Abstract
Acting through cell surface receptors, “extracellular” lysophosphatidic acid (LPA) influences cell growth, differentiation, apoptosis and development in a wide spectrum of settings [1–5]. Within the vasculature, smooth muscle cells [6, 7], endothelial cells [8] and platelets [9, 10] display notable responses to LPA [11, 12], which likely regulate blood vessel development and contribute to vascular pathology. The bioactive effects of LPA are mediated by a family of G-protein coupled receptors with at least six members (termed LPA1-6 that are encoded by the LPAR genes in humans and Lpar in mice) [1–3]. LPA may also serve as a ligand for the receptor for advanced glycation end products (RAGE) [13]. This review summarizes evidence to support a role for LPA signaling in vascular biology based on studies of LPA receptors and enzymes that produce or metabolize the lipid (Figure 1).
LPA receptors
The receptors for LPA are widely distributed on blood and vascular cells. In preclinical animal models, targeting the LPA receptors genetically and pharmacologically suggests that they may contribute to vascular injury and inflammatory responses, as well as endothelial barrier function and vascular stability. Single and multiple deletions of LPA receptors in mice produce differing vascular phenotypes. Deficiency of Lpar1, which results in 50% neonatal lethality, gives rise to the development of spontaneous frontal hematomas [14]. This suggests a role for LPA1 in stabilization of vessels, as no defect in hemostasis has been observed in these animals. In experimental arterial injury models, LPA1 regulates the development of intimal hyperplasia, a complex response involving inflammation and smooth muscle cell proliferation and migration. LPA1 may influence the vascular remodeling response via the Gα12/Gα13 pathway that couples to RhoGEF to activate RhoA, given the similarities in development on intimal hyperplasia after injury in the Lpar1−/− mice [6] and those lacking the Gα12/Gα13 and Rho pathways [15] in smooth muscle cells. The lack of LPA1 disrupts the endothelial barrier and results in increased vascular permeability in response to inflammatory stimuli in the lung [16] and the skin [17]. Conversely, LPA1 antagonists prevent inflammation in response to peritoneal injection of lipopolysaccharide [18]. Whether either a defect in smooth muscle or endothelial cell function accounts for the bleeding observed in the Lpar1−/− mice remains unknown. Knockout of both Lpa1 and Lpa2 increases the incidence of prefrontal hematomas [19], impairs the response to vascular injury [6], and results in the development of pulmonary hypertension with age [20]; the latter phenotype in not observed in mice with deficiency of either of the receptors alone. Together, these results suggest some redundancy or overlap between the 2 receptor systems. Likewise, LPA1 and LPA3 antagonists reduce arterial remodeling elicited by denudation injury [7] in mice, perhaps due to attenuated signaling through both G12/G13 and Gq/G11 signaling pathways, which appear to regulate vascular remodeling antagonistically. Lpar4-deficient mice display a genetic background-dependent defect in formation of vasculature. On the C57Bl/6 background, the mice develop hemorrhage and edema due to a maturation defect from lack of smooth muscle cell and pericytes recruitment vessels [21]. As described in more detail below, studies in zebrafish also support a role for several of the canonical LPA receptors in blood vessel formation. Additionally, LPA signaling through RAGE may also affect SMC function [13].
LPA synthesis pathways
LPA is present in many biologic fluids, including plasma, ascites, and bronchoalveolar lavage fluid. In the circulation, LPA turns over rapidly [22] and therefore must be maintained by constant production. Certain conditions, such as acute coronary syndromes [23] [24] and chronic liver disease [25] are associated with higher levels of plasma LPA. Whether this is due to increased production or reduced clearance/breakdown or both is currently not known. There are several pathways that can generate LPA. The secreted lysophospholipase D autotaxin (ATX) generates extracellular LPA by hydrolysis of lysophosphatidylcholine (LPC) [26,27][28]. ATX is an is an ecto-nucleotide pyrophosphatase/phospho-diesterase family member (encoded by ENPP2 in humans and Enpp2 in mice) that is synthesized as a pre-proenzyme and undergoes sequential signal peptide removal and proprotein convertase cleavage before being secreted from cells. Of five functional isoforms (ATXα, ATXβ, ATXγ, ATXδ, ATXε) generated through alternatively splicing, ATXβ is the most abundant and appears to account for most of the lysophospholipase D activity in plasma. The cellular source(s) of plasma ATX are incompletely understood, however, adipocytes likely secrete a substantial portion [29]. ATX is also stored in platelets and released during their activation [28] [30]. Circulating ATX is rapidly taken up by the scavenger receptors of liver sinusoidal endothelial cells, and then degraded in the liver [31]. Thus, much like hormones, including insulin, ATX is largely removed from the circulation during first passage through the liver. While ATX is normally a major source of plasma LPA levels [22, 32], other minor pathways may contribute to increases in LPA in certain situations, such as in the setting of acute myocardial infarction [23, 24, 33].
Studies of organisms that lack or express catalytically-inactive ATX have shed light on its role, and by inference, the role of LPA signaling, in vascular biology. ATX expression is required for normal vascular development in mice. Enpp2-deficient mice [34] [35] die between embryonic days 9.5–10.5 with blood vessel formation defects in the yolk sac and embryo. In the absence of ATX, initial blood vessel formation appears normal, but vessels fail to maturate, suggesting that ATX is critical for extension and stabilization of blood vessels but perhaps not for the initial endothelial cell differentiation and migration. The phenotype of Enpp2−/− mice resembles that observed in Gα13 knockout embryos, which would be consistent with ATX-generated LPA signaling through Gα13-coupled receptors. To date, no single or multiple LPA receptor knock-out mice have fully recapitulated the phenotype observed in embryos lacking ATX or Gα13. However, mice expressing a functionally inactive ATX (T210A) also die embryonically [36], indicating that the catalytic activity of ATX, and likely LPA synthesis, is essential for vascular development. Enpp2-heteroxygous mice are viable and express half of normal levels of ATX and LPA. However, they are hyper-responsive to hypoxia-induced vasoconstriction and remodeling, and prone to develop pulmonary hypertension [20]. Knockdown of ATX in zebrafish embryos by morpholino antisense oligonucleotides also causes aberrant vascular connections [20] with normal initial sprouting from the dorsal aorta. A similar defect occurs in fish embryos lacking functional LPA1 and LPA4. Together these results suggest that the ATX/LPA signaling nexus, likely acting through multiple receptors coupled to Gα13, is required for normal blood vessel maturation.
ATX, originally identified as a motility factor in serum, can modulate angiogenesis directly and indirectly [19]. In angiogenesis models, ATX stimulates cultured endothelial cells to form tubules in vitro and new blood vessel formation within Matrigel™ plugs in vivo. ATX promotes the motility in human coronary artery smooth muscle cells, a function that may also support vessel maturation and remodeling [37]. Finally, ATX may promote blood vessel maturation by recruiting pericytes [38] and/or smooth muscle cells to developing blood vessels. While most of the ATX effects may be attributed to the production of LPA, ATX may have LPA-independent effects. ATX binds integrins and heparan sulfates on the cell surface through its somatomedin-B-like (SMB) domain and may promote non-LPA receptor mediated signaling. Finally, ATX has been reported to hydrolyze sphingosylphosphorylcholine (SPC) to sphingosine-1-phosphate (S1P) [39], another potent bioactive lipid mediator implicated in vascular signaling.
LPPs regulate LPA signaling
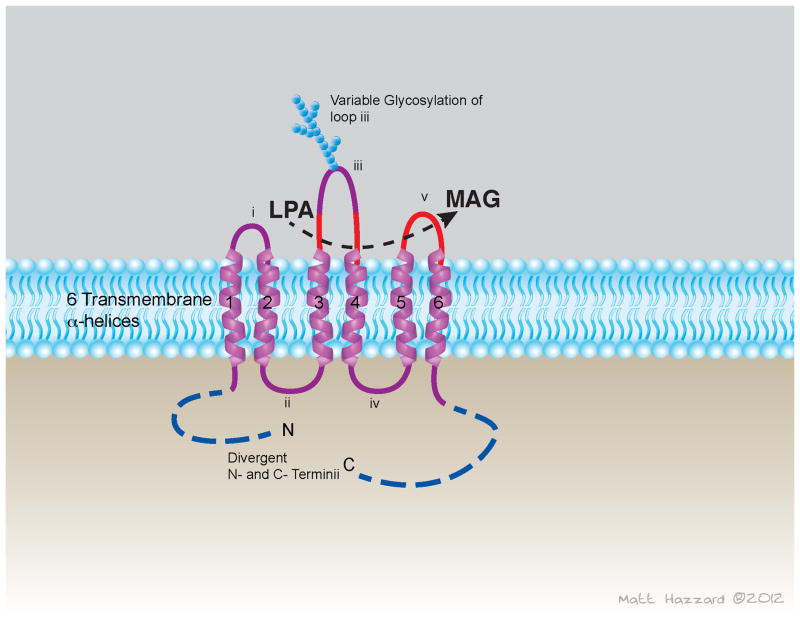
Degradation of LPA by removal of the phosphate group eliminates signaling through LPA receptors. Lipid phosphate phosphatases (LPPs) constitute a family of three enzymes that dephosphorylate a broad range of lipid phosphates, including LPA. LPPs are encoded by the PPAP2 genes: LPP1 by the PPAP2A gene, LPP2 by the PPAP2C gene, and LPP3 by PPAP2B [40]. LPPs share a common structure with a core of six predicted transmembrane helices linked by extra membrane loops. The enzymes are oriented with their N- and C- termini in the cytoplasm and their active sites on the extracellular or luminal surface of the membrane (Figure 2). Subcellular localization of these enzymes is both dynamic and cell-specific. Although the three LPP enzymes demonstrate similar catalytic activities and substrate preferences in vitro, the phenotypes of mice with targeted inactivation of the Ppap2 genes establishes that their functions are non-redundant. Primary cells isolated from mice harboring an exon trap inactivated allele of Ppap2a gene display a reduced ability to dephosphorylate exogenously provided LPA, indicating a role for LPP1 as a cell surface “ecto” LPA phosphatase [41]. Mice homozygous for an insertionally inactivated allele of the Ppap2c gene, encoding murine LPP2, are phenotypically indistinguishable [42]. By contrast, inactivation of Ppap2b results in embryonic lethality in part to due to failure of extra-embryonic vasculature [43]. Media from cultured embryonic fibroblasts isolated from Ppap2b-null mice contains higher levels of LPA suggesting that the other LPPs cannot compensate for the regulation of extracellular accumulation of LPA. Recent evidence from the LPP3 homologs in Drosophila, Wunen and Wunen2, demonstrated that LPPs generate and regulate phospholipid gradients in vivo, and that the establishment of a phospholipid gradient may drive migration of germ cells. The maximum range of influence of Wunen-expressing cells on germ cells is approximately 33μm [44].
Figure 2.
Proposed topology of lipid phosphate phosphatase (LPP) transmembrane enzymes. LPA = lysophosphatidic acid; MAG = monoacyl glycerol.
Lack of LPP3 in endothelial cells results in embryonic lethality with extra-embryonic vascular defects similar to those observed in germline Ppap2b-null mice [17]. In adult mice, inducible deletion of Ppap2b in endothelial cells increases vascular leak, especially in settings of inflammation. Thus, LPP3 may function to attenuate the ATX/LPA-mediated permeability described above. LPP3 has also been implicated as a negative regulator of Wnt pathway potentially through interactions with β-catenin that may not require the LPP’s phosphatase activity [43]. In human dermal microvascular endothelial cells, siRNA mediated knockdown of LPP3 results in reduced VE cadherin, p120 catenin and fibronectin levels as well as displayed reduced branch point formation in a collagen matrix – a finding which implicates LPP3 in the β-catenin/LEF-1 signaling pathway regulating endothelial cell migration as well as cell-to-cell adhesion. LPP3 also contains an RGD cell adhesion sequence in its third extracellular loop which has been postulated to regulate its interactions with α5β1 and αvβ3 integrins [45] [46].
Mice that lack LPPs in smooth muscle cells display exaggerated development of neointimal formation associated with heightened inflammatory cell accumulation [47]. Isolated Ppap2b−/− smooth muscle cells display reduced LPA metabolism and heightened cell proliferation, ERK activity, Rho activation, and cell migration in response to serum and LPA. These exaggerated responses are attenuated by lentiviral expression of human or mouse LPP3, but not a catalytically inactive LPP3 mutant. Taken together these results indicate that LPP3 normally functions to attenuate SMC proliferation and vascular inflammation, possibly due to its ability to degrade LPA (and/or S1P) and thereby limit signaling effects.
Role of the ATX/LPA/LPP3 signaling nexus in atherosclerosis
Levels of vessel-associated LPA increase during progression of atherosclerosis [48, 49] and are substantially higher in advanced lesions [50]. Cholesterol feeding influences levels and distribution of plasma LPA [51]. Additionally, feeding mice a chow diet supplemented with unsaturated LPA mimics the inflammatory effects of Western diets [52]. LPA that is generated during atherosclerosis may have pro-atherosclerotic, pro-inflammatory, and pro-thrombotic effects [53]. Genome wide association studies of patients with coronary artery disease revealed single nucleotide polymorphisms in PPAP2B, that are associated with disease risk [54] [55]. The identified risk SNPs predict lower LPP3 expression in human vascular endothelial cells, and the effect is exacerbated by exposing the cells to oxidized PAPC [56]. The risk allele also attenuates the upregulation of PPAP2B by oxLDL in macrophages, at least in part by reducing enhancer activity and decreasing binding of C/EBP beta. Together, these findings suggest that variation in the PPAP2B gene regulates gene – environment interactions by influencing the response to lipid risk factors.
In summary, the evidence reviewed above implicates LPA signaling in a range of important events during blood cell development and in the response to inflammation and environmental injury. Observations in experimental models support a role for LPA in regulating vascular biology and pathology. These concepts need to be tested using strategies in preclinical animal models and in translational studies in humans.
Figure 1.
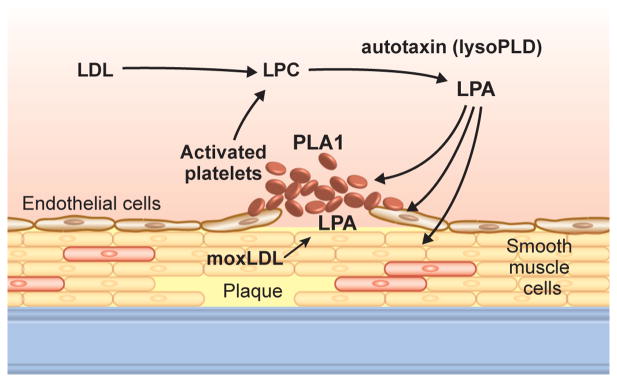
Autotaxin (ATX) and LPA actions in blood and vascular cells.
LDL = low density liprotein; LPC = lysophosphatidylcholine; moxLDL = minimally oxidized LDL; PLA = phospholipase A.
Acknowledgments
This work was supported in part by the National Heart Lung and Blood Institute (R01HL078663); the National Institute of General Medical Sciences (P20GM103527); the National Center for Research Resources (P20RR021954); and the National Center for Advancing Translational Sciences (UL1TR000117) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This material is the result of work supported in part with resources and the use of facilities at the Lexington VA Medical Center. PM was supported in part by a grant from the Heart Lung and Blood Institute (T32HL072743) and National Center for Advancing Translational Sciences (TL1TR000115). SY was supported in part by a grant from the Heart Lung and Blood Institute (T32HL091812)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Choi JW, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–86. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 2.Moolenaar WH, Hla T. SnapShot: Bioactive lysophospholipids. Cell. 2012;148(1–2):378–378 e2. doi: 10.1016/j.cell.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev. 2011;111(10):6299–320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tigyi G. Physiological responses to lysophosphatidic acid and related glycero-phospholipids. Prostaglandins Other Lipid Mediat. 2001;64(1–4):47–62. doi: 10.1016/s0090-6980(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 5.Tigyi G. Preface to the special issue: Lysophospholipids in health and disease. Biochim Biophys Acta. 2008;1781(9):423. doi: 10.1016/j.bbalip.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Panchatcharam M, et al. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103(6):662–70. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian P, et al. Lysophosphatidic acid receptors LPA1 and LPA3 promote CXCL12-mediated smooth muscle progenitor cell recruitment in neointima formation. Circ Res. 2010;107(1):96–105. doi: 10.1161/CIRCRESAHA.109.212647. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z, et al. Lipoprotein-Derived Lysophosphatidic Acid Promotes Atherosclerosis by Releasing CXCL1 from the Endothelium. Cell Metab. 2011;13(5):592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Pamuklar Z, et al. Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler Thromb Vasc Biol. 2008;28(3):555–61. doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- 10.Haseruck N, et al. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103(7):2585–92. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 11.Morris AJ, et al. Blood relatives: dynamic regulation of bioactive lysophosphatidic acid and sphingosine-1-phosphate metabolism in the circulation. Trends Cardiovasc Med. 2009;19(4):135–40. doi: 10.1016/j.tcm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris AJ, et al. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thromb Haemost. 2009;7(Suppl 1):38–43. doi: 10.1111/j.1538-7836.2009.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai V, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209(13):2339–50. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contos JJ, et al. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97(24):13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Althoff TF, et al. Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J Exp Med. 2012;209(12):2277–90. doi: 10.1084/jem.20120350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tager AM, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 17.Panchatcharam M, et al. Mice With Targeted Inactivation of Ppap2b in Endothelial and Hematopoietic Cells Display Enhanced Vascular Inflammation and Permeability. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.302335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, et al. Lysophosphatidic acid receptor 1 antagonist ki16425 blunts abdominal and systemic inflammation in a mouse model of peritoneal sepsis. Transl Res. 2015 doi: 10.1016/j.trsl.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contos JJ, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22(19):6921–9. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HY, et al. Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling. Arterioscler Thromb Vasc Biol. 2012;32(1):24–32. doi: 10.1161/ATVBAHA.111.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida H, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116(23):5060–70. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- 22.Albers HM, et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci U S A. 2010;107(16):7257–62. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurano M, et al. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic Acid in acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2015;35(2):463–70. doi: 10.1161/ATVBAHA.114.304748. [DOI] [PubMed] [Google Scholar]
- 24.Dohi T, et al. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 2013;229(1):192–7. doi: 10.1016/j.atherosclerosis.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41(6):616–23. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- 26.Moolenaar WH, Perrakis A. Insights into autotaxin: how to produce and present a lipid mediator. Nat Rev Mol Cell Biol. 2011;12(10):674–9. doi: 10.1038/nrm3188. [DOI] [PubMed] [Google Scholar]
- 27.Hausmann J, et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol. 2011;18(2):198–204. doi: 10.1038/nsmb.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pamuklar Z, et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem. 2009;284(11):7385–94. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dusaulcy R, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52(6):1247–55. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leblanc R, et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood. 2014;124(20):3141–50. doi: 10.1182/blood-2014-04-568683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen S, et al. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284(2):216–21. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Gierse J, et al. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J Pharmacol Exp Ther. 2010;334(1):310–7. doi: 10.1124/jpet.110.165845. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, et al. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand J Clin Lab Invest. 2003;63(7–8):497–503. doi: 10.1080/00365510310003265. [DOI] [PubMed] [Google Scholar]
- 34.van Meeteren LA, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26(13):5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281(35):25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 36.Ferry G, et al. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581(18):3572–8. doi: 10.1016/j.febslet.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 37.Nam SW, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61(18):6938–44. [PubMed] [Google Scholar]
- 38.Motiejunaite R, Aranda J, Kazlauskas A. Pericytes prevent regression of endothelial cell tubes by accelerating metabolism of lysophosphatidic acid. Microvasc Res. 2014;93:62–71. doi: 10.1016/j.mvr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Clair T, et al. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003;63(17):5446–53. [PubMed] [Google Scholar]
- 40.Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem J. 2005;387(Pt 2):281–93. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomsig JL, et al. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem J. 2009;419(3):611–8. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris AJ, et al. Lysophospholipid Receptors: Signaling and Biochemistry. John Wiley; 2012. Lipid phosphate phosphatases: Recent progress and assay methods. [Google Scholar]
- 43.Escalante-Alcalde D, et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130(19):4623–37. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee A, Neher RA, Renault AD. Quantifying the range of a lipid phosphate signal in vivo. J Cell Sci. 2013;126(Pt 23):5453–64. doi: 10.1242/jcs.136176. [DOI] [PubMed] [Google Scholar]
- 45.Humtsoe JO, et al. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 2003;22(7):1539–54. doi: 10.1093/emboj/cdg165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humtsoe JO, et al. Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures. Mol Cell Biol. 2010;30(7):1593–606. doi: 10.1128/MCB.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panchatcharam M, et al. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33(1):52–9. doi: 10.1161/ATVBAHA.112.300527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siess W, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96(12):6931–6. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582(1–3):204–15. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 50.Bot M, et al. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am J Pathol. 2010;176(6):3073–84. doi: 10.2353/ajpath.2010.090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokumura A, et al. Increased formation of lysophosphatidic acids by lysophospholipase D in serum of hypercholesterolemic rabbits. J Lipid Res. 2002;43(2):307–15. [PubMed] [Google Scholar]
- 52.Navab M, et al. Transgenic 6F Tomatoes Act on the Small Intestine to Prevent Systemic Inflammation and Dyslipidemia Caused by Western Diet and Intestinally Derived Lysophosphatidic Acid. J Lipid Res. 2013 doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smyth SS, et al. Arguing the case for the autotaxin-lysophosphatidic Acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(3):479–86. doi: 10.1161/ATVBAHA.113.302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43(4):339–44. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 55.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erbilgin A, et al. Identification of CAD candidate genes in GWAS loci and their expression in vascular cells. J Lipid Res. 2013;54(7):1894–905. doi: 10.1194/jlr.M037085. [DOI] [PMC free article] [PubMed] [Google Scholar]