Abstract
The common marmoset (Callithrix jacchus) has been valuable as a primate model in biomedical research. Interest in this species has grown recently, in part due to the successful demonstration of transgenic marmosets. Here we examine the prospects of the marmoset model for visual neuroscience research, adopting a comparative framework to place the marmoset within a broader evolutionary context. The marmoset’s small brain bears most of the organizational features of other primates, and its smooth surface offers practical advantages over the macaque for areal mapping, laminar electrode penetration, and two-photon and optical imaging. Behaviorally, marmosets are more limited at performing regimented psychophysical tasks, but do readily accept the head restraint that is necessary for accurate eye tracking and neurophysiology, and can perform simple discriminations. Their natural gaze behavior closely resembles that of other primates, with a tendency to focus on objects of social interest including faces. Their immaturity at birth and routine twinning also makes them ideal for the study of postnatal visual development. These experimental factors, together with the theoretical advantages inherent in comparing anatomy, physiology, and behavior across related species, make the marmoset an excellent model for visual neuroscience.
Keywords: Primate, Marmoset, Vision, Comparative, Cognition, Behavior
1. Introduction
Vision is central to human cognition and has long been an important model system for studying principles of brain function. Humans, like other primates, depend upon vision extensively for navigation, interaction with other individuals, manipulation of objects, and many other aspects of daily life. The coevolution of the eye and visual brain in our distant primate ancestors brought with it many adaptations that benefit diurnal and arboreal living as well as social living in larger extended family groups. These changes are manifest as a pattern of specific features of the visual system that support unique perceptual and behavioral abilities (for review, see Kaas 2013). Visual neuroscience has benefitted from decades of comparative studies, as the parallax afforded by studying multiple species has helped to identify traits that are core features of the mammalian brain and other traits that are unique to primates, including humans.
The present article reviews primate vision from a comparative standpoint and places focus on the common marmoset (Callithrix jacchus), an arboreal, small-bodied New World primate. The review is motivated by growing interest in the marmoset as a model species to complement the rhesus monkey (Macaca mulatta) and laboratory mouse (Mus musculus), which are commonly used to study neural circuits supporting human vision. Humans’ most recent common ancestor with the marmoset lived approximately 35–40 million years ago, before our most recent ancestor with the macaque (25–30 million years ago) and long after our most recent ancestor with the mouse (80–90 million years ago) (Janecka et al., 2007; Springer et al., 2011). Thus from a purely phylogenetic standpoint, the marmoset offers an intermediate point of comparison between these species (Figure 1). For visual neuroscience, the marmoset also provides a number of distinct experimental advantages over each of these model systems, and we point to areas where a fully developed marmoset animal model promises to cast new light on mechanisms of visual cognition in the human brain.
Figure 1.
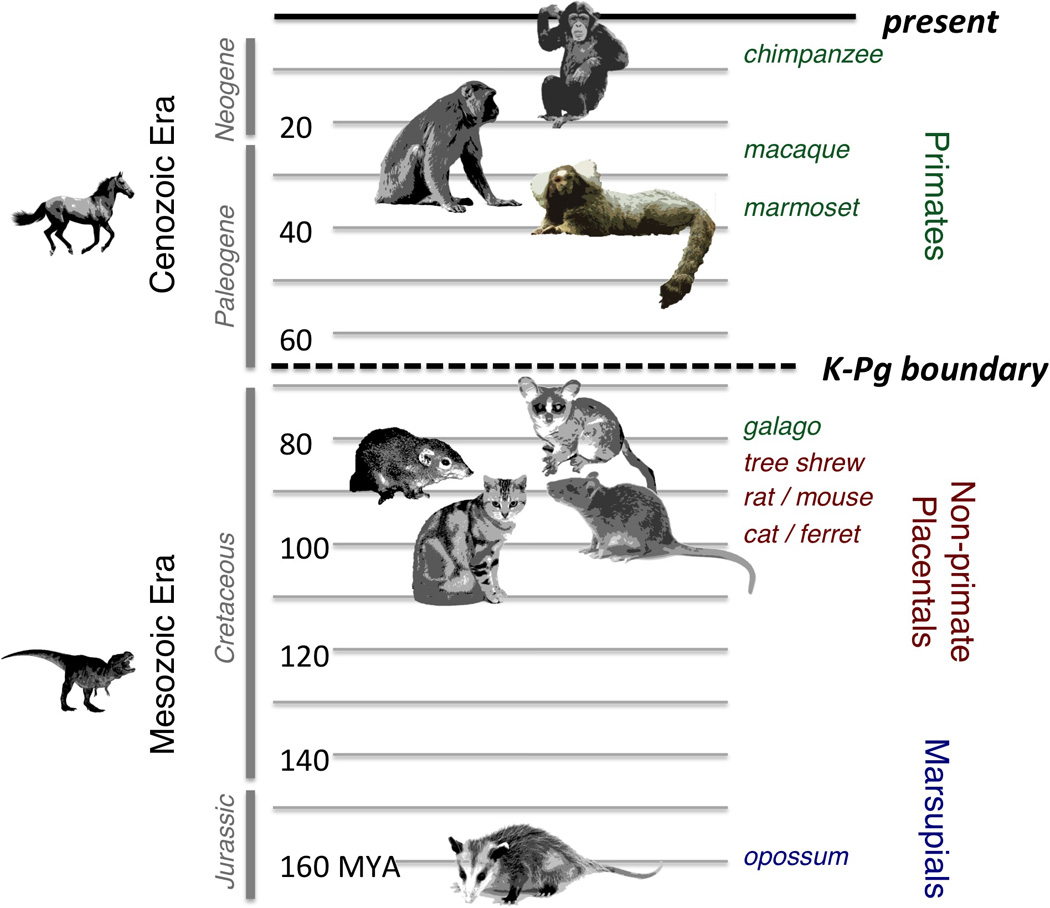
Phylogenetic portrait of common mammalian experimental models for visual neuroscience, now and from previous scientific generations. Representative species are arranged with respect to human ancestry. The vertical timeline indicates for each species the period of which the most recent common ancestor with humans lived. For the macaque and marmoset, this ancestor lived near the end of the Paleogene Period, long after the so-called K-Pg boundary that marked the end of the Mesozoic Era. However, for other mammalian models, including prosimian primates, the most recent common ancestor lived during the Cretaceous Period, in the Mesozoic Era. MYA, million years ago.
It makes sense to begin by hailing a success story in neuroscience: the recent flourishing of the mouse model, the facility of its genetic manipulation, and its use as a tool to probe the exquisite detail of the brain’s functional circuitry. Advances in the mouse have set new standards for the precision with which animal models can contribute to the investigation of the brain (Callaway, 2005; Deisseroth et al, 2006; Bernstein and Boyden, 2011). In particular, the development of transgenic lines, such as CRE lines, combined with viral-based optogenetics, have made it possible to express light sensitive opsins such as Channelrhodopsin (ChR2) in highly specific neuronal classes and then causally manipulate their activity with light (Livet et al, 2007; Cardin et al, 2012). This approach can be used to study functioning of specific anatomical pathways and has been used to link activity of specific cell types in the mouse visual cortex to distinct functional roles (Wilson et al, 2012) and to perceptual decisions (Lee et al, 2012; Zhang et al, 2014). There is at present a concerted push toward assembling a comprehensive description of visual circuitry in the mouse brain (Huberman and Niell, 2011).
However, over many decades the Old World rhesus macaque monkey (and closely related macaque species, referred to here collectively as “macaque”) has emerged as the species of choice for modeling human vision. The macaque is a logical choice owing to its evolutionary proximity to humans, which is reflected in the similarity of its brain’s basic anatomical layout as well as its perceptual capacities and ability to perform complex tasks. As a result, great detail is known about the anatomical connections and electrophysiological responses properties of its brain (e.g. Felleman and Van Essen, 1991; Markov et. al, 2014). Comparison with the human brain demonstrates that the basic layout of the visual system is similar between the two species (Orban et al., 2004). Moreover, because macaques can learn and perform diverse tasks under constrained experimental conditions, researchers have investigated neural responses throughout the brain and have related them to many high-level perceptual and visually guided behaviors, as well as the impact of focal lesions on such behaviors (e.g. Shadlen and Kiani, 2013; Rudebeck et al, 2013).
In some ways, the mouse and macaque models each stand on their own so successfully that they can obscure a very important aspect of neuroscience: comparative studies. As noted by Preuss (2000), “the concentration of effort on such a few species would be defensible if cortical organization were basically uniform across mammals, as is commonly believed … phyletic variation in cortical organization is far more extensive than has generally been appreciated or acknowledged.” Neuroscience in previous eras was characterized by investigation of a much broader range of species with some research directed toward understanding, for example, the organization of the visual cortex of the cat, ferret, tree shrew, ground squirrel, and many other mammalian and nonmammalian models. Some studies were explicitly comparative and allowed for a contextualization of neural features found in macaques species, such as those related to the anatomical connections between two visual structures (Harting et al., 1991) or the level of direct cortical control over movement (Nudo and Masterton, 1988). This breadth of study provided a foundation for describing the pathways of the primate brain from an evolutionary perspective, for example making the important distinction between ancestral and derived traits. The more recent focus on just two principal species, the mouse and macaque, threatens to diminish this perspective.
In this article, we argue that the marmoset provides a strategic choice, based on its specific experimental advantages and phylogenetic relationship to humans, to complement the mouse and the macaque for the study of vision. The marmoset model is already well established for biomedical research in fields such as infectious disease, reproduction, and aging, thus practical issues such as housing and breeding are well understood (Mansfield, 2003; Tardif et al, 2011; Carrion and Patterson, 2012). Some of this research has focused on the central nervous system, including the manipulation of cognition through pharmacological intervention and selective ablation (Robbins and Roberts, 2007). Marmosets are an important animal model for human audition and active vocal communciation (Miller et al, 2010; Takahasi et al, 2013; Wang, 2013). Although less studied in vision, a small number of groups have made great progress over the last two decades and built a foundation for understanding the basic organization of their visual system, primarily through visual field mapping and assessment of stimulus selectivity in anesthetized animals (for review, see Solomon and Rosa, 2014). We submit that expanding the role of marmosets in visual neuroscience requires little effort and has the potential to significantly advance our understanding of primate vision. To make our case, we begin in Section 2 by reviewing several important features of primate vision. Specifically, we show that the primate eye and brain are adapted in ways that are categorically different from other mammals, with many of the adaptations being consequences of high retinal acuity. In Section 3, we place our focus on the marmoset to compare and contrast features of its eye, brain, and behavior with the macaque. In Section 4, we review and discuss experimental aspects of the marmoset model that offer new and exciting opportunities for visual neuroscience. We end by briefly summarizing the main points of the review and look ahead to the opportunities and challenges afforded by the marmoset model in the neuroscientific investigation of the human brain.
2. The primate brain: a commitment to vision
2.1 Primates in evolutionary context
Primates have brains that share much in common with other mammalian species, most notably the prominent cerebral cortex. At the same time, primate brains have unique features that reflect how they interact with the world and with one another (Preuss, 2007). In this section we review the specific adaptations that have made the primate visual system distinct from that of other mammals. This provides the appropriate context for understanding, first, that marmoset vision is highly conserved with other primates, and second, that is differs in important ways from mammals of similar small size. Those readers familiar with the evolution of primate vision may prefer to begin in Section 3, where we focus on the marmoset and its distinctions from other primates.
To best understand the value of the non-human primate model it is important to consider the evolutionary context from which the primate lineage emerged. The diversity of the modern mammal was shaped by a volatile period of evolution in the late Mesozoic Era, when dinosaurs were still the predominant large animals on Earth (see Figure 1). During this time, the major radiations of mammals diverged, including the Euarchontoglires superorder containing rodents, rabbits, flying lemurs, tree shrews and primates. The specialization of early primates, which is thought to have been a set of adaptations for a nocturnal arboreal niche, may have strongly shaped the brain of modern extant primates, including humans (Cartmill, 1992). A pivotal point in mammalian evolution occurred approximately 66 million years ago (MYA), commonly termed the K-Pg boundary, which marks the time at which a large asteroid impacted the earth and is believed to have led to the extinction of the dinosaurs (Renne et al., 2013). Before the K-Pg event, the primate line diverged from other members of Euarchontoglires, followed a few million years later by its division into strepsirrhine (e.g. the prosimian Galago), and haplorhine (e.g. New and Old World monkeys and apes) branches (Janecka et al., 2007; Kaas, 2013). After the K-Pg event, a range of diurnal niches were opened up that were gradually filled by mammals, including primates. Much more recently, some 35–40 MYA, New World monkeys (e.g. marmosets) diverged from Old World monkeys (e.g. macaques) and apes (e.g. humans). The divergence of New World monkeys has been traced to an incredible example of geographical isolation, where a group of haplorhine monkeys appears to have rafted across the Atlantic Ocean from Africa to South America. This monkey, which is the most recent common ancestor of humans and marmosets, was a small to medium-sized arboreal fruit and seed eater (Fleagle, 1988; Ross, 1996). In Africa, Old World monkeys subsequently diverged from apes and humans approximately 25–30 MYA. When considering the evolutionary relationship between any pair of species, it is important to keep in mind that evolutionary changes can and do occur along both branches emanating from the most recent common ancestor. Thus monkeys and apes should not be treated as evolving from modern prosimians, which have undergone their own evolutionary adaptations. Similarly, humans and other apes did not evolve from rhesus monkeys or any other extant species.
The neural and behavioral evolution of primates is strongly tied to adaptations in the domain of vision, which we describe in detail below. However, it is worth emphasizing here that the importance of vision is in no way unique to primates. It is a vital sense for nearly all vertebrates, with its critical value being that it provides information about the environment from a distance. In many species, vision is important for navigation, interspecies interactions (e.g. predation), intraspecies interactions (e.g. mate selection), and the selection of nesting and foraging sites. In birds, the surviving descendents of dinosaurs whose most recent common ancestor with primates lived approximately 320 MYA, vision is paramount and there are many examples of convergent evolution affecting primate and bird vision, for example in the specialization of a retinal fovea (Fite and Rosenfield-Wessels, 1975; Ross, 1996; Kirk and Kay, 2004), or in aspects of their visual cognition (Emery and Clayton, 2004). Among mammals, primates are unusually reliant on vision, as they do not conform to the more typical mammalian pattern of using olfactory cues for social interaction and foraging. Prosimians show less reliance on vision than simians (monkeys, apes, and humans) and may be regarded as intermediate in this respect. The primate commitment to vision has evolved along with specific adaptations of the eye and brain, which we describe in the following sections along with how they uniquely shape primate behaviors.
2.2 Adaptations of the eyes
While cause and effect relationships are notoriously difficult to specify in evolutionary biology, it appears evident that a major force driving innovation of the primate brain was changes to the eye. This section identifies three such changes and briefly discusses the importance of each.
2.2.1 Foveal high acuity vision
Perhaps the most distinguishing feature of primate vision is its high spatial acuity, or the ability to resolve fine detail. Among mammals humans rank highest in their visual acuity, which commonly exceeds 50 cycles/degree, and this is followed closely by apes and monkeys (Kirk and Kay, 2004). In fact, this aspect of primate vision is unmatched among mammals and only exceeded by a few species of large, predatory birds (Kirk and Kay, 2004).
Primates’ unusually high acuity stems from multiple adaptations, including the large size of the eye, its optics, the high density of retinal photoreceptors and ganglion cells in central vision, and the low amount of spatial pooling of photoreceptor signals onto individual gangion cells (Provis et al., 2013). In simian primates (i.e. monkeys and apes), the most unique feature of the retina is the fovea mediating central vision. The fovea is a pit in the inner retina caused by the local absence of cell processes, creating a window of optical clarity for light reaching photoreceptors situated along the outer circumference of the retinal epithelium (Figure 2A). The high density of cone photoreceptors that populate the fovea is, quite remarkably, free of blood vessels (Figure 2B). Across primates, the fovea has a roughly constant size of somewhat less than 0.5 mm despite large variations in eye size. The fact that larger eyes do not have larger foveas may suggest that its size is limited by the diameter within which the overlying cell processes and vasculature can be cleared without harming the photoreceptors themselves (Franco et al., 2000). Some prosimian primates such as the nocturnal Galago have a more primitive region of photoreceptor concentration mediating central vision, termed the area centralis. However, the increase in receptor density in this region is notably less than in the simian primate fovea, with only a 2–3 fold increase compared to a 20 or more fold increase in New and Old World monkeys (Wikler and Rakic, 1990). Thus the spatial acuity of the galago (4.8–6.0 cycles/deg) is much lower than most monkeys and humans (Langston et al., 1986).
Figure 2.
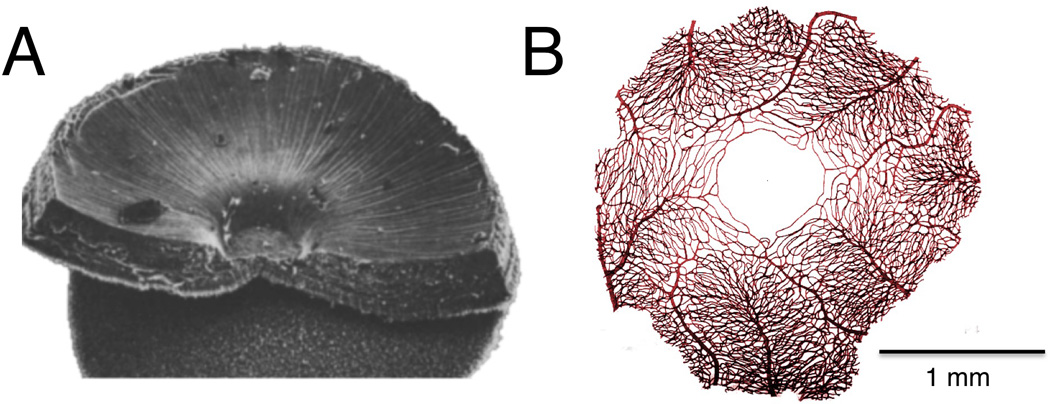
The unique foveal specialization of the primate retina, which has shaped the organization of the primate brain and much of primate behavior. A. Scanning electron micrograph of portion of the macaque retina showing the foveal slope and pit (adapted from Borwein, 1983). B. Pattern of vascularization surrounding the avascular foveal region of the macaque retina (adapted from Snodderly et al., 1992).
Signals arising from the high density of foveal cones in monkeys and apes are carried by tightly packed midget retinal ganglion cells into to the brain. In the foveal region of the retina, midget ganglion cells outnumber the cone photoreceptors and each midget ganglion cell samples from only a single cone photoreceptor via one-to-one connections with midget bipolar cells (Boycott and Dowling, 1969; Schein, 1988; Wassle et al, 1990; Wilder et al, 1996). By comparison, the corresponding classes of small ganglion cells in other species, such as the cat β cells, pool from up to 30 cone photoreceptors (Goodchild et al, 1996). At more peripheral regions of the retina, midget ganglion cells in monkeys show a higher level of spatial pooling from up to 10 cone photoreceptors (Goodchild et al, 1996). Inherent in the spatial pooling of photoreceptor information is a trade-off between light sensitivity and spatial acuity. The demands of night vision seem to have pushed at least two nocturnal species, the Galago and owl monkey, to a level of photoreceptor pooling that is unusually high among primates (Yamada et al, 1998; Yamada et al, 2001; Kilavik et al, 2007), but still much less than in the cat.
The evolution of high acuity in primates may have been gradual. One hypothesis holds that high acuity in early diurnal primates benefitted from the unusually large eyes of their nocturnal ancestors, which was itself an adaptation to low light conditions (Ross, 2000). For a given cone density, larger eyes translate directly into higher acuity, since a given visual angle subtended on the retina is projected onto more photoreceptors. Another set of adaptations led to the intense concentration of photoreceptors and clearing of vasculature at the central part of the retina to create the foveal pit. How and when this came about is far from clear, but it may be linked to the requirements for visually guided insect predation, much like in some insectivorous birds (see Kirk and Kay, 2004).
For primates, the possession of a fovea has important implications for both near and far vision, both of which critically shape primate behavior. For near vision the resulting high acuity allows for perception of fine detail in objects and textures, which can be important for manipulating or selecting objects, food, or, in the case of humans, tools. For far vision high acuity translates to the ability to see details about conspecifics and their movements even at a distance. For primates, this latter capacity is closely related to a unique aspect of primate social behavior: primates constantly use their vision to make judgments about individuals, their actions, emotional state, and attentional focus, in order to “read” the complex social landscape within a large and hierarchical group.
2.2.2 Trichromacy
Trichromatic color vision is another perceptual capacity that stems directly from adaptations to the retina. Most mammals have two cone types, short (S) and long (L), with the wavelength-sensitivity of each cone determined by opsin genes that lead to the selective expression of either S or L photopigment within each cone’s outer segment. However, many primates have an additional cone type (medium, M), offering trichromatic vision that other mammals lack (for review see Jacobs, 2008). The expression of these L and M photopigments is highest in the foveal region of the retina, from which ganglion cells carry signals to the brain about the relative stimulation of the different cone types. These internal, comparative signals, often termed red/green opponency, are superimposed on the high acuity information described above and are thought to be critically important for color perception.
All Old World monkeys and apes are routine trichromats. However, many New World monkey species are marked by genetic polymorphisms that render some individuals as dichromats, similar to other mammals, and others as trichromats, similar to Old World monkeys. In fact, in those species it is only a subset of females that have the capacity for trichromatic vision. This odd relationship between gender and trichromacy can be understood in terms of the underlying molecular genetics. As described above, to be a trichromat one needs three distinct types of cones, each with a different spectral sensitivity. These are the S cones, coded by an autosomal gene that is highly conserved across mammals, and the L and M cones. In Old World monkeys and apes, two separate L and M genes reside on the X chromosome. Since both males and females have at least one X chromosome, all members of those Old World species are trichromats (Jacobs and Deegan, 1999). Humans are the only marginal exception to this rule, where roughly 8% of males have a mutation of one of these genes and are thus dichromats (Sharpe et al., 1999). However, in New World monkeys, only a single opsin gene locus exists on the X chromosome, but there are multiple alleles in the population with varying wavelength sensitivity in the L and M range (Jacobs et al., 1993). As a result a female, having two X chromsomes, can by chance inherit X chromosome alleles that are sensitive to different wavelengths. In such a case, this female will be a trichromat. Since males from the same species only have one X chromosome, they are obligatory dichromats. As we discuss later, this difference allows for an interesting test of the role played by trichromacy in shaping visual processing.
How did trichromatic vision evolve? Unlike high acuity, primate trichromacy seems to have arisen abruptly. At some point after the divergence of New World and Old World primates (see Figure 1), two genetic events are thought to have occurred in line leading to extant Old World monkeys and apes. First there was a gene duplication event leading to two opsin copies on the X chromosome. This was followed by the mutation of one of the genes that led to a shift in its chromatic sensitivity. The result was the routine presence of both M and L opsins on the X chromosome, allowing vision to capitalize on three distinct cone types (Nathans et al., 1986; Surridge et al., 2003). The stable maintenance of trichromacy among Old World primates is thought to reflect selective advantages. Most often, it is linked to advantages in foraging, since the discrimination of green and red hues can lead to better selection of fruit or leaves (Dominy and Lucas, 2001; Mollon, 1989; Regan et al., 2001). However recent genetic evidence suggests that the emergence of trichromatic vision also affected primate social behavior. Interestingly, this hypothesis stems from a peculiar evolutionary trade-off between photopigment opsin genes and olfactory receptor genes. Most mammals use chemical olfactory signals to convey identity and other social information. To some extent, prosimians and New World monkeys use such signaling mechanisms. However, the genetic potential supporting this mode of social communication virtually disappeared in trichromatic primates, with many olfactory receptor genes mutating into nonfunctional pseudogenes (Mundy, 2006). This deterioration of olfaction is suspiciously coincident with the emergence of trichromacy (Gilad et al., 2004; Liman and Innan, 2003; Zhang and Webb, 2003), and this linkage may be reflected in the dominance of vision over olfaction for important types of primate social exchange (Liman, 2006), which we discuss further in Section 2.4.2 below.
2.2.3 Binocular visual field
A third peripheral adaptation is the convergence of the orbits in the primate skull and the resulting high degree of binocular field overlap. These changes are thought to have been part of a larger array of craniofacial changes that, like visual acuity, may have originally benefitted the requirements of nocturnal predation (Ross, 2000). It has also been suggested that the larger binocular overlap could have developed to meet optical constraints in focusing images for the larger nocturnal eyes of early primates, a feature found in prosimians but also fruit bats and owls (see Rosa, 1993). Primates have a higher degree of binocular convergence than other mammals, a condition that affords advantages for vision, including stereoscopic depth perception (Parker, 2007) and redundant sampling to aid perception in a cluttered environment (Changizi and Shimojo, 2008). The requirements placed on the brain for reconciling high-resolution images from the two eyes into a coherent representation appears to have strongly influenced the organization of the visual system. For one, it is hypothesized that such central reconciliation may have eliminated the advantages of feature extraction in the retinal periphery and led to a shift in which such features are first computed in the cortex (Pettigrew, 1986a). While the diversity of retinal cell types found in other mammals remains present in the primate (Dacey, 2004), and may support some complex feature extraction, it is notable that what have become the numerically dominant retinal classes in the primate, the parasol and midget cells, lack such feature selectivity. For example, neurons in the rodent retina are readily observed to have directional selectivity, but this is much less common in the primate. In the primate, even the input layers of the primary visual cortex have circularly symmetric, undifferentiated monocular receptive fields (Schiller et al, 1976; Ringach et al, 2002), with higher order features, such as orientation or directional selectivity, computed subsequently (Hubel and Wiesel, 1968). That this organization can be related to the challenges of binocular overlap is supported by the fact analagous differences are observed in species of birds with differing levels of binocular overlap (Pettigrew, 1986a).
In primates, the need for binocular reconciliation strongly shapes the development and function of the early visual system, for example in the high degree of crossing at the optic chiasm, the strong lamination of the lateral geniculate nucleus, and the prominent ocular dominance columns in the primary visual cortex. Ultimately it impacts brain function in many other ways, such as in the support of high resolution stereoscopic vision and need for precise orienting mechanisms to compensate for the restricted visual field. In the next section we examine more specifically how high acuity, trichromatic, and binocular vision may have shaped the brain and behavior of primates.
2.3 The visual brain
The primate brain has a number of unique characteristics that distinguish it from that of other mammals (Figure 3, for review, see Preuss, 2007). Most of these features are shared throughout the primate Order, despite a wide diversity of sizes and habitats of individual species. Here we describe several brain regions related to vision, focusing on those features that distinguish primates from other mammals.
Figure 3.
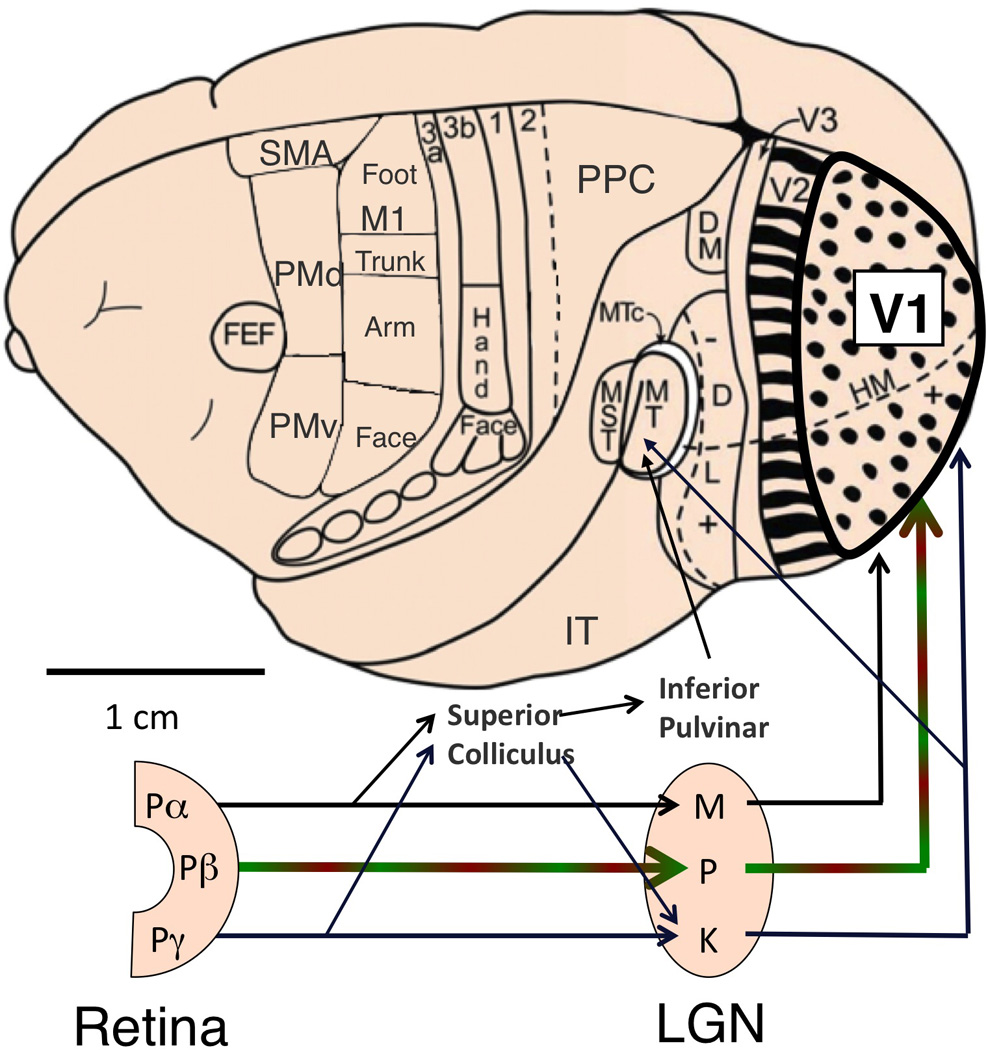
Schematic drawing of retinal pathways to the cerebral cortex for a prototypical adult primate, shown on the brain of a New World monkey. Parallel streams of visual information leave the retina through three principal classes of projecting ganglion cells (Pα, Pβ and Pγ), whose LGN projection targets are magnocellular (M), parvocellular (P) and koniocellular (K), respectively. The parvocellular pathway dominates, carrying high-resolution foveal information in all primates and also red/green opponent signals in trichromats. In contrast to other mammals, nearly all visual information enters the visual cortex through V1. Only major feedforward projections are depicted. Adapted from Kaas (2012) and Preuss (2007).
2.3.1 Superior colliculus
The most distal target of retinal ganglion cell axons growing in the brain is the superior colliculus (Huerta and Harting, 1984). This structure, which is homologous to the retinorecipient optic tectum in other vertebrates, contains organized maps of visual space derived directly from the layout of the retina. The primate superior colliculus receives inputs that include magnocellular- and koniocellular-type signals from the retina, arising from the parasol (P) and diverse types of bistratified (P) ganglion cells, respectively (Rodieck and Watanabe, 1993; Schiller and Malpeli, 1977). Magnocellular and koniocellular pathways carry visual information of relatively low spatial acuity, but good temporal acuity and sensitivity at low light levels. They can be contrasted with the higher acuity information present in parvocellular signals, which we will discuss in the context of the geniculate pathway shortly.
One notable feature of retinotopic maps in the colliculus that distinguishes primates from other mammals is that input to each colliculus is exclusively from the contralateral visual field. In most other mammals studied to date, visual maps in the colliculus correspond to the complete visual extent of the contralateral eye, including both contralateral and ipsilateral visual fields. The ipsilateral visual field, which is often much smaller, is represented rostrally. The contralateral field occupies the larger portion of the colliculus and extends caudally. The level of orbital convergence determines the level of ipsilateral visual field representation, with more binocular overlap leading to a higher degree of ipsilateral field representation (Rosa and Schmid, 1994). In primates, however, the retinal input to each superior colliculus is very different and derives almost exclusively from the contralateral visual field. Although there must always be a limited overlap near the midline, there is otherwise no rostral ipsilateral field representation despite the large binocular overlap (Kaas and Huerta, 1988). This strict field segregation in the colliculus is a strong feature that distinguishes primates from all other mammals (Allman, 1977). In fact, it is such a primate-unique feature that when a somewhat similar organization was found in fruit-eating bats (megachiroptera), it was proposed that these bats must be descended from primates (Pettigrew, 1986b). This proposition has been challenged by subsequent electrophysiological (Thiele et al., 1991) and molecular (Murphy et al, 2001) evidence, though debate continues. The functional implication of this anatomical feature remains unknown, but suggests that in our early primate ancestors there was a significant and lasting rerouting of retinal ganglion cell projections to the superior colliculus within the optic chiasm (Allman, 1977).
2.3.2 Lateral geniculate nucleus
In mammals the lateral geniculate nucleus (LGN) is the principal recipient of retinal ganglion cell afferents. The primate LGN is composed of multiple, layered cells that form parallel maps of the visual world (Casagrande and Norton, 1991; for a review see Casagrande et al, 2006). These layers are named based on the size of their constitutent cells. The large projection neurons in the magnocellular layers (“magno” = large) receive their input primarily from the parasol, or P, ganglion cells. The smaller projection neurons in the parvocellular layers (“parvo” = small) receive their input from the midget, or P, ganglion cells. Finally, the smallest projection neurons in the koniocellular layers (“konio” = dust) receive their input from diverse sets of ganglion cells, collectively termed P (Nassi and Callaway, 2009). In the primate LGN, the inputs from left and right eyes further segregate the cell classes functionally into distinct layers. Thus each LGN contains retinotopically registered maps corresponding to left eye magnocellular, right eye magnocellular, left eye parvocellular, right eye parvocellular, giving four layers, a number that is shared by all primates. In larger primates, the parvocellular layers are further subdivided into incomplete folds or “subleaflets”, giving the appearence of six layers in the macaque, or even eight in the human (see Kaas et. al., 1978). Among primates, the parallel projection of retinal ganglion cell types into distinct LGN layers is highly conserved (Itoh et al, 1982) as is the segregated projection of LGN cell classes into the input layers of the visual cortex (Diamond et al, 1985). It is not until the primary visual cortex that information from the different retinal cell classes, and from the two eyes, is mixed together.
Functional segregation is also a feature of the LGN of other mammals, though is generally not as strict as in primates. Ocular segregation is common, where retinogeniculate terminations from the contralateral eye show limited overlap with those from the ipsilateral eye (Kaas et al., 1972). The compartmentalization of physiological types is more varied. In cats and some diurnal rodents (e.g. grey squirrels) LGN cells are layered and receive selective ganglion cell input. Layers of X-like cells (A and A1 layers in the cat) have tonic sustained responses that pool linearly across space, whereas layers of Y-like cells (C and C1 layers in the cat) have transient non-linear response properties. An additional category of W-like cells is more distributed and carries low-acuity information from diverse bistratified retinal ganglion cells about luminance (Enroth-Cugell and Robson, 1966; Wilson et al, 1976; Van Hooser et al, 2005). These classes are physiologically similar to those originating in the Pα, Pβ, and Pγ retinal classes in macaques. Importantly, in mammals where the LGN does not exhibit obvious laminar segregation, similar classes still exist. For example, in some nocturnal rodents such as rats (Lennie and Perry, 1981) and mice (Grubb and Thompson, 2003; Piscopo et al., 2013), similar physiological classes exist in the LGN (e.g. sustained and transient) despite minimal evidence for their laminar segregation (Drager UC, 1974; Hughes HC, 1977; Harting and Huerta, 1983). Even in the marsupial opossum, neurons fall into distinct classes that resemble the X, Y, and W responses of other mammals (Kirby and Wilson, 1986), suggesting that this retinogeniculate division of labor evolved before the split between marsupials and placental mammals in the Jurassic period (Luo et al., 2011) (see Figure 1), and perhaps much earlier. Importantly, comparative studies of the mammalian LGN reveal a clear dissociation between the existence of physiological cell classes and the extent of their laminar compartmentalization. The fact that all extant species evaluated in this way have multiple classes suggests these physiological ganglion cell classes are more fundamental than the compartmentalization of their target neurons within the LGN, which is highly variable across mammals.
In addition to the strict segregation of its physiological cell classes, at least two additional features distinguish the primate LGN, both of which can be traced to retinal specialization. The first feature is the dominance of the parvocellular pathway, which constitutes approximately 90% of the LGN in some primate species. This is the channel for high-resolution vision, which transmits information from the small midget ganglion cells to the primary visual cortex. Parvocellular neurons respond to fine image details and modulate their activity approximately linearly with contrast, particularly in central vision (Malpeli et al, 1996). Although the homology of these classes across species remains unclear, parvocellular neurons have response properties that resemble X-type signals in othe mammals and magnocellular neurons have properties that resemble Y-type signals. This physiological connection is most evident by the similar sustained versus transient nature of the X- and Y-type neural responses, respectively. At the same time, other physiological characteristics of these classes can differ significantly. For example, in rodents the spatial acuity of the sustained X class is no greater than that of transient Y class, and the contrast linearity of response of X class is no greater than Y cells (Cacieri et al, 2003; Grubb and Thompson, 2003; Van Hooser et al, 2005). In cats, the distinction between these classes lies somewhere in between with regard to both acuity and response linearity (Derrington and Fuchs, 1979; Bullier and Norton, 1979). Taken together, it appears that the most widely shared property that distinguishes physiologilogical cell classes among mammals is response transience, suggesting that this is a core principle governing parallel transmission through this structure. When there is retinal specialization for high acuity, which is extreme in primates, the parvocellular pathway is much expanded to carry that information.
The second feature distinguishing of the primate LGN is the red/green opponency that is transmitted using the same parvocellular pathway. This feature of the parvocellular system has previously been featured as a possible primate specialization for trichromacy (Shapley and Perry, 1986), though given the broad range of dichromatic species that have the same parallel sustained and transient LGN channels it is unlikely to have evolved specifically for color vision. Experiments in different primate species reveals that the parvocellular channel is most obviously related to conveying high acuity, sustained visual information, rather than color per se. For example, comparing LGN responses in dichromatic male versus trichromatic female members of the same New World monkey species has revealed essentially identical responses of parvocellular neurons to achromatic stimuli (Martin et al, 2011). Similarly, parvocellular neurons in the Galago, a prosimian nocturnal primate that has much higher ratios of rod photoreceptors than cones, were shown to respond in a similar fashion to parvocellular neurons in other primates (Yamada et al, 1998).
Taken together, this comparative analysis points to the order of events in the evolution of the LGN, shaping our understanding of human vision. It is likely that the parallel retinogeniculate pathways evolved first, carrying sustained and transient signals through parallel channels emanating from each position on the retina. The laminar segregation of these channels within the LGN probably came at a later point. Then in some early primate species, regions of exceptionally high photoreceptor density in the retina selectively adopted the sustained pathway for high-resolution signals. Finally, in trichromatic primates this same pathway was further utilized to relay red/green opponent signals, which were most pronounced in the high resolution fovea.
2.3.3 Early retinotopic visual cortex
Neurons in the LGN send long-range projections that transmit organized maps of visual space to the cerebral cortex, preserving the topological layout of the retina. This “retinotopy” has analogy in other sensory systems, such as audition and somatosensation, where the layout of the sensory epithelium is also preserved in the cortex. The visual cortex has multiple retinotopic maps. In the early retinotopic maps, a given position responds to input from a unique region of the retina, and a line running along the cortical surface of each map traces out a continuous trajectory of corresponding positions on the retina.
In primates, nearly all LGN projections are directed to area 17, also termed the primary visual cortex or V1. The long thalamocortical axons that run through the optic radiations to V1 remain highly organized until their primary innervation of layer 4C and secondary innervation of layer 6. Parvocellular LGN inputs selectively innervate neurons in layer 4C and magnocellular inputs selectively innervate neurons in layer 4C (Hubel and Wiesel, 1972; Blasdel and Lund, 1983; Blasdel and Fitzpatrick, 1984), whereas the koniocellular inputs terminate in the supragranular layers (Hendry and Reid, 2000). Likewise, the magnocellular pathway provides collateral inputs to layer 6B, whereas the parvocellular pathway provides collateral inputs to layer 6A. Running perpendicular to these cell layers is a different type of functional organization, in which radial units of similar functional properties are assembled into columns. The columnar structure of the early cortex is superimposed upon its most conspicuous feature, which is the systematic mapping of visual space. At least one major feature of the tangential organization of the cortex is derived directly from segregation within the LGN. Namely, the the ocular dominance columns are a product of the spatially interleaved input, primarily in layer 4C, from the ipsilateral and contralateral eye LGN cell layers (Hubel and Wiesel, 1968).
In non-primate mammals, the organization of projections from the LGN to the cerebral cortex is quite different. First, in most species that have been studied the LGN projects divergently, not only to area 17 but significantly also to secondary visual areas (e.g. area 18 and 19) (Dreher and Cottee, 1975; Olavarria and Torrealba, 1978). This difference in anatomical projections is obvious following the effects of area 17 lesions, which in primates causes a condition that approximates complete blindness but in other mammals is much less severe (Killackey et al, 1971, 1972; Funk and Rosa, 1998). Also, the subdivisions within the input layers, and presumed segregation of innervation to sublayers containing different cell classes, varies in other mammals from prominent (Freund et al., 1985; Wong and Kaas, 2008) to weak or absent (Wong and Kaas, 2009). It is notable that in at least one species, the tree shrew, the geniculostriate terminations in layer 4 convey not transient and sustained responses, but rather “on” and “off” responses, which are similarly segregated in the retina and in the LGN (Conley et al., 1984; Van Hooser et al., 2013). Together, these findings suggest that the sublamination of the input layers of the primary visual cortex is closely tied to the segregation of information within the LGN.
Similarly, the columnar organization perpendicular to the V1 input layers is more pronounced in primates than in other mammals. Regarding ocular dominance columns, the tangentially alternating pattern of ocular segregation in layer 4 is less pronounced in the cat (Hubel and Wiesel, 1972). It is also weak or absent in species closely related to macaques such as tree shrews (Humphrey et al, 1977) and rodents, including diurnal visual rodents like squirrels that have laminated LGN (Van Hooser et al, 2005) (reviewed in (Horton and Hocking, 1996)). Ocular dominance columns almost certainly pertain to the reconciliation of images from two frontally positioned eyes. As all primates have considerable binocular overlap, ocular dominance columns are common and have been found in all prosimians and Old World monkeys tested to date. However, it is not a distinguishing feature of the primate brain and is not obviously present in some New World monkeys (Casagrande and Boyd, 1996). At present the basis of ocular dominance columns and relationship to the LGN and other features of V1 organization remains a puzzle (Adams and Horton, 2009).
Another notable specialization of primate V1 is the extremely high density and cellular morphology of its primary geniculorecipient layer, which is almost certainly tied to high visual acuity. While considerable evidence suggests that the areal density of neurons across the cortex in different mammalian species is approximately the same (but see Collins et al, 2010), a consistent finding is that primate V1 stands out as having the highest neuronal density (Rockel et al, 1980; Collins et al, 2010; Carlo and Stevens, 2012). One factor contributing to this tighter packing is the evolution of a specialized class of pyramidal neurons that have an unusually compact stellate morphology (Lund, 1990). Such stellate neurons are not found in the input layers of rodent visual cortex (Peters and Kara, 1985), although a similar type of cell exists in rodent somatosensory cortex (Woolsey and Van der Hoos, 1970). These distinctions in the input layer structure are also reflected in the local patterns of gene expression in Old World primates, and also appear in New World monkeys but are less pronounced, and are totally absent among rodents (Takahata et al, 2006; Takahata et al, 2011).
In the extragranular layers of primate V1, the parvo-, magno-, and koniocellular information becomes less segregated and is to some extent reorganized to meet the requirements of dorsal and ventral processing streams. For example, information is segregated between the “blobs” and “interblobs” of the superficial layers (see Figure 3), which can be visualized using cytochrome oxidase staining (Horton and Hubel, 1981). Neurons in blobs and interblobs have different visual response properties and different projection targets (Federer et al., 2009). Cytochrome oxidase blobs are present in primates but absent in other orders of Euarchontoglires, suggesting that this feature evolved early in primate evolution (Kaas, 2012). At the same time, similar blobs have been described in cats (Boyd and Matsubara, 1996; Shoham et. al., 1997), thus the evolutionary origins of this V1 feature remain unclear. Neurons in the primate interblob regions are organized into columns tuned for a particular orientation with the preferred orientation progressing smoothly across the cortical surface. Orientation columns are also present in certain other mammalian species such as cats (Hubel and Wiesel, 1963) and tree shrews (Humphrey and Norton, 1980). They are, however, curiously absent in rodents (Metin et al, 1988), including highly visual rodents such as squirrels (Von Hooser et al, 2005), despite the fact that individual neurons in their area 17 show orientation tuned responses. The dissociation of orientation tuning and orientation columns is a comparative finding that draws attention to the fact that these two functional characteristics are not fundamentally linked (Reid RC, 2012).
The segregation of input continues into a second, strongly retinotopic visual area (V2). In area V2, cytochrome oxidase staining reveals a different set of functional zones that take the form of stripes of different intensity and width and run perpendicularly to the V1/V2 border (see Figure 3). The progression of these stripes is superimposed upon the map of retinal space and takes the form of a repeated sequence of pale-thick and pale-thin sets of stripes (Tootell et al, 1983; Federer et al, 2009). Neurons within the thick and pale stripes, receiving input from the interblob regions, are selective to binocular disparity and orientation. The thin and pale stripes receive afferents from blob regions, and are selective to luminance and color. These anatomical features in V1 and V2 are shared across primates, including New World monkeys such as the marmoset (Federer et al, 2009) as well as prosimians such as the Galago (Kaas, 2012), though the pattern in V2 of prosimians is more patch-like than stripe-like (Collins et. al., 2001). The stripes also appear to project differentially to an array of common extra-striate visual areas that analyze dorsal (DM, MT/MST, FST, PPC) and ventral (V3, V4, and IT) stream information. The shared projections among different primate species, with thick stripes projecting to visual area MT and the other band projecting to visual area DL (or V4), suggests that this basic organization was present in an early common ancestor (Kaas, 2012).
2.3.4 High-level visual cortex
Higher-level visual cortex in primates is typically described as consisting of dorsal and ventral visual pathways, in which different types of visual information are extracted from the retinal input and used for behavior (Ungerlieder and Mishkin, 1982; Goodale and Milner, 1992). The dorsal stream gains much of its input through projections from area MT. Major projections to subregions of the posterior parietal cortex (PPC) are critical for guiding visually directed actions to mediate specific behaviors and spatial judgments, whereas other projections carry visual information further to retrosplenial and parahippocampal regions and are thought to be important for navigation (Kaas et al, 2011; Kravitz et al, 2011). The ventral stream is also composed of multiple pathways, in this case passing visual information to different subregions of the inferotemporal cortex. The ventral pathways receive much of their input through area V4, though signals from MT are also important in shaping responses in many ventral stream areas. The ventral pathway is thought to be involved in the processing of complex structure for recognizing objects and social information (Kravitz et al, 2013). While high-level visual cortex has been most studied in primates, other mammals appear to have an analagous division of labor in their cerebral cortex. Lesion studies in cats reveal a dissociation in the deficits following parietal and temporal lesions that approximates that seen in monkeys (Lomber et al, 1996). Sheep have abundant face-selective neurons in their temporal cortex that resemble those found in monkeys (Tate et al, 2006). In rodents, there is some evidence to suggest that a dedicated dorsal visual stream conducts spatial analysis (Kolb, 1990; Reep et al, 1994), with evidence for a separate ventral visual stream analog being less obvious (Preuss and Goldman-Rakic, 1991; Wang et al, 2012). Thus much evidence supports the origination of dorsal and ventral visual pathways in the Mesozoic period, before the first primate (see Figure 1). Nonetheless, there is good reason to believe that the dorsal and ventral systems underwent considerable evolution within the primate radiation. This is because the two most prominent behavioral adaptations related to vision, which we discuss next, derive directly from parietal and temporal cortex function.
The first prominent adaptation is visually guided reaching and grasping, a behavior at which primates excel and which is unmatched among mammals. This faculty allows for efficient movement through the arboreal environment as well as the manipulation of food and other types of objects, including tools in humans. These abilities are thought to have drawn, at least in part, upon expansion and specialization of the posterior parietal cortex (PPC), together with the development of motor and premotor cortex (Kaas et al, 2012). Distinct sub-networks mediate different visually guided behaviors that include reaching, defensive, and grasping movements (for review, see Kaas et al, 2011). These areas have been extensively studied in macaques, and include the parietal reach region (PRR) for reaching movements (Batista et al, 1999), the lateral intra-parietal area (LIP) for eye movements (Colby et al, 1996), the anterior intra-parietal area (AIP) for grasping movements (Sakata et al, 1995), and the ventral intraparietal area (VIP) for defensive movements of the head and arm (Cooke et al, 2003). Areas with similar functions have been found in the Galago (Stepniewska et al, 2005, 2009a) and in New World monkeys (Gharbawie et al, 2010, 2011), including the marmoset (Rosa et al, 2009; Paxinos et al, 2012; Reser et al, 2013). Each subregion of the parietal cortex projects to corresponding subregions of premotor and motor cortex that specialize in similar movements, and thus form distinct networks for particular categories of actions (Stepniewska et al, 2009b; Gharbawie et al, 2010, 2011). Among rodents and the tree shrew the PPC is much smaller and appears to play a less direct role in guiding movement. For example, while the tree shrew has a greatly expanded visual cortex as compared to rodents, most of its visual and somatosensory information still reaches motor cortex through direct projections rather than through the PPC, unlike in primates (Remple et al, 2007; Kaas et al, 2011). While comparative physiological studies have been relatively rare in this area, it seems likely that at least some of these PPC regions have no clear homolog in mammals that do not use their vision to guide reaching and grasping.
The specialization of the parietal cortex is particulary important for understanding the human brain. Human bipedalism has fundamentally changed the manner in which extrapersonal space is encoded and has further led to exceedingly complex visually guided actions, and associated brain specializations, that facilitate tool use (Orban and Caruana, 2014). Humans have an expansion of the PPC that includes areas that appear to be absent in other primates (Chaplin et al, 2013a). This expansion is thought to give rise to a more sophisticated repertoire of motor behaviors including the fine maniupation of tools and other objects (Orban et al, 2006; Peeters et al, 2009).
The second prominent behavioral adaptation in primates is the use of vision for complex social exchange. As mentioned above, the routine use of vision for individual recognition, sexual selection, and social monitoring is facilitated by specialization of the retina that improved visual acuity and led to trichromatic color vision. The impact of these peripheral specializations on social behavior has been accompanied by massive expansion of the ventral visual cortex, including the large proportion of inferotemporal tissue apparently dedicated to the processing of faces and bodily actions (Leopold and Rhodes, 2010).
Ventral visual pathway expansion in the primate appears to be strongly linked with the focus on foveal processing and the use of vision to observe individuals from a distance. Multiple cortical pathways in the inferotemporal cortex project to distinct cortical and subcortical targets and specialize in different aspects of processing including object recognition, scene recognition, and emotional or affective valence (for review, see Kravitz et al 2013). Along the ventral stream there is a division of labor between cortical areas that lie anterior to, and receive input from, the foveal portions of early visual areas and those which lie anterior to, and receive input from, peripheral field representations. Peripheral regions feed into parahippocampal cortex, where neurons have peripheral receptive fields (Sato and Nakamura, 2003) and are thought to be involved in the spatial understanding of a scene (Landis et al, 1986; Park et al, 2011; Kravitz et al, 2011). Foveal regions feed into the inferior temporal cortex, which contributes to complex form vision, including the analysis of objects and social stimuli. Nearly all neurons in the inferotemporal cortex respond most strongly to stimuli when they are presented at the fovea (Gross et al, 1972; Desimone and Gross, 1979; Tanaka et al, 1991; Op De Beeck and Vogels, 2000). As rodents lack a foveal specialization, it may be difficult to identify a homologous, or even analagous, processing pathway (Preuss and Goldman-Rakic, 1991). However, a recent study has identified extrastriate visual areas in the mouse whose function appears to map onto dorsal and ventral streams in the primate, with the ventral stream involved in object or landmark recognition (Wang et al, 2012). Other work demonstrates that rodents can learn to distinguish between visually complex objects (Zoccolan et al, 2009; Alemi-Neissi et al, 2013). Thus, while there seems to be no clear homolog to inferior temporal cortex, rodents do show some features of visual cognition commonly associated with the ventral processing pathway. There is still much to be learned from comparative research about the origin and evolution of the dorsal and ventral visual pathways that play such a prominent role in understanding the organization of the human brain.
2.4 Visually guided behaviors
The evolved changes to the primate eye and brain can be linked directly to an array of specialized behaviors. Here we review three visually guided behaviors at which primates excel: the detailed visual exploration of a scene, the perception and interpretation of social information, and the precise guidance of reaching and grasping.
2.4.1 Natural exploratory behavior
Monkeys’ reputation for being curious is well deserved, and is supported by neural systems that promote the efficient acquisition of visual information from the world. More than other mammals, primates have a fast and precise means of directing their gaze from point to point in the form of rapid eye movements called saccades. Saccades, along with smooth pursuit for tracking moving objects, are highly developed in primates and coordinated with actions of the head and body. These movements are tightly coordinated with the processing of the visual input and are controlled by an elaborate network of cortical and subcortical structures (for a review, see Krauzlis, 2005). The purpose of each saccade is to reposition the high acuity fovea to a new location. Because the eyes are lighter than the head and can be turned more easily, most primates use saccades rather than head movements to scan and orient to elements in their environment. This sequential sampling of visual space is used to accumulate information about the structure of a scene. As such, visual exploration can be compared to tactile exploration, such as a person moving their fingertips over an object’s surface or a rodent using its whiskers to feel and recognize objects in the dark. In humans, the exploration of visual scenes by eye movements can vary in highly complex ways depending on the context and motivation of the observer (Yarbus, 1967; Hayhoe and Ballard, 2005). With on average 2–3 saccades issued each second, saccadic eye movements consititute our most frequent overt behavior.
Oculomotor behavior among mammals is determined by the density distribution of photoreceptors on the retina, which is in turn determined by ecology. The small and round foveal pit in the primate retina requires exquisite control over saccadic eye movements to optimally redirect gaze and gather information about a scene. Other species have very different retinal density patterns and correspondingly different eye movement patterns. Mammals such as rabbits and horses with horizontal streaks on their retina tend to reside in open environments, as the higher acuity in the visual streak allows them to monitor the horizon without the need for continual scanning (Ahnelt et al., 2006). In rabbits, gaze shifts are infrequent and saccades are strongly coupled with head movements (Tegetmeyer, 1996). The cat retina lies somewhere in between, as does its oculomotor behavior. The cat area centralis is much lower in its resolution than the primate fovea and is also elongated horizontally (Rapaport and Stone, 1984). The saccades of cats are less frequent, slower, and more variable than those of primates (Moeller et al, 2004). Mice, whose central portion of the retina shows minimal specialization for higher acuity vision, make even fewer saccade-like eye movements and rely primarily on head movements to orient their gaze (Sakatani and Isa, 2007).
It is notable that even closely related species can have very different profiles of retinal receptor density. For example, within the Carnivore order, the area centralis found in the domestic cat and other felines is absent in the closely related cheetah, which has a visual streak much like that of a horse. This adaptation has been attributed to living on the open savannah (Ahnelt et al., 2006). Similarly, the plains kangaroo has a streak whereas the tree kangaroo has area centralis (Hughes, 1975). An important principle in comparative neuroscience is that sensory systems can evolve quickly and that peripheral adaptations can strongly influence the organization of the brain (Krubitzer and Kaas, 2005). In primates, the invention of the fovea led to optimization of circuits for directing gaze and processing visual detail. One domain of behavior particularly affected by these features is social interaction, which we discuss next.
2.4.2 Social behavior
One feature that sets monkeys and apes apart from other mammals is the complexity and expression of their behavior toward other individuals in the group (de Waal and Waal, 2007; Maestripieri, 2008). This widely shared feature of primate life provides good reason to believe that the primate brain is highly adapted to accommodate complex social interaction. All mammals need to engage in certain forms of social interaction, for example related to the rearing of offspring, mate selection, territorial disputes, cooperation in foraging and defense. However, primate social interaction involves a higher degree of visual specialization than that of other mammals, starting with their use of high acuity vision to analyze faces, bodies, and actions from a distance (Allman, 1977). From several meters away, primates are able to observe and monitor the detailed actions of other individuals, giving them an advantage in the social group. However, the rules of the dominance hierarchy of the group also influence how vision can be used, placing certain restrictions on gaze behavior. Because one individual can determine where another individual is looking, dominant males prohibit subordinate animals from looking directly at them. Thus, in the primate competition for power, not all animals are allowed equal access to the social benefits of high acuity vision.
Another important aspect of social behavior is sexual selection, which, as discussed in Section 2.2.2, has been linked in Old World monkeys to the emergence of trichromatic color (Fernandez and Morris, 2007). Comparative analysis suggests that the capacity to see reds and yellows may have gradually shaped sexual selection because of the increased perceptual sensitivity to level of blood perfusion and oxygen saturation level in the skin (Changizi et al., 2006). In the millions of years after genetic mutations that introduced a new cone type in the retina and broadened the capacity to distinguish between different shades of red, Old World monkeys became increasingly bare-faced, and bore an increasing yellow and red coloration on their face, skin, and pelage. It is suggested that trichromatic females increasingly were able to choose males based on the quality of their red markings, and trichromatic males likewise were able to detect ovulating females on the basis of their red perineal skin swellings (Dixson, 2012; Surridge et al., 2003). Statistical analysis based on extant primate species suggests that not only did color patterns become more prevalent after the advent of routine trichromacy, but that primate mating also became more gregarious (Fernandez and Morris, 2007).
The high level of social monitoring, hierarchical reinforcement, and sexual selection exhibited by primates figures prominently into an aspect of primate social behavior that has been called “Machiavellian intelligence” (de Waal and Waal, 2007; Humphrey, 1976; Maestripieri, 2008). This term refers to the high level of social maneuvering among primates, much of which is based upon observing one another and predicting their behavior. Larger groups entail a higher degree of social complexity, since there are more possible pair-wise social relationships. It has been suggested that the number of members in a primate social group is ultimately constrained by the capacity of the cerebral cortex, including those regions specialized for high-level vision (Baron, 1998; Dunbar, 1992), as well as by brain regions such as the amygdala and hypothalamus that underlie the expression of social behavior in all mammals (Lewis and Barton, 2006).
2.4.3 Visually guided reaching and grasping
Primates are also unique among mammals in their use of visually guided reaching and grasping, precision grip, and object manipulation. Humans represent the pinnacle of this behavior in their use of tools, or, for example, playing a musical instrument. As mentioned in Section 2.3.4, precise visually guided actions are linked to refinement of mammalian dorsal visual stream, with a large expansion of posterior parietal cortex, particularly in humans, and an elaborated domain in the primary motor cortex representing direct cortical control over the hands. Manual manipulation also benefits from stereoscopic vision, along with vergence eye movements that coordinate the two fovea towards targets in depth (Hadjidimitrakis et al, 2012).
The evolution of visually guided reaching and grasping is closely associated with primate ecology and the need to move through the forest canopy. Apes and monkeys that use brachiation rely upon vision to select, reach for, and secure their grip onto appropriate branches. These actions are often rapid and coordinated with other self-generated movements that strongly affect the visual input, such as the motion of the body, turning of the head, and frequent saccades. Since a miscalculation can have fatal consequences, primates have evolved under a strong selective pressure for accuracy in this domain. The origins of precise manual behavior may stem from specific aspects of the feeding behaviors of early primates. One influential hypothesis holds that early primates were nocturnal predators who were aided by a wide field of stereoscopic vision for catching insects in their hands (Cartmill, 1992). An alternative view is that precision reaching and grasping evolved for the purposes of extracting small fruits from terminal branches of angiosperms (Bloch and Boyer, 2002; Sussman et al., 2013).
Rats and other members of Euarchontoglires exhibit some visually guided reaching and grasping, but with much less precision than primates. For example, a rat reaching for a piece of food will not preshape its hand based on visual cues. Instead it will draw upon tactile cues for posturing its forepaw, much like the behavior of a blindfolded human subject who is aware of the position of an object but not its shape (Karl and Whishaw, 2013). Note that much of this deficit is specifically related to the use of vision. Rats have sufficient motor control to preshape their forepaws, as they do so when reaching up to grasp a piece of food already sitting in their mouth. The use of vision to guide reaches and enable manipulation is a difficult computational problem for which special circuits have evolved in primates.
2.5 Primate adaptations: summary
In this section, we have specified a number of characteristics of the eyes, visual brain, and visual behavior that are shared among primates and in some cases distinguish primates from other mammals. Perhaps the most important are the peripheral adaptations, including the dense packing of photoreceptors in the fovea, the three retinal cone types, and the convergent orbits. These can be linked to many of the specializations of the brain that underlie fundamental primate behaviors, including exploration, social complexity, and precise manual motor behavior.
3. Comparing marmoset and macaque vision
We now turn our attention to one particular primate, the common marmoset (Callithrix jacchus), a small New World monkey that has recently attracted much interest as an animal model for the neurosciences. We continue to apply a comparative analysis of vision as above, here placing greater emphasis on comparing the marmoset with the better-studied macaque.
3.1 Visual system
The gross appearance of the marmoset brain differs from that of larger monkeys such as the macaque. The most obvious difference is that its surface is nearly free of sulci and in that sense is similar to a rodent. However, visual comparison reveals that in most other respects the marmoset brain more closely resembles the brain of a macaque than that of a rat (Figure 4). For example, the rat telencephalon lacks a Sylvian fissure, has a prominent olfactory bulb and piriform cortex, and bears a different angular relationship to the hindbrain. Beyond its gross anatomy, the detailed anatomy and basic electrophysiology of the marmoset’s eye and brain are categorically those of a primate, with many aspects of the visual system nearly indistinguishable from that of the macaque (see Solomon and Rosa, 2014). At the same time, there are notable differences in their eye and brain that affect their visual behavior. These differences reflect the evolutionary changes that have impacted each species since the time of their most recent common ancestor 35–40 MYA.
Figure 4.
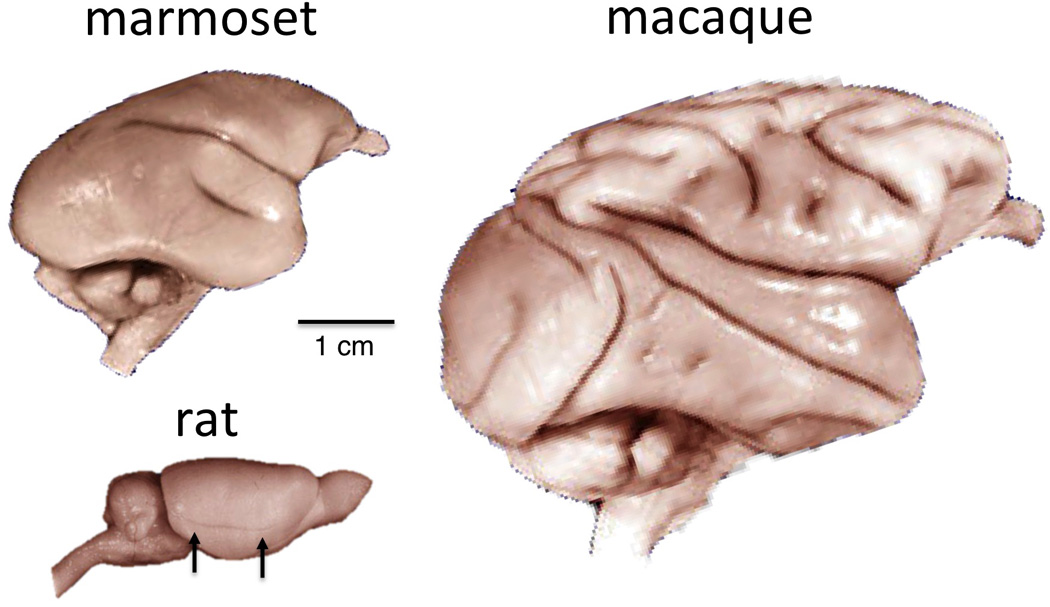
Comparison of marmoset, rat, and macaque brains, lateral view. Unlike the primate, the rat brain has no Sylvian fissure separating the frontoparietal and occipitotemporal cortical regions. The faint horizontal sulcus in the rat brain (arrows) is the rhinal sulcus, which separates the neocortex from the more primitive piriform (olfactory) cortex. This feature is much smaller in monkeys and not visible from the lateral view. The overall geometry of the marmoset brain is much more similar to the macaque than the rat.
3.1.1 Eyes and retina
In the marmoset, the organization of the retina and placement of the eyes, including the high level of binocular overlap, is typical for a primate. The marmoset’s retina has a well-defined fovea with tightly packed cones and dense midget ganglion cells that carry high-resolution visual information out of the eye and into the brain. The estimated visual acuity of marmosets at 30 cycles/deg is somewhat less than the 50 cycles/deg of macaques (Kirk and Kay, 2004), but this can be largely ascribed to the size of the marmoset eyes, whose axial length is 11 mm (Troilo et al., 1993) compared to the macaque’s 18 mm (Lapuerta and Schein, 1995). For a given cone density, larger eyes translate to a higher visual acuity as the same arc of visual angle subtends a larger region of retinal epithelium. The peak cone density at the fovea is highly similar between the species (Figure 5A). A more detailed analysis of the marmoset eye further reveals that the visual optics and basic topographic cone density pattern also closely resemble those of macaques and humans (Troilo et al., 1993). One difference between the marmoset and the other species is a notably higher cone density in the peripheral retina (Figure 5A). Whether this increased density has a measurable effect on the peripheral visual acuity of the marmoset is unknown, since other factors such as the pooling of cone signals by individual ganglion cells, also contributes to acuity.
Figure 5.
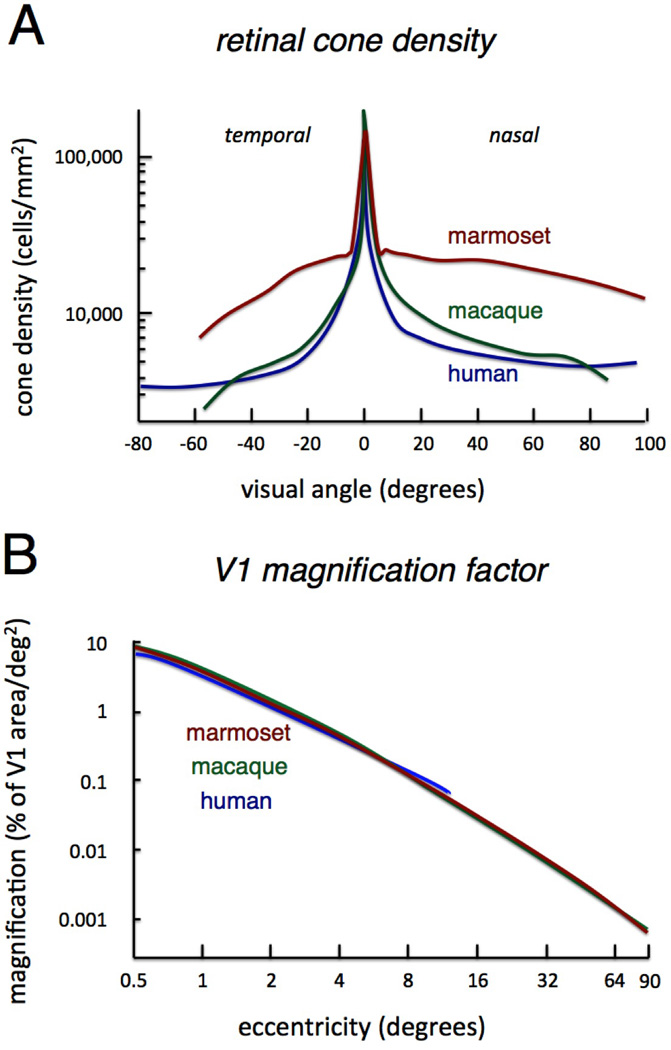
Comparison of low level features related to foveal specialization in marmosets, macaques, and humans. A. Cone density as a function of retinal position for each species. Note the similarity in peak cone density in the fovea. The marmoset has notably higher cone density in the retinal periphery. (Adapted from [Troilo et al, 1993].) B. V1 magnification factor expressed as proportion of V1 dedicated to processing visual space at a given eccentricity. The three species are nearly identical in this regard, despite the fact that the absolute size and relative proportion of V1 in the cerebral cortex is substantially different. (Adapted from [Chaplin et al, 2013b]).
Color vision in marmosets, like other New World monkeys, is complex and depends upon gender. A genetic polymorphism in the longer-wavelength sensitive (L) cone type results in different individuals having different chromatic sensitivity. Moreover, because the corresponding gene is on the X chromosome, this polymorphism differentially affects males and females. Both genders have an autosomal short-wavelength sensitive (S) opsin that is present in most mammals. However, since males have only one X chromosome, they have only one of the L alleles. Thus they are only able to produce two cone types (S + L) and are obligatory dichromats. Females, on the other hand, having two X chromosomes, can possess two different L alleles (for simplicity, we call them here L and M) with somewhat different wavelength sensitivities. For this subset of females, the additional opsin results in three cone types (S + M + L) giving them trichromatic vision similar to that of Old World monkeys. Trichromatic New World monkeys are able to perceive colors differently than their dichromatic conspsecifics (Pessoa et al., 2005). However, while there may be distinct survival advantages to the third cone type (Mollon, 1989; Osorio et al., 2004), these may actually be matched by advantages to dichromatic vision, such as in foraging under low light conditions, and these complementary advantages appear to have resulted in a stable genetic balance of dichromats and trichromats in New World monkey populations (Caine et al., 2010). This is not the case in Old World monkeys, where both males and females possess fixed L and M genes on each X chromosome and are therefore always trichromats. In fact, among Old World primates, only humans substantially deviate from this pattern, with ~8% of males being dichromats due to a mutation rendering one of their X-linked opsins dysfunctional.
3.1.2 Lateral geniculate nucleus
The functional organization of the marmoset LGN closely resembles that of the macaque, with some differences in the layering of the different cell classes. The paired layers of magnocellular, koniocellular, and parvocellular neurons fit within the larger pattern of LGN structure that differentiates primates from other mammals (Kaas et al., 1978). As noted earlier, all primates have four layers in their LGN, although in the macaque the parvocellular layers are folded giving six layers in cross-section whereas the marmoset has four in cross-section (Kaas et. al., 1978). One difference with the macaque is that the marmoset has a more defined lamination of the zones containing koniocellular neurons. This latter feature makes the marmoset particularly useful in the study of koniocellular pathways (Cheong and Pietersen, 2014; Goodchild and Martin, 1998; White et al., 2001). For example, a recent study demonstrated a small proportion of neurons in its koniocellular layers that respond both binocularly and with a relatively strong orientation selectivity (Cheong et al., 2013), two features that are not normally associated with the primate LGN. However, as there have been rare reports of similar phenomena in the macaque (e.g. (Smith et al., 1990)), it remains to be determined whether this is a general feature of the primate koniocellular system.
Studying LGN responses in marmosets has also benefited from the coexistence of dichromatic and trichromatic individuals, as mentioned briefly in Section 2.3.2. Parvocellular LGN responses of the two groups differ in their red/green opponency, with responses in trichromatic marmosets resembling those in the macaque (White et al, 1998; Kremers and Lee, 1998). However, aside from this feature, the spatial and temporal charactersitics of the parvocellular LGN was found to be virtually identical in the two groups of marmosets (Martin et al., 2011) and also similar to the monochromatic Galago (Yamada et al, 1998). This evidence suggests that the parvocellular specialization did not specifically emerge to carry red/green opponent signals, which is sometimes assumed. Instead, the parvocellular pathway is built upon the mammalian X pathway, which expanded and specialized to support high resolution central vision early in primate evolution (Kaas et al., 1978). This modified X pathway transmits red/green opponent signals as long as they are provided by the retina (Martin et al., 2011). This realization is underscored by recent work showing that a third cone type artificially introduced into the retina of adult male New World monkeys induces trichromatic color vision (Mancuso et al, 2009).
In summary, the marmoset LGN differs somewhat from that of macaques and humans, with the largest differences being its layering and the chromatic signals it transmits. However, it bears the key hallmarks of the primate LGN, including most prominently the thick parvocellular layers that transmit high actuity visual information from the fovea to an expanded region of the primary visual cortex, which we review in the next section.
3.1.3 Primary visual cortex
The organization of the marmoset visual cortex is well understood based on experiments carried out by a relatively small number of laboratories. The detailed anatomy and physiology has been recently reviewed and is in most respects very similar to that of the macaque (for a recent comprehensive review, see Solomon and Rosa, 2014). Area V1 has been studied in great detail (Sengpiel et al, 1996; Webb et al, 2003; Bourne et al, 2002; Tinsley et al, 2003; Bourne et al, 2004; Forte et al, 2005; Barraclough et al, 2006; Guo et al, 2006; Zinke et al, 2006; Buzas et al, 2008; Hashemi-Nezhad, 2008; Nowak and Barone, 2009; Cheong et al, 2013; Yu et al, 2010; Yu et al, 2014; Solomon et al, 2014). Electrophysiological mapping has revealed that the visual field layout and basic neural selectivity of this area are similar to that of macaques. Anatomical features, such as the cytochrome oxidase blobs in the supragranular layers, are also present. At the same time, there are some notable differences between the primary visual cortex of the marmoset, macaque, and human.
One difference is the proportion of cortex V1 occupies in the different species. By mass, area V1 is ~14.5% of the cortical gray matter in the marmoset, ~8.8% in the macaque, and only ~1.5% in the human (Collins et al., 2013). These values may reflect that an absolute amount of cortical tissue is required to support high-resolution vision, and appears to derive directly from the number of neurons providing input to V1 from the LGN (Stevens, 2001). Despite differences in the overall size, the percentage of cortex dedicated to different retinal eccentricities, or magnification factor, is nearly identical between the species (Figure 5B) (Chaplin et al., 2013b). That the magnification factor matches so closely may reflect an important constraint for high resolution central vision, and is in part surprising for eyes of different sizes given the conserved absolute size of the foveal pit (Franco et al, 2000). How the marmoset’s small eye size, high peripheral cone density, and conserved magnification factor together contribute to the marmoset’s perception of visual detail in central and peripheral vision remain an open question.
Another distinguishing feature of marmosets is their lack of strongly defined ocular dominance columns in area V1 (Roe et al., 2005), which are present in macaques (Hubel and Wiesel, 1968) and probably also in humans (Yacoub et al., 2007). Ocular dominance is strongest in the input layer, where afferents from the two eyes remain segregated. Whether the absence of ocular dominance columns has any functional consequence is unclear. Interestingly, marmoset ocular dominance columns do appear transiently in early development, but then nearly disappear in adulthood (Spatz, 1989).
A third difference between marmoset and macaque V1 is that, in contrast to the macaque, those neurons in layer 4B that project to area MT have pyramidal rather than stellate morphology (Elston and Rosa, 2006). In the macaque, stellate neurons of layer 4B project to MT while neurons of pyramidal morphology project to area V2 (Nassi and Callaway, 2007). Stellate neurons do have different functional properties, showing greater sensitivity to small inputs and overall higher firing rates (Klink and Alonso, 1993), and pooling inputs over more limited spatial extents (Schubert et al, 2003). Thus it is possible that stellate projection neurons in 4B of the macaque might mediate faster or higher precision information to motion-selective area MT than other channels. It is also possible that this species difference stems from modification of the layer 4B developmental trajectory, since cortical stellate cells initially take the form of pyramidal neurons before they lose their apical dendrites as they mature (Callaway and Borell, 2011). It is worth emphasizing that the locally projecting stellate cells in the input layers, as well as layer 4B, are abundant in the marmoset, as is typical among primates.
3.1.4 Extrastriate visual cortex
The electrophysiological responses of other visual areas in the marmoset have been studied in detail and generally resemble those of the macaque, including V2 (Rosa et al, 1997; Lui et al, 2005; Roe et al, 2005; Barraclough et al, 2006; Federer et al, 2009), DM (Rosa and Schmid, 1995; Lui et al, 2006; Lui et al, 2013), V3 (Rosa and Tweedale, 2000) and MT (Rosa and Elston, 1998; Solomon et al, 2011; Lui et al, 2007a; Lui et al, 2007b; Lui et al, 2012; Lui et al, 2013; McDonald et al, 2014; Solomon et al, 2014).
Area MT is of particular interest for comparative studies because there is a question as to whether its evolution is unique to the primate line (Krubitzer and Kahn, 2003). It is a compact, motion-selective area containing both upper and lower visual field representations and is very well studied in the macaque because of its conspicuous extraction of direction and stereoscopic depth from retinotopic visual space (Albright et al., 1984; DeAngelis and Newsome, 1999). In the marmoset, the basic topographic organization and response properties appear similar to the macaque, including for example the selective neural responses for higher-order, or pattern, motion selectivity (Solomon et al, 2011; McDonald et al, 2014). Sitting on the cortical surface, area MT can be studied more easily in the marmoset, a fact that has been exploited to address questions that have are difficult to study in macaque MT. For example, a recent study used planar surface arrays to measure correlated activity of populations of MT neurons (Solomon et al, 2014). Another set of studies has focused on the early maturation of MT (Bourne and Rosa, 2006) and the existence of a transient retino-pulvinar-MT visual pathway early in development (Warner et al., 2012). This putative pathway, which has not been previously described in the macaque, can be well studied in the marmoset due to the surface location of MT and the extended period of postnatal development.
Beyond basic mapping, much less is known about higher order visual cortex of the temporal and parietal lobes of the marmoset (see Figure 6). This gap in our knowledge is due in part to the paucity of electrophysiological studies in awake marmosets, as activity in these higher visual areas is dependent on behavioral state. While the marmoset visual cortex is generally believed to possess dorsal and ventral visual pathways similar to the macaque, the extent to which the specific functional specializations resemble those of macaques and humans is unknown. This topic is important because, as reviewed in Sections 2.3 and 2.4, these areas are closely associated with primate-specific behaviors, such as visually guided grasping and social perception. Learning whether the physiological responses in the marmoset are similar to those in the macaque may provide insight into the evolution of these behaviors.
Figure 6.
Flat map comparison between the marmoset and macaque visual cortex (adapted from Rosa et al. (2009), and Felleman and Van Essen (1991)). The layout of the visual cortex is generally similar in the two species, including the highlighted features, which are either unique to primates (Middle Temporal Area) or greatly expanded in primates (Posterior Parietal and Inferotemporal Cortex).
Similarly, relatively little is known about responses in inferotemporal cortex of the marmoset. No previous study has systematically studied IT response properties of the marmoset, though there have been indications that neurons there are selective to faces (Tamura and Fujita, 2007). Several studies have examined the impact of lesions to IT, and find it causes severe impairments for recognizing visual objects in simple discrimination tasks (Ridley et al, 2001). A recent study has demonstrated that functional specialization of the marmoset inferotemporal (IT) cortex bears striking resemblance to that of the macaque (Hung et al, 2015). In the macaque, neurons throughout IT cortex respond with a high degree of selectivity for complex stimuli, and there are a number of regions where neurons are particularly selective for faces (for a review, see (Tsao and Livingstone, 2008)). Marmoset IT cortex is likewise composed of multiple areas that show selective fMRI and electrophysiological responses to faces over other objects (Hung et al., 2015).
The parietal visual cortex in marmosets and its role in visually guided actions are even less explored. Most of knowledge of parietal cortex function in New World monkeys comes indirectly, from microstimulation studies in anesthetized animals (Gharbawie et al, 2010, 2011). Specifically, long-train electrical microstimulation provides a means to study the contribution of an area to particular aspects of behavior (Graziano et al., 2002). In the posterior parietal cortex, such microstimulation in anesthetized squirrel and owl monkeys leads to coordinated reaching, grasping, and defensive actions. The sites of stimulation, and the pattern of complex motor action, show good correspondence across the prosimian galago, squirrel and owl monkeys, and also macaques but not in non-primate Euarchontoglires species, such as tree shrews or rodents (Kaas et al., 2013). No studies have examined marmoset parietal cortex using similar micro-stimulation paradigms, although anatomical evidence suggests similar connectivity between parietal and premotor areas as found in other primates (Burman et. al., 2014). This frontal-parietal machinery may be unique to the reaching and grasping behavior exhibited by primates, which is under most conditions guided by visual input.
3.1.5 Oculomotor structures
The cortical and sub-cortical areas controlling eye movements have been identified in the marmoset and are largely homologous to the macaque. The superior colliculus (SC) is the main subcortical structure initiating goal directed eye movements, and receives cortical inputs from early visual areas (V1, V2, and MT) and from four visuomotor fields which include the frontal eye fields (FEF), frontal visual area (FV), an area in the region of the supplementary eye fields (SEF), and parietal eye fields (Collins et al, 2005). Projections from the SEF itself appear either weak or absent in the marmoset. The frontal eye field (FEF), or area 8aV, was identified in early studies, and micro-stimulation was shown to evoke either saccade eye movements or slow eye movements of varying speeds (Blum et al, 1982). Recent anatomical studies have found that the cortical inputs to FEF in the marmoset resemble those of the macaque (Burman et al, 2006; Reser et al, 2013). No studies have yet examined the projections from the FEF to the SC or to oculomotor nuclei in the marmoset. In other New World species, such as squirrel and owl monkeys, these projections to lower oculomotor control centers appear similar to that in macaques (Huerta et al, 1986).
3.2 Visual behavior
Like other primates, marmosets are skillful in their use of vision. Most of what is known about marmoset visual behavior derives from either natural ethological observations or neuropsychological experiments carried out in a small number of laboratories (Ridley et al, 1986; Roberts et al, 1990; Derrington et al, 2002; Burkart and Heschl, 2006; Schiel and Huber; 2006; Hook and Rogers, 2008; Kemp and Kaplan, 2013). Recent work has shown that it is also possible to carry out controlled experiments in head-fixed, awake marmosets, where oculomotor behavior and visual stimulation can be precisely determined (Mitchell et al, 2014; Hung et al, 2015). Here we briefly review several aspects of visual cognitive, social, and motor behavior that have been studied in this species.
3.2.1 Visual cognitive behavior
In experiments allowing unrestrained movement, marmosets reliably make complex visual discriminations, for example choosing between pairs of distinct real objects or visual patterns based on a previously established association with food reward (Ridley et al, 1981; Ridley et al, 1984; Ridley et al, 1986). Several researchers have used the Wisconsin General Test Apparatus in which the animal may use its hand to displace an object it believes to be associated with a food reward in a well underneath. Between trials, the investigator manually changes the choice objects, fills the food well, or sometimes handles the animal, repositioning it to begin the next trial. In this mode of testing, which is often used to study the effect of pharmacological agents or brain lesions on higher order aspects of cognition, a marmoset will typically perform 40–50 trials in a session. Marmosets can also be trained in more automated testing setups, where they respond to stimuli on visual displays by pushing a touch-sensitive screen or depressing a mechanical lever for receipt of reward (Dias et al, 1996; Crofts et al, 1999; Clarke et al, 2004; Spinelli et al, 2004). Together, these paradigms have been able to tap into aspects of visual perception, for example, by demonstrating that marmosets can distinguish reliably between subtly different colors (Derrington et al, 2002; Pessoa et al, 2005) and complex patterns (Ridley et al, 1984; Ridley et al, 1986; Maclean et al, 2001; Easton et al, 2003; Ridley et al, 2005). Moreover, large lesions to the inferotemporal cortex, which are commonly associated with object agnosia in macaques and humans, similarly impair visual discrimination capacity in marmosets (Ridley et al, 2001). Like macaques, marmosets are able to flexibly use visual context, or the rewarding properties of a visual feature, to alter their response strategy (Barefoot et al, 2002; Ridley et al, 2001; Barefoot et al, 2003; Dias et al, 1996; Roberts et al, 1990; Roberts et al, 1992). Touch screen testing has further shown that marmosets can perform visuospatial memory tasks well when the delay is short (e.g. 3 seconds) but struggle with longer delays (e.g. 12 seconds) (Spinelli et al, 2004). Attempts to further explore visual memory with delayed match to sample (DMS) and related tasks suggests that marmosets are more challenged by this mode of testing than the macaque (Ridley et al, 1988; Ridley et al, 1991; Ridley et al, 1993; Spinelli et al,2004), although the reason for this difference is not well understood and could depend ondifferences in the methods for training and testing the species.
3.2.2 Gaze behavior and visual attention
Like all primates, marmosets use saccadic eye movements to direct their high-resolution fovea to objects of interest within a visual scene. Methods for the monitoring and conditioning of behavior under head restraint have been recently established in the marmoset (Remington et al, 2013). One study has begun to use video-based eye tracking to record scan paths and other aspects of saccadic gaze behavior (Mitchell et al, 2014). Other categories of eye movements such as vergence and visual pursuit have not been studied in the marmoset, though pursuit movements in another New World species, the squirrel monkey, are generally comparable to the macaque (Heiney and Blazquez, 2011). Figure 7A shows typical scan patterns of marmosets viewing a static, natural scene, including the frequent shifts in gaze position induced by saccadic eye movements (yellow lines) punctuated by extended periods of fixation (red circles). The analysis of scan patterns to many such natural stimuli revealed a number of gaze characteristics that closely resemble those demonstrated previously in macaques (Mitchell et al, 2014). First, the general mechanics of the saccades, including for example the well-described relationship between peak velocity and amplitude, was closely matched between the species. Second, other aspects of free gaze behavior, such as fixation duration, and mean intersaccade interval, were broadly similar, albeit at a somewhat faster pace in the marmoset as compared to the macaque. Third, marmosets, like macaques and humans, target regions of social and biological interest, and particularly faces (Mitchell et al, 2014; Keating and Keating, 1982).
Figure 7.
Marmoset scan paths during the viewing of complex natural images. A. Scan paths of a marmoset viewing two images for 20s each, with one image depicting a conspecific and the other a macaque. In both cases, gaze is systematically directed toward the body, and particularly toward the face. B. Comparison of scan paths between a marmoset and macaque viewing the same scene of consisting of baboons. Eye position is indicated by yellow lines with red points marking fixations longer than 200 ms (adapted from Mitchell et al., 2014).
In one experiment, Mitchell et al. (2014) showed both macaques and marmosets the same natural images while recording gaze behavior. Aside from the many similarities, there were also certain differences of comparative interest. One difference relates to the overall spatial distribution of saccades. Because the inertial weight of the smaller marmoset’s head is low compared to larger primates, marmosets rely more on head movements to redirect their gaze than do macaques and humans, a feature shared by squirrel monkeys (McCrea and Gdowski, 2003; Heiney and Blazquez, 2011). While head-restrained macaques tend to make large saccadic eye movements, head restrained marmosets seldom make saccades that deviate more than 10 degrees from the head-defined center of view. Similarly restricted saccade behavior has been reported previously in the squirrel monkey (McCrea and Gdowski, 2003; Heiney and Blazquez, 2011). Another difference that is apparent in the example in Figure 7B is the reluctance of macaques to look directly at certain faces. Mitchell and colleagues noticed a trend across images that macaque subjects spent less time looking at faces, particularly for those faces in close view or that were directed at the camera. As mentioned in Section 2.4.2, the social rules of primate gaze behavior are strongly influenced by the fact that one animal is able to monitor and enforce the gaze behavior of another. This difference may thus have its origins in deeply ingrained macaque social rules that restrict extended direct gaze toward faces. In macaques, direct gaze can be seen as a challenge or threat towards social dominance, particularly if directed toward an individual higher in the dominance hierarchy (Chance, 1967). Macaques frequently monitor dominant animals not through direct gaze but instead using covert visual attention. The observation that marmosets are less inhibited to directly foveate the faces of large monkeys in images such as in Figure 7B may in part reflect the fact that gaze aversion and covert attention is not a pronounced feature of marmoset social behavior.
Understanding marmoset gaze behavior under head restraint has practical considerations for electrophysiological and functional imaging experiments. It is clear that marmosets are less willing than macaques to maintain fixation on a small point for many seconds at a time. Nonetheless, Mitchell and colleagues (2014) trained them to reliably perform several tasks involving fixation. In one task, they were required to look repeatedly at a small fixation point for up to two seconds while ignoring other stimuli flashed peripherally onto the screen. In a second task, the animals readily performed an orientation discrimination task by directing their eyes to the stimulus having an orientation that differed from an array of identical distracters of the same orientation. This combination of tasks provides the necessary conditions for controlled electrophysiological testing in behaving marmosets, including the initial calibration of video-based eye tracking, the presentation of a range of visual stimuli, and the comparison with behavioral decisions. The last of these is particularly important. In the orientation discrimination task, psychometric functions, along with each animal’s orientation discrimination threshold, were reliably measured and appeared similar to data been previously reported from macaques (Vogels and Orban, 1990). Interestingly, the marmosets’ behavior was much more enduring in the discrimination task than the ostensibly simpler visual fixation task, suggesting that marmosets perform better when tasks do not require focused inhibitory control of overt orienting responses.
Although this early behavioral study is likely to represent a lower bound on marmoset task performance that will improve with time, it does appear that marmosets require greater effort to suppress overt motor and orienting responses. This difference to the macaque is likely to be a real one and may reflect one cost of having a small brain. Brain functionality varies with absolute brain size, even among closely related species (Kaas, 2000). It is also the case that of those areas which have expanded most in total brain proportion from New to Old World primates, and then again from Old World primates to humans, include those prefrontal areas that are involved in executive and inhibitory control (Chaplin et. al., 2013a). It is thus possible that the focused and controlled behavior of macaques during a fixation task stems from a proportionally large number of neurons in the prefrontal cortex or elsewhere that are able to exert downstream inhibitory control. This same type of control contributing to a fixation task may also explain their natural use of covert attention in the social group. The cerebral cortex of the marmoset, having fewer such projections, may simply be unable to exert sustained inhibitory control for similar periods. In summary, while much still remains unknown about marmoset behavior under constrained conditions, it is clear that they display similarities and differences with the macaque. Their active and exploratory viewing of natural scenes resembles macaques in most respects. They can also be trained for repeated, brief fixations and to perform visual discriminations, albeit for relatively short periods at a time.
3.2.3 Visual social behavior
Marmosets are a highly social and hierarchical species in which individuals are continually interacting with members of their family groups (Stevenson and Poole, 1976). Unlike Old World species, marmosets use cooperative breeding strategies in which the father and older siblings participate in rearing young, which is some ways, more closely resembles the socialization in human families (French, 2013). Some of this interaction relies on complex visual analysis, from the reading of faces and bodily postures to the observational learning of adult foraging skills by juvenile observers (Schiel and Huber, 2006). While it is now clear that marmosets routinely direct their gaze to faces and bodies, even under artificial experimental conditions (Mitchell et al, 2014), the specific types of information that they utilize based on visual inspection of one another remains an open area of research.
An important category of such information is the following of another’s orientation and gaze direction, which is a capacity that has been shown in a handful of species (Emery and Clayton, 2009). The ability to follow the gaze of one’s conspecifics can place one at an advantage for identifying locations, events, and objects of interest, for example related to food and predators. Marmosets appear to exhibit some gaze following behavior, but to date this capacity has only been demonstrated with respect to human gaze cues (Burkart and Heschl, 2006; Rosati and Hare, 2009). While more research needs to be done on the marmoset in this regard, it is notable that the ringtailed lemur, a prosimian primate, interprets and uses conspecific gaze direction to orient its own gaze during natural visual behavior (Sheperd and Platt, 2008). Some known features of marmoset group behavior suggest indirectly that they are able closely monitor and follow certain types of conspecific orienting. For example when faced with a visually identified predator, members of a family synchronize their actions through a mobbing behavior that involves facing the threat and engaging in local tsik vocalizations (Clara et al, 2008). In fact, the eye traces in the right panel of Figure 7A are at least suggestive of gaze following in the marmoset, since they appear to show a high density of fixations in the region where the macaque in the picture is looking, though more experiments clearly need to be done.
For animals such as primates that use their vision as their main social sense, faces and bodily actions have the capacity to convey a wide range of important information (for a review, see (Leopold and Rhodes, 2010)). For example, the visual determination of individual identity allows animals living in a hierarchical group to track one anothers’ actions and interactions from a distance. Unlike other forms of identification, such as the reciprocal acoustic phee calling used by marmosets (Miller and Wren Thomas, 2012), visual identification does not require participation of the subject being identified. While it may seem intuitive that most primate species are able to visually recognize their conspecifics as individuals, direct evidence has only been demonstrated in a few species, including Old World macaques and New World capuchin monkeys, each of which is more skilled at discriminating conspecific than heterospecific faces (Dufour et al., 2006). Visual recognition of individuals has not been tested systematically in the marmoset, though it is now well established that this species recognizes individuals through their vocalizations (Miller and Wren Thomas, 2012).
It is also an open question whether marmosets systematically use facial expressions for social signaling. Like other primates, marmosets have elaborated facial musculature to support a range of expressions exceeding what is possible in most mammals (Preuschoft and van Hooff, 1995; Burrows, 2008; Kemp and Kaplan, 2013). While their use of faces is less conspicuous than that of macaques and humans, some evidence suggests that they produce and perceive certain types of expressions. For example, marmosets and closely related tamarins use their tongues in a sociosexual context as a visual display signal (Heymann and Lage, 2009). They also respond to video-recorded facial expressions of fear, disgust, and pleasure, of their cage mates (Kemp and Kaplan, 2013). In this sense, marmosets appear similar to other primates (Cook and Mineka, 1989), and some other non-primate mammals (Langford, 2006; Tate et al., 2006) in readily interpreting coarse facial expressions. Whether this ability extends to more nuanced aspects of facial signaling is not known. However, some observations, such as the interest in viewing faces (Mitchell et al, 2014) and the apparent specialization of several regions in their inferotemporal cortex for faces (Hung et al., 2015), suggests that there may be more to be discovered. Again the pattern of gaze is informative. Close inspection of Figure 7A reveals that gaze is primarily directed to internal facial features, which are likely to contain information about identity and affect, rather than to species- identifying features such as the ear tufts.
3.2.4 Visually guided manual behavior
As discussed in Section 2.4.3, primates are unique among mammals in their use of vision to guide reaching and grasping. Marmosets often perform such actions from an upright posture, standing on their hind limbs, and with their eyes binocularly focused on targets (see Figure 8). In experimental testing, marmosets can adapt this behavior to a variety of tasks and conditions, demonstrating great postural control and problem solving. For example, from a variety of bodily postures they learn quickly to steer their hand through holes and around obstacles to grasp food rewards (Hook and Rogers, 2008). Their capacity make detour reaches around visible obstacles is adversely affected by damage to the prefrontal cortex (Wallis and Roberts, 2001; Walker et al, 2006). While individual marmosets may show right or left hand preferences, no dominant hand preference is evident across the wider population (Hook-Costigan and Rogers, 1994; Guerra et al, 1997; Hook and Rogers, 2008). For those with hand preferences, the preferred hand often depends on their postural position (Hook and Rogers, 2008; Hashimoto et al, 2013). Marmosets have also been taught to perform memory-guided sequences of reaching movements to positions on a touch screen (Nakako et al, 2013) and to perform reaches to eccentric positions while under head and body restraint (Pohlmeyer et al, 2013).
Figure 8.
Visually guided reaching in the marmoset. A. Frontally positioned eyes allow for stereoscopic near vision typical of primates. B, C. Reaching movements in marmosets are often performed from an upright posture. Vision is used to guide and preshape the hand or hands based upon the features of the target object (adapted from Hashimoto et al, 2013).
Marmosets can also learn to perform sophisticated visually guided actions using tools, such as learning to use a special rake with which they can obtain food placed beyond their reach (Yamazaki et al, 2011). Marmosets probably pre-shape their hand to facilitate grasping of objects, though this has not been tested in detail. Hand pre-shaping in macaques exploits three-dimensional structure, orientation, texture, and movement of a target object based on visual cues (Galletti et al, 2003). The cotton-top tamarin, a close Callithrichidae relative of the marmoset, pre-shapes its hand based on the orientation of an object it is about to grasp (Weiss et al, 2007). However, it is important to point out that despite marmosets’ capacity for this type primate-unique manual behavior, their skills in this domain are much less than macaques, which are in turn less than humans. For example, in learning to use a rake to reach food, marmosets required more than an order of magnitude more trials to achieve the same level of proficiency as Japanese macaques (Macaca fuscata) (>7000 trials in marmosets versus ~500 trials in macaques) (Yamazaki et al, 2011). Regarding their dexterity, a key difference from macaques is that marmosets do not have a precision grip. Old World primates have opposable thumbs that allow them to grasp small objects between the thumb and fingers. The precision grip may have been critical for important advances involving fine motor behavior, including tool use among apes and humans (Marzke, 1997). This ability is absent among most New World monkeys, including the marmoset, who make a “power” grip in which all of the fingers close in one sweep.
From a comparative standpoint, it is worth noting that at least one New World monkey, the Cebus or capuchin monkey, has a degree of manual control that includes a precision grip and rivals macaques (Fragazy, 1983). This skill is considered to have evolved independently in the Cebus, particularly since the specific changes in hand morphology used to achieve the fine motor actions differs distinctly from that of Old World monkeys (Napier and Napier, 1967; Fleagle and Simons, 1995; Rose, 1996; Padberg et al, 2007). This example of convergent evolution in the motor system is thought to have arisen from adaptations to the hand, fitting well with a major theme of this article that peripheral adaptations can strongly impact the organization and function of the brain. What is interesting about this example is that changes in the hand, and the capacity for precision grip, profoundly affected the grasping-related regions of Cebus brain. In fact, the posterior parietal cortex of the Cebus bears a much stronger resemblance to that of the macaque than to phylogenetically closer New World monkeys (Padberg et al, 2007). In addition to a major expansion of parietal and frontal areas related to grasping (Huffman and Krubitzer, 2001; Padberg et al, 2005; Padberg et al, 2007), the Cebus brain is able to control fine manual movements through direct cortical projections to the ventral spinal cord. For example, like the macaque, but unlike other New World primates that have been studied, Cebus monkeys have large Betz cells in layer 5 of what is sometimes termed “new” M1 that synapse onto primary motor neurons in the lateral ventral horn that control of digits (Bortoff and Strick, 1993; Lemon and Griffiths, 2005). This control may reflect a more general principle of mammalian brain organization, that as the motor cortex grows relative to the size of the spinal cord, it commands more synaptic territory in the ventral horn (Streidter, 2005). This example also underscores the point that peripheral adaptations, in altering the brain, can also influence cognitive behavior. For example, the Cebus monkey has an ability to use tools in acquiring food, which appears to be much greater than the marmoset (Cummins-Sebree and Fragaszy, 2005). Thus for the study of the neural mechanism of fine motor control, the Cebus is likely to be a considerably better model for human manual control than the marmoset, despite their sharing the same most recent common ancestor with humans.
3.3 Summary of marmoset versus macaque vision
As distinct species of the same mammalian Order, the marmoset and macaque clades can be considered rather different variations of a common primate theme. The differences between their brains, and the corresponding behaviors, provide an interesting and important parallax for understanding human brain function. In the case of basic visual perception, the two species are likely very similar. Marmosets have a slightly lower visual acuity, most are dichromats, and their use of vision in social contexts may differ in several subtle ways. Other aspects of their brain and behavior, such as the fine control of the hand, differ considerably from macaques. The most notable physical aspect of the marmoset is its size. For a primate, it is atypically small, and in that sense adapted in a manner very different from macaques and particularly from humans. This also affords several new experimental possibilities, which we consider in the following section.
4. Opportunities afforded by the marmoset model for visual neuroscience
In this final section, we highlight areas where the marmoset model offers theoretical and practical advantages for visual neuroscience. While there are many useful similarities between the macaque and the marmoset, the two primate species are adapted very differently in their size and in multiple aspects of their behavior. While phylogenetic proximity to humans has made the macaque the model of choice in providing analogy to the human brain, certain features of the marmoset brain, breeding, and behavior offer new opportunities for modern experimental neurobiology.
4.1 Comparative neurobiology
Advancing the marmoset as a second major non-human primate model has inherent value simply because it offers an additional perspective on the complex relationship between brain organization and behavior. Throughout this review, we have emphasized points of comparison between species that inform our knowledge of brain organization. Since opinions differ widely on the importance of such a comparative perspective, we discuss this issue briefly here, including why the marmoset is a particularly valuable complement to the macaque.
Though often overlooked in modern neuroscience curricula, it is undeniable that information gained from studying multiple species has always shaped our understanding of the human brain. It may be useful to consider a thought experiment: consider what might have happened if, from the beginning, the macaque were the only species studied. In this scenario, a major challenge would be to determine which of the innumerable observable details of macaque’s brain are important for understanding its function. For example, how critical is the lamination of the LGN for visual perception? How important is the lamination of the cerebral cortex? Are the specific positions of the sulci important? What about cytochrome oxidase features or orientation columns in V1, or anatomical projections from the visual cortex to the superior colliculus and pulvinar? Are alpha rhythms, gamma synchrony, or extraclassical receptive field details essential aspects of neural function? For any species, a comprehensive list of brain features is limitless and continues to expand with the availaibility of new measurement and analytical methods.
Comparative studies have the capacity to focus researchers’ attention on details of brain organization that are most likely to be important for the behavior of the species. For example, owing to comparative studies, we know that the six-layer cerebral cortex is a feature that is present in all mammals and is a very important conserved aspect of the brain; however, the lamination of the LGN is variable even among primates and probably less important. The positions of the sulci do not contribute much to our mechanistic understanding of brain function. For some of the questions raised above, and many more, the answers are still unknown and can actively benefit from comparative research. Sometimes a single observation in a different species is sufficient to call into question long-standing beliefs. We mentioned one such example earlier, in which recordings from the dichromatic marmosets and monochromatic prosimian primates led researchers to conclude that the fundamental function of the LGN parvocellular pathway is not the support the perception of color but rather of detail. We also mentioned a second example, in which the observed absence of V1 orientation columns in nocturnal and diurnal rodent species, despite abundant orientation tuned neurons, overturned notions that these two features are necessarily coupled through the columnar architecture of the cerebral cortex. These types of comparative observations from decades past, though largely forgotten, have worked their way into our common understanding of what is important in the primate brain.
The marmoset is a strategic counterpoint to the macaque for studying the human brain, in part because these three primate species are adapted so differently. The small marmoset brain is on the extreme opposite end of the spectrum compared to humans, while still being closely related. Thus the features that it shares with humans are likely to be essential, shared features of diurnal simian primates, and can be clearly distinguished from other smaller brained species of comparable size. Similarly, the ways in which marmoset behavior differs from the macaque and human may cast light on specific neural mechanisms critical to support them. For example, differences in visually guided manual behavior or sustained inhibitory control can be linked to specific changes neural circuits taken place along the marmoset, macaque, or human branches since their common ancestry. Finally, in certain domains the behavior of marmosets is quite similar to humans, particularly in aspects of social behavior such as the biparental rearing of offspring (French, 2013). Here, studying the relevant circuitry and its connection to social behavior in macaques and marmosets may reveal areas in which the marmoset is a better animal model for studying the healthy or diseased human brain.
In drawing concusions from interspecies comparisons, it is also important to recall that since the most recent common ancestor, that in the case of marmosets, humans, and macaques lived more than 35 MYA, each extant species under consideration has undergone profound evolutionary changes. As such, any particular species such as the macaque will have been shaped in a way that is not representative of other primates, including humans. Focusing only on one particular branch of this division, such as that leading to the macaque, thus has the potential to lead researchers astray in their thinking. Studying brain circuits with the appropriate balance of species provides a guard against falling into this trap. Thus the marmoset, as a small New World species with well-lain path for breeding and housing, would be a logical choice to complement the macaque model for studying the human brain even if it were not for the several experimental advantages outlined in the next subsections.
4.2 Model for visual system development
Just as a comparative perspective provides added depth for understanding principles of brain organization, so does neurodevelopment. Marmosets offer an ease of breeding and postnatal maturation time line that makes them a good model for studying primate brain development. In the past decade, the mouse has opened new vistas for understanding the brain’s various developmental events, including the birth and migration of different types of neurons (Marín and Rubenstein, 2003), the transient expression of developmental genes (Lein et al., 2007), the spatial gradients of secreted morphogens (Sansom and Livesey, 2009), and the membrane expression of guidance molecules (Kolodkin and Tessier-Lavigne, 2011). Thousands of molecular mapping experiments, and their assembly into data bases such as the Allen Brain Atlas (Lein et al., 2007), now provide an essential reference for understanding how the brain assembles itself and how alterations of this process might lead to brain dysfunction. This work also appears to be leading, quite remarkably, to the overturning of prevailing ideas about the basic axial layout of the brain, which has been in place since it was put forth by neuroembryologists more than a century ago (Puelles et al., 2013). This revolution is directly relevant for humans, since there is a strong conservation in the basic sequence of neurodevelopmental events among all mammals (Workman et al., 2013).
At the same time, many features that distinguish the primate brain from that of other mammals are not well modeled by the mouse and need a robust primate model (Homman-Ludiye and Bourne, 2013). In vision, for example, our understanding of the areal layout of the primate cortex, its specialization for high acuity input, or its regions ostensibly devoted to the processing of faces, would all benefit from a neurodevelopmental perspective. Most of what is known about primate visual system development derives from experiments that preceded the modern molecular era (Barone et al., 1995; Rakic, 1977). Much could be gained from a renewed focus on primate neurodevelopment, taking advantage of the technological and conceptual advances gleaned from work in mice (Homman-Ludiye and Bourne, 2014).
Marmosets have qualities that make them excellent candidates to study brain development. Notably, they are, like the cat or ferret, born at a comparatively immature developmental stage relative to macaques. While the corresponding time line has not yet been worked out in great detail as it has in the mouse, their caecal period, or the period between conception and eye opening, indicates that their visual system is considerably less developed at birth compared to macaques or humans (Robinson and Dreher, 1990; Warner et al., 2012). As a result, there is an unusually wide postnatal window for studying basic development of the visual system. Recent work exploiting this window has, for example, investigated the transition from the retinopulvinar pathway to the retinogeniculate pathway during the first months of life, as mentioned in Section 3.1.4 (Warner et al., 2012). Other advantages of the marmoset are a gestation period of 4–5 months, a transition to full sexual maturity in approximately 18 months, and the routine production of twins or triplets in each litter, resulting in the highest birth rate of any simian primate (Tardif et al., 2003). These features, combined with increasing availability of molecular and genetic tools in this species (Goldshmit et al., 2014; Sasaki et al., 2014), raises hopes that the marmoset will soon be a principal model to study human neurodevelopment.
4.3 Experimental advantages of a lissencephalic brain
The smooth surface of the marmoset lissencephalic brain offers unique opportunities to study the distribution of activity over cortical areas, uninterrupted or obscured by the sulci present in other species. Nearly all of the marmoset visual cortex sits on the brain’s lateral surface, as do important oculomotor areas such as the frontal eye fields. By comparison, many areas of visual cortex in macaques lie buried inside sulci, such as V2 and V3 that sit in the lunate sulcus or area MT that sits at the bottom of the superior temporal sulcus. The absence of sulci in the marmoset allows for functional mapping of visual areas that cannot be easily done in the macaque. For some methods, the sulci and gyri of the macaque brain pose insurmountable challenges. For example, optical imaging methods, which have been highly informative in the study of the primary visual cortex (Grinvald, 1992; Fitzpatrick, 2000), rely critically on access to the cortical surface. Most optical imaging experiments in the macaque have been carried out in area V1 (Chen and Seidemann, 2012), with a few studies in dorsal area V4 (Tanigawa et al., 2010), and other cortical regions on the surface (Raffi and Siegel, 2005; Seidemann et al., 2002; Wang et al., 1996). Many areas of prime interest for visual neuroscientists, including V2, V3, V3A, MT, LIP and several areas in the superior temporal sulcus (STS), are currently inaccessible. In the marmoset, each of these areas is on the surface and is therefore open to optical investigation.
The continuity of surface cortex is also critical for certain types of electrophysiological investigation, such as large-scale surface recordings using electrocorticography (ECoG) arrays (Rubehn et al., 2009; Shimoda et al., 2012) or multiple arrays of penetrating electrodes. Only rarely have large electrode arrays been introduced into sulci (Fukushima et al., 2012; 2014). In macaques, one well-studied primate area that is particularly affected by its sulcal location is the motion-sensitive area MT. While its functional architecture has been carefully charted using single electrode mapping methods (Albright et al, 1984; DeAngelis and Newsome, 1999), and its role in motion perception has been studied in great detail (Newsome and Salzman, 1993; Shadlen and Newsome, 1994), its inaccessibility has prohibited investigation with optical methods and large-scale implanted microelectrode arrays. In contrast, area MT of the marmoset sits directly on the cortical surface, and its activity has recently been studied in the anesthetized animal using large, implanted surface arrays (Solomon et al., 2014). Of course, it remains unknown to what extent marmosets might perform complicated motion perception tasks under head-restraint, though recent work indicates at least simple tasks should be feasible (Mitchell et al, 2014).
The convoluted surface of the macaque brain also poses difficulties for the systematic investigation of layer-specific cortical signals, which is of increasing interest to neurophysiologists. The popular method of current source density analysis, which identifies concentrations of synaptic activity in different cortical layers, makes certain assumptions that almost certainly do not hold when a multicontact linear array of electrodes is not inserted perpendicular to the cortical surface (Mitzdorf, 1985). While it is possible to approach sulcal cortex from different angles in the macaque to apply this method (Schroeder et al., 1998), such measurements are more straightforward in the marmoset, where a much larger proportion of the visual cortex lends itself to perpendicular penetration.
Of these opportunities in the marmoset that stem from its smooth brain, optical imaging is likely to benefit the most. In addition to the large-scale mapping described above, the flat and compact cortex of the marmoset is ideally suited for spatially restricted methods, such as two-photon imaging. Two-photon calcium imaging has become a powerful method for studying visual processing at the single cell level in the mouse, but for other species has also been applied to cats (Ohki and Reid, 2014) and more recently to macaque area V1 (Nauhaus et al, 2012). Two-photon microscopy can resolve individual neurons at different depths (though the maximal achievable depth is currently also one of its limitations (Ustione and Piston, 2011)). It also can reveal aspects of functional architecture expressed over spatial scales less than one millimeter (Nauhaus et al, 2012). Finally, the unchanging geometry of the neural positions relative to fixed blood vessels, makes it possible to use 2-photon imaging to track neural responses not just within a session, but also longitudinally across days (Huber et al., 2012). This last point may be critical, since it allows one to apply this method to study learning and plasticity within the marmoset visual system. It is not yet known the extent to which other features of the marmoset, including its small size, thin skull, and more transparent dura, offer additional advantages to two-photon imaging. Nor is it known to what extent viral and transgenic methods in the marmoset will facilitate 2-photon imaging in the species, making such experimens as feasible and reliable as they now are in the mouse. Several groups are at present pursuing genetic and molecular approaches for the marmoset (see commentary in Nature, Shen, 2013)), and thus it seems likely some of these tools will be available to vision scientists soon.
4.4 Prospect of genetic manipulation
Marmosets appear on the verge of becoming the consensus model for the creation of primate transgenic lines (see commentary in Nature, Cyranoski, 2014). Proof-of-concept experiments in this species have already demonstrated the feasibility of transgenesis germline transmission, using lentiviral infection of embryos (Sasaki et al, 2009). These initial successes have brought with them a cautious optimism that transgenic marmosets might soon play as important a role in neuroscience research as transgenic mice. Several international groups are now moving forward to build genetic models of human mental disease in the marmoset (Shen, 2013), primarily because of the strong overlap with the human in aspects of its primate-specific brain development, as well as its cognitive and social behavior (Tokuno et al, 2012).
Lentiviral methods are limited by the size of gene that can be delivered and the lack of control over the insertion point of base-pair seqeuences into the host DNA. For some diseases such as Parkinson’s disease, where there is overexpression of a small mutant gene, a lentivirusbased method can be used to create disease models (Kishi, Sato, Sasaki, and Okano, 2014). However for more precise or extensive genetic manipulations, advanced gene editing methods are needed. Several such methods have recently been developed in the mouse, allowing for precise targeting of genes in the genome and ultimately making expression in the cell more reliable. These methods, including TALEN and CRISPR systems, do not rely on a viral payload capacity and thus are conducive to the insertion of much larger genes (Kishi, Sato, Sasaki, and Okano, 2014). For disease models of schizophrenia and autism, where an extensive manipulation of genes is envisioned, such methods are of particularly high value. For basic neuroscience, these methods may facilitate the creation of marmoset Cre lines that allows for the restricted expression of a target gene in specific neuronal cell types selected by large promotors, the technology that has proven so successful in the mouse.
How might transgenic marmosets be used to study the neurobiology of vision? One can divide the applications into models of disease, and models for basic neurobiology research. With respect to schizophrenia and autism, mouse models are impoverished in their capacity to assay the some of the most obvious behavioral symptoms in humans. As one example, these afflictions are associated with abnormal active vision and eye movement behavior, particularly for complex stimuli like faces (Williams et al, 1999; Pelphrey et al, 2002; Kennedy and Adoplhs, 2012), as well as deficits in visual perception more generally (Tadin et al, 2006; Ben-Sasson et al, 2009; Yang et al, 2013; Foss-Feig et al, 2013). As mice use their vision less broadly than primates, and attend to different features of the environment, mouse models are not optimal to study important aspects of these diseases. A marmoset model would allow more direct comparison to the human phenotype, especially as tests for marmoset visual perception continue to improve. Even at present, the analysis of marmosets’ eye movements, and their targeting of regions of social interest including faces (Mitchell et al, 2014), could provide insights into the link between genes, neural circuits, and behavioral deficits in disease. As a strong model for neurodevelopment, the marmoset may further provide concrete information about what processes fail in neuropsychiatric or neurological disorders, for example relating to the failure of late migrating cells to deliver secreted signals, or to confer the proper balance of inhibition and excitation, in cortical circuits (Rubenstein and Merzenich, 2003; Lewis, 2014). The same line of reasoning holds for other mental disorders that involve higher-level cognitive or social processing, where a primate transgenic model might mimic a disease state much better than any mouse model. With regard to basic visual neuroscience research, transgenic marmosets promise to be invaluable for studying the functional anatomy of the brain, its cell type specific neurophysiology, and its circuit principles.
4.5 Opportunities and challenges in marmoset behavior
All primate species share aspects of their behavior, such as their inherent curiosity and use of vision to mediate social interaction. However, there is also great diversity in the primate Order, and particularly with respect to apparent cognitive behaviors. While the macaque has proven to be a highly valuable research organism for controlled testing, the marmoset is still relatively unexplored. Under freely moving conditions, marmosets can be trained to perform tasks that tap into diverse aspects of their cognition, as reviewed in Section 3.2.1. However, a fully developed marmoset model for studying mechanisms of visual cognition requires physical restraint amenable to various neural measurements as well as robust behavioral performance by cooperative marmoset participants. What is clear from even a short period working with marmosets is that their “personality” differs from macaques, as does their work ethic. A well-trained macaque can carry out sequences of hundreds or even thousands of consecutive behavioral trials, stopping to take only a few breaks over a period of several hours. They can navigate a large repertoire of psychophysical and behavior paradigms, learn complex rules, and even perform task that require a high level of concentration, such as sustaining attentional focus away from their center of gaze during extended fixation. These are key factors that have propelled macaques to such success as a model species for studying the neural basis of perception, cognition, and behavior.
The capacity of macaques for such a wide range of tasks may stem from the fact that they are highly adaptable under natural conditions, making them the most abundant and successful primate species second only to humans (Maestripieri, 2008). The natural ecology of marmosets is very different, and is closely tied to arboreal living in coherent family groups. Marmosets use vocal communication to constantly monitor the positions of their family members in the thick forest canopy (Rylands, 1993; Norcross and Newman, 1993; Miller et al, 2010). They are highly emotional, and will act against intruders as a group with a mobbing behavior. As codependent individuals, they easily become anxious when they are separated from their family members. This ecology provides a basis for understanding some aspects of their behavior in the laboratory. Mitchell and colleagues (2014) found that the marmoset was able to routinely perform sessions of 700–800 trials over the course of 1–2 hours. This is approximately one quarter to one half as many trials as a macaque might perform during analogous testing. However, the finding that marmosets can overcome their natural anxiety associated with removal from their social group and work routinely in visual tasks under restrained conditions was an important step, and perhaps the first of many in using and refining the awake marmoset paradigm.
The most successful tasks were those in which the marmosets were required to actively participate in the task, for example performing a visual discrimination by making an eye movement to a nonmatching stimulus. That they were worse for tasks that involved only prolonged fixation can be understood by the fact that this is the most unnatural of conditions for them. While the work ethic of the marmoset may not ever match that of the macaque, it is important to point out that two visual studies to date have been able to obtain approximately one hour’s worth of data from each of their subjects on average (Hung et al., 2015; Mitchell et al., 2014). It also bears mentioning that new methods of neural data collection may place fewer demands on long sessions, since neural activity can be tracked and accumulated across sessions. This possibility was mentioned briefly in Section 4.3 in the context of two-photon imaging with calcium indicators. Similar longitudinal recordings are also possible with chronic micro-wire electrodes, which can provide the stable isolation of single-units across sessions (McMahon et al, 2014).
An important challenge for the future may be to align testing paradigms more closely with marmosets’ natural behavioral repertoire. Marmosets are naturally engaged when allowed to explore visual scenes. Their frequent saccades and selection of particular scene content resembles similar active vision processes in humans (Yarbus, 1967; Hayhoe and Ballard, 2005). It might be seen as ironic that, because macaques are so disciplined in their capacity to fixate a small point, thus allowing for the precise visual stimulation of the retina, we know rather little about how the primate brain receives and processes stimuli during active, natural vision. With each shift of gaze, which typically occurs 2–3 times per second, the primate brain redirects the location of its high acuity fovea to seek new information about the scene. Through these programmed movements, the visual brain must constantly contend with massive changes in retinal stimulation. Our understanding of how the brain reconciles its sensory input and motor actions under natural conditions sorely lags our understanding of concepts of visual processing obtained from fixed-gaze experiments, such as the receptive field, stimulus selectivity, gain fields, and covert attentional orienting. While the marmoset is, at least for now, a weaker candidate model than the macaque for fixed gaze tasks, they may be just as strong for “active vision” tasks. This may gradually lead to stronger consideration of these topics by a community eager to exploit the many experimental advantages listed above.
Given the rate of emerging technology, studying active vision may soon be possible in animals whose heads, and maybe even bodies, are entirely free. In the rodent, where head-restraint is less common than in monkeys, paradigms involving spatial navigation and active whisking have revealed that during active sensing the brain behaves in a way that differs entirely from passive stimulation. For example, certain rhythmic synchronous activity appears only during active exploration of the environment and is now thought to play important roles for sensory coding or memory (Ferezou and Petersen, 2006; Curtis and Kleinfeld, 2009; Buszaki and Moser, 2013). In the visual domain, recent study in the New World Cebus monkey found that active sensing through eye movements similarly changed the neural dynamics associated with early visual processing, in this case involving synchronous neural activity time-locked to saccadic events (Ito et al, 2011). Another recent study examining visual responses in the macaque hippocampus found that during active sensing, oscillatory activity was evident and predictive of subsequent recognition performance (Jutras et al, 2013). Marmosets seem a natural primate candidate model for studying the brain under natural conditions. This may be particularly useful in the domain of social neuroscience, where activity in the brain can be studied under relatively natural familial contexts. Such experiments may reveal aspects of brain activity never before observed, but highly relevant to understanding neural circuits in the human.
4.6 Experimental opportunities: summary
For visual neuroscience experiments, the marmoset and macaque have complementary strengths. In the domain of behavior, marmosets fall short of macaques on some counts, such as in the capacity to direct fixation for extended periods or perform the same task continuously for several hours. However, it is important to point out that the experimental behavioral repertoire is, at present, largely unexplored and we might expect considerable improvements in this domain with further research. The marmoset clearly excels in other areas. Its small lissencephalic brain, its suitability for studying neurodevelopment, and the prospect of creating transgenic lines, provide unique avenues with which to study the human brain. The similarity of natural viewing behavior in this species compared to that of humans promises to spur creative new directions for understanding brain function. These factors, combined with the comparative benefits of systematically studying the brain and behavior of a New World monkey species, make the marmoset a worthy investment for visual neuroscience.
5. Conclusions
In this review we considered marmosets as a potential model for visual neuroscience from a broad range of theoretical and practical perspectives. At the heart of this consideration is a deep appreciation of comparative experimental approaches that help navigate the complexity of the brain through recognition of ancestral and derived traits (Kaas, 2013). The marmoset is first and foremost a primate, and thus shares many unique traits with other primates, including humans, particularly in the visual and social domains. Moreover, the organization of the primate brain differs in important ways from its closest mammalian relatives.
A critically important visual adaptation in primates is high acuity foveal vision, which is associated with specializations of the parvocellular pathway in early vision as well as the emphasis for foveal processing along the ventral stream into inferior temporal cortex. Foveal vision has strongly shaped visual cognition, giving us the ability to recognize fine detail and read the expressions and intentions of other individuals from a safe distance. It also determines how primates use their vision, with frequent, rapid eye movements serially positioning the fovea on points of interest. The target of each saccade is often the target for a reaching movement or the face of a conspecific, all of which are critical in a primate’s life. The frontal placement of eyes in the primate also affords binocular focusing of the left and right fovea towards objects, enabling depth information to guide the precise use of the hands. Together these unique visual and behavioral primate adaptations are foundations upon which much of human cognition and intelligence is built. Though we may learn much from studying of mammals such as the mouse whose adaptations are very different, it is essential to consider those evolved elements in the brain that so strongly shape our own cognition, and are most obviously is similar in other primates. The marmoset offers a much needed point of comparison to the growing body of circuit specific knowledge that is now accumulating in the mouse and the array of anatomical, physiological, and behavioral knowledge that has already been obtained from the highly studied macaque.
There are several areas of inquiry where we believe the marmoset will make an important contribution towards furthering our understanding of vision and primate brain organization. Due to its smaller and geometrically tractable brain, the marmoset is ideal for optical imaging, surface electrode arrays, and laminar recordings. Owing to their ease of breeding, inherent fecundity and rapid maturation, the marmoset is an excellent primate candidate to take advantage of methods developed in the mouse for molecular manipulation. Advances in transgenesis or viral methods will valuable for developing disease models and enable more precise methods to manipulate neuronal activity. The relative immaturity of the marmoset at birth is also likely to make it important as a model for studying primate brain development, including vision. The natural visual and social behaviors of marmosets are also highly overlapping with macaques and humans, and there is thus great potential to develop behavioral paradigms that exploit this similarity.
In considering whether to turn their attention to the marmoset brain, researchers are presented with trade-offs. This is true for any animal model, including the macaque and the mouse, and we have discussed many of these trade-offs in this article. If modern trends continue, marmosets are poised to provide an important experimental bridge by which advances in the mouse community can be applied to the primate brain. Marmosets are at the forefront of this push and are likely to play an important and exciting role for understanding our own brains using recent molecular and genetic techniques, including how circuits go awry in disease. In this journey, it is vital that the neurobiologist researchers retain a comparative and evolutionary perspective, as history teaches us that the deeper insights about the brain over the long term arise from consideration of these points.
Highlights.
The marmoset offers an excellent complement to the macaque as a model for visual neuroscience.
The marmoset brain bears most of the organizational features of other primates.
Marmoset natural gaze behavior closely resembles that of other primates, and can learn visual tasks.
Marmosets provide advantages for modern neurophysiology techniques including optical imaging.
Acknowledgments
We thank Drs. James Bourne, Yogita Chudasama, Greg Field, Rebecca Berman, Lindsey Glickfeld, and Sam Nummela for their comments on an earlier version of this manuscript. This work was supported by R21-MH104756 and the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DL, Horton JC. Ocular dominance columns: enigmas and challenges. Neuroscientist. 2009;15:62–77. doi: 10.1177/1073858408327806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnelt PK, Schubert C, Kübber-Heiss A, Schiviz A, Anger E. Independent variation of retinal S and M cone photoreceptor topographies: A survey of four families of mammals. Vis. Neurosci. 2006;23:429–435. doi: 10.1017/S095252380623342X. [DOI] [PubMed] [Google Scholar]
- Alemi-Neissi A, Rosselli FB, Zoccolan D. Multifeatural shape processing in rats engaged in invariant visual object recognition. J. Neurosci. 2013;33(14):5939–5956. doi: 10.1523/JNEUROSCI.3629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J. Neurophysiol. 1984;51:16–31. doi: 10.1152/jn.1984.51.1.16. [DOI] [PubMed] [Google Scholar]
- Allman JM. In: Evolution of the visual system in early primates. In Progress in Psychobiology and Physiological Psychology. Sprague JM, Epstein AM, editors. New York: Academic Press; 1977. pp. 1–53. [Google Scholar]
- Barefoot HC, Baker HF, Ridley RM. Crossed unilateral lesions of temporal lobe structures and cholinergic cell bodies impair visual conditional and object discrimination learning in monkeys. Eur. J. Neurosci. 2002;15(3):507–516. doi: 10.1046/j.0953-816x.2001.01888.x. [DOI] [PubMed] [Google Scholar]
- Barefoot HC, Maclean CJ, Baker HF, Ridley RM. Unilateral hippocampal and inferotemporal cortex lesions in opposite hemispheres impair learning of single-pair visual discriminations as well as visuovisual conditional tasks in monkeys. Behav. Brain Res. 2003;141(1):51–62. doi: 10.1016/s0166-4328(02)00320-0. [DOI] [PubMed] [Google Scholar]
- Barone P, Dehay C, Berland M, Bullier J, Kennedy H. Developmental remodeling of primate visual cortical pathways. Cereb. Cortex. 1995;5:22–38. doi: 10.1093/cercor/5.1.22. [DOI] [PubMed] [Google Scholar]
- Barraclough N, Tinsley C, Webb B, Vincent C, Derrington A. Processing of first-order motion in marmoset visual cortex is influenced by second-order motion. Vis. Neurosci. 2006;23:815–824. doi: 10.1017/S0952523806230141. [DOI] [PubMed] [Google Scholar]
- Barton RA. Visual specialization and brain evolution in primates. Proc. Biol. Sci. 1998;265:1933–1937. doi: 10.1098/rspb.1998.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009;39:1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011;15(12):592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Lund JS. Termination of afferent axons in macaque striate cortex. J. Neurosci. 1983;3(7):1389–1413. doi: 10.1523/JNEUROSCI.03-07-01389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Fitzpatrick D. Physiological organization of layer 4 in macaque striate cortex. J. Neurosci. 1984;4(3):880–895. doi: 10.1523/JNEUROSCI.04-03-00880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B, Kulikowski JJ, Carden D, Harwood D. Eye movements induced by electrical stimulation of the frontal eye fields of marmosets and squirrel monkeys. Brain Behav. Evol. 1982;21(1):34–41. doi: 10.1159/000121613. [DOI] [PubMed] [Google Scholar]
- Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Tweedale R, Rosa MGP. Physiological responses of New World monkey V1 neurons to stimuli defined by coherent motion. Cereb. Cortex. 2002;12:1132–1145. doi: 10.1093/cercor/12.11.1132. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Lui L, Tweedale R, Rosa MGP. First- and second-order stimulus length selectivity in New World monkey striate cortex. Eur. J. Neurosci. 2004;19:169–180. doi: 10.1111/j.1460-9568.2004.03082.x. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Rosa MGP. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT) Cereb. Cortex. 2006;16:405–414. doi: 10.1093/cercor/bhi119. [DOI] [PubMed] [Google Scholar]
- Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticoneuronal connections provide part of the neural substrate for manual dexterity. J. Neurosci. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borwein B. Scanning electron microscopy of monkey foveal photoreceptors. Anat. Rec. 1983;205(3):363–373. doi: 10.1002/ar.1092050313. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: Light microscopy. Philos. Trans. R. Soc. B (Lond) 1969;255:109–184. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Matsubara JA. Laminar and columnar patterns of geniculocotrical projections in the cat: relationship to cytochrome oxidase. J. Comp. Neurol. 1996;365:659–682. doi: 10.1002/(SICI)1096-9861(19960219)365:4<659::AID-CNE11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bullier J, Norton TT. Comparison of receptive-field properties of X and Y ganglion cells with X and Y lateral geniculate cells in the cat. J. Neurophysiol. 1979;42(1):274–291. doi: 10.1152/jn.1979.42.1.274. [DOI] [PubMed] [Google Scholar]
- Burkart J, Heschl A. Geometrical gaze following in common marmosets (Callithrix jacchus) J. Comp. Psychol. 2006;120(2):120–130. doi: 10.1037/0735-7036.120.2.120. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Palmer SM, Gamberini M, Rosa MG. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J. Comp. Neurol. 2006;495(2):149–172. doi: 10.1002/cne.20837. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Bakola S, Richardson KE, Reser DH, Rosa MG. Patterns of afferent input to the caudal and rostral areas of the dorsal premotor cortex (6DC and 6DR) in the marmoset monkey. J. Comp. Neurol. 2014;522:3683–3716. doi: 10.1002/cne.23633. [DOI] [PubMed] [Google Scholar]
- Burrows AM. The facial expression musculature in primates and its evolutionary significance. Bioessays. 2008;30(3):212–225. doi: 10.1002/bies.20719. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampalentorhinal system. Nat. Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzás P, Szmajda BA, Hashemi-Nezhad M, Dreher B, Martin PR. Color signals in the primary visual cortex of marmosets. J. Vis. 2008;8:7. doi: 10.1167/8.10.7. [DOI] [PubMed] [Google Scholar]
- Caine NG, Osorio D, Mundy NI. A foraging advantage for dichromatic marmosets (Callithrix geoffroyi) at low light intensity. Biol. Lett. 2010;6:36–38. doi: 10.1098/rsbl.2009.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28(4):196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Borrell V. Developmental sculpting of dendritic morphology of layer 4 neurons in visual cortex: influence of retinal input. J. Neurosci. 2011;31(20):7456–7470. doi: 10.1523/JNEUROSCI.5222-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA. Dissecting local circuits in vivo: integrated optogenetic and electrophysiology approaches for exploring inhibitory regulation of cortical activity. J. Physiol. Paris. 2012;106:104–111. doi: 10.1016/j.jphysparis.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcieri SM, Jacobs AL, Nirenberg S. Classification of retinal ganglion cells: a statistical approach. J. Neurophysiol. 2003;90(3):1704–1713. doi: 10.1152/jn.00127.2003. [DOI] [PubMed] [Google Scholar]
- Carlo CN, Stevens CF. Structural uniformity of neocortex, revisited. Proc. Natl. Acad. Sci. U.S.A. 2013;110(4):1488–1493. doi: 10.1073/pnas.1221398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, Jr, Patterson JL. An animal model that reflects human disease: the common marmoset (Callithrix jacchus) Curr. Opin. Virol. 2012;2(3):357–362. doi: 10.1016/j.coviro.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmill M. New views on primate origins. Evol. Anthropol. 1992;1:105–111. [Google Scholar]
- Casagrande VA, Norton TT. Lateral geniculate nucleus: a review of its physiology and function. In: Leventhal A, editor. The Neural Basis of Visual Function: Vision and Visual Dysfunction. London: McMillan Press; 1991. pp. 41–84. [Google Scholar]
- Casagrande VA, Boyd JD. The neural architecture of binocular vision. Eye (Lond) 1996;10(Pt 2):153–160. doi: 10.1038/eye.1996.40. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Khaytin I, Boyd J. The evolution of parallel visual pathways in the brains of primates. In: Preuss TM, Kaas J, editors. Evolution of the Nervous System. Vol. 4. 2006. pp. 87–108. [Google Scholar]
- Chance MRA. Attention Structure as the Basis of Primate Rank Orders. Man. 1967;2:503. [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. Bare skin, blood and the evolution of primate colour vision. Biol. Lett. 2006;2:217–221. doi: 10.1098/rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi MA, Shimojo S. “X-ray vision" and the evolution of forward-facing eyes. J. Theor. Biol. 2008;254(4):756–767. doi: 10.1016/j.jtbi.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Chaplin TA, Yu HH, Soares JG, Gattass R, Rosa MG. A conserved pattern of differential expansion of cortical areas in simian primates. J. Neurosci. 2013a;33(38):15120–15125. doi: 10.1523/JNEUROSCI.2909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TA, Yu HH, Rosa MG. Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J. Comp. Neurol. 2013b;521(5):1001–1019. doi: 10.1002/cne.23215. [DOI] [PubMed] [Google Scholar]
- Chen Y, Seidemann E. Attentional modulations related to spatial gating but not to allocation of limited resources in primate V1. Neuron. 2012;74:557–566. doi: 10.1016/j.neuron.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. J. Neurosci. 2013;33:6864–6876. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SK, Pietersen ANJ. Antidromic latency of magnocellular, parvocellular, and koniocellular (Blue-ON) geniculocortical relay cells in marmosets. Vis. Neurosci. 2014;31:263–273. doi: 10.1017/S0952523814000066. [DOI] [PubMed] [Google Scholar]
- Clara E, Tommasi L, Rogers LJ. Social mobbing calls in common marmosets (Callithrix jacchus): effects of experience and associated cortisol levels. Anim. Cogn. 2008;11(2):349–358. doi: 10.1007/s10071-007-0125-0. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304(5672):878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J. Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in a prosimian primate (Galago garnetti) J. Comp. Neurol. 2001;431(2):155–167. [PubMed] [Google Scholar]
- Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;285(1):619–627. doi: 10.1002/ar.a.20207. [DOI] [PubMed] [Google Scholar]
- Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc. Natl. Acad. Sci. U.S.A. 2010;107(36):15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Leitch DB, Wong P, Kaas JH, Herculano-Houzel S. Faster scaling of visual neurons in cortical areas relative to subcortical structures in non-human primate brains. Brain Structure and Function. 2013;218:805–816. doi: 10.1007/s00429-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M, Fitzpatrick D, Diamond IT. The laminar organization of the lateral geniculate body and the striate cortex in the tree shrew (Tupaia glis) J. Neurosci. 1984;4:171–197. doi: 10.1523/JNEUROSCI.04-01-00171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M, Mineka S. Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. J. of Abnormal Psychology. 1989;98:448–459. doi: 10.1037//0021-843x.98.4.448. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Muggleton NG, Bowditch AP, Pearce PC, Nutt DJ, Scott EA. Home cage presentation of complex discrimination tasks to marmosets and rhesus monkeys. Lab Anim. 1999;33(3):207–214. doi: 10.1258/002367799780578174. [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat. Neurosci. 2009;12(4):492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins-Sebree SE, Fragaszy DM. Choosing and using tools: capuchins (Cebus apella) use a different metric than tamarins (Saguinus oedipus) J. Comp. Psychol. 2005;119(2):210–219. doi: 10.1037/0735-7036.119.2.210. [DOI] [PubMed] [Google Scholar]
- Cyranoski D. Marmosets are stars of Japan’s ambitious project. Nature. 2014;514:151–152. doi: 10.1038/514151a. [DOI] [PubMed] [Google Scholar]
- Dacey DM. 20 Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways. The cognitive neurosciences. 2004;281 [Google Scholar]
- de Waal F, Waal FBM. Chimpanzee Politics: Power and sex among apes. JHU Press; 2007. [Google Scholar]
- DeAngelis GC, Newsome WT. Organization of disparity-selective neurons in macaque area MT. J. Neurosci. 1999;19(4):1398–1415. doi: 10.1523/JNEUROSCI.19-04-01398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006;26(41):10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Fuchs AF. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J. Physiol. 1979;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Parker A, Barraclough NE, Easton A, Goodson GR, Parker KS, Tinsley CJ, Webb BS. The uses of colour vision: behavioural and physiological distinctiveness of colour stimuli. Philos. Trans. R Soc. Lond. B Biol Sci. 2002;357(1424):975–985. doi: 10.1098/rstb.2002.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Conley M, Itoh K, Fitzpatrick D. Laminar organization of geniculocortical projections in Galago senegalensis and Aotus trivirgatus. J. Comp. Neurol. 1985;242(4):584–610. doi: 10.1002/cne.902420408. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Primate sexuality: Comparative studies of the prosimians, monkeys, apes, and humans. Oxford University Press; 2012. [Google Scholar]
- Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- Dräger UC. Autoradiography of tritiated proline and fucose transported transneuronally from the eye to the visual cortex in pigmented and albino mice. Brain Res. 1974;82(2):284–292. doi: 10.1016/0006-8993(74)90607-6. [DOI] [PubMed] [Google Scholar]
- Dreher B, Cottee LJ. Visual receptive-field properties of cells in area 18 of cat's cerebral cortex before and after acute lesions in area 17. J. Neurophysiol. 1975;38(4):735–750. doi: 10.1152/jn.1975.38.4.735. [DOI] [PubMed] [Google Scholar]
- Dufour V, Pascalis O, Petit O. Face processing limitation to own species in primates: a comparative study in brown capuchins, Tonkean macaques and humans. Behav. Processes. 2006;73(1):107–113. doi: 10.1016/j.beproc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Dunbar R. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;20:469–493. [Google Scholar]
- Easton A, Parker K, Derrington AM, Parker A. Behaviour of marmoset monkeys in a T-maze: comparison with rats and macaque monkeys on a spatial delayed non-match to sample task. Exp. Brain Res. 2003;150(1):114–116. doi: 10.1007/s00221-003-1409-5. [DOI] [PubMed] [Google Scholar]
- Elston GN, Rosa MGP. Ipsilateral corticocortical projections to the primary and middle temporal visual areas of the primate cerebral cortex: area-specific variations in the morphology of connectionally identified pyramidal cells. Eur. J. Neurosci. 2006;23:3337–3345. doi: 10.1111/j.1460-9568.2006.04847.x. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Clayton NS. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–1907. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Clayton NS. Comparative social cognition. Annu. Rev. Psychol. 2009;60:87–113. doi: 10.1146/annurev.psych.60.110707.163526. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 1966;187:517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federer F, Ichida JM, Jeffs J, Schiessl I, McLoughlin N, Angelucci A. Four projection streams from primate V1 to the cytochrome oxidase stripes of V2. J. Neurosci. 2009;29:15455–15471. doi: 10.1523/JNEUROSCI.1648-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50(4):617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Fernandez AA, Morris MR. Sexual selection and trichromatic color vision in primates: statistical support for the preexisting-bias hypothesis. The American Naturalist. 2007;170:10–20. doi: 10.1086/518566. [DOI] [PubMed] [Google Scholar]
- Fite KV, Rosenfield-Wessels S. A comparative study of deep avian foveas. Brain, Behav. Evol. 1975;12:97–115. doi: 10.1159/000124142. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. Cortical imaging: capturing the moment. Curr. Biol. 2000;10(5):R187–R190. doi: 10.1016/s0960-9822(00)00348-1. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate evolution and adaptation. N.Y.: Academic Press; 1988. [Google Scholar]
- Fleagle JG, Simons EL. Limb skeleton and locomotor adaptations of Apidium phiomense, an Oligocene anthropoid from Egypt. Am. J. Phys. Anthropol. 1995;97:235–289. doi: 10.1002/ajpa.1330970303. [DOI] [PubMed] [Google Scholar]
- Forte JD, Hashemi-Nezhad M, Dobbie WJ, Dreher B, Martin PR. Spatial coding and response redundancy in parallel visual pathways of the marmoset Callithrix jacchus. Vis. Neurosci. 2005;22:479–491. doi: 10.1017/S0952523805224094. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J. Neurosci. 2013;33(19):8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy DM. Preliminary quantitative studies of prehension in squirrel monkeys (Saimiri sciureus) Brain Behav. Evol. 1983;23(3–4):81–92. doi: 10.1159/000121499. [DOI] [PubMed] [Google Scholar]
- Franco ECS, Finlay BL, Silveira LCL, Yamada ES, Crowley JC. Conservation of absolute foveal area in New World monkeys. Brain. Beh. Evol. 2000;56:276–286. doi: 10.1159/000047211. [DOI] [PubMed] [Google Scholar]
- French JA. The role of androgenic steroids in shaping social phenotypes across the lifespan in male marmosets (Callithrix spp.) Am. J. Primatol. 2013;75(3):212–221. doi: 10.1002/ajp.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. I. Arborization patterns and quantitative distribution of postsynaptic elements. J. Comp. Neurol. 1985;242:263–274. doi: 10.1002/cne.902420208. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Saunders RC, Leopold DA, Mishkin M, Averbeck BB. Spontaneous high-gamma band activity reflects functional organization of auditory cortex in the awake macaque. Neuron. 2012;74:899–910. doi: 10.1016/j.neuron.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Saunders RC, Mullarkey M, Doyle AM, Mishkin M, Fujii N. An electrocorticographic electrode array for simultaneous recording from medial, lateral, and intrasulcal surface of the cortex in macaque monkeys. J. Neurosci. Methods. 2014;233:155–165. doi: 10.1016/j.jneumeth.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk AP, Rosa MG. Visual responses of neurones in the second visual area of flying foxes (Pteropus poliocephalus) after lesions of striate cortex. J. Physiol. 1998;513(Pt 2):507–519. doi: 10.1111/j.1469-7793.1998.507bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp. Brain Res. 2003;153(2):158–170. doi: 10.1007/s00221-003-1589-z. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Burish MJ, Kaas JH. Thalamocortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb. Cortex. 2010;20:2391–2410. doi: 10.1093/cercor/bhp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in NewWorld monkeys. Cereb. Cortex. 2011;21(9):1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Wiebe V, Przeworski M, Lancet D, Paabo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS. Biol. 2004;2:E5. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Homman-Ludiye J, Bourne JA. EphA4 is associated with multiple cell types in the marmoset primary visual cortex throughout the lifespan. Eur. J. Neurosci. 2014;39:1419–1428. doi: 10.1111/ejn.12514. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Ghosh KK, Martin P. A comparison of photoreceptor spatial density and ganglion cell morphology in the retina of human, macaque monkey, cat, and the marmoset Callithrix jacchus. J. Comp. Neurol. 1996;366:55–75. doi: 10.1002/(SICI)1096-9861(19960226)366:1<55::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Martin PR. The distribution of calcium-binding proteins in the lateral geniculate nucleus and visual cortex of a New World monkey, the marmoset, Callithrix jacchus. Vis. Neurosci. 1998;15:625–642. doi: 10.1017/s0952523898154044. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Grinvald A. Optical imaging of architecture and function in the living brain sheds new light on cortical mechanisms underlying visual perception. Brain Topogr. 1992;5(2):71–75. doi: 10.1007/BF01129033. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the macaque. J. Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J. Neurophysiol. 2003;90:3594–3607. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- Guerra RF, da Silveira NL, Bernardi N, Legal EJ. Hand preference during behavioral tests and spontaneous activity in two species of common marmoset (Callithrix jacchus and Callithrix penicillata) Rev. Bras. Biol. 1997;57(4):563–570. [PubMed] [Google Scholar]
- Guo K, Robertson R, Nevado A, Pulgarin M, Mahmoodi S, Young MP. Primary visual cortex neurons that contribute to resolve the aperture problem. Neuroscience. 2006;138:1397–1406. doi: 10.1016/j.neuroscience.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Hadjidimitrakis K, Breveglieri R, Bosco A, Fattori P. Three-dimensional eye position signals shape both peripersonal space and arm movement activity in the medial posterior parietal cortex. Front. Integr. Neurosci. 2012;6:37. doi: 10.3389/fnint.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Huerta MF. The geniculostriate projection in the grey squirrel: preliminary autoradiographic data. Brain Res. 1983;272(2):341–349. doi: 10.1016/0006-8993(83)90581-4. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, van Lieshout DP. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J. Comp. Neurol. 1991;304(2):275–306. doi: 10.1002/cne.903040210. [DOI] [PubMed] [Google Scholar]
- Hashemi-Nezhad M, Blessing EM, Dreher B, Martin PR. Segregation of short-wavelength sensitive ("blue") cone signals among neurons in the lateral geniculate nucleus and striate cortex of marmosets. Vision Res. 2008;48:2604–2614. doi: 10.1016/j.visres.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Yamazaki Y, Iriki A. Hand preference depends on posture in common marmosets. Behav. Brain Res. 2013;248:144–150. doi: 10.1016/j.bbr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Ballard D. Eye movements in natural behavior. Trends Cogn. Sci. 2005;9(4):188–194. doi: 10.1016/j.tics.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Heiney SA, Blazquez PM. Behavioral response of trained squirrel and rhesus monkeys during oculomotor tasks. Exp. Brain Res. 2011;212(3):409–416. doi: 10.1007/s00221-011-2746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu. Rev. Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Heymann EW, von der Lage F. Brief communication: noninvasive measuring of operational tongue length in callitrichids. Am. J. Phys. Anthropol. 2009;139:430–433. doi: 10.1002/ajpa.21038. [DOI] [PubMed] [Google Scholar]
- Homman-Ludiye J, Bourne JA. The guidance molecule Semaphorin3A is differentially involved in the arealization of the mouse and primate neocortex. Cereb. Cortex. 2013;24(11):2884–2898. doi: 10.1093/cercor/bht141. [DOI] [PubMed] [Google Scholar]
- Homman-Ludiye J, Bourne JA. Mapping arealisation of the visual cortex of non-primate species: lessons for development and evolution. Front. Neural Circuits. 2014;8:79. doi: 10.3389/fncir.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand, mouth and eye preferences in the common marmoset (Callithrix jacchus) Folia Primatol. (Basel) 1995;64(4):180–191. doi: 10.1159/000156851. [DOI] [PubMed] [Google Scholar]
- Hook MA, Rogers LJ. Visuospatial reaching preferences of common marmosets (Callithrix jacchus): an assessment of individual biases across a variety of tasks. J. Comp. Psychol. 2008;122(1):41–51. doi: 10.1037/0735-7036.122.1.41. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. Anatomical demonstration of ocular dominance columns in striate cortex of the squirrel monkey. J. Neurosci. 1996;16:5510–5522. doi: 10.1523/JNEUROSCI.16-17-05510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Niell CM. What can mice tell us about how vision works? Trends Neurosci. 2011;34(9):464–473. doi: 10.1016/j.tins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Albano JE, Norton TT. Organization of ocular dominance in tree shrew striate cortex. Brain Res. 1977;134(2):225–236. doi: 10.1016/0006-8993(77)91069-1. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Shape and arrangement of columns in cat's striate cortex. J. Physiol. 1963;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J. Physiology. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculocortical fibers in macaque monkey. J. Comp. Neurology. 1972;146:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O'Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus: Studies of its morphology and connections. In: Vanegas H, editor. Comparative Neurology of the Optic Tectum. New York: Plenum; 1984. pp. 687–773. [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. Subcortical connections. J. Comp. Neurol. 1986;253(4):415–439. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb. Cortex. 2001;11(9):849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Hughes A. A comparison of retinal ganglion cell topography in the plains and tree kangaroo. J. Physiology. 1975;244:61–63. [PubMed] [Google Scholar]
- Hughes HC. Anatomical and neurobehavioral investigations concerning the thalamocortical organization of the rat's visual system. J. Comp. Neurol. 1977;175(3):311–336. doi: 10.1002/cne.901750306. [DOI] [PubMed] [Google Scholar]
- Humphrey N. Growing Points in Ethology. Cambridge: Cambridge Univeristy Press; 1976. The social function of intellect; pp. 303–317. [Google Scholar]
- Humphrey AL, Norton TT. Topographic organization of the orientation column system in the striate cortex of the tree shrew (Tupaia glis). I. Microelectrode recording. J. Comp. Neurol. 1980;192(3):531–547. doi: 10.1002/cne.901920311. [DOI] [PubMed] [Google Scholar]
- Hung CC, Yen CC, Ciuchta JL, Papoti D, Bock NA, Leopold DA, Silva AC. Functional mapping of face-selective regions in the extrastriate visual cortex of the marmoset. 2015 doi: 10.1523/JNEUROSCI.2659-14.2015. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Maldonado P, Singer W, Grün S. Saccade-related modulations of neuronal excitability support synchrony of visually elicited spikes. Cereb. Cortex. 2011;21(11):2482–2497. doi: 10.1093/cercor/bhr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Conley M, Diamond IT. Retinal ganglion cell projections to individual layers of the lateral geniculate body in Galago crassicaudatus. J. Comp. Neurol. 1982;205(3):282–290. doi: 10.1002/cne.902050308. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Neitz M. Genetic basis of polymorphism in the color vision of platyrrhine monkeys. Vision Res. 1993;33:269–274. doi: 10.1016/0042-6989(93)90083-9. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF. Uniformity of colour vision in Old World monkeys. Proc. Biol. Sci. 1999;266:2023–2028. doi: 10.1098/rspb.1999.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH. Primate color vision: a comparative perspective. Vis. Neurosci. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- Janecka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM, Springer MS, Murphy WJ. Molecular and genomic data identify the closest living relative of primates. Science. 2007;318(5851):792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl. Acad. Sci. U.S.A. 2013;110(32):13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav. Evol. 1972;6:253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Huerta MF, Weber JT, Harting JK. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J. Comp. Neurol. 1978;182:517–553. doi: 10.1002/cne.901820308. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Huerta MF. The subcortical visual system of primates. In: Steklis HD, Erwin J, editors. Comparative Primate Biology, Vol 4: Neurosciences. New York: Alan R. Liss; 1988. pp. 327–391. [Google Scholar]
- Kaas JH. Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front. Neuroanat. 2011;5:34. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Evolution of columns, modules, and domains in the neocortex of primates. Proc. Natl. Acad. Sci. 2012;109:10655–10660. doi: 10.1073/pnas.1201892109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Stepniewska I, Gharbawie O. Cortical networks subserving upper limb movements in primates. Eur. J. Phys. Rehabil. Med. 2012;48(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. Cortical networks for ethologically relevant behaviors in primates. Am. J. Primatol. 2013;75:407–414. doi: 10.1002/ajp.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The evolution of brains from early mammals to humans. Wiley Interdiscip. Rev. Cogn. Sci. 2013;4(1):33–45. doi: 10.1002/wcs.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl JM, Whishaw IQ. Different evolutionary origins for the reach and the grasp: an explanation for dual visuomotor channels in primate parietofrontal cortex. Front. Neurol. 2013;4:208. doi: 10.3389/fneur.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C, Keating E. Visual scan patterns of rhesus monkeys viewing faces. Perception. 1982;11:211–219. doi: 10.1068/p110211. [DOI] [PubMed] [Google Scholar]
- Kemp C, Kaplan G. Facial expressions in common marmosets (Callithrix jacchus) and their use by conspecifics. Anim. Cogn. 2013;16(5):773–788. doi: 10.1007/s10071-013-0611-5. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 2012;16(11):559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Silveira LC, Kremers J. Spatial receptive field properties of lateral geniculate cells in the owl monkey (Aotus azarae) at different contrasts: a comparative study. Eur. J. Neurosci. 2007;26(4):992–1006. doi: 10.1111/j.1460-9568.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Killackey H, Snyder M, Diamond IT. Function of striate and temporal cortex in the tree shrew. J. Comp. Physiol. Psychol. 1971;74(2):1–29. doi: 10.1037/h0020531. [DOI] [PubMed] [Google Scholar]
- Killackey H, Wilson M, Diamond IT. Further studies of the striate and extrastriate visual cortex in the tree shrew. J. Comp. Physiol. Psychol. 1972;81:45–63. doi: 10.1037/h0033320. [DOI] [PubMed] [Google Scholar]
- Kirby MA, Wilson PD. Receptive field properties and latencies of cells in the lateral geniculate nucleus of the North American opossum (Didelphis virginiana) J. Neurophysiol. 1986;56:907–933. doi: 10.1152/jn.1986.56.4.907. [DOI] [PubMed] [Google Scholar]
- Kirk EC. Comparative morphology of the eye in primates. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;281(1):1095–1103. doi: 10.1002/ar.a.20115. [DOI] [PubMed] [Google Scholar]
- Kirk EC, Kay RF. The evolution of high visual acuity in the Anthropoidea. In: Ross CF, Kay RF, editors. Anthropoid Origins: New Visions. New York: Kluwer/Plenum Publishing; 2004. pp. 539–602. [Google Scholar]
- Kishi N, Sato K, Sasaki E, Okano H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev. Growth Differ. 2014;56(1):53–62. doi: 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J. Neurophysiol. 1993;70(1):144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Kolb B. Posterior parietal and temporal association cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. Cambridge, MA: MIT Press; 1990. pp. 459–471. [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb. Perspect. Biol. 2011;3(6) doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ. The control of voluntary eye movements: new perspectives. Neuroscientist. 2005;11(2):124–137. doi: 10.1177/1073858404271196. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Peng CS, Baker CI. Real-world scene representations in high-level visual cortex: it's the spaces more than the places. J. Neurosci. 2011;31(20):7322–7333. doi: 10.1523/JNEUROSCI.4588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn. Sci. 2013;17(1):26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers J, Lee BB. Comparative retinal physiology in anthropoids. Vision Res. 1998;38(21):3339–3344. doi: 10.1016/s0042-6989(97)00343-x. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Kaas J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr. Opin. Neurobiol. 2005;15:444–453. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Kahn DM. Nature versus nurture revisited: an old idea with a new twist. Prog. Neurobiol. 2003;70:33–52. doi: 10.1016/s0301-0082(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Landis T, Cummings JL, Benson DF. Loss of topographic familiarity. Arch. Neurol. 1986;43:132–136. doi: 10.1001/archneur.1986.00520020026011. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312(5782):1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Langston A, Casagrande VA, Fox R. Spatial resolution of the Galago. Vision Res. 1986;26:791–796. doi: 10.1016/0042-6989(86)90094-5. [DOI] [PubMed] [Google Scholar]
- Lapuerta P, Schein SJ. A four-surface schematic eye of macaque monkey obtained by an optical method. Vision Res. 1995;35:2245–2254. doi: 10.1016/0042-6989(94)00320-l. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488(7411):379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32(3):261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Lennie P, Perry VH. Spatial contrast sensitivity of cells in the lateral geniculate nucleus of the rat. J. Physiol. 1981;315:69–79. doi: 10.1113/jphysiol.1981.sp013733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Rhodes G. A comparative view of face perception. J. Comp. Psychol. 2010;124:233–251. doi: 10.1037/a0019460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: a comparative analysis using independent contrasts. J. Comp. Psychology. 2006;120:31–37. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr. Opin. Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER. Use it or lose it: molecular evolution of sensory signaling in primates. Pflugers Arch. 2006;453:125–131. doi: 10.1007/s00424-006-0120-3. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P, Long KD. Perceptual and cognitive visual functions of parietal and temporal cortices in the cat. Cereb. Cortex. 1996;6(5):673–695. doi: 10.1093/cercor/6.5.673. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. Single-unit responses to kinetic stimuli in New World monkey area V2: physiological characteristics of cue-invariant neurones. Exp. Brain Res. 2005;162:100–108. doi: 10.1007/s00221-004-2113-9. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. Functional response properties of neurons in the dorsomedial visual area of New World monkeys (Callithrix jacchus) Cereb. Cortex. 2006;16:162–177. doi: 10.1093/cercor/bhi094. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. Spatial and temporal frequency selectivity of neurons in the middle temporal visual area of new world monkeys (Callithrix jacchus) Eur. J. Neurosci. 2007a;25:1780–1792. doi: 10.1111/j.1460-9568.2007.05453.x. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. Spatial summation, end inhibition and side inhibition in the middle temporal visual area (MT) J. Neurophysiol. 2007b;97:1135–1148. doi: 10.1152/jn.01018.2006. [DOI] [PubMed] [Google Scholar]
- Lui LL, Dobiecki AE, Bourne JA, Rosa MGP. Breaking camouflage: responses of neurons in the middle temporal area to stimuli defined by coherent motion. Eur. J. Neurosci. 2012;36:2063–2076. doi: 10.1111/j.1460-9568.2012.08121.x. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. Relationship between size summation properties, contrast sensitivity and response latency in the dorsomedial and middle temporal areas of the primate extrastriate cortex. PLoS One. 2013;8:e68276. doi: 10.1371/journal.pone.0068276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Excitatory and inhibitory circuitry and laminar mapping strategies in primary visual cortex of the monkey. In: Edelmann GM, Gall WE, Cowan WM, editors. Signal and Sense: local and global order in perceptual maps. New York, NY: John Wiley and Sons; 1990. pp. 51–66. [Google Scholar]
- Luo ZX, Yuan CX, Meng QJ, Ji Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature. 2011;476:442–445. doi: 10.1038/nature10291. [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Gaffan D, Baker HF, Ridley RM. Visual discrimination learning impairments produced by combined transections of the anterior temporal stem, amygdala and fornix in marmoset monkeys. Brain Res. 2001;888(1):34–50. doi: 10.1016/s0006-8993(00)02998-x. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Macachiavellian Intelligence. University of Chicago Press; 2008. [Google Scholar]
- Malpeli JG, Lee D, Baker FH. Laminar and retinotopic organization of the macaque lateral geniculate nucleus: magnocellular and parvocellular magnification functions. J. Comp. Neurol. 1996;375(3):363–377. doi: 10.1002/(SICI)1096-9861(19961118)375:3<363::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461(7265):784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K. Marmoset models commonly used in biomedical research. Comp. Med. 2003;53(4):383–392. [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Cell migration in the forebrain. Annu. Rev. Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markov NT, Vezoli J, Chameau P, Falchier A, Quilodran R, Huissoud C, Lamy C, Misery P, Giroud P, Ullman S, Barone P, Dehay C, Knoblauch K, Kennedy H. Anatomy of hierarchy: feedforward and feedback pathways in macaque visual cortex. J. Comp. Neurol. 2014;522:225–259. doi: 10.1002/cne.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzke MW. Precision grips, hand morphology, and tools. Am. J. Phys. Anthropol. 1997;102(1):91–110. doi: 10.1002/(SICI)1096-8644(199701)102:1<91::AID-AJPA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Martin PR, Blessing EM, Buzás P, Szmajda BA, Forte JD. Transmission of colour and acuity signals by parvocellular cells in marmoset monkeys. J Physiol. 2011;589(Pt 11):2795–812. doi: 10.1113/jphysiol.2010.194076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT. Firing behavior of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. J Physiol. 2003;546:207224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JS, Clifford CW, Solomon SS, Chen SC, Solomon SG. Integration and segregation of multiple motion signals by neurons in area MT of primate. J. Neurophysiol. 2014;111:369–378. doi: 10.1152/jn.00254.2013. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Jones AP, Bondar IV, Leopold DA. Face-selective neurons maintain consistent visual responses across months. Proc. Natl. Acad. Sci. U.S.A. 2014;111(22):8251–8256. doi: 10.1073/pnas.1318331111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin C, Godement P, Imbert M. The primary visual cortex in the mouse: receptive field properties and functional organization. Exp. Brain Res. 1988;69:594–612. doi: 10.1007/BF00247312. [DOI] [PubMed] [Google Scholar]
- Miller CT, Mandel K, Wang X. The communicative content of the common marmoset phee call during antiphonal calling. Am. J. Primatol. 2010;72(11):974–980. doi: 10.1002/ajp.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Wren Thomas A. Individual recognition during bouts of antiphonal calling in common marmosets. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012;198(5):337–346. doi: 10.1007/s00359-012-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Reynolds JH, Miller CT. Active vision in marmosets: a model system for visual neuroscience. J. Neurosci. 2014;34(4):1183–1194. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 1985;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Moeller GU, Kayser C, Knecht F, König P. Interactions between eye movement systems in cats and humans. Exp. Brain Res. 2004;157(2):215–224. doi: 10.1007/s00221-004-1835-z. [DOI] [PubMed] [Google Scholar]
- Mollon JD. "Tho' she kneel'd in that place where they grew…" The uses and origins of primate colour vision. J. Exp. Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- Mundy NI. Genetic basis of olfactory communication in primates. Am. J. Primatol. 2006;68:559–567. doi: 10.1002/ajp.20252. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Eizirik E, O’brien SJ, Madsen O, Scally M, Douady CJ, et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- Nauhaus I, Nielsen KJ, Disney AA, Callaway EM. Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex. Nat. Neurosci. 2012;15(12):1683–1690. doi: 10.1038/nn.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakako T, Murai T, Ikejiri M, Ishiyama T, Taiji M, Ikeda K. Effects of a dopamine D1 agonist on ketamine-induced spatial working memory dysfunction in common marmosets. Behav. Brain Res. 2013;249:109–115. doi: 10.1016/j.bbr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Napier JR, Napier PH. Morphology, ecology and behaviour of nonhuman primates. London: Academic; 1967. A handbook of living primates. [Google Scholar]
- Nassi JJ, Callaway EM. Specialized circuits from primary visual cortex to V2 and area MT. Neuron. 2007;55:799–808. doi: 10.1016/j.neuron.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10(5):360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Salzman CD. The neuronal basis of motion perception. Ciba Found. Symp. 1993;174:217–230. doi: 10.1002/9780470514412.ch11. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. Am. J. Primatology. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Barone P. Contrast adaptation contributes to contrast-invariance of orientation tuning of primate V1 cells. PLoS One. 2009;4:e4781. doi: 10.1371/journal.pone.0004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord: a comparative study of 22 mammals. J. Comp. Neurol. 1988;N277(1):53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- Ohki K, Reid RC. In vivo two-photon calcium imaging in the visual system. Cold Spring Harb Protoc. 2014;4:402–416. doi: 10.1101/pdb.prot081455. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Torrealba F. The effect of acute lesions of the striate cortex on the retinotopic organization of the lateral peristriate cortex in the rat. Brain Res. 1978;151(2):386–391. doi: 10.1016/0006-8993(78)90894-6. [DOI] [PubMed] [Google Scholar]
- Op De Beeck H, Vogels R. Spatial sensitivity of macaque inferior temporal neurons. J. Comp. Neurol. 2000;426:505–518. doi: 10.1002/1096-9861(20001030)426:4<505::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 2004;8(7):315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and nonhuman primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Orban GA, Caruana F. The neural basis of human tool use. Front. Psychol. 2014;5:310. doi: 10.3389/fpsyg.2014.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Smith AC, Vorobyev M, Buchanan Smith HM. Detection of fruit and the selection of primate visual pigments for color cision. The American Naturalist. 2004;164:696–708. doi: 10.1086/425332. [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb. Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JG, Rosa MG, Fiorani M, Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J. Neurosci. 2007;27(38):10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Brady TF, Greene MR, Oliva A. Disentangling scene content from its spatial boundary: complementary roles for the PPA and LOC in representing real-world scenes. J. Neurosci. 2011;31(4):1333–1340. doi: 10.1523/JNEUROSCI.3885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AJ. Binocular depth perception and the cerebral cortex. Nat. Rev. Neurosci. 2007;8(5):379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Petrides M, Rosa M, Tokuno H. The Marmoset Brain in Stereotaxic Coordinates. London: Academic Press, Elsevier; 2012. [Google Scholar]
- Peeters R, Simon L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J. Autism Dev. Disord. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pessoa DM, Tomaz C, Pessoa VF. Color vision in marmosets and tamarins: behavioral evidence. Am. J. Primatol. 2005;67(4):487–495. doi: 10.1002/ajp.20202. [DOI] [PubMed] [Google Scholar]
- Peters A, Kara DA. The neuronal composition of area 17 of rat visual cortex. I. The pyramidal cells. J. Comp. Neurol. 1985;234(2):218–241. doi: 10.1002/cne.902340208. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. Evolution of binocular vision. Vis. Neurosci. 1986a:208–222. [Google Scholar]
- Pettigrew JD. Flying primates? Megabats have the advanced pathway from eye to midbrain. Science. 1986b;231:1304–1306. doi: 10.1126/science.3945827. [DOI] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 2013;33:4642–4656. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Geng S, Prins NW, Sanchez JC. Deciding to take action: Striatum activation during reaching to targets of varying reward value. Soc. Neurosci. Abstr. 2013 [Google Scholar]
- Preuschoft S, van Hooff JA. Homologizing primate facial displays: a critical review of methods. Folia Primatologica. 1995;65(3):121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J. Comp. Neurol. 1991;310(4):475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Taking the measure of diversity: comparative alternatives to the model-animal paradigm in cortical neuroscience. Brain Behav. Evol. 2000;55(6):287–299. doi: 10.1159/000006664. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Evolutionary specializations of primate brain organization. In: Ravosa M, Dagosto M, editors. Primate Origins. New York: Kluwer; 2007. pp. 625–675. [Google Scholar]
- Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Prog. Retin. Eye. Res. 2013;35:63–81. doi: 10.1016/j.preteyeres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Harrison M, Paxinos G, Watson C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 2013;36(10):570–578. doi: 10.1016/j.tins.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Raffi M, Siegel RM. Functional architecture of spatial attention in the parietal cortex of the behaving monkey. J. Neurosci. 2005;25:5171–5186. doi: 10.1523/JNEUROSCI.5201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos. Trans. R. Soc. Lond. Biol. Sci. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Stone J. The area centralis of the retina in the cat and other mammals: focal point for function and development of the visual system. Neuroscience. 1984;11(2):289–301. doi: 10.1016/0306-4522(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Regan BC, Julliot C, Simmen B, Vienot F, Charles-Dominique P, Mollon JD. Fruits, foliage and the evolution of primate colour vision. Philos. Trans. R. Soc. Lond B Biol. Sci. 2001;356:229–283. doi: 10.1098/rstb.2000.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: Topography of corticocortical and thalamic connections. Exp. Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Reid RC. From functional architecture to functional connectomics. Neuron. 2012;75(2):209–217. doi: 10.1016/j.neuron.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington ED, Osmanski MS, Wang X. An operant conditioning method for studying auditory behaviors in marmoset monkeys. PLoS One. 2012;7(10):e47895. doi: 10.1371/journal.pone.0047895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J. Comp. Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Renne PR, Deino AL, Hilgen FJ, Kuiper KF, Mark DF, Mitchell WS, Morgan LE, Mundil R, Smit J. Time scales of critical events around the Cretaceous-Paleogene boundary. Science. 2013;339:684–687. doi: 10.1126/science.1230492. [DOI] [PubMed] [Google Scholar]
- Reser DH, Burman KJ, Yu HH, Chaplin TA, Richardson KE, Worthy KH, Rosa MG. Contrasting patterns of cortical input to architectural subdivisions of the area 8 complex: a retrograde tracing study in marmoset monkeys. Cereb. Cortex. 2013;23(8):1901–1922. doi: 10.1093/cercor/bhs177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF, Crow TJ. A new approach to the role of noradrenaline in learning: problem-solving in the marmoset after alpha-noradrenergic receptor blockade. Pharmacol. Biochem. Behav. 1981;14(6):849–855. doi: 10.1016/0091-3057(81)90373-7. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Bowes PM, Baker HF, Crow TJ. An involvement of acetylcholine in object discrimination learning and memory in the marmoset. Neuropsychologia. 1984;22(3):253–263. doi: 10.1016/0028-3932(84)90073-3. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Murray TK, Johnson JA, Baker HF. Learning impairment following lesion of the basal nucleus of Meynert in the marmoset: modification by cholinergic drugs. Brain Res. 1986;376(1):108–116. doi: 10.1016/0006-8993(86)90904-2. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Murray TK. Basal nucleus lesions in monkeys: recognition memory impairment or visual agnosia? Psychopharmacology (Berl) 1988;95:289–290. doi: 10.1007/BF00174527. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF. A critical evaluation of monkey models of amnesia and dementia. Brain Res. Rev. 1991;16:15–37. doi: 10.1016/0165-0173(91)90018-4. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF. Assessing memory in monkeys. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. Vol. 1. Oxford: IRL Press at Oxford Univ. Press; 1993. pp. 149–163. [Google Scholar]
- Ridley RM, Warner KA, Maclean CJ, Gaffan D, Baker HF. Visual agnosia and Klüver-Bucy syndrome in marmosets (Callithrix jacchus) following ablation of inferotemporal cortex, with additional mnemonic effects of immunotoxic lesions of cholinergic projections to medial temporal areas. Brain Res. 2001;898(1):136–151. doi: 10.1016/s0006-8993(01)02187-4. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Cummings RM, Green ME, Leow-Dyke A. Mild topographical memory impairment following crossed unilateral lesions of the mediodorsal thalamic nucleus and the inferotemporal cortex. Behav. Neurosci. 2005;119(2):518–525. doi: 10.1037/0735-7044.119.2.518. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J. Neurosci. 2002;22(13):5639–5651. doi: 10.1523/JNEUROSCI.22-13-05639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Jones GH, Sirkia TE, Wilkinson J, Page K. The effects of excitotoxic lesions of the basal forebrain on the acquisition, retention and serial reversal of visual discriminations in marmosets. Neuroscience. 1990;34(2):311–329. doi: 10.1016/0306-4522(90)90142-q. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47(2):251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb. Cortex. 2007;17:151–160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dreher B. The visual pathways of eutherian mammals and marsupials develop according to a common timetable. Brain Behav. Evol. 1990;36:177–195. doi: 10.1159/000115306. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103(2):221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Watanabe M. Survey of the morphology of macaque retinal ganglion cells that project to the pretectum, superior colliculus, and parvicellular laminae of the lateral geniculate nucleus. J. Comp. Neurol. 1993;338:289–303. doi: 10.1002/cne.903380211. [DOI] [PubMed] [Google Scholar]
- Roe AW, Fritsches K, Pettigrew JD. Optical imaging of functional organization of V1 and V2 in marmoset visual cortex. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;287:1213–1225. doi: 10.1002/ar.a.20248. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Schmid LM, Krubitzer LA, Pettigrew JD. Retinotopic organization of the primary visual cortex of flying foxes (Pteropus poliocephalus and Pteropus scapulatus) J. Comp. Neurol. 1993;335:55–72. doi: 10.1002/cne.903350105. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Schmid LM. Topography and extent of visual-field representation in the superior colliculus of the megachiropteran Pteropus. Vis. Neurosci. 1994;11:1037–1057. doi: 10.1017/s0952523800006878. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Schmid LM. Visual areas in the dorsal and medial extrastriate cortices of the marmoset. J. Comp. Neurol. 1995;359:272–299. doi: 10.1002/cne.903590207. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Fritsches KA, Elston GN. The second visual area in the marmoset monkey: visuotopic organisation, magnification factors, architectonical boundaries, and modularity. J. Comp. Neurol. 1997;387:547–567. doi: 10.1002/(sici)1096-9861(19971103)387:4<547::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J. Comp. Neurol. 1998;393:505–527. [PubMed] [Google Scholar]
- Rosa MGP, Tweedale R. Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. J. Comp. Neurol. 2000;422:621–651. doi: 10.1002/1096-9861(20000710)422:4<621::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Palmer SM, Gamberini M, Burman KJ, Yu HH, Reser DH, Bourne JA, Tweedale R, Galletti C. Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J. Neurosci. 2009;29:4548–4563. doi: 10.1523/JNEUROSCI.0529-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Hare B. Looking past the model species: diversity in gaze-following skills across primates. Curr. Opin. Neurobiol. 2009;19(1):45–51. doi: 10.1016/j.conb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Rose MD. Functional morphological similarities in the locomotor skeleton of miocene catarrhines and platyrrhine monkeys. Folia Primatol. 1996;66:7–14. doi: 10.1159/000157180. [DOI] [PubMed] [Google Scholar]
- Ross CF. Adaptive explanation for the origins of the Anthropoidea (Primates) Am. J. Primatol. 1996;40:205–230. doi: 10.1002/(SICI)1098-2345(1996)40:3<205::AID-AJP1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ross CF. Into the Light: The Origin of Anthropoidea. Annual Review of Anthropology. 2000:147–194. [Google Scholar]
- Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 2009;6:036003. doi: 10.1088/1741-2560/6/3/036003. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLRR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat. Neurosci. 2013;16(8):1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylands AB. Marmosets and tamarins: systematics, behavior, and ecology. Oxford University Press; 1993. [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb. Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sakatani T, Isa T. Quantitative analysis of spontaneous saccade-like rapid eye movements in C57BL/6 mice. Neurosci Res. 2007;58(3):324–331. doi: 10.1016/j.neures.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Livesey FJ. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb. Perspect. Biol. 2009;1:a002519. doi: 10.1101/cshperspect.a002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Oga T, Nakagaki K, Sakai K, Sumida K, Hoshino K, Miyawaki I, Saito K, Suto F, Ichinohe N. Developmental expression profiles of axon guidance signaling and the immune system in the marmoset cortex: potential molecular mechanisms of pruning of dendritic spines during primate synapse formation in late infancy and prepuberty (I) Biochem. Biophys. Res. Commun. 2014;444:302–306. doi: 10.1016/j.bbrc.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Schein SJ. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. J. Comp. Neurol. 1988;269(4):479–505. doi: 10.1002/cne.902690403. [DOI] [PubMed] [Google Scholar]
- Schiel N, Huber L. Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus) Am. J. Primatol. 2006;68(12):1150–1160. doi: 10.1002/ajp.20284. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Finlay BL, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. II. Orientation specificity and ocular dominance. J. Neurophysiol. 1976;39(6):1320–1333. doi: 10.1152/jn.1976.39.6.1320. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Malpeli JG. Properties and tectal projections of monkey retinal ganglion cells. J. Neurophysiol. 1977;40:428–445. doi: 10.1152/jn.1977.40.2.428. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb. Cortex. 1998;8(7):575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J. Neurosci. 2003;23(7):2961–2970. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Troilo D, Kind PC, Graham B, Blakemore C. Functional architecture of area 17 in normal and monocularly deprived marmosets (Callithrix jacchus) Vis. Neurosci. 1996;13:145–160. doi: 10.1017/s0952523800007197. [DOI] [PubMed] [Google Scholar]
- Seidemann E, Arieli A, Grinvald A, Slovin H. Dynamics of depolarization and hyperpolarization in the frontal cortex and saccade goal. Science. 2002;295:862–865. doi: 10.1126/science.1066641. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Kiani R. Decision making as a window on cognition. Neuron. 2013;80(3):791–806. doi: 10.1016/j.neuron.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Curr. Opin. Neurobiol. 1994;4(4):569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Shapley R, Hugh Perry V. Cat and monkey retinal ganglion cells and their visual functional roles. Trends in Neurosciences. 1986;9:229–235. [Google Scholar]
- Sharpe LT, Stockman A, Jaegle H, Nathans J. Opsin genes, cone pigments, color vision, and color blindness. In: Gegenfurtner KR, Sharpe LT, editors. Color vision: from genes to perception. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Shen H. Precision gene editing paves way for transgenic monkeys. Nature. 2013;503(7474):14–15. doi: 10.1038/503014a. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Platt ML. Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta) Anim. Cogn. 2008;11(1):13–20. doi: 10.1007/s10071-007-0083-6. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Nagasaka Y, Chao ZC, Fujii N. Decoding continuous three-dimensional hand trajectories from epidural electrocorticographic signals in Japanese macaques. J. Neural Eng. 2012;9:036015. doi: 10.1088/1741-2560/9/3/036015. [DOI] [PubMed] [Google Scholar]
- Shoham D, Hübener M, Schulze S, Grinvald A, Bonhoeffer T. Spatio-temporal frequency domains and their relation to cytochrome oxidase staining in cat visual cortex. Nature. 1997;385:529–533. doi: 10.1038/385529a0. [DOI] [PubMed] [Google Scholar]
- Smith EL, Chino YM, Ridder WH, Kitagawa K, Langston A. Orientation bias of neurons in the lateral geniculate nucleus of macaque monkeys. Vis. Neurosci. 1990;5:525–545. doi: 10.1017/s0952523800000699. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis) J. Neurosci. 1992;12(4):1169–1193. doi: 10.1523/JNEUROSCI.12-04-01169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Tailby C, Gharaei S, Camp AJ, Bourne JA, Solomon SG. Visual motion integration by neurons in the middle temporal area of a New World monkey, the marmoset. J. Physiol. 2011;589:5741–5758. doi: 10.1113/jphysiol.2011.213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SS, Chen SC, Morley JW, Solomon SG. Local and global correlations between neurons in the middle temporal area of primate visual cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Solomon SG, Rosa MG. A simpler primate brain: the visual system of the marmoset monkey. Frontiers in Neural Circuits. 2014;8(96):91–24. doi: 10.3389/fncir.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz WB. Loss of ocular dominance columns with maturity in the monkey, Callithrix jacchus. Brain Res. 1989;488:376–380. doi: 10.1016/0006-8993(89)90734-8. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain Res. Cogn. Brain Res. 2004;19(2):123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Springer MS, Meredith RW, Janecka JE, Murphy WJ. The historical biogeography of Mammalia. Philos. Trans. R. Soc. B Biol. Sci. 2011;366:2478–2502. doi: 10.1098/rstb.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J. Comp. Neurol. 2009a;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J. Comp. Neurol. 2009b;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. An evolutionary scaling law for the primate visual system and its basis in cortical function. Nature. 2001;411:193–195. doi: 10.1038/35075572. [DOI] [PubMed] [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim. Behav. 1976;24(2):428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Striedter GF. Principles of brain evolution. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- Surridge AK, Osorio D, Mundy NI. Evolution and selection of trichromatic vision in primates. Trends in Ecology and Evolution. 2003;18:198–205. [Google Scholar]
- Sussman RW, Tab Rasmussen D, Raven PH. Rethinking primate origins again. Am. J. Primatol. 2013;75(2):95–106. doi: 10.1002/ajp.22096. [DOI] [PubMed] [Google Scholar]
- Tadin D, Kim J, Doop ML, Gibson C, Lappin JS, Blake R, Park S. Weakened center-surround interactions in visual motion processing in schizophrenia. J. Neurosci. 2006;26(44):11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi DY, Narayanan DZ, Ghazanfar AA. Coupled oscillator dynamics of vocal turn-taking in monkeys. Curr. Biol. 2013;23(21):2162–2168. doi: 10.1016/j.cub.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Takahata T, Komatsu Y, Watakabe A, Hashikawa T, Tochitani S, Yamamori T. Activity-dependent expression of occ1 in excitatory neurons is a characteristic feature of the primate visual cortex. Cereb. Cortex. 2006;16(7):929–940. doi: 10.1093/cercor/bhj034. [DOI] [PubMed] [Google Scholar]
- Takahata T, Shukla R, Yamamori T, Kaas JH. Differential expression patterns of striate cortex-enriched genes among Old World, New World, and prosimian primates. Cereb. Cortex. 2012;22(10):2313–2321. doi: 10.1093/cercor/bhr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Fujita I. Complex-image selective visual responses of IT neurons in a new world monkey. Proc. 7th IBRO World Congr. Neurosci.; Paris. 2007. p. 304. [Google Scholar]
- Tanaka K, Saito H, Fukada Y, Moriya M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 1991;66:170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nat. Neurosci. 2010;13:1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate A, Fischer H, Leigh A, Kendrick K. Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philos. Trans. R Soc. B Biol. Sci. 2006;361:2155. doi: 10.1098/rstb.2006.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegetmeyer H. Influence of visual signals on the coordination of spontaneous eye and head movements in infant rabbits. Brain Reseach Bull. 1996;40(5–6):359–362. doi: 10.1016/0361-9230(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Thiele A, Vogelsang M, Hoffmann KP. Pattern of retinotectal projection in the megachiropteran bat Rousettus aegyptiacus. J. Comp. Neurol. 1991;314:671–683. doi: 10.1002/cne.903140404. [DOI] [PubMed] [Google Scholar]
- Tinsley CJ, Webb BS, Barraclough NE, Vincent CJ, Parker A, Derrington AM. The nature of V1 neural responses to 2D moving patterns depends on receptive field structure in the marmoset monkey. J. Neurophysiol. 2003;90:930–937. doi: 10.1152/jn.00708.2002. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Moriya-Ito K, Tanaka I. Experimental techniques for neuroscience research using common marmosets. Exp. Anim. 2012;61(4):389–397. doi: 10.1538/expanim.61.389. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Silverman MS, De Valois RL, Jacobs GH. Functional organization of the second cortical visual area in primates. Science. 1983;220:737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Troilo D, Howland HC, Judge SJ. Visual optics and retinal cone topography in the common marmoset (Callithrix jacchus) Vision Res. 1993;33(10):1301–1310. doi: 10.1016/0042-6989(93)90038-x. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annu. Rev. Neurosci. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DG, Goodale MA, Mansfield RJQ, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Ustione A, Piston DW. A simple introduction to multiphoton microscopy. J. Microsc. 2011;243(3):221–226. doi: 10.1111/j.1365-2818.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Heimel JA, Nelson SB. Functional cell classes and functional architecture in the early visual system of a highly visual rodent. Prog. Brain Res. 2005;2005(149):127–145. doi: 10.1016/S0079-6123(05)49010-X. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Roy A, Rhodes HJ, Culp JH, Fitzpatrick D. Transformation of receptive field properties from lateral geniculate nucleus to superficial V1 in the tree shrew. J Neurosci. 2013;33(28):11494–11505. doi: 10.1523/JNEUROSCI.1464-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Orban GA. How well do response changes of striate neurons signal differences in orientation: a study in the discriminating monkey. J. Neurosci. 1990;10:3543–3558. doi: 10.1523/JNEUROSCI.10-11-03543.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Dias R, Robbins TW, Roberts AC. Dissociable contributions of the orbitofrontal and lateral prefrontal cortex of the marmoset to performance on a detour reaching task. Eur. J. Neurosci. 2001;13(9):1797–1808. doi: 10.1046/j.0953-816x.2001.01546.x. [DOI] [PubMed] [Google Scholar]
- Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. Eur. J. Neurosci. 2006;23(11):3119–3123. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Tanaka K, Tanifuji M. Optical imaging of functional organization in the monkey inferotemporal cortex. Science. 1996;272:1665–1668. doi: 10.1126/science.272.5268.1665. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sporns O, Burkhalter A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 2012;32(13):4386–4399. doi: 10.1523/JNEUROSCI.6063-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. The harmonic organization of auditory cortex. Front. Syst. Neurosci. 2013;17(7):114. doi: 10.3389/fnsys.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner CE, Kwan WC, Bourne JA. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J. Neurosci. 2012;32:17073–17085. doi: 10.1523/JNEUROSCI.3269-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res. 1990;30(11):1897–1911. doi: 10.1016/0042-6989(90)90166-i. [DOI] [PubMed] [Google Scholar]
- Webb BS, Tinsley CJ, Barraclough NE, Parker A, Derrington AM. Gain control from beyond the classical receptive field in primate primary visual cortex. Vis. Neurosci. 2003;20:221–230. doi: 10.1017/s0952523803203011. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Wark JD, Rosenbaum DA. Monkey see, monkey plan, monkey do: the end-state comfort effect in cotton-top tamarins (Saguinus oedipus) Psychol. Sci. 2007;18(12):1063–1068. doi: 10.1111/j.1467-9280.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J. Neurosci. 1990;10(10):3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder HD, Grünert U, Lee BB, Martin PR. Topography of ganglion cells and photoreceptors in the retina of a New World monkey: the marmoset Callithrix jacchus. Vis. Neurosci. 1996;13(2):335–352. doi: 10.1017/s0952523800007586. [DOI] [PubMed] [Google Scholar]
- Williams LM, Loughland CM, Gordon E, Davidson D. Visual scanpaths in schizophrenia: is there a deficit in face recognition? Schizophr. Res. 1999;40(3):189–199. doi: 10.1016/s0920-9964(99)00056-0. [DOI] [PubMed] [Google Scholar]
- Wilson PD, Rowe MH, Stone J. Properties of relay cells in cat's lateral geniculate nucleus: a comparison of W-cells with X- and Y-cells. J. Neurophysiol. 1976;39(6):1193–1209. doi: 10.1152/jn.1976.39.6.1193. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488(7411):343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Wilder HD, Goodchild AK, Sefton AJ, Martin PR. Segregation of receptive field properties in the lateral geniculate nucleus of a New-World monkey, the marmoset Callithrix jacchus. J. Neurophysiol. 1998;80(4):2063–2076. doi: 10.1152/jn.1998.80.4.2063. [DOI] [PubMed] [Google Scholar]
- White AJ, Solomon SG, Martin PR. Spatial properties of koniocellular cells in the lateral geniculate nucleus of the marmoset Callithrix jacchus. J. Physiol. 2001;533:519–535. doi: 10.1111/j.1469-7793.2001.0519a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis) Anat. Rec. (Hoboken) 2008;291:1301–1333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica) Brain Behav. Evol. 2009;73:206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PC. The nocturnal primate niche in the New World. J. Human Evolution. 1989;18(7):635–658. [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. NeuroImage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Marshak DW, Silveira LC, Casagrande VA. Morphology of P and M retinal ganglion cells of the bush baby. Vision Res. 1998;38(21):3345–3352. doi: 10.1016/s0042-6989(97)00412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Silveira LC, Perry VH, Franco EC. M and P retinal ganglion cells of the owl monkey: morphology, size and photoreceptor convergence. Vision Res. 2001;41:119–131. doi: 10.1016/s0042-6989(00)00244-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comp. Med. 2003;53:364–368. [PubMed] [Google Scholar]
- Yamazaki Y, Echigo C, Saiki M, Inada M, Watanabe S, Iriki A. Tool-use learning by common marmosets (Callithrix jacchus) Exp. Brain Res. 2011;213(1):63–71. doi: 10.1007/s00221-011-2778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Tadin D, Glasser DM, Hong SW, Blake R, Park S. Visual context processing in schizophrenia. Clin. Psychol. Sci. 2013;1(1):5–15. doi: 10.1177/2167702612464618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus AL. In: Eye Movements and Vision. Haigh B, translator. New York: Plenum Press; 1967. [Google Scholar]
- Yu HH, Verma R, Yang Y, Tibballs HA, Lui LL, Reser DH, Rosa MGP. Spatial and temporal frequency tuning in striate cortex: functional uniformity and specializations related to receptive field eccentricity. Eur. J. Neurosci. 2010;31:1043–1062. doi: 10.1111/j.1460-9568.2010.07118.x. [DOI] [PubMed] [Google Scholar]
- Yu HH, Rosa MGP. Uniformity and diversity of response properties of neurons in the primary visual cortex: selectivity for orientation, direction of motion, and stimulus size from center to far periphery. Vis. Neurosci. 2014;31:85–98. doi: 10.1017/S0952523813000448. [DOI] [PubMed] [Google Scholar]
- Zhang J, Webb DM. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8337–8341. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Phong Hoang Do J, Chang WC, Jenvay S, Miyamich K, Luo L, Dan Y. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke W, Roberts MJ, Guo K, McDonald JS, Robertson R, Thiele A. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized marmoset monkeys. Eur. J. Neurosci. 2006;24:314–328. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolan D, Oertelt N, DiCarlo JJ, Cox DD. A rodent model for the study of invariant visual object recognition. Proc. Natl. Acad. Sci. 2009;106(21):8748–8753. doi: 10.1073/pnas.0811583106. [DOI] [PMC free article] [PubMed] [Google Scholar]


