We also collected a high-resolution 3D MPRAGE sequence and diffusion data (reported elsewhere) for anatomic localization.
We performed preprocessing and statistical analyses using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included motion correction, normalization to MNI stereotactic space, and spatial smoothing with an 8 mm Gaussian kernel. We calculated a GLM (general linear model) design matrix for each subject, including all 18 pain functional runs (1 run before and 1 run after treatment on each of the 9 “Lidocaine”, “Capsaicin” and neutral sites, see Figure 1), modeling each pain stimulus and rating scale as events. We used this to generate the following contrast maps: 1) all pre-treatment pain functional runs; 2) comparisons of before and after treatment on “Lidocaine,” “Capsaicin,” and control sites when identical pain stimuli were applied; and 3) contrasts comparing pre minus post differences in response to identical pain stimuli among each different condition (“Lidocaine,” “Capsaicin,” and control) separately.
We performed group analysis using a random-effects model and a paired t-test to determine group activation for each generated contrast as described above. We also performed a regression analysis between each subject’s fMRI signal changes and the corresponding subjective pain rating changes. We set a threshold of family-wise error (FWE) correction at p<0.05 after small volume correction (svc). We used brain regions implicated in reward processing, anxiety, and pain regulation as independent regions of interest (ROIs) for svc.
For the reward network, we used the bilateral ventral striatum (±14, 10, −10, radius of the sphere =6 mm) obtained from a study of another research group (O’Doherty et al., 2004), rostral anterior cingulate cortex (rACC) (±2, 44, 10, radius of the sphere = 12 mm) (Kong et al., 2006), as well as the caudate and putamen defined using the corresponding AAL (automatic anatomical labeling) mask (Tzourio-Mazoyer et al., 2007). For the anxiety network, we used the hippocampus and amygdala, defined using the corresponding AAL mask. For the pain modulation network, we used the periaqueductal gray (PAG) (±6, −26, −10, radius of the sphere =6 mm) (Behrens et al., 2003), orbito-frontal cortex (OFC) (±38, 46, −4, radius of the sphere =12 mm) (Ochsner et al., 2006) dorsal-medial prefrontal cortex (dmPFC) (±4, 23, 27, radius of the sphere = 12 mm) (Wager et al., 2004), the dorsal ACC (±2, 32, 19, radius of the sphere = 12 mm) (Wager et al., 2004), and anterior insula using the corresponding AAL mask. For non-ROI brain regions, we used a voxel-wise threshold p<0.005 uncorrected with 25 contiguous voxels and p<0.05 FWE corrected at cluster level. All coordinates were reported in MNI coordinates, as used by SPM.
Results
Behavioral results
Thirty-eight volunteers consented to participate in the study. Twenty-four healthy adults (12 male) aged 21 to 49 completed the study. Three subjects withdrew from the study, one due to discomfort with the heat pain and two due to scheduling issues. Eleven subjects were excluded after Session 1 or 2, seven due to the inability to reliably distinguish between high and low pain intensities, and four due to equipment malfunctions. Data from all 24 subjects who completed Session 3 were included in the analysis.
We present the subjective pain ratings from Session 3 in Table 1 and Figure 2. A 2×3 (time by condition) repeated measures ANOVA indicated a significant main effect for condition, F (1,23) = 22.83, p < .001, eta2 = .50, qualified by a significant time by condition interaction, F(2,46) = 26.37, p < .001, eta2 = .53. Post hoc comparisons using Bonferroni corrections indicated that “Lidocaine” lowered pain (p < .001) and “Capsaicin” increased pain ratings (p = .002) compared to the neutral cream. Similarly, within-condition t-tests indicated that pain ratings decreased in the “Lidocaine” condition, t(23) = −4.83, p < .001, and increased in the “Capsaicin” condition, t(23) = 3.18, p = .004, but did not change significantly in the neutral cream condition (p = .459).
Table 1.
Subjective pain ratings in response to identical pain stimuli pre- and post- cream application in Session 3 (Mean ± SD).
| Positive Expectancy (“Lidocaine”) | Negative Expectancy (“Capsaicin”) | Neutral condition | ||||
|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | |
| Pain rating (0–20) | 10.7±2.4 | 8.1±3.0 | 11.0 ±1.8 | 12.2±2.6 | 11.2±2.0 | 10.8±2.3 |
Figure 2.
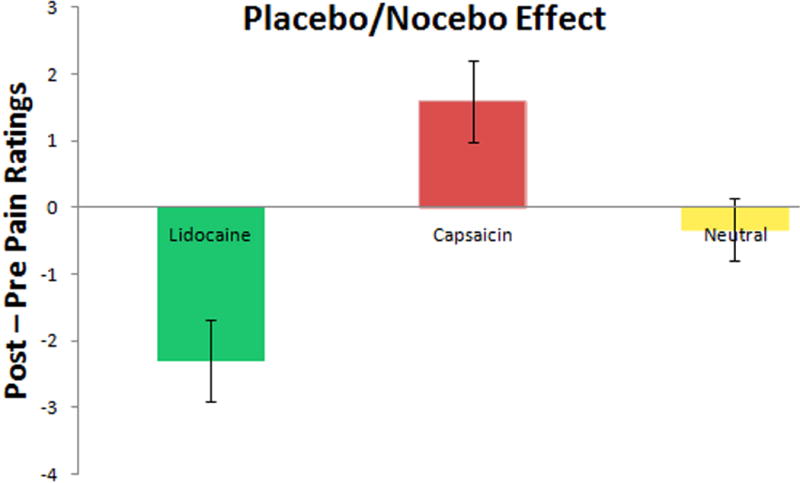
Subjective pain rating differences (mean ± SD) in session 3.
Expectancy ratings for pain relief from “Lidocaine” and pain exacerbation from “Capsaicin” are shown in Table 2. To check the effect of the conditioning procedure on expectancy, we performed a 5×2 (time by “Lidocaine”/”Capsaicin”) repeated measures analysis of variance (ANOVA) on these ratings. The ANOVA revealed a significant main effect for time, F (4,92) = 26.60, p < .001, eta2 = .54. Post hoc comparisons with Bonferroni corrections indicated that expectancy ratings increased following the conditioning procedure in Session 2 (p < .001) and did not change significantly until after the post-treatment test in Session 3 when they decreased (p < .001) to a level that was not significantly different from pre-conditioning expectancy ratings. There was no significant difference between Session 2 and Session 3 in expected pain relief from “Lidocaine” or expected pain exacerbation from “Capsaicin,” nor did the interaction between type of cream and time of assessment approach significance.
Table 2.
Expectancy ratings before and after conditioning in Sessions 2 and 3. (Mean ± SD).
| Positive Expectancy (“Lidocaine”) | Negative Expectancy (“Capsaicin”) | |||
|---|---|---|---|---|
| pre | Post | pre | post | |
| Session 2 | 5.2±1.8 | 8.0±1.8 | 5.3±1.8 | 8.3±1.3 |
| Session 3 | 7.6±1.7 | 8.0±1.9 | 7.9±1.2 | 7.9±1.4 |
We also conducted a paired sample t-test on pre- and post-treatment values in the “Lidocaine” and “Capsaicin” conditions to directly compare the magnitude of positive and negative expectancy. The results indicated that the positive expectancy effect was significantly greater than the negative expectancy effect, t(23) = 2.10, p = .047.
To explore the within subject association between the pre- and post-treatment pain rating change after application of “Capsaicin”, “Lidocaine” and neutral creams, we applied Pearson correlation among the three conditions. We found a significant correlation between the “Capsaicin” and neutral cream (r = 0.56, p = 0.006) and between “Lidocaine” and neutral cream (r = 0.54 p = 0.005) conditions, but no significant correlation between the “Capsaicin” and “Lidocaine” conditions (r = −0.08, p = 0.72). This pattern of correlation suggests that neutral cream ratings were functioning as a suppressor variable, decreasing the association between the effects of “Capsaicin” and “Lidocaine.” We tested this idea further by regressing “Capsaicin” induced pain rating changes on neutral and “Lidocaine” induced pain rating changes. With neutral cream ratings controlled, the partial correlation between the “Lidocaine” response and the “Capsaicin” response was −.54 (p = .008), indicating that a greater positive expectancy effect was associated with a greater negative expectancy effect.
In this study, we also measured several psychological variables including the Life orientation test (LOT), Behavioral Appetitive Scale (BAS), and Tellegen Absorption Scale (TAS). To explore the association of these psychological measurement and the placebo/nocebo effects, we also performed a Pearson correlation analysis. The results showed no significant association between the psychological variables and the placebo/nocebo effects [LOT: Placebo (p=0.13), Nocebo p=0.73; BAS: Placebo (p=0.57), Nocebo (p=0.94); TAS: Placebo (p=0.14), Nocebo (p=0.78)]. We speculate that this finding may be due to the small sample size of the study.
fMRI results
Main effect of pain perception
To delineate pain responsive brain regions we calculated a contrast between all pre-treatment pain stimuli (12 seconds each) and baseline (pain minus implicit baseline) using all nine scans. The comparison yielded significant activations (voxel-wise p>0.005, uncorrected with 20 contiguous voxels) in the entire predicted network of pain-responsive regions including the bilateral insular/opercular cortices, dorsal ACC (dACC)/dmPFC), caudate, putamen, and cerebellum (Figure 3). The opposite contrast revealed significant activation in the bilateral ventro-medial dorsolateral prefrontal cortex (vmPFC), precunus/posterior cingulate cortex, hippocampus, and cerebellar cortex (Figure 3). These main effects are consistent with previous findings on the patterns of pain-elicited neural activation and deactivation (Kong et al., 2010a).
Figure 3.
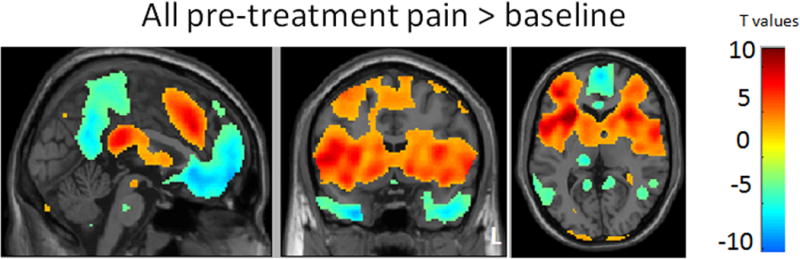
Brain activation (red) and deactivation (blue) evoked by all pre-treatment pain. L: left side.
Direct comparison between positive expectancy (“Lidocaine”) and negative expectancy (“Capsaicin”) conditions
We performed paired t-tests to directly compare pre-/post fMRI signal change differences in response to identical moderate intensity pain stimuli administered under positive (“Lidocaine”) and negative (“Capsaicin”) expectancy conditions (Table 3 and Figures 4 & 5). The contrast of “Lidocaine” (pre > post) > “Capsaicin” (pre > post) identified brain regions that showed enhanced activation during pain administration in positive expectancy relative to negative expectancy (Table 3, Figure 4). This contrast revealed significant differences in the dACC, right orbitoprefrontal cortex (OPFC), and left anterior insula. We extracted the beta values from those brain regions and found significant fMRI signal decreases in the “Lidocaine” condition and signal increases in the “Capsaicin” condition (Figure 4). Interestingly, the dACC region showed a unique pattern of enhanced activation in the pre-treatment “Lidocaine” condition relative to the pre-treatment “Capsaicin” condition that reversed post treatment. In contrast, the left anterior insula and right DLPFC showed comparable pre-treatment activation in the two conditions that diverged post-treatment in the direction consistent with the response of the pain responsive networks, e.g. increased after “Capsaicin” and decreased after “Lidocaine.” The opposite contrast [“Capsaicin” (pre > post) > “Lidocaine” (pre > post)] revealed no regions above the threshold.
Table 3.
Results of paired t-tests for the direct comparison between pre-/post fMRI signal change differences in response to identical moderate intensity pain stimuli given under positive (“Lidocaine”) and negative (“Capsaicin”) expectancy conditions.
| Contrast | Voxels | Brain area | Peak Coordinate (x, y, z) | Z value |
|---|---|---|---|---|
| Positive expectancy (pre>post) > Negative expectancy (pre>post) | 47 | L anterior insula | 36, 16, −6 | 4.15 svc |
| 312 | R dorsal ACC | −4, 32, 14 | 3.45 | |
| 189 | R dorsolateral PFC | −34, 48, 18 | 3.72 | |
| Negative expectancy (pre>post) > Positive expectancy (pre>post) | no brain region above the threshold | |||
| Positive expectancy (pre>post) > Negative expectancy (post>pre) | 99 | L ventral striatum | 12, 8, −10 | 3.85 svc |
| Negative expectancy (post>pre) > Positive expectancy (pre>post) | 143 | R anterior insula | −26, 20, −2 | 3.79 svc |
| 158 | Bilateral precuneus | 0, −14, 60 | 3.44 | |
| 296 | R orbitofrontal cortex | −38, 46, −4 | 3.83 svc | |
| 9 | R periaqueductal grey | 2, −28, −6 | 3.17 svc | |
Figure 4.
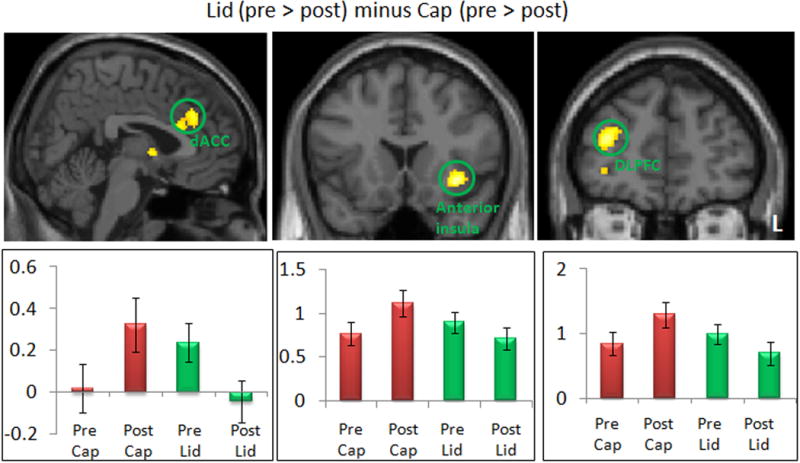
Comparison between positive (Lid) (pre > post) and negative (Cap) expectancy conditions (pre > post). The bar indicates the peak beta of each brain regions pre- and post-treatment. dACC: dorsal ACC; DLPFC: dorsal lateral prefrontal cortex; L: left side.
Figure 5.
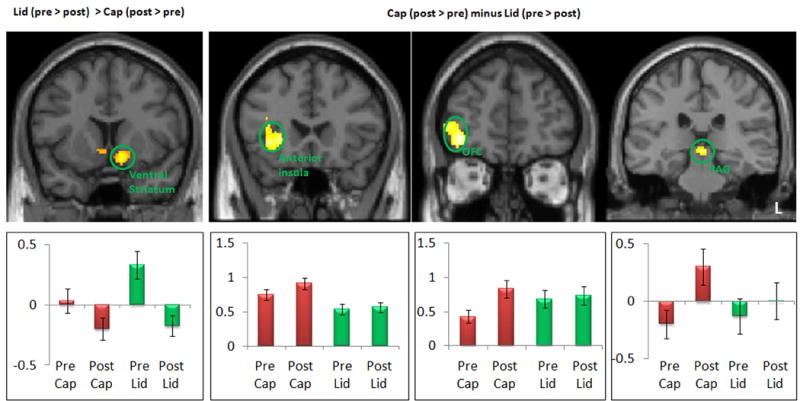
Comparison between positive (Lid) (post > pre) and negative (Cap) expectancy conditions (pre > post). The bar indicates the peak beta of each brain regions pre- and post-treatment. OFC: orbitoprefrontal cortex; PAG: periaqueduct gray; L: left side.
To identify brain regions that exhibited activity in opposite directions during pain administration in the “Lidocaine” and “Capsaicin” conditions, we performed two additional contrasts (Table 3, Figure 5). First, we compared “Lidocaine” (pre > post) to “Capsaicin” (post > pre). The left ventral striatum was significantly activated in this comparison. The opposite contrast [(“Capsaicin” (post > pre) minus “Lidocaine” (pre > post)] revealed activity in the right anterior insula, OFC, and PAG (Figure 5).
To identify any brain regions commonly activated in both the “Lidocaine” and “Capsaicin” conditions during pain administration, we performed four conjunction analyses (Friston et al., 2005; Nichols et al., 2005); 1) “Capsaicin” (post>pre) and “Lidocaine” (post>pre), 2) “Capsaicin” (pre>post) and “Lidocaine” (pre>post), 3) “Capsaicin” (post>pre) and “Lidocaine” (pre>post), and 4) “Capsaicin” (pre>post) and “Lidocaine” (post>pre). The null hypothesis for the Conjunction analysis is: “not all subjects/contrasts activated this pixel.” If any one of the conjunction results is significant, the null hypothesis is rejected and we can conclude that all subjects/contrasts activated the pixel for that pair of contrasts. The threshold for each individual conjunction contrast is P<0.005 uncorrected. A logical AND requires that all the comparisons in the conjunction are individually significant. None of these four conjunction analyses revealed any significant activation, indicating that no common brain regions were observed between these contrasts. For exploratory purposes, we lowered the threshold to voxel-wise p < 0.05 with 50 continuous voxels. At this more liberal threshold, the results did show overlapping activity in the “Lidocaine” (pre minus post) and “Capsaicin” conditions (post minus pre) in pain-related brain regions, including the bilateral dACC (x, y, z, voxel size, peak z value: 0, 32, 12, 50 voxels, z = 2.1), left insula / OPFC (35, 32, −40, 250 voxels, z = 2.69), and left operculum / putaman (32, 2, 6, 70 voxels, z = 2.36).
Pre minus post in positive expectancy, negative expectancy, and no manipulation neutral conditions
To explore the fMRI signal changes under each of the different conditions, we analyzed post-treatment pain vs. pre-treatment pain in the “Capsaicin,” “Lidocaine,” and neutral conditions separately (Table 4, Figure 6). In the “Capsaicin” condition, a pre minus post contrast revealed activation in the frontal pole. The opposite contrast in the “Capsaicin” condition (post > pre) showed activation in the bilateral insula, and right OPFC and PAG (Figure 6). In the “Lidocaine” condition, pre minus post contrast showed activation in the bilateral ventral striatum. The post minus pre contrast revealed no brain regions activated above the threshold. In the neutral condition, post minus pre contrast indicated activation in the posterior insula, hippocampus, amygdala and precuneus. The opposite contrast revealed no significant activation.
Table 4.
Results of paired t-tests comparing fMRI signal change differences (post > pre and pre>post) for positive, negative and control (neutral) expectancy conditions separately.
| Contrast | Voxels | Brain area | Peak Coordinate (x, y, z) | Z value |
|---|---|---|---|---|
| Negative Expectancy (pre>post) | 164 | L frontal pole | 12, 62, 30 | 3.48 |
| Negative Expectancy (post>pre) | 254 | R anterior insula | −32, 22, 2 | 3.80 svc |
| 70 | L anterior insula | 32, 22, 14 | 3.22 svc | |
| 262 | Rorbitofrontal cortex | −36, 44, −4 | 3.83 svc | |
| 8 | R periaqueductal grey | 4, −24, −6 | 2.80 svc | |
| Positive Expectancy (pre>post) | 96 | L ventral striatum | 12, 8, −10 | 4.05 svc |
| 6 | R ventral striatum | −10, 8, −6 | 2.77 svc | |
| Positive Expectancy (post>pre) | no brain region above the threshold | |||
| Neutral (post>pre) | 348 | R posterior insula | −42, −16, −2 | 3.66 svc |
| 126 | R hippocampus | −30, −10, −28 | ||
| 63 | R amygdala | −24, −4, −28 | 3.42 svc | |
| 9 | L amygdala | 16, −2, −20 | 3.12 svc | |
| 1479 | R precuneus | −4, −34, 54 | 3.32 | |
| Neutral (pre>post) | no brain region above the threshold | |||
PFC=prefrontal cortex, L=left, R=right. For regions of interest (ROI), results were significant at PFWE<0.05 after small volume correction (svc). Other results were significant at voxel-wise p<0.005, uncorrected for 20 contiguous voxels.
Figure 6.
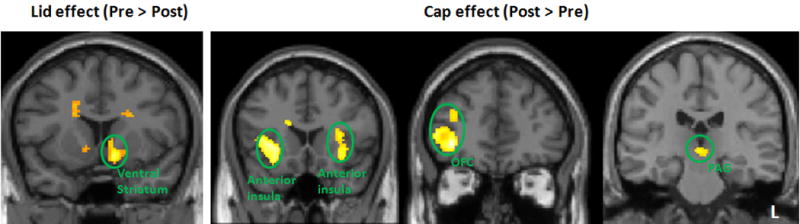
The positive (Lid) and negative expectancy (Cap) effect. The bar indicates the peak beta of each brain regions pre- and post-treatment. OFC: orbitoprefrontal cortex; PAG: periaqueduct gray; L: left side.
When we further explored the data by comparing post minus pre differences in response to identical pain stimuli in the “Capsaicin” and neutral sites [“Capsaicin” (post > pre) > neutral (post > pre)], no brain regions passed the significance threshold. The opposite contrast, [neutral (post > pre) > “Capsaicin” (post > pre)], revealed significant brain activation differences in the bilateral amygdala, hippocampus and superior temporal gyrus (Table 5). When we subtracted the fMRI signal difference of pre-treatment from post-treatment during pain application in the “Lidocaine” condition from the same difference in the neutral condition [i.e. Lidocaine (post > pre) > neutral (post > pre)], no brain regions passed the significance threshold. The opposite contrast revealed significantly greater fMRI signal changes in the bilateral ventral striatum, right putamen, left vmPFC, precentral gyrus, and occipital cortex (Table 5).
Table 5.
Results of a paired t-test comparing fMRI signal change differences (post-treatment pain>pre-treatment pain) between nocebo and neutral control, as well as between placebo and neutral control.
| Contrast | Voxels | Brain area | Peak Coordinate (x, y, z) | Z value |
|---|---|---|---|---|
| Neutral > Negative Exp | 75 | L hippocampus | 14, −10, −22 | 3.30 svc |
| 100 | R hippocampus | −28, −10, −24 | 3.36 svc | |
| 38 | L amygdala | 16, −2, −20 | 3.47 svc | |
| 100 | R amygdala | −26, −10, −22 | 3.22 svc | |
| 402 | L superior temporal sulcus | 32,0, −42 | 3.71 | |
| 218 | R superior temporal sulcus | −54,14, −26 | 3.69 | |
| Negative Exp > Neutral | no brain region above the threshold | |||
| Neutral > Positive Exp | 151 | R putamen | −28,2, −6 | 3.86 svc |
| 37 | L caudate | 8,10,6 | 3.35 svc | |
| 8 | R ventral striatum | −10,8, −6 | 2.83 svc | |
| 26 | L ventral striatum | 10,8, −12 | 3.16 svc | |
| 217 | L ventromedial PFC | 8,58, −2 | 3.34 svc | |
| 4848 | L occipital cortex | 14, −72,18 | 4.04 | |
| L precentral gyrus | 44, −16,30 | 4.41 | ||
| Positive Exp >Control | no brain region above the threshold | |||
| Negative Exp > Positive Exp | 5 | R periaqueductal grey | −2,−28,−6 | 2.97 svc |
| 143 | R anterior insula | −26,20,−2 | 3.79 svc | |
| 61 | R orbitofrontal cortex | −38,46,−4 | 3.83 svc | |
| Positive Exp > Negative Exp | 99 | L ventral striatum | 12,8,−10 | 3.85 svc |
PFC=prefrontal cortex, L=left, R=right. For regions of interest (ROI), results were significant at PFWE<0.05 after small volume correction (svc). Other results were significant at voxelwise p<0.005, uncorrected for 20 contiguous voxels.
Association between positive and negative expectancy-evoked brain response in pain related brain regions
To explore the within subject association between the brain response changes in the pain matrix after application of “Capsaicin,” “Lidocaine,” and neutral creams, similar to our behavioral analysis, we extracted the average beta values of all pain-sensitive brain regions (from the initial contrast as shown in Figure 3) (Eippert et al., 2009; Wager et al., 2013) across different conditions and performed a similar analysis to the one we conducted for subjective pain rating changes.
We first applied a Pearson correlation among the three conditions. We found a non-significant trend for the association between “Capsaicin” and neutral conditions (r = .35, p = .093), but no other correlations approached significance. These results are different from the results of the behavioral analysis. We also regressed the “Capsaicin” brain response to calibrated heat pain in the pain matrix on neutral and “Lidocaine” brain response changes. With neutral cream brain response in the pain matrix controlled, the partial correlation between the “Lidocaine” response and the “Capsaicin” response was 0.13 (p=.605), indicating that there is no significant association in brain response to identically calibrated heat pain in the pain matrix between the positive and negative expectancy conditions. These findings are also different from the results based on subjective pain rating changes.
Brain and behavior association
To explore the association between the behavioral and brain responses, we also performed a whole brain voxel-wise regression analysis between subjective pain rating differences and the corresponding brain response in positive and negative expectancy conditions separately. We found that dmPFC [x=2, y=32, z=36, PFWE<0.05, Z=3.67] activity from the pre minus post contrast was positively correlated with self-reported pain rating reduction in the “Lidocaine” condition, i.e. stronger reduction in pain post treatment was associated with weaker activity in the dmPFC post treatment. The activity in the rostral ACC [x=12, y=38, z=12; PFWE<0.05, Z=3.88] showed a negative correlation with the placebo effect (Figure 7).
Figure 7.
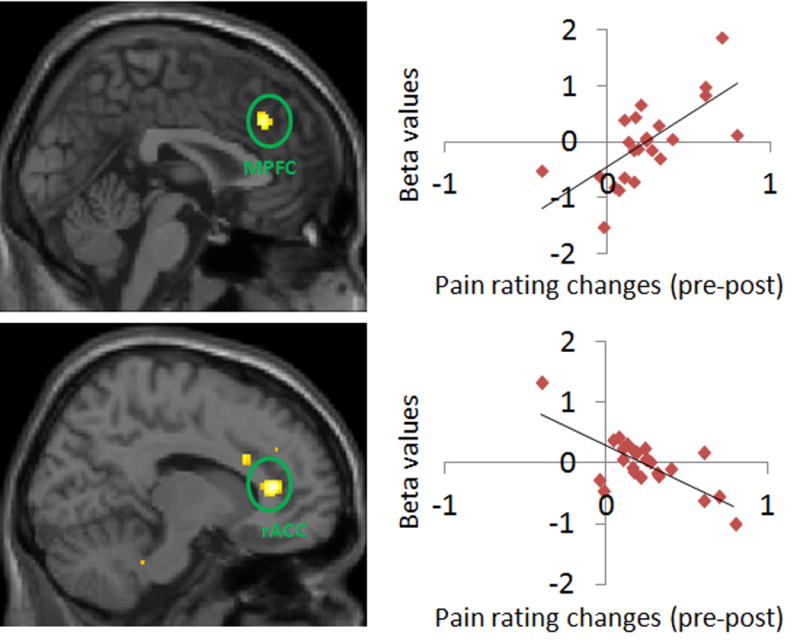
Brain regions showed significant association between fMRI signal change (Pre > Post) and corresponding pain rating changes in positive expectancy condition. MPFC: medial prefrontal cortex; rACC: rostral anterior cingulate cortex.
We did not find a significant association between changes in brain activity and changes in subjective pain ratings in other conditions.
Discussion
In the present study, using a within-subject design, we found that the application of inert “Lidocaine” cream with expectation of pain relief evoked a significant reduction in subjective pain ratings and fMRI signal changes in the striatum, whereas inert “Capsaicin” cream with expectancy of pain enhancement evoked a significant increase in subjective pain ratings and fMRI signal changes in the insula, OFC, and PAG. No overlapping brain regions were identified in response to both the “Capsaicin” and “Lidocaine” conditions at the threshold we set. Regression analysis showed that after controlling for the response bias (changes in the neutral condition), the subjective pain rating changes evoked by positive and negative expectancy were significantly associated. However, we did not observe this association in brain responses to calibrated heat pain in the regions that were activated by the pain. Our findings suggest that while self-reported placebo and nocebo responses are highly associated, this association does not exist in the corresponding brain activity changes in pain-related brain regions; positive and negative expectancy seem to engage different brain networks to modulate the experience of pain.
Independence of positive and negative expectancy in neural responses
To date, there have been many investigations concerning the placebo effect, but only a few studies have focused on within-subject responses to both placebo and nocebo effects. In one placebo analgesia study, Scott and colleagues (Scott et al., 2008) asked subjects to undergo a 20-minute pain challenge and found placebo-enhanced opioid neurotransmission in the anterior cingulate, orbitofrontal and insular cortex, nucleus accumbens, amygdala, and periaqueductal gray, as well as dopamine activation (DA) in the ventral basal ganglia, including the nucleus accumbens. In the same study, five subjects who responded negatively to the pain challenge showed the opposite changes in brain activity: a deactivation of DA, and decreased opioid release in the brain regions mentioned above. In a more recent study, Benedetti and colleagues (Benedetti et al., 2014) found that nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway, which suggests that placebo and nocebo modulation of this type of headache may share the same biochemical pathways, specifically those affected by the non-steroidal anti-inflammatory class of analgesic drugs. In another study, investigators from the same group (Benedetti et al., 2006) found that a cholecystokinin (CCK) antagonist counteracted nocebo-induced hyperalgesia and enhanced placebo analgesia in humans. Overall, these studies suggest that some placebo and nocebo responses may arise from common brain networks.
Results from previous brain imaging studies imply that the brain mechanisms associated with placebo and nocebo may not be entirely the same. For instance, results from one of our previous studies (Kong et al., 2007) suggest that the placebo effect results mainly from top-down processing involving the emotional network, including the rostral ACC, and is mediated by reward-related genes (COMT), personality, and intrinsic resting state brain activity (Yu et al., 2014). In contrast, the nocebo effect is largely associated with the hippocampus and other anxiety-related brain regions (Gondo et al., 2012; Kong et al., 2008; Ploghaus et al., 2001). However, no study has directly compared placebo and nocebo responses in the absence of an active treatment in the same cohort of subjects.
One of the strengths of the present study is that we used a within subject design, which allowed a direct comparison of how positive and negative expectancy can modulate the subjective experience of pain. We found that both positive and negative expectancy effects were correlated with changes in reported pain intensity in the neutral cream condition, suggesting reliable individual differences in response biases across the three conditions. With responses to the neutral cream held constant, within-subject positive expectancy responses were strongly associated with negative expectancy responses. In other words, the placebo effect was highly correlated with the nocebo effect. Our finding that the strong positive association between placebo and nocebo responses was masked by changes in the neutral condition is of methodological importance in designing future studies. Unless a natural history condition is included as a control, as was done in our study, measures of association between placebo and nocebo may be spuriously low.
Conversely, brain responses to identical calibrated heat pain in the pain matrix in the placebo and nocebo conditions controlling for the neutral cream response, did not show association between positive and negative expectancy. Similarly, no overlapping brain regions were identified in the conjunction analysis between the placebo and nocebo effects at the threshold we set. At a liberal threshold of voxel-wise p < 0.05 with 50 continuous voxels, we found overlapping activity in the “Lidocaine” (pre minus post) and “Capsaicin” conditions (post minus pre) in pain-related brain regions, including the bilateral dACC, left insula / OPFC, and left operculum / putaman. These findings are consistent with results from previous studies showing that activity in the insula is modulated by both positive and negative treatment expectations in visceral pain (Schmid et al., 2013). The results suggest that despite that our observation that nocebo effects are distinct from placebo effects at the neural level, it is possible that expression of the two cognitive constructs may share some pain related brain regions as the result of context modulation.
In this study, we did not find the same association between the brain response to calibrated heat pain in pain related brain regions and subjective pain rating changes. We speculate this may be due to complicated mechanisms underlying the placebo and nocebo effects. Theoretically, an inert treatment could function through three interrelated processes to produce placebo and nocebo effects (Amanzio et al., 2013; Kong et al., 2007). In the first stage, prior to the experience of pain, the expectation or anticipation of pain relief/enhancement can modulate the perception of subsequent pain stimuli. In the second stage, during administration of painful stimuli, an inert treatment may inhibit/enhance the incoming signals of noxious stimuli. Finally, in the third stage, when the pain stimulus has ended and when subjects are required to evaluate the pain intensity, memory of previous experience/context may subconsciously distort the decision-making process, a construct we have described as selective distortion of pain intensity evaluation (Kong et al., 2007).
Overall, placebo and nocebo effects aggregate the contribution of all three stages. The extent to which each stage contributes varies under different circumstances and by individual. Distinguishing between the aforementioned placebo and nocebo contributions under different conditions remains challenging. Since the brain responses in the pain-related brain regions to calibrated heat pain only represent the response in one stage, fMRI mainly assesses phasic, i.e. stimulus-evoked, responses. It is quite possible that more tonic, modulatory neuronal changes are correlated between nocebo and placebo conditions. This may be the reason that the association observed in subjective pain rating cannot be observed in brain responses in the pain matrix to calibrated pain.
Positive expectancy and the reward network
Results from recent studies suggest that placebo administration with expectation of pain relief can be regarded as a specific form of reward processing that recruits activity in reward regions such as the striatum (Benedetti, 2009; de la Fuente-fernandex et al., 2002; Leknes et al., 2011; Petrovic et al., 2005; Schweinhardt et al., 2009; Scott et al., 2007). It has been shown that individual variation in ventral striatal response to reward expectation accounted for 28% of the variance in the magnitude of placebo analgesia (Scott et al., 2007). Individual differences in placebo response were significantly associated with dopamine release under placebo and subsequent activity in reward tasks (Schweinhardt et al., 2009). One of our previous studies (Yu et al., 2014) showed that dopamine-related measurements including baseline ventral striatum coherence, functional variation in the COMT gene, and openness to experience together could predict conditioning cue responses in healthy individuals, which highlights a strong link between placebo responsiveness and brain reward processing. In the present study, after the expectancy manipulation in Session 2, participants believed that the inert “Lidocaine” cream could reduce their pain sensation. Conversely, during post-treatment pain application, this positive expectancy was eliminated as a result of very subtle pain reduction. This may explain why we found ventral striatum activation to be stronger in the pre-treatment “Lidocaine” condition. This result is consistent with a recent study, in which Wrobel and colleagues (Wrobel et al., 2014) found that the ventral striatum might not be causally involved in placebo analgesia, but rather linked to phenomena associated with placebo analgesia, such as reward processing and learning.
We also found a correlation between the placebo effect and activity in the dmPFC, which is a part of the pain matrix, implicated emotion regulation (Kong et al., 2007) and pain modulation (Fields, 2000; Kong et al., 2013; Kong et al., 2007; Kong et al., 2010b). This finding suggests that the dmPFC could signal pain relief as a result of the positive expectancy effect. Furthermore, we observed a correlation with the rACC, another region involved in pain regulation, indicating that increased activity in the rACC during conditioning may be associated with a stronger placebo effect. This result is partly consistent with previous studies (Eippert et al., 2009; Wager et al., 2004) suggesting that the descending pain modulatory system is involved in placebo analgesia. Also, unlike previous studies (Eippert et al., 2009; Wager et al., 2004), which used a relatively long duration of 30 seconds (Eippert et al., 2009; Wager et al., 2004) and only post-treatment pain (in these studies, there is no pre-treatment pain applied, authors compared the brain responses evoked by identical pain at different spots with placebo and control creams), we used a much shorter stimulation duration of 12 seconds and compared pre- and post-treatment differences at different spots. We speculate that this may be the reason for this discrepancy between the present study and previous studies.
Negative expectancy response and the anxiety network
Although there are accumulating studies investigating the neural basis of positive expectancy effect, far less research is available on the negative effect. During expectation of high pain at the nocebo site, subjects are likely to feel anxious. Behavioral studies have highlighted a dominant role of cholecystokinin (CCK) in nocebo hyperalgesia via anticipatory anxiety mechanisms (Benedetti et al., 1997; Benedetti et al., 2006). Our current study shows the relevance of a wider network of regions including the insula, OFC, dmPFC, and PAG in the nocebo response. Investigators have previously shown that anxiety is associated with the functional connectivity between the amygdala and the insula (Baur et al., 2013; Shah et al., 2009; Stein et al., 2007) as well as the coupling between the amygdala and the OFC (Hahn et al., 2011; Sladky et al., 2013). The activity in the PAG and the OFC has also been often related to the processing of anxiety associated with anticipating nociceptive stimuli (Brodersen et al., 2012; Fairhurst et al., 2007). Our results provide further neural evidence to support a close link between nocebo and anxiety.
Previous neuroimaging studies have also shown that nocebo effects are mediated by the hippocampus and regions involved in anticipatory anxiety processing (Bingel et al., 2011; Kong et al., 2008; Ploghaus et al., 2001). In this study, we did not find activation of the hippocampus. We speculate that this may be due to the strength of nocebo expectancy. In our previous study (Kong et al., 2008), during Session 2 (the manipulation session), we applied mild pain before administration of sham acupuncture treatment, and applied high intensity pain after sham acupuncture treatment. In this study, since both positive and negative expectancy manipulations were involved, the pre-treatment stimuli were moderate intensity and the post-treatment stimuli were high intensity. Thus, the anxiety level evoked in this study may be weaker compared to our previous experiment.
Neutral cream condition applied in a positive and negative expectancy context
It is important to note that the post minus pre contrast in the neutral condition in our study activated both the anxiety network and the reward network. This might be due to the unique design we used in which all participants experienced nocebo, neutral, and placebo conditions. In this within-subject design, the perception of a neutral condition might have been context-dependent. Previous studies have shown that outcome processing is highly sensitive to the range of possible outcomes from which the final outcome is selected (Akitsuki et al., 2003; Nieuwenhuis et al., 2005). Thus, we speculate that the neutral condition in our study was processed as nocebo if individuals used placebo as a reference.
One potential limitation of this study is that creams were colored and that this color-coding was not randomized. The colors of the creams were very light and only served to differentiate them when the containers were opened. When the creams were applied to the skin, there was no visible difference between them in terms of color. Furthermore, in Session 3 (the fMRI session), subjects could not see the cream when it was applied to their arm due to their placement in the MRI scanner. Thus, it is unlikely that observations of the colors of the creams influenced the responses of the subjects. Additionally, since we did not have an anticipation stage in our study, we were not able to explore brain networks associated with anticipation.
In conclusion, we found that positive and negative expectancy can significantly modulate pain experience. Our behavioral results suggest that after controlling the neutral condition, the subjective pain rating changes evoked by positive and negative expectancy were significantly associated, suggesting some common psychological / cognitive processes underlying them. However, this association was not observed in brain responses to calibrated heat pain in the pain matrix. No significant overlap was revealed in the brain networks activated by placebo and nocebo conditions either, suggesting that positive expectancy and negative expectancy engage distinct neural networks rather than acting in opposite directions within a common network.
Acknowledgments
This work was supported by R01AT006364 (NCCAM) to Jian Kong, P01 AT006663 to Bruce Rosen / Randy Gollub, and R01AT005280 (NCCAM) to Randy Gollub.
Footnotes
There is no conflict of interest for any of the authors.
References
- Akitsuki Y, Sugiura M, Watanabe J, Yamashita K, Sassa Y, Awata S, Matsuoka H, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Context-dependent cortical activation in response to financial reward and penalty: an event-related fMRI study. Neuroimage. 2003;19:1674–1685. doi: 10.1016/s1053-8119(03)00250-7. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34:738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Corazzini LL, Vase L, Benedetti F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain. 2009;146:261–269. doi: 10.1016/j.pain.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett. 2012;520:140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. Jama. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Langer N, Jancke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Placebo Effects: Understanding the Mechanism in Health and Disease. Oxford University Press; New York: 2009. [Google Scholar]
- Benedetti F, Amanzio M, Casadio C, Oliaro A, Maggi G. Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain. 1997;71:135–140. doi: 10.1016/s0304-3959(97)03346-0. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Durando J, Vighetti S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain. 2014;155:921–928. doi: 10.1016/j.pain.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Brodersen KH, Wiech K, Lomakina EI, Lin CS, Buhmann JM, Bingel U, Ploner M, Stephan KE, Tracey I. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage. 2012;63:1162–1170. doi: 10.1016/j.neuroimage.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H, Miller FG. Lessons from recent research about the placebo effect–from art to science. Jama. 2011;306:2612–2613. doi: 10.1001/jama.2011.1850. [DOI] [PubMed] [Google Scholar]
- Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Carlino E, Frisaldi E, Benedetti F. Pain and the context. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- Cleophas TJ. The importance of placebo effects. Jama. 1995;273:283. doi: 10.1001/jama.273.4.283b. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. Jama. 2012;307:567–568. doi: 10.1001/jama.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- de la Fuente-fernandex R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet-Neurology. 2002;1:85–91. doi: 10.1016/s1474-4422(02)00038-8. [DOI] [PubMed] [Google Scholar]
- Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357:757–762. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128:101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain. 2005;114:3–6. doi: 10.1016/j.pain.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Geuter S, Buchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci. 2013;33:13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo M, Moriguchi Y, Kodama N, Sato N, Sudo N, Kubo C, Komaki G. Daily physical complaints and hippocampal function: an fMRI study of pain modulation by anxiety. Neuroimage. 2012;63:1011–1019. doi: 10.1016/j.neuroimage.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath PA, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978a;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath PA, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978b;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6:218ra215. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–1725. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan T, Rosen B, Smoller JS, Kaptchuk TJ, Gollub R. Functional connectivity of frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154:459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci. 2007;18:173–190. doi: 10.1515/revneuro.2007.18.3-4.173. [DOI] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub RL. An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage. 2009a;47:1066–1076. doi: 10.1016/j.neuroimage.2009.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Webb JM, Kong JT, Sasaki Y, Polich GR, Vangel MG, Kwong K, Rosen B, Gollub RL. Functional neuroanatomical investigation of vision-related acupuncture point specificity–a multisession fMRI study. Hum Brain Mapp. 2009b;30:38–46. doi: 10.1002/hbm.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: Activations, deactivations and their relation. Pain. 2010a;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010b;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspect Biol Med. 2009;52:518–539. doi: 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25:1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen GL, Finnerup NB, Colloca L, Amanzio M, Price DD, Jensen TS, Vase L. The magnitude of nocebo effects in pain: A meta-analysis. Pain. 2014 doi: 10.1016/j.pain.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P. Placebo analgesia and nocebo hyperalgesia – two sides of the same coin? Pain. 2008;136:5–6. doi: 10.1016/j.pain.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing–induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Schmid J, Theysohn N, Gass F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. 2013;154:2372–2380. doi: 10.1016/j.pain.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The Anatomy of the Mesolimbic Reward System: A Link between Personality and the Placebo Analgesic Response. J Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Hoflich A, Kublbock M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C. Disrupted Effective Connectivity Between the Amygdala and Orbitofrontal Cortex in Social Anxiety Disorder During Emotion Discrimination Revealed by Dynamic Causal Modeling for fMRI. Cereb Cortex. 2013 doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Thomas KB. The placebo in general practice. Lancet. 1994;344:1066–1067. doi: 10.1016/s0140-6736(94)91716-7. [DOI] [PubMed] [Google Scholar]
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Herve PY, Mazoyer B. Neuroanatomy: tool for functional localization, key to brain organization. Neuroimage. 2007;37:1059–1060. doi: 10.1016/j.neuroimage.2007.02.007. discussion 1066–1058. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wagner K, Frings L, Quiske A, Unterrainer J, Schwarzwald R, Spreer J, Halsband U, Schulze-Bonhage A. The reliability of fMRI activations in the medial temporal lobes in a verbal episodic memory task. Neuroimage. 2005;28:122–131. doi: 10.1016/j.neuroimage.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wrobel N, Wiech K, Forkmann K, Ritter C, Bingel U. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex. 2014;57:60–73. doi: 10.1016/j.cortex.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: Integrating genetics, personality, and intrinsic brain activity. Hum Brain Mapp. 2014;35:4583–4593. doi: 10.1002/hbm.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


