Abstract
Rhythmic brain activity at low frequencies (<12 Hz) during rest are thought to increase in neurodegenerative disease, but findings in healthy neurocognitive aging are mixed. Here we address two reasons conventional spectral analyses may have led to inconsistent results. First, spectral-power measures are compared to a baseline condition; when resting activity is the signal of interest, it is unclear what the baseline should be. Second, conventional methods do not clearly differentiate power due to rhythmic versus non-rhythmic activity. The Better OSCillation detection method (BOSC; [10], [65]) avoids these problems by using the signal’s own spectral characteristics as a reference to detect elevations in power lasting a few cycles. We recorded electroencephalographic (EEG) signal during rest, alternating eyes open and closed, in healthy younger (18–25 years) and older (60–74 years) participants. Topographic plots suggested the conventional and BOSC analyses measured different sources of activity, particularly at frequencies, like delta (1–4 Hz), at which rhythms are sporadic (but topographies were more similar in the 8–12 Hz alpha band). There was little theta-band activity meeting the BOSC method’s criteria, suggesting prior findings of theta power in healthy aging may reflect non-rhythmic signal. In contrast, delta oscillations were present at higher levels than theta in both age groups. In sum, applying strict and standardized criteria for rhythmicity, slow rhythms appear present in the resting brain at delta and alpha, but not theta frequencies, and appear unchanged in healthy aging.
Keywords: Rhythms, resting activity, electroencephalography, aging, theta oscillations, delta oscillations, alpha oscillations
1 Introduction
Oscillations, rhythmic fluctuations in neuronal-population activity recorded with electroencephalographic (EEG) or magnetoencephalographic (MEG) sensors, are thought to play important roles in cognitive brain function [57]. Here we consider oscillations at delta (1–4 Hz), theta (4–8 Hz) and alpha (8–12 Hz) frequencies, which, in rest (the absence of a task), have been of particular interest in studies of neurocognitive aging. When reviewing studies of slow, rhythmic activity (under ~12 Hz), a paradox can be seen: task-related oscillations (particularly theta-band) are thought to support cognitive function, whereas oscillations at similar frequencies during rest may signify reduced brain function. However, closer inspection shows that our picture of slow oscillations in neurocognitive aging is unclear.
For example, theta oscillations [62] are of particular interest, as they accompany a rich set of higher cognitive functions including memory, spatial, exploratory and motor tasks and they modulate induction of synaptic plasticity [8]. Theta oscillations are likely important for multiple distinct neurocognitive functions (e.g., [11], [46], [64], [67]). Although evidence of task-related theta oscillations has almost exclusively been collected in young adult participants, there has been a recent surge of interest in slow rhythms, in both the theta, and the slower, delta band, in older adults during rest. There is accumulating evidence that slow rhythms could signal neurocognitive decline and predict the future course of neurodegeneration, but findings are somewhat inconsistent. With regard to theta activity in particular, it is unclear whether resting theta is a sign of healthy cognitive function or not. Our goal here was to understand slow EEG activity in healthy aging. In addition to elucidating healthy brain activity, this could also potentially serve as the electrophysiological comparison point for clinical populations. Specifically, we sought to distinguish rhythmic from non-rhythmic activity, which prior studies of resting EEG in aging have not explicitly done, to evaluate whether resting theta activity, as well as activity in the delta band and at other frequencies, is rhythmic or not, and to test for possible effects of healthy aging on both rhythmic and non-rhythmic resting brain activity.
Theta activity during rest
Increased amplitude and extent of resting theta-band activity may reflect the presence of Alzheimer’s Disease (AD) and may predict when individuals progress from healthy aging to Mild Cognitive Impairment (MCI) and probable AD, with a range of topographies, and using a variety of resting tasks and methods of quantifying power [13], [19], [21], [27], [26], [32], [47], [51]. However, the relationship between theta activity and cognitive function is less clear. For example, [32] correlated theta power (relative to total power) during eyes-closed rest with scores on neuropsychological tests administered at a different time. They found that, in a sample including participants with probable AD, MCI and healthy controls, the correlation was negative in various regions for visuospatial and attention functions, but non-significant for memory tests. However, [23] found frontal theta power (in the 4–6.5 Hz band, normalized by total power) during eyes-closed rest correlated positively with cognitive function as measured by a separate behavioral battery in healthy older adults. [52] found mixed results, with absolute theta power correlating negatively with various cognitive scores at frontal sites, but at temporal and occipital sites, correlating positively with perceptual organization and processing speed tasks. Their relative-power measures correlated negatively with verbal comprehension and working-memory tests with a widespread topography. [3], recording during a 5-minute eyes-closed condition, found no significant correlations between theta (4–8 Hz) power measured across the scalp and their separately administered neuropsychological tests, although they found statistically robust correlations at other frequencies, including delta and alpha. [37] found reduced, not increased, theta (4–8 Hz) absolute power in left frontal and posterior recording sites in participants with the ε4 allele of the Apolipoprotein E gene (ApoE ε4), a risk factor for AD, compared to non-carriers. [25] found that hippocampal atrophy associated with dementia was accompanied by increases in frontal theta (4–8 Hz) power (without normalizing by total power) during a 10-minutes eyes-closed rest condition. [16] found that theta (band defined relative to each participant’s alpha frequency as suggested by) [34] power (not normalized by total power) did not differ during a 3-minute eyes-open rest period, but was greater in their healthy older control group than in their amnestic MCI group during a recognition-memory task.
These mixed results about whether resting theta activity is a sign of neurodegenerative disease or healthy cognitive function are even more confusing, considering that studies of healthy neurocognitive aging also produce mixed results. [17] found frontal and central theta (4.88–6.84 Hz) power (not normalized) during a 3-minute eyes-open condition increased in young (18–27 years) compared to older (60–80 years) participants, both in rest and during a recognition-memory task. They did not report other frequencies, so these effects could possibly be broadband. [66] measured theta (4–7.5 Hz) power as well as other frequency bands, both absolute and relative measures, and during an eyes-closed condition as well as an eyes-open fixation condition. The eyes-closed condition produced no significant age effects, but during eyes-open, theta relative power was greater in young (mean=29) than older (mean=73 years) participants during rest. The picture changed when comparing rest to various cognitive tasks; for the young participants, theta relative power (distributed across electrodes) was lower in rest than during the tasks, but the reverse held for the older participants.
Clearly part of the cause of this variable set of findings is that parameters including rest conditions, recording sites and frequency band limits and ages vary across studies. However, we next consider an additional possible cause of mixed results: that measures of power may include both rhythmic and non-rhythmic signals.
Rhythmic versus non-rhythmic sources of power
One possible cause of ambiguous results is that spectral power observed during rest might not in fact be rhythmic, a question that has often been tagged as an important theme in EEG research (e.g., [34], [39]). According to Fourier’s Theorem, any signal, whether rhythmic or not, can be re-expressed as a weighted sum of periodic functions with varying frequency, amplitude and phase [6]. Consequently, for any EEG signal, there will be some non-zero value for power at any given frequency, but that does not confirm that there are any rhythms present. Narrow peaks in the power spectrum can be very good tell-tale signs of the presence of rhythmic activity [34], [39]. However, this has its limits. First, it is difficult to come up with a clear criterion for how narrow the peak must be to signify a rhythmicity. More seriously, a clear peak in the spectrum would only be expected for oscillations that are both prevalent a large portion of the recording time, and that, over the times they occur, have little variability in frequency. Oscillations that are sporadic may get washed out by power at neighbouring frequencies. Oscillations that are clearly rhythmic for numerous consecutive cycles but can appear at a broad range of central frequencies would be expected to produce a very wide-band peak, which, for example, might be what produces some of the so-called “broadband gamma” activity (e.g. [7], [14], [29]). The Better OSCillation detection method (BOSC) was designed to impose more conservative criteria for identifying segments of EEG signal as rhythmic. It requires that power at a frequency, f, be elevated above a minimum threshold power value, PT(f) for a minimum duration, DT(f) thus requiring that the signal show some evidence of repeating. The thresholds are derived by modeling the statistical properties of the signal (see Methods). One advantage of the BOSC method is that it corrects for the “colored-noise” form of the power spectrum, wherein lower frequency components of the signal tend to be larger than higher frequency components. The BOSC method is sensitive to oscillations even when they are rare and fail to produce a clear peak in the power spectrum, and it is robust to electrode location, species, state of alertness and task condition [10], [12], [9], [15], [20], [30], [33], [43], [44], [48], [60], [63], [65].
Need for a baseline period
Next, consider that conventional measures of spectral power require some reference value for comparison. A resting baseline is often used as the reference, but if resting EEG includes rhythms, these rhythms will be subtracted from task-related activity. Moreover, what happens in the present situation, when the research question is about the resting state itself? Because absolute power depends on factors unrelated to brain-activity per se (e.g., geometry of the head and brain), it is uninformative to compare absolute power values between groups of participants. To eliminate these confounds, researchers often normalize (e.g., [3], [13], [19], [31]) by dividing each power value by the total power (the approach we take here for this conventional measure) or compute ratios of power (e.g., [56]) in one frequency to another (e.g., theta:alpha). Thus, prior measures of slow rhythmic activity during rest have always been relative to power at other frequencies. This introduces an ambiguity: an increase in a ratio could be due to an increase in the numerator or a decrease in the denominator. The BOSC method has an advantage in this regard: no additional “baseline” period is required, and the reference is based on the statistical characteristics of the signal itself. Granted, in one sense, the BOSC method also uses other frequencies as a reference. However, the way in which the power spectrum is used to generate the amplitude threshold values is highly constrained by prior knowledge of the form of the spectrum (1/fα form, and χ2(2) form of the distribution of power values at each frequency), keeping the cross-talk between frequencies to a minimum, and it is relatively robust to the presence of peaks in the spectrum [65].
We suggest that normalization and ratio-measures may be partly responsible for the mixed and even sometimes, contradictory, evidence about resting rhythmic activity in healthy older and younger participants. Thus, one reason to compare the BOSC method to a conventional power measure is to obtain a less confounded measure of rhythms during rest, which could then be comparison states in studies of task-related rhythms (in both young and older participants), and the control data for investigations of resting rhythms in neurodegenerative disease.
Choice of rest “task”
Resting conditions have varied across studies. Some investigators ask participants to keep their eyes closed (e.g., [3], [23], [25], [47], [52], [53]), whereas others ask participants to keep their eyes open (e.g., [17], [16]). This procedural choice could be important, as the very large-amplitude posterior alpha rhythm increases in amplitude when the eyes close [5]. Because conventional spectral analyses compare power across frequencies, a large alpha peak could have a sizeable effect on measures of power at other frequencies. In contrast, the BOSC method should be relatively more robust to modulations in alpha power. We adopted a protocol [4], [45], [65], in which participants are asked to alternate eyes-open with eyes-closed. In addition to enabling a comparison between resting conditions, asking participants to alternate eyes-open with eyes-closed also prevented participants falling asleep, a problem researchers have noted when using eyes-closed conditions [58].
Delta oscillations
Unlike theta activity, there is currently no consistent picture about whether delta activity increases or decreases with cognitive demands [57], although there are plenty of results suggesting that delta-band activity plays a role in cognition. For example, [59] found delta activity progressively reducing with repeated presentations of words in a continuous recognition-memory task [28], [35], see also. Such findings could mean that delta activity indicates poor performance, or alternatively, that delta activity reflects task difficulty, which reduces with repeated trials. Delta-band activity during rest also shows promise as an early marker of AD. Resting delta-band activity has been found to be greater in individuals with MCI and probable AD than healthy controls [3], [21], [27], [51] and may increase with the progression to MCI [13]. Some studies find no differences in resting delta activity [66]. And, [2] found increased delta activity in healthy older (51–85 years) than healthy young (18–50 years) participants during rest, but [38] found decreased delta activity in older than younger participants. Clearly, our understanding of delta oscillations during rest in neurocognitive aging is just as unclear as it is for theta oscillations. We were therefore also interested in the delta band, asking whether or not delta-band signal during rest is rhythmic, and if delta rhythms or delta power differed between young and older participants.
Design of the study
We recorded EEG during rest, alternating 5-s intervals of eyes-open and eyes-closed, in Young adult participants and Older adult participants. We asked three research questions: 1) When do conventional spectral measures and the BOSC method agree, and when do they diverge? We predicted that for more dominant rhythms (larger amplitude and present for a greater proportion of the time), the measures would be most consistent, and for more sporadic rhythms, the two methods may diverge considerably. 2) Is there any detectable and meaningful rhythmic theta activity during rest? 3) Do healthy older adults have increased theta or delta power, and if so, is this low-frequency signal rhythmic?
2 Methods
2.1 Participants
The human EEG is thought to stabilize by age 18, and then change further after age 60 [57]. We therefore recruited 18–30-year-old (“Young”) and 60–74-year-old (“Older”) participants. Participants were screened in a pre-intake phone interview for gross brain injury and head trauma, epilepsy, nervous system or psychiatric disorder (past, present, and family history), diabetes, and major heart attack. Younger participants were recruited from the introductory psychology pool at the University of Alberta, in exchange for course credit. N = Young participants were included in the analyses, after participants were excluded due to subjectively reported anxiety disorders and due to excessive artifacts. Older participants were recruited through ad placements in a local newsletter and by word of mouth, and were paid an honorarium. Older participants were pre-screened and were not tested if they reported anxiety disorders. N = Older participants were included in the analyses after exclusion due to excessive artifacts and were excluded because their Mini Mental State Examination (MMSE) score was in the impaired range (< 27/30). Procedures, including informed consent, were approved by a University of Alberta ethical review board.
2.2 Experimental procedure
Participants were administered a Personal Data Sheet and the Mini Mental State Examination (MMSE).
The final Young group had significantly higher MMSE scores than the final Older group, t(27)= 2.26, p < 0.05, mean ± standard deviation=29.6 ± 1.2 (Young) and 28.7 ± 1.2 (Older). Years of education did not differ significantly between groups, t(27)= −0.95,p > 0.1. The Young group had equal numbers of male and female participants (8 each), whereas the Older group had more females (N = 9) than males (N = 4). Participants next performed a recognition-memory task, not reported here.
Finally, to examine resting EEG, participants performed an alternating eyes-open/eyes-closed task, which was a longer version of the procedure used by [65], presented in E-Prime (Psychology Software Tools Inc., Pittsburgh, PA). Participants received verbal and written instructions to begin with their eyes open, and to close (or open) their eyes gently upon hearing a beep. The beep was presented every 5 s, with 61 beep presentations in total, constituting 5 minutes.
2.3 Data collection
Participants were fitted with a 256-channel HydroCell geodesic sensor net (EGI; Electrical Geodesics Inc., Eugene, OR) with electrode impedances kept below 50 kΩ [22]. Participants were shown the recording artefacts produced by eye blinks, jaw clenching, limb movement, and other common tics to demonstrate the importance in remaining still. Signal was initially recorded referenced to Cz, amplified by the EGI NetAmps 300 amplifier with a 400-Hz anti-aliasing hardware filter, digitized at 250 Hz and acquired via NetStation software using a 24-bit A/D converter.
2.4 Data analysis
Analyses were conducted in MATLAB (The Mathworks, Natick, MA), supplemented by EEGLAB [18]. Signal was digitally filtered (bandpass: 0.1–50 Hz). Bad channels were interpolated when necessary. Signal at each channel was re-referenced to the average across electrodes. Independent component analysis [41] was conducted to identify and remove artifacts such as eye blinks and saccades.
2.4.1 Conventional power analysis
The continuous recording was analyzed (without epoching) with a Morlet wavelet transform [24], with a wave number of 6 cycles and frequency sampled logarithmically over the 1–45 Hz range. Wavelet power values were log-transformed, and normalized by dividing each mean log-power value by the sum of log-power across the 1–45 Hz range. For each participant, one power value was obtained at each frequency, at each electrode, averaging over all central time samples in the 30 epochs of a given condition (eyes open or closed), and statistical tests were then done at the subject level.
2.4.2 BOSC analysis
The BOSC method identifies segments of signal during which wavelet power at a given frequency, f, exceeds a power threshold, PT(f), for a minimum duration, of DT(f) consecutive time samples. DT(f) scales with frequency: DT = 3/f. PT(f) is derived from the theoretical background distribution of power values at each frequency as follows. The background EEG spectrum is assumed to be colored noise, Power(f) = Af−α typical of natural autocorrelated signals [55]. This assumption means we can estimate the background spectrum by fitting it with a linear regression in log–log coordinates. PT(f was set to the 95th percentile of the expected χ2(2) distribution of power values at each frequency with the distribution mean set to the estimated mean from the linear regression step. The reason for the χ2(2) distribution is that wavelet-power values are squared wavelet coefficients, which in turn, are complex numbers. The real and imaginary parts of these complex numbers inherit the approximately Gaussian-distribution of voltage values in the time domain. Squaring these complex numbers produces values that are sums of pairs of squared Gaussian values, one for the real part and one for the imaginary part of the complex number, hence a χ2 distribution with 2 degrees of freedom [50]. DT was calculated based on the duration of three complete oscillation cycles at each frequency, 3/f. Episodes for which both PT(f) and DT(f) were exceeded were tagged as oscillations. An open-source MATLAB library is freely available in the supplementary materials associated with [65]. Our primary measure, Pepisode(f) was the proportion of time during which oscillations at a given frequency, f, were detected. To avoid signal due to the auditory event-related potential response to the beep stimuli, the 1000 ms following each beep was excluded from all analyses. As with power, for each participant, one Pepisode value was obtained at each frequency, at each electrode, averaging over all central time samples in the 30 epochs of a given condition (eyes open or closed), and statistical tests were then done at the subject level.
Topographic maps were rendered with interpolated 3-D splines, in a 2-D spherical view, using EEGLAB’s topolot.m function [18].
3 Results
We examine rhythmic activity at two electrodes of interest, comparing conventional spectral analysis with the BOSC method, and comparing Older versus Young participants (Figures 1–4) to evaluate all our research questions. We then examine the topographic patterns of activity (Figures 5–7) to test if the two methods may be sensitive to different underlying sources, further speaking to research question #1.
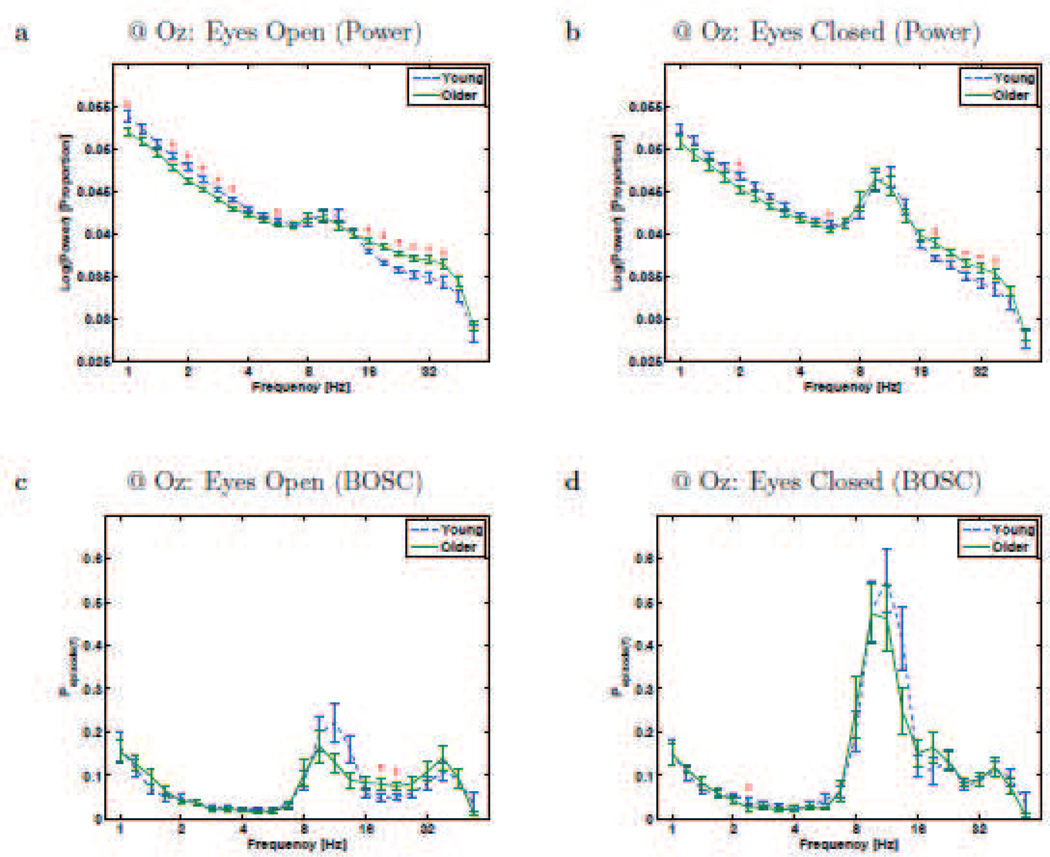
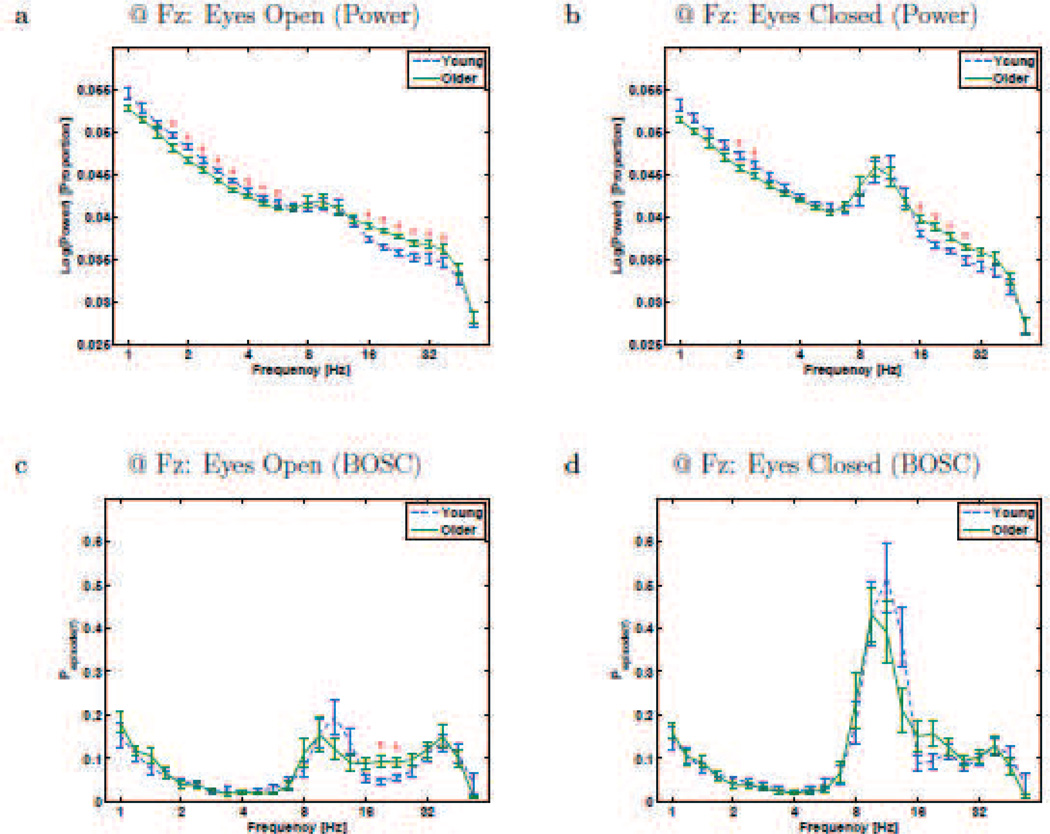
Figure 1.
Resting-state rhythms at Electrode Oz for Young and Older participants. a and b plot conventional power-analysis measure: wavelet power (log-transformed and then normalized by total log-power) as a function of frequency (also on a logarithmic scale). c and d plot the BOSC measure, Pepisode, or proportion of time occupied by oscillations at each frequency. Error bars are standard error of the mean. * - significant difference between Young and Older groups (Mann-Whitney U test, df = 26, p < 0.05, uncorrected).
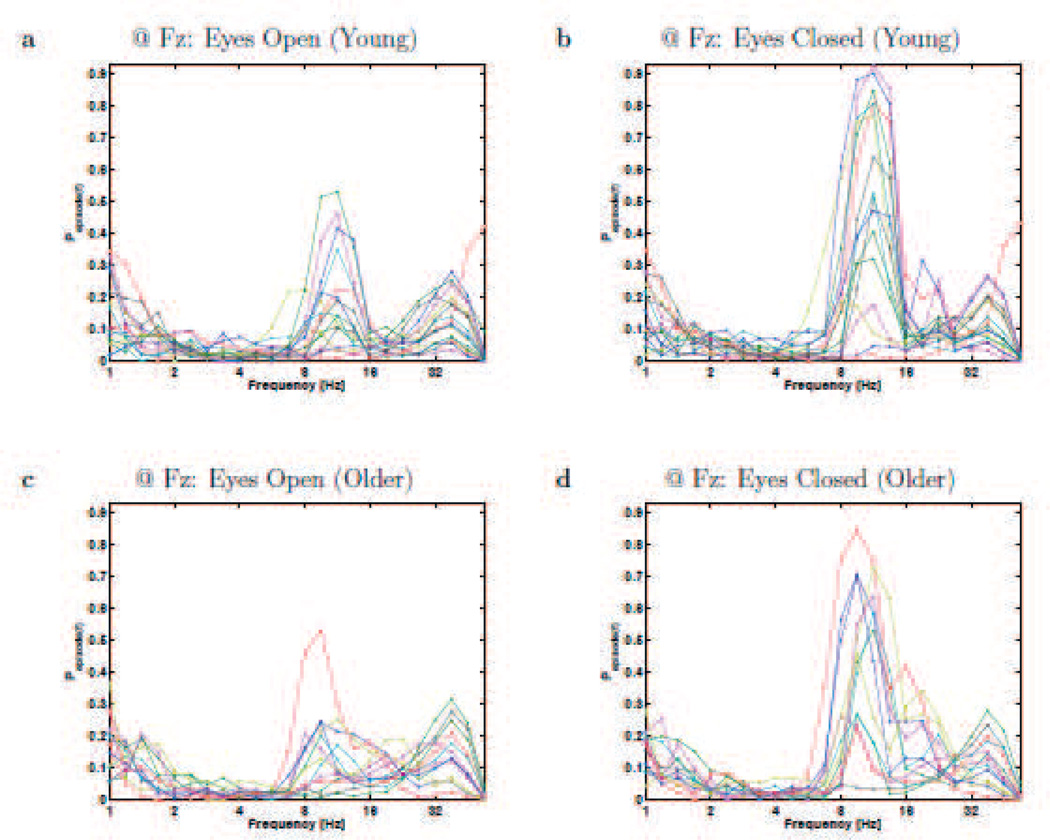
Figure 4.
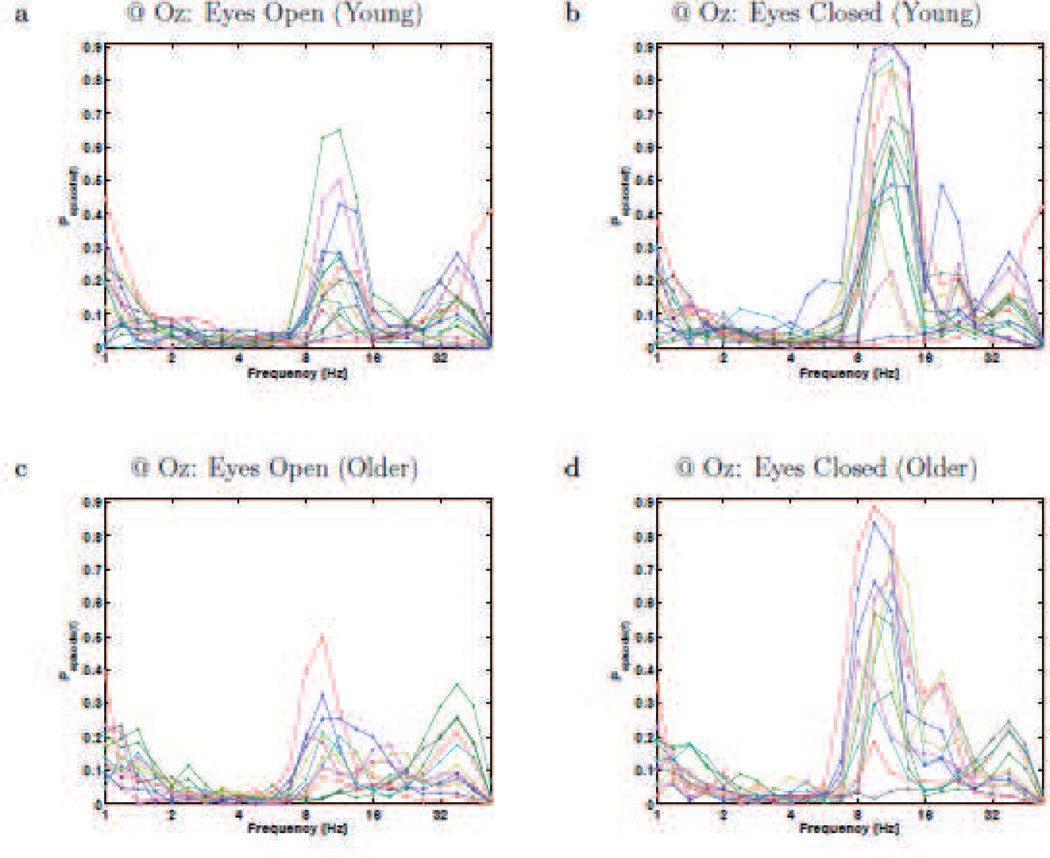
Resting-state rhythms plotted for individual participants at electrode Fz. Each panels plots the BOSC measure as a function of frequency (Pepisode, or proportion of time occupied by oscillations at each frequency), for Young (a,b) and Older (c,d) participants, during eyes-open (a,c) and eyes-closed (b,d). These plots correspond to the means and standard errors that are plotted in Figure 3c,d. Each line graph is for a different participant, and colours cycle through Matlab’s default colour sequence.
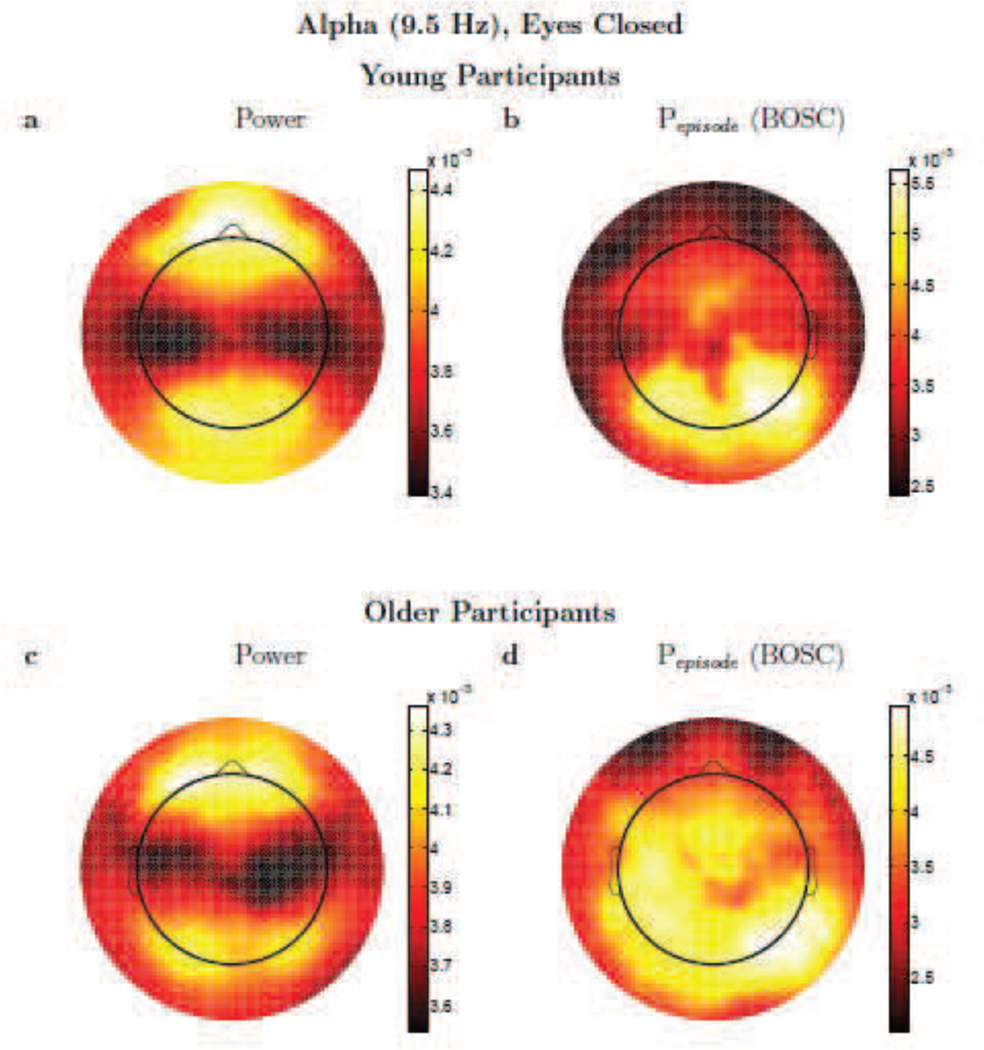
Figure 5.
Alpha activity. Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing alpha (9.51 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log(power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
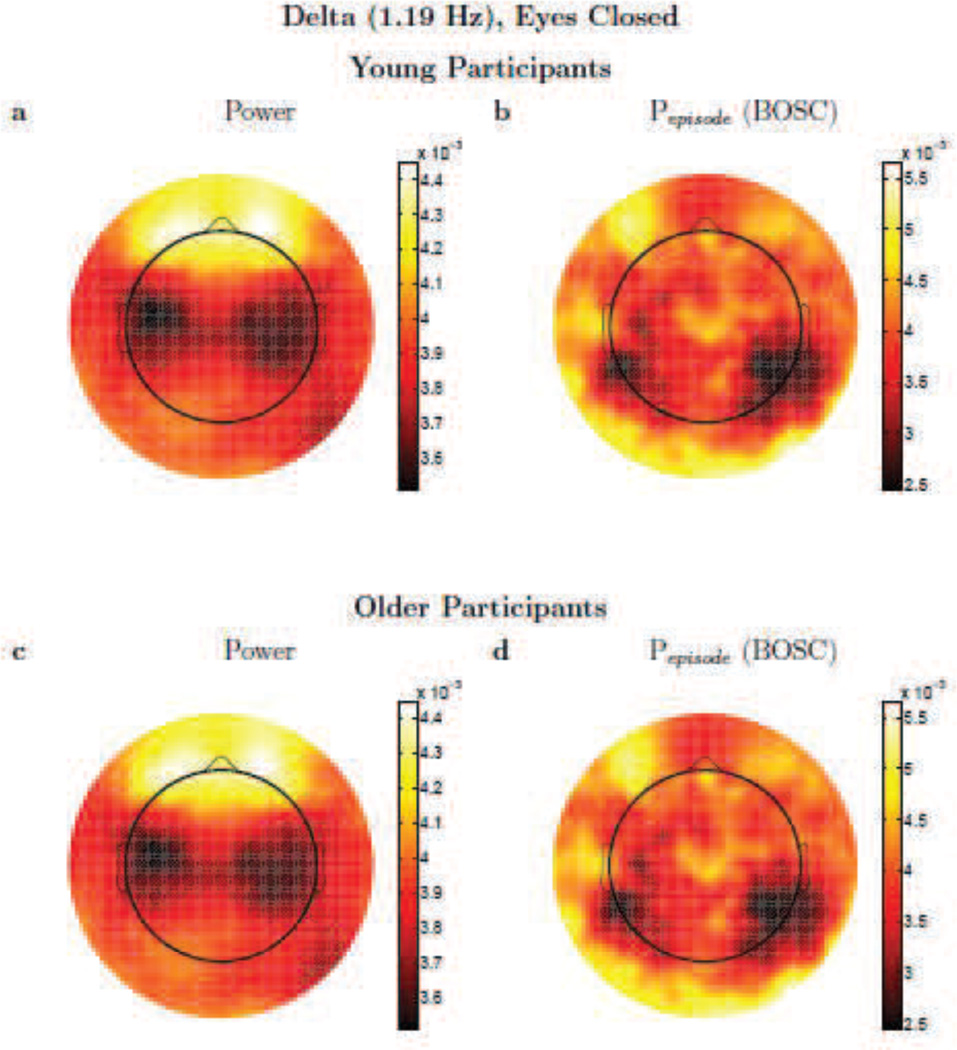
Figure 7.
Delta activity. Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing delta (1.19 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log(power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
3.1 Single-electrode analyses
The upper panels in Figures 1 and 3 plot spectra based on a conventional analysis: the logarithm of power (normalized by total power), measured with wavelets, as a function of frequency, at two example electrodes (Oz and Fz, respectively). One can clearly see the so-called colored-noise form of the power spectrum, where power values are higher for slower frequencies than faster frequencies. Both groups also exhibited the expected alpha-band peak (8–12 Hz, typically ~10 Hz) which was larger with eyes closed than open. The delta and theta bands clearly had non-zero power values, but without a plainly visible peak in the power spectrum, it is unclear if all or any of the power at those frequencies reflects actual rhythms. The BOSC method was then applied to the signal (lower panels). To visualize individual variability, we include single-participant plots in Figures 2 and 4. The measure, Pepisode(f), is the proportion of recording time during which oscillations were detected, as a function of frequency, f. The BOSC plots are much flatter than the power spectra (apart from the peaks), showing that the colored-noise background has been corrected for. The alpha peak remains, suggesting that it indeed reflected rhythmic activity.
Figure 3.
Resting-state rhythms at Electrode Fz for Young and Older participants. a and b plot conventional power-analysis measure: wavelet power (log-transformed and then normalized by total log-power) as a function of frequency (also on a logarithmic scale). c and d plot the BOSC measure, Pepisode, or proportion of time occupied by oscillations at each frequency. Error bars are standard error of the mean. * - significant difference between Young and Older groups (Mann-Whitney U test, df = 26, p < 0.05, uncorrected).
Figure 2.
Resting-state rhythms plotted for individual participants at electrode Oz. Each panels plots the BOSC measure as a function of frequency (Pepisode, or proportion of time occupied by oscillations at each frequency), for Young (a,b) and Older (c,d) participants, during eyes-open (a,c) and eyes-closed (b,d). These plots correspond to the means and standard errors that are plotted in Figure 1c,d. Each line graph is for a different participant, and colours cycle through Matlab’s default colour sequence.
Alpha band (~10 Hz)
The “alpha rhythm,” associated with visual idling or inattention, is one of the most dominant features of the human EEG [57]. Thus, we expected it to be detected comparably with both spectral analysis methods (research question #1). It appeared as a peak at Oz (Figure 1), both in the conventional spectrum and with the BOSC measure, and as expected, it synchronized further when the eyes closed. As in [65], the BOSC method confirmed that there were long runs of alpha-band rhythms, lasting around 50% of the time during eyes-closed. The peak frequency was not dependent on age, and during eyes-closed, we found no significant difference between Older and Young participants, in line with prior studies [57].
Theta band (4–8 Hz)
As can be seen in Figures 1 and 3, the conventional power measure did not show a clear theta-band peak, as usual [34], but neither did the BOSC measure. At Oz during both eyes-open and eyes-closed, the conventional measure showed significantly greater theta-band power (at 5.66 Hz at Oz, and at both 5.66 Hz and 4.76 Hz at Fz) for Young than Older participants; however, this difference did not survive the additional criteria applied with the BOSC method. In addition, the Pepisode values within the theta band were low, quite near the false-detection rate one expects due to the 95th percentile power threshold, which caps the false-detection rate at 0.05, followed by the duration threshold, which reduces that upper limit a bit further. Therefore, our recordings showed little evidence of the presence of theta rhythms at all, answering research question #2, and suggests that prior reports of age-differences in theta power are due to non-repeating signal (research question #3).
Delta band (1–4 Hz)
The wavelet power spectrum was sizeable in the delta-frequency range. This could also be observed with the BOSC method, suggesting that activity at low frequencies may indeed reflect rhythms. Interestingly, at both Fz and Oz, there was significantly more power at numerous delta frequencies in the Young group. However, this effect did not remain when the BOSC measure was used. This may be in part due to the normalization in the conventional method over-correcting for power differences at other (beta, in this case) frequencies. This demonstrates how conventional methods can be misleading. In fact, although non-significant, the BOSC measure during eyes-open was nominally higher in the Older group at frequencies under 2 Hz. It is possible that this is related to prior reports of “slowing” of the EEG in healthy aging [57]. Unexpectedly, at 2.38 Hz, at Oz during eyes-closed, the BOSC measure showed more oscillations in Young than Older participants. Overall, we found no evidence of increased delta activity, rhythmic or non-rhythmic, in older participants (research question #3).
Beta/Gamma band (>12 Hz)
Although we had not anticipated it (but not unprecedented; see Discussion), there were age differences in the beta band. Older participants exhibited more beta-band power during both eyes-open and eyes-closed, although only significantly with the BOSC measure in the eyes-open condition. One can see in Figure 4 that the beta effects are not due to one or two participants, but also that not all Older participants have elevated levels of beta oscillations. Further, this figure shows that the beta oscillations do not appear as a clearly distinct peak; rather, some participants’ beta oscillations might be an extension of either of the two peaks (alpha and gamma) that sandwich it.
3.2 Topographic patterns and presumed sources
The BOSC method is more selective for rhythmic (repeating) activity than conventional power-spectrum measures. We reasoned (research question #1) that for oscillations that are high-amplitude and present for much of the recording time, the two measures should agree, since the power would clearly be mainly measuring power of that rhythmic activity. For frequencies for which oscillations are sporadic, we expected the two measures to diverge. Thus, for sporadic oscillations, power may be predominantly measuring a characteristic of non-rhythmic (non-repeating) activity (within a narrow spectral band), and thus, potentially different sources of brain-activity as well. The topographic plot of the measure gives some indication of its source.1
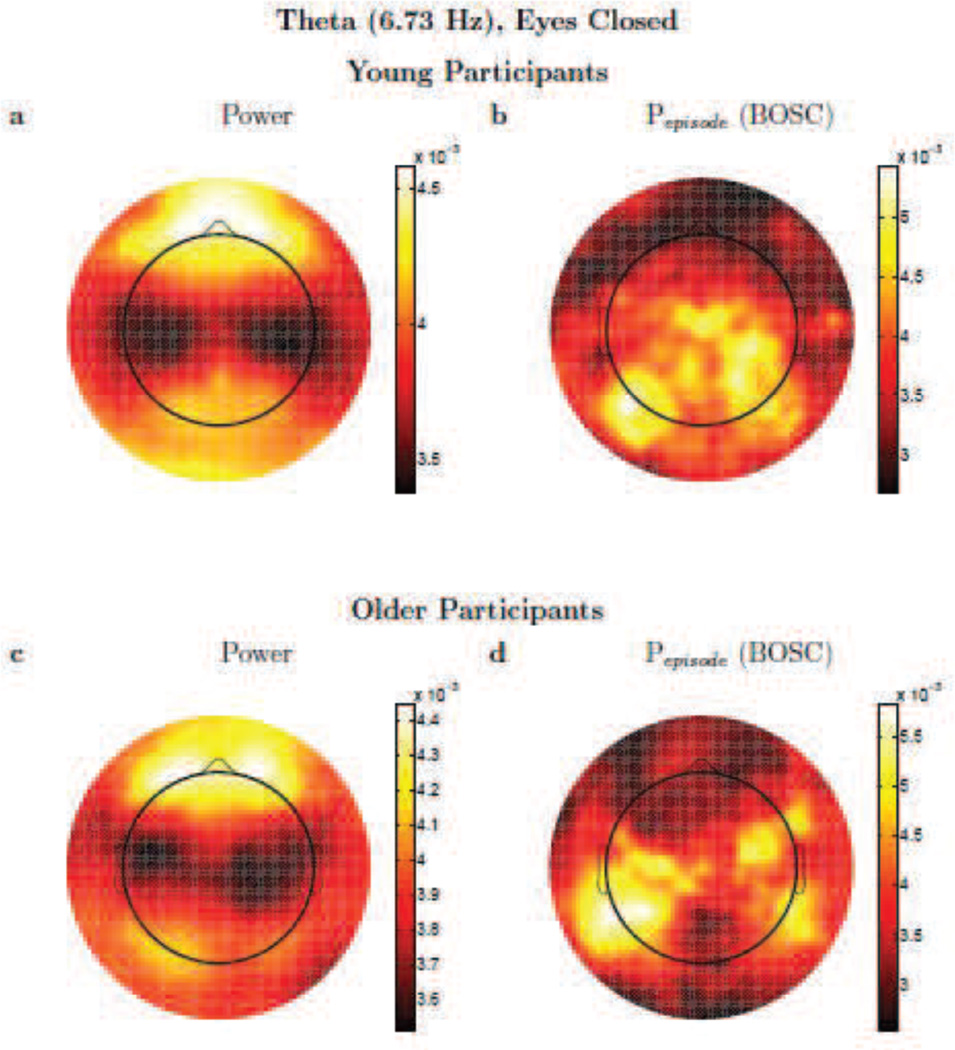
One can see in Figure 5 that the topographic patterns, for eyes-closed alpha activity, are moderately similar between the power and BOSC measures. This holds for both Young and Older participants, but the topographies did differ in potentially important ways, suggesting that the two measures may also be sensitive to different sources, even when the very robust alpha rhythm is present. Unexpectedly, there was considerable alpha-band power at anterior electrodes, but much of that anterior power apparently was not due to rhythmic, repeating signals, as Pepisode values were quite low at anterior sites. In Figure 6, one can see what happens when rhythmic activity is deemed to be rare; in this case, the topographic profile of theta power is quite different than the topographic profile of theta oscillations as detected with the BOSC method. Finally, in the delta band (Figure 7), the topographic plots were also quite different between measures. Although the BOSC method detected levels of rhythmic delta-band activity that were comfortably above the false-positive rate, suggesting that delta rhythms were in fact present, they were still somewhat rare. At such low frequencies, delta power was evidently mostly measuring something very different than the delta-band rhythmic activity detected by the BOSC method.
Figure 6.
Theta activity. Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing theta (6.73 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log(power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
To quantify the similarity of these topographic patterns (expressed as value as a function of electrode), we use the mean-centered (mean value across all electrodes is subtracted from the value at each electrode), normalized dot product.2 This normalized dot product between two topographies can thus take on values from −1 to +1, and can be interpreted much like a Pearson correlation. The higher the dot product, the greater the similarity. Mean dot products between the power and BOSC measures for Young participants were (mean ± SD) alpha-band: 0.43 ± 0.27; theta-band: 0.05 ± 0.26, and these were significantly different by a paired-samples Mann-Whitney U test: U = 213, df =15, z = 3.20, p < 0.01. The delta-band also had relatively low dot products: 0.11 ± 0.32, also significantly below the dot products in the alpha band, U = 203, df =15, z = 2.83, p < 0.005. For Older participants, mean dot products between the power and BOSC measures were, alpha-band: 0.38 ± 0.32; theta-band: 0.11 ± 0.31; and these were also significantly different, U =124, df =11, z = 2.03, p < 0.05. Dot products for the delta-band were also somewhat low: 0.28 ± 0.32 but not significantly lower than the alpha-band dot products.
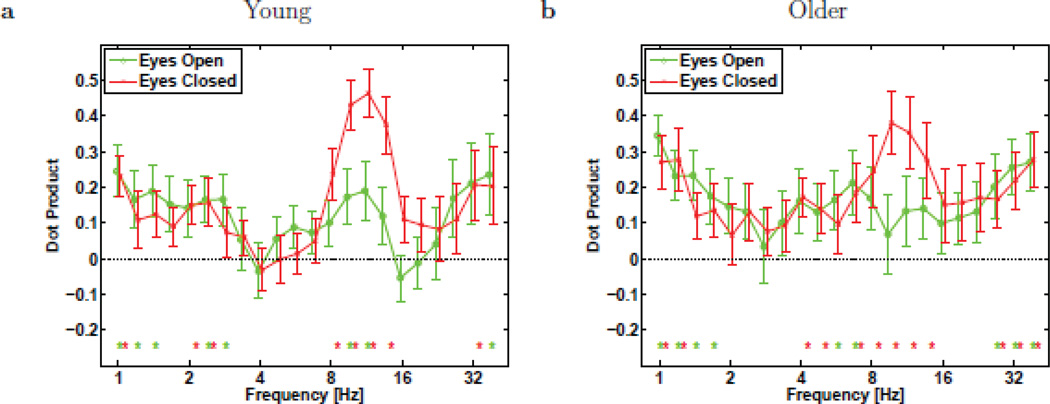
To get a more complete picture of how the topographic similarity depends on frequency, Figure 8 plots the dot products between wavelet power and BOSC topographic maps at all frequencies, for both eyes-open and eyes-closed, and for both Young and Older participants. The dot products were fairly constant, rising somewhat at low delta frequencies, and were highest during eyes-closed at alpha frequencies. This is consistent with the idea that the more prevalent an oscillation is, the more the conventional power measure will be driven primarily by that rhythmic activity, whereas conversely, the more sporadic rhythmic activity is, the more conventional power measures and the BOSC method must be measuring different brain-activity, as we had predicted for our research question #1 (see similar reasoning by [49]).
Figure 8.
Mean dot product of topographic patterns (e.g., Figs. 5–7) between the log(power) and BOSC measures, for Young (a) and Older (b) participants, computed separately for eyes-open (o) and eyes-closed (×) conditions. Error bars denote standard error of the mean. Asterisks denote dot product significantly non-zero (p < 0.05, Mann-Whitney U test with df = 15 and 11 for Young and Older groups, respectively).
4 Discussion
The BOSC method, with its improved selectivity for rhythmic (repeating) signals, and lack of need to normalize by total power, was able to confirm the presence of oscillations in the alpha, delta and beta bands, but cast doubt on the presence of truly rhythmic theta-band activity during rest. Age differences using the conventional power measure appear to be largely due to normalization artifacts [31], a problem avoided by the BOSC measure. Our findings suggest that low-frequency rhythmic activity during rest remains stable across healthy adulthood.
Theta activity in rest
Theta-band resting activity is being investigated as an early marker of neurodegeneration, decline or other sources of cognitive dysfunction. Because this seemed at odds with the more common findings linking task-related theta activity to effective cognitive function, we asked if there were theta rhythms during rest in healthy young and older adult brains (research question #2). Without a clear peak in the theta band [34], conventional power measures cannot speak directly to this question. With the BOSC method, our findings suggest that there are no theta oscillations exceeding the false-positive rate. This may explain failures to find correlations between theta power and fMRI signal during rest (e.g., [36]). Interestingly, [54] found frontal theta was inversely related to BOLD signal in the default mode network, which may be due to methodological differences, but we note that these authors defined the theta band as 2–9 Hz, which overlaps both with the conventional delta band and the conventional alpha and delta bands. It is unclear if their results were due to power in the theta band at all. In any case, our findings suggest that, at least in healthy young and older adults, theta-band power during rest may not reflect rhythmic activity, and perhaps not activity in a single, simple source. Our findings thus inform our understanding of applied studies; if rhythmic theta were to be confirmed as a feature of AD or MCI (i.e., satisfying the BOSC method’s criteria for rhythmicity), our findings suggest that this should be understood as an additional brain-activity pattern that is absent in healthy aging, rather than an exaggeration of a signal associated with healthy aging.
Delta activity during rest
Unlike theta activity, delta activity satisfied the BOSC method’s additional criteria to be considered rhythmic, and at a rate that comfortably exceeded the false-positive rate. Delta-band power did differ between ages, but in the “wrong” direction, with more power in Young than Older participants. We suggest this was due to the normalization. By normalizing by total power, one allows measures of one frequency (in our case, beta-band power) to influence the measure at other frequencies. Interestingly, recording MEG during a fixation eyes-open resting task, [61] found an unusual result: low-frequency power (< 7 Hz) correlated inversely with age. Inspection of their power spectra, however, suggests that this might be an artifact of their normalization, as we discussed in the introduction. They normalized by mean power (broadband), and their older participants seem to have increased beta-band activity (which the authors believed was noise due to a nearby railway). Whether or not this beta activity is noise (and our findings and others suggest that it may be legitimate), this would clearly introduce a confound, because older participants’ mean power would be greater than younger participants’ power, due to the older participants’ excess beta activity. It is hard to know for sure, but it is possible that the low-frequency effects are due, at least in part, to the influence of the beta-band activity on the normalization step.
According to the BOSC criteria, delta oscillations did not differ significantly between our two healthy age groups. Our findings suggest that, assuming no confounds due to normalization procedures, conventional measures of power might, in part, be measuring delta rhythms, but also other sources of potentially non-rhythmic, delta-band power, as the topographic patterns differed considerably between the conventional power measure and the BOSC measure. In turn, prior findings of increased delta-band activity associated with the onset or progression of AD may either reflect an increase in the prevalence or amplitude of those same delta rhythms, or the addition of a different source of delta rhythms, or it could reflect increases in non-repeating signals with energy in the delta band. Future clinical studies could resolve this ambiguity by quantifying both power and specifically rhythmic activity, using the BOSC method. One might even obtain a purer measure of bandpass activity in non-rhythmic sources by first excluding segments of the recording during which the BOSC method deems oscillations to be present.
Choice of resting protocol
As mentioned in the introduction, resting-state studies differ in whether they ask participants to keep their eyes open or closed. [58] used a classifier to detect neural signatures of sleep in a number of studies. They found that resting states lasting at least a few minutes have a high prevalence of participants presumably falling asleep. This was true for eyes-open as well as eyes-closed conditions. They cited this as a problem because sleep is associated with patient populations for which resting activity is being considered as a diagnostic measure or early marker. We chose our procedure, alternating several seconds of eyes open with eyes closed, in part to guard against participants falling asleep. [58] noted that resting states wherein participants are asked to maintain visual fixation (e.g., [21], [66]) guard against falling asleep. However, one could argue that active fixation conditions go against the spirit of resting-state research by asking participants to maintain vigilance. Although that may also be partly true for our resting condition, we suggest that it may strike a good balance between unconstrained eyes-open or eyes-closed resting conditions in which participants tend to fall asleep [58] and actual tasks. Our participants could not fall asleep, and yet their eye opening and closing was signalled by an auditory tone, so participants had no need to stay vigilant, only to respond when the tone arrived. Five-second intervals are long enough that one can avoid the auditory evoked potential and still have enough recording time in between eye opening and closing events to be able to adequately analyze oscillations. And, we can confirm that in at least one respect, our task resembles other resting tasks: alpha oscillations were present at high rates, particularly during the eyes-closed periods.
It is important to note that the five-second time scale limits our frequency resolution at the low end. Importantly, we did not epoch the data before wavelet-transforming, which would have been even more limiting, as well as introducing edge artifacts. Still, the 5-second alternations may have introduced non-stationarity at about 0.2 Hz. That is, with this protocol, we would be unable to measure power or oscillations at ultra-low frequencies, which may correspond to resting activity found in other studies (e.g., [1]), particularly with the duration threshold, requiring multiple cycles of sustained, elevated power. For theta frequencies, the window of analysis was sufficiently long, and for the frequencies we report (down to 1 Hz), we found little sign of slow rhythms increasing in prevalence in healthy aging. However, it is nonetheless possible that large aging effects would emerge at even lower frequencies, beyond our bandwidth. For this reason, future studies may find it advisable to slow down the protocol to alternate every 10 seconds or more, to be measure such lower-frequency power and oscillations.
Beta oscillations
We did not expect increased beta oscillations in Older than Young participants, but there are, in fact, precedents in the literature. [27] found increased beta activity in older compared to younger participants, but then no difference in beta activity between older participants and patients with Alzheimer’s-like dementia. That suggests that elevated beta activity may be a sign of healthy aging, and not a marker of dementia. Our findings add to this, suggesting that beta activity associated with healthy aging is indeed rhythmic. [19] found an increase in beta power (percent of total power) in healthy elderly (65–80 years) compared to a healthy younger group (40–65 years). [42] similarly found an increase in beta activity during eyes-closed rest in one group of older participants (mean=61 years) compared to a younger control group (mean=30 years) but beta activity was lower again for an even older group (mean=79 years), although beta activity correlated with age across the 50–70 year range of their “young–old” group. Thus, the relationship between beta rhythms and age may not be straight-forward. Finally, these findings should be interpreted with caution, because beta-band activity can reflect muscle-movement artifact rather than brain activity [40], [57].
Conclusion
In sum, we did not observe a greater presence of low-frequency oscillations in healthy aging. Rather, low-frequency rhythmic activity, in both the delta and alpha bands, was present during rest and remained stable between our two healthy age groups. The clearest age effect we found was increased oscillations in the beta band in healthy Older compared to healthy Young participants. The BOSC method offered two chief benefits to the goal of studying resting EEG. First, because rest is, in a sense, its own “baseline” condition, power normalization is challenging. Normalizing by total power can introduce confounds [31], allowing activity at one frequency to produce spurious influences of measures of activity at other frequencies. The BOSC method avoided this problem by using the statistical properties of the signal itself to set oscillation-detection criteria modeled on the non-rhythmic, background, colored-noise spectrum. Second, the BOSC method provides principled criteria that are consistent with other studies to classify signals as rhythmic, in the sense that power exceeds a threshold, derived from the theoretical distribution of power values, for a minimum duration of several cycles. This produced a measure of brain activity that, being more selective for rhythmicities, can be distinguished from conventional measures of power, which are more inclusive; consequently, topographic patterns, which are indicative of the neural source, differed substantially between the BOSC measure and the conventional power measure for frequencies at which rhythms were sporadic. By including both conventional power measures and BOSC-derived measures in a resting EEG study, one can obtain greater specificity in the measure, potentially fine-tuning early markers of AD and improving our understanding of healthy resting EEG across the lifespan.
Table 1.
Resting-state rhythms at Electrode Oz for Young and Older participants.
| a | @ Oz: Eyes Open (Power) | b | @ Oz: Eyes Closed (Power) |
| c | @ Oz: Eyes Open (BOSC) | d | @ Oz: Eyes Closed (BOSC) |
a and b plot conventional power-analysis measure: wavelet power (log-transformed and then normalized by total log-power) as a function of frequency (also on a logarithmic scale). c and d plot the BOSC measure, Pepisode, or proportion of time occupied by oscillations at each frequency. Error bars are standard error of the mean.
* - significant difference between Young and Older groups
(Mann-Whitney U test, df = 26, p < 0.05, uncorrected).
Table 2.
Resting-state rhythms plotted for individual participants at electrode Oz.
| a | @ Oz: Eyes Open (Young) | b | @ Oz: Eyes Closed (Young) |
Each panels plots the BOSC measure as a function of frequency (Pepisode, or proportion of time occupied by oscillations at each frequency), for Young (a,b) and Older (c,d) participants, during eyes-open (a,c) and eyes-closed (b,d). These plots correspond to the means and standard errors that are plotted in Figure 1c,d. Each line graph is for a different participant, and colours cycle through Matlab’s default colour sequence.
Table 3.
Resting-state rhythms at Electrode Fz for Young and Older participants.
| a | @ Fz: Eyes Open (Power) | b | @ Fz: Eyes Closed (Power) |
| c | @ Fz: Eyes Open (BOSC) | d | @ Fz: Eyes Closed (BOSC) |
a and b plot conventional power-analysis measure: wavelet power (log-transformed and then normalized by total log-power) as a function of frequency (also on a logarithmic scale). c and d plot the BOSC measure, Pepisode, or proportion of time occupied by oscillations at each frequency. Error bars are standard error of the mean.
* - significant difference between Young and Older groups
(Mann-Whitney U test, df = 26, p < 0.05, uncorrected).
Table 4.
Resting-state rhythms plotted for individual participants at electrode Fz.
| a | @ Fz: Eyes Open (Young) | b | @ Fz: Eyes Closed (Young) |
| c | @ Fz: Eyes Open (Older) | d | @ Fz: Eyes Closed (Older) |
Each panels plots the BOSC measure as a function of frequency (Pepisode, or proportion of time occupied by oscillations at each frequency), for Young (a,b) and Older (c,d) participants, during eyes-open (a,c) and eyes-closed (b,d). These plots correspond to the means and standard errors that are plotted in Figure 3c,d. Each line graph is for a different participant, and colours cycle through Matlab’s default colour sequence.
Table 5.
Alpha activity.
| Alpha (9.5 Hz), Eyes Closed | |||
|---|---|---|---|
| Young Participants | |||
| a | Power | b | Pepisode (BOSC) |
| Older Participants | |||
| c | Power | d | Pepisode (BOSC) |
Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing alpha (9.51 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log (power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
Table 6.
Theta activity.
| Theta (6.73 Hz), Eyes Closed | |||
|---|---|---|---|
| Young Participants | |||
| a | Power | b | Pepisode (BOSC) |
| Older Participants | |||
| c | Power | d | Pepisode (BOSC) |
Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing theta (6.73 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log (power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
Table 7.
Delta activity.
| Delta (1.19 Hz), Eyes Closed | |||
|---|---|---|---|
| Young Participants | |||
| a | Power | b | Pepisode (BOSC) |
| Older Participants | |||
| c | Power | d | Pepisode (BOSC) |
Topographic plots (spline-interpolated) of conventional, wavelet power (a,c) versus the Pepisode of oscillations based on the BOSC method (b,d) comparing delta (1.19 Hz) activity for Young (a,b) and Older (c,d) participants. Colour scale denotes log(power) (a,c) or Pepisode (b,d), but these are dimensionless, since the topographic patterns were normalized to unit vector-length for each participant prior to averaging. Scales are adjusted to the range of values for each panel separately, to best visualize the topographic patterns. Note that although sources of EEG signal are usually dipoles, because power and the BOSC method start by squaring voltage, a dipole appears not as a positive pole next to a negative pole, but like two positive poles.
Table 8.
Mean dot product of topographic patterns (e.g., Figs. 5–7) between the log(power) and BOSC measures, for Young (a) and Older (b) participants, computed separately for eyes-open (o) and eyes-closed (×) conditions.
| a | Young | b | Older |
Error bars denote standard error of the mean. Asterisks denote dot product significantly non-zero (p < 0.05, Mann-Whitney U test with df =15 and 11 for Young and Older groups, respectively).
Highlights.
Rhythmic EEG during rest may increase in aging and age-related neurodegeneration.
Previous studies have not clearly distinguished rhythmic from non-rhythmic signal.
We use a rhythm-selective method (BOSC) to disentangle these in healthy aging.
Theta oscillations were not present above expected false-positive rates.
Delta rhythms were present but showed little evidence of age effects.
Acknowledgements
We thank Stanislau Hrybouski for help testing participants and Jill Friesen for advice on recruitment and testing procedures. Supported in part by a grant from the Natural Sciences and Engineering Research Council of Canada to author JC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This deserves some caveats. First, measures derived from power are all positive. Thus, one does not expect to see dipole patterns, in which polarity changes across the dipole; rather, both poles would have the same sign. Second, due to the well known inverse problem, there is always some degree of ambiguity about the generators of a signal recorded extracranially.
For two topographic patterns, written as vectors, x and y, each with n dimensions, where n =the number of electrodes, the dot product is defined as and the normalized dot product is defined as (x · y)/(|x‖ y|), where ‖ denotes the vector magnitude, . This is equivalent to the cosine of the (n-dimensional) angle between the two vectors.
References
- 1.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Romani Gian L, Rossini PM. Sources of cortical rhythms in adults during physiological aging: a multicentric EEG study. Human Brain Mapping. 2006;27(2):162–172. doi: 10.1002/hbm.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babiloni C, Cassetta E, Binetti G, Tombini M, Del Percio C, Ferreri F, Ferri R, Frisoni G, Lanuzza B, Nobili F, Parisi L, Rodriguez G, Frigerio L, Gurzì M, Prestia A, Vernieri F, Eusebi F, Rossini PM. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer’s disease. European Journal of Neuroscience. 2007;25(12):3742–3757. doi: 10.1111/j.1460-9568.2007.05601.x. [DOI] [PubMed] [Google Scholar]
- 4.Balsters JH, O’Connell RG, Galli A, Nolan H, Greco E, Kilcullen SM, Bokde ALW, Lai R, Upton N, Robertson IH. Changes in resting connectivity with age: a simultaneous electroencephalogram and functional magnetic resonance imaging investigation. Neurobiology of Aging. 2013;34(9):2194–2207. doi: 10.1016/j.neurobiolaging.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Berger H. Über das Elektroenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrank. 1929;87:527–570. [Google Scholar]
- 6.Brigham EO. The Fast Fourier Transform. Englewood Cliffs, NJ: Prentice-Hall; 1974. [Google Scholar]
- 7.Burke JF, Ramayya AG, Kahana MJ. Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Current Opinion in Neurobiology. 2015;31:104–110. doi: 10.1016/j.conb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzsáki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- 9.Caplan JB, Glaholt MG. The roles of EEG oscillations in learning relational information. NeuroImage. 2007;38(3):604–616. doi: 10.1016/j.neuroimage.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 10.Caplan JB, Kahana MJ, Raghavachari S, Madsen JR. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. Journal of Neurophysiology. 2001;86(1):368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- 11.Caplan JB, Kahana MJ, Sekuler R, Kirschen MP, Madsen JR. Task dependence of human theta: the case for multiple cognitive functions. NeuroComputing. 2000;32–33:659–665. [Google Scholar]
- 12.Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. Journal of Neuroscience. 2003;23(11):4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coben LA, Danziger W, Storandt M. A longitudinal EEG study of mild senile dementia of Alzheimer type: changes at 1 year and at 2.5 years. Electroencephalography and Clinical Neurophysiology. 1985;61(2):101–112. doi: 10.1016/0013-4694(85)91048-x. [DOI] [PubMed] [Google Scholar]
- 14.Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. International Journal of Psychophysiology. 2011;79(1):9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruikshank LC, Singhal A, Hueppelsheuser M, Caplan JB. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. Journal of Neurophysiology. 2012;107(1):65–77. doi: 10.1152/jn.00893.2010. [DOI] [PubMed] [Google Scholar]
- 16.Cummins TDR, Broughton M, Finnigan S. Theta oscillations are affected by amnestic mild cognitive impairment and cognitive load. International Journal of Psychophysiology. 2008;70(1):75–81. doi: 10.1016/j.ijpsycho.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Cummins TDR, Finnigan S. Theta power is reduced in healthy cognitive aging. International Journal of Psychophysiology. 2007;66(1):10–17. doi: 10.1016/j.ijpsycho.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;15(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 19.d’Onofrio F, Salvia S, Petretta V, Bonavita V, Rodriguez G, Tedeschi G. Quantified-EEG in normal aging and dementias. Acta Neurologica Scandanavica. 1996;93(5):336–345. doi: 10.1111/j.1600-0404.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15(7):881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- 21.Fernández A, Maestú F, Amo C, Gil P, Fehr T, Wienbruch C, Rockstroh B, Elbert T, Ortiz T. Focal temporoparietal slow activity in Alzheimer’s Disease revealed by magnetoencephalography. Biological Psychiatry. 2002;52(7):764–770. doi: 10.1016/s0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- 22.Ferree TC, Luu P, Russell GSMTD. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- 23.Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology. 2011;48(8):1083–1087. doi: 10.1111/j.1469-8986.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 24.Grossmann A, Morlet J. Decomposition of functions into wavelets of constant shape, and related transforms. In: Streit L, editor. Mathematics + Physics. Vol. 1. Singapore: World Scientific; 1985. pp. 135–165. [Google Scholar]
- 25.Grunwald M, Busse F, Hensel A, Kruggel F, Riedel-Heller S, Wolf H, Arendt T, Gertz H. Correlation between cortical θ activity and hippocampal volumes in health, mild cognitive impairment, and mild dementia. Journal of Clinical Neurophysiology. 2001;18(2):178–184. doi: 10.1097/00004691-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Grunwald M, Busse F, Hensel A, Riedel-Heller S, Kruggel F, Arendt T, Wolf H, Gertz H-J. Theta-power differences in patients with mild cognitive impairment under rest condition and during haptic tasks. Alzheimer disease and associated disorders. 2002;16(1):40–48. doi: 10.1097/00002093-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Günther W, Giunta R, Klages U, Haag C, Steinberg R, Satzger W, Jonitz L, Engel R. Findings of electroencephalographic brain mapping in mild to moderate dementia of the Alzheimer type during resting, motor and music-perception conditions. Psychiatry Research: Neuroimaging. 1993;50(3):163–176. doi: 10.1016/0925-4927(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 28.Harmony T, Fernández T, Silva Ja, Bernal J, Díaz-Comas L, Reyes A, Marosi E, Rodríguez M, Rodríguez M. EEG delta activity: an indicator of attention to internal processing during performance of mental tasks. International Journal of Psychophysiology. 1996;24(1–2):161–171. doi: 10.1016/s0167-8760(96)00053-0. [DOI] [PubMed] [Google Scholar]
- 29.Hermes D, Miller KJ, Wandell BAW. Stimulus dependence of gamma oscillations in human visual cortex. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes AM, Whitten TA, Caplan JB, Dickson CT. BOSC: a Better OSCillation detection method, extracts both sustained and transient rhythms from rat hippocampal recordings. Hippocampus. 2012;22(6):1417–1428. doi: 10.1002/hipo.20979. [DOI] [PubMed] [Google Scholar]
- 31.Jelic V, Johansson S, Almkvist O, Shigeta M, Julina P, Nordberg A, Winblad B, Wahlund L. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiology of Aging. 2000;21(4):533–540. doi: 10.1016/s0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 32.Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO. Quantitative electroencephalography power and coherence in Alzheimer’s disease and mild cognitive impairment. Dementia. 1996;7(6):314–323. doi: 10.1159/000106897. [DOI] [PubMed] [Google Scholar]
- 33.Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proceedings of the National Academy of Sciences, USA. 2013;110(32):13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 35.Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clinical Neurophysiology. 2000;111(5):781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- 36.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences, USA. 2003;100(19):11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee T-W, Yu YW, Hong C-J, Tsai S-J, Wu H-C, Chen T-J. The influence of apolipoprotein E epsilon4 polymorphism on qEEG profiles in healthy young females: a resting EEG study. Brain Topography. 2012;25(4):431–442. doi: 10.1007/s10548-012-0229-y. [DOI] [PubMed] [Google Scholar]
- 38.Leirer VM, Wienbruch C, Kolassa S, Schlee W, Elbert T, Kolassa I-T. Changes in cortical slow wave activity in healthy aging. Brain Imaging and Behavior. 2011;5(3):222–228. doi: 10.1007/s11682-011-9126-3. [DOI] [PubMed] [Google Scholar]
- 39.Lopes Da Silva FH. EEG and MEG: relevance to neuroscience. Neuron. 2013;80(5):1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Luck SJ. An introduction to the event-related potential technique. Cambridge, MA, USA: MIT Press; 2005. [Google Scholar]
- 41.Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Marciani MG, Maschio M, Spanedda F, Caltagirone C, Gigli GL, Bernardi G. Quantitative EEG evaluation in normal elderly subjects during mental processes: age-related changes. International Journal of Neuroscience. 1994;76(1–2):131–140. doi: 10.3109/00207459408985998. [DOI] [PubMed] [Google Scholar]
- 43.Marzano C, Ferrara M, Mauro F, Moroni F, Gorgoni M, Tempesta D, Cipolli C, De Gennaro L. Recalling and forgetting dreams: theta and alpha oscillations during sleep predict subsequent dream recall. Journal of Neuroscience. 2011;31(18):6674–6683. doi: 10.1523/JNEUROSCI.0412-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marzano C, Moroni F, Gorgoni M, Nobili L, Ferrara M, De Gennaro L. How we fall asleep: regional and temporal differences in electroencephalographic synchronization at sleep onset. Sleep Medicine. 2013;14(11):1112–1122. doi: 10.1016/j.sleep.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Missonnier P, Herrmann FR, Rodriguez C, Deiber M-P, Millet P, Fazio-costa L, Gold G, Giannakopoulos P. Age-related differences on event-related potentials and brain rhythm oscillations during working memory activation. Journal of Neural Transmission. 2011;118(6):945–955. doi: 10.1007/s00702-011-0600-2. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Progress in Neurobiology. 2008;86(3):156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Moretti DV, Frisoni GB, Fracassi C, Pievani M, Geroldi C, Binetti G, Rossini PM, Zanetti O. MCI patients’ EEGs show group differences between those who progress and those who do not progress to AD. Neurobiology of Aging. 2011;32(4):563–571. doi: 10.1016/j.neurobiolaging.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Moroni F, Nobili L, De Carli F, Massimini M, Francione S, Marzano C, Proserpio P, Cipolli C, De Gennaro L, Ferrara M. Slow EEG rhythms and inter-hemispheric synchronization across sleep and wakefulness in the human hippocampus. NeuroImage. 2012;60(1):497–504. doi: 10.1016/j.neuroimage.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 49.Olbrich E, Landolt HP, Achermann P. Effect of prolonged wakefulness on electroencephalographic oscillatory activity during sleep. Journal of Sleep Research. 2014;23(3):255–262. doi: 10.1111/jsr.12123. [DOI] [PubMed] [Google Scholar]
- 50.Percival DB, Walden AT. Spectral Analysis for Physical Applications. Cambridge, U. K.: Cambridge University Press; 1993. [Google Scholar]
- 51.Prichep LS. Quantitative EEG and electromagnetic brain imaging in aging and in the evolution of dementia. Annals of the New York Academy of Science. 2007;1097:156–167. doi: 10.1196/annals.1379.008. [DOI] [PubMed] [Google Scholar]
- 52.Roca-Stappung M, Fernández T, Becerra J, Mendoza-Montoya O, Espino M, Harmony T. Healthy aging: relationship between quantitative electroencephalogram and cognition. Neuroscience Letters. 2012;510(2):115–120. doi: 10.1016/j.neulet.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Rossini PM, Del Percio C, Pasqualetti P, Cassetta E, Binetti G, Dal Forno G, Ferreri F, Frisoni G, Chiovenda P, Miniussi C, Parisi L, Tombini M, Vecchio F, Babiloni C. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143(3):793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 54.Scheeringa R, Bastiaansen MCM, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology. 2008;67(3):242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 55.Schlesinger MF, West BJ. 1/f versus 1/fα noise. In: Stanley EH, Ostrowsky N, editors. Random fluctuations and pattern growth: experiments and models. Dordrecht: Kluwer Academic Publishers; 1988. pp. 320–324. [Google Scholar]
- 56.Schmidt MT, Kanda PAM, Basile LFH, da Silva Lopes HF, Baratho R, Demario JLC, Jorge MS, Nardi AE, Machado S, Ianof JN, Nitrini R, Anghinah R. Index of alpha/theta ratio of the electroencephalogram: a new marker for Alzheimer’s disease. Frontiers in Aging Neuroscience. 2013;5(60) doi: 10.3389/fnagi.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schomer DL, Lopes da Sliva FH, editors. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 6th Edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011. [Google Scholar]
- 58.Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82(3):695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Van Strien JW, Hagenbeek RE, Stam CJ, Rombouts SARB, Barkhof F. Changes in brain electrical activity during extended continuous word recognition. NeuroImage. 2005;26(3):952–959. doi: 10.1016/j.neuroimage.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 60.van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. Journal of Neuroscience Methods. 2007;162(1–2):49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vlahou EL, Thurm F, Kolassa I-T, Schlee W. Resting-state slow wave power, healthy aging and cognitive performance. Scientific Reports. 2014;4(5101) doi: 10.1038/srep05101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter WG, Dovey VJ. Electroencephalography in cases of sub-cortical tumour. Journal of Neurology, Neurosurgery and Psychiatry. 1944;7:57–65. doi: 10.1136/jnnp.7.3-4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watrous AJ, Fried I, Ekstrom AD. Behavioral correlates of human hippocampal delta and theta oscillations during navigation. Journal of Neurophysiology. 2011;105(4):1747–1755. doi: 10.1152/jn.00921.2010. [DOI] [PubMed] [Google Scholar]
- 64.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low frequency oscillations during spatial navigation. Hippocampus. 2013;23(8):656–661. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitten TA, Hughes AM, Dickson CT, Caplan JB. A better oscillation detection method robustly extracts EEG rhythms across brain state changes: the human alpha rhythm as a test case. NeuroImage. 2011;54(2):860–874. doi: 10.1016/j.neuroimage.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 66.Widagdo MM, Pierson JM, Helme RD. Age-related changes in qEEG during cognitive tasks. International Journal of Neuroscience. 1996;95(1–2):63–75. doi: 10.3109/00207459809000650. [DOI] [PubMed] [Google Scholar]
- 67.Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Frontiers in Human Neuroscience. 2010;4(1):1–13. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]