Abstract
The current approach to treating HER2-overexpressed breast cancer is the use of monoclonal antibodies or a combination of antibodies with traditional chemotherapeutic agents or kinase inhibitors. Our approach is to target clinically validated HER2 domain IV with peptidomimetics and inhibit the protein-protein interactions (PPI) of HERs. Unlike antibodies, peptidomimetics have advantages in terms of stability, modification, and molecular size. We have designed peptidomimetics (compounds 5 and 9) that bind to HER2 domain IV, inhibit protein-protein interactions, and decrease cell viability in breast cancer cells with HER2 overexpression. We have shown, using enzyme fragment complementation and proximity ligation assays, that peptidomimetics inhibit the PPI of HER2:HER3. Compounds 5 and 9 suppressed the tumor growth in a xenograft mouse model. Furthermore, we have shown that these compounds inhibit PPI of HER2:HER3 and phosphorylation of HER2 as compared to control in tissue samples derived from in vivo studies. The stability of the compounds was also investigated in mouse serum, and the compounds exhibited stability with a half-life of up to 3 h. These results suggest that the novel peptidomimetics we have developed target the extracellular domain of HER2 protein and inhibit HER2:HER3 interaction, providing a novel method to treat HER2-positive cancer.
Keywords: HER2, HER3, protein-protein interaction, breast cancer, peptidomimetic
Introduction
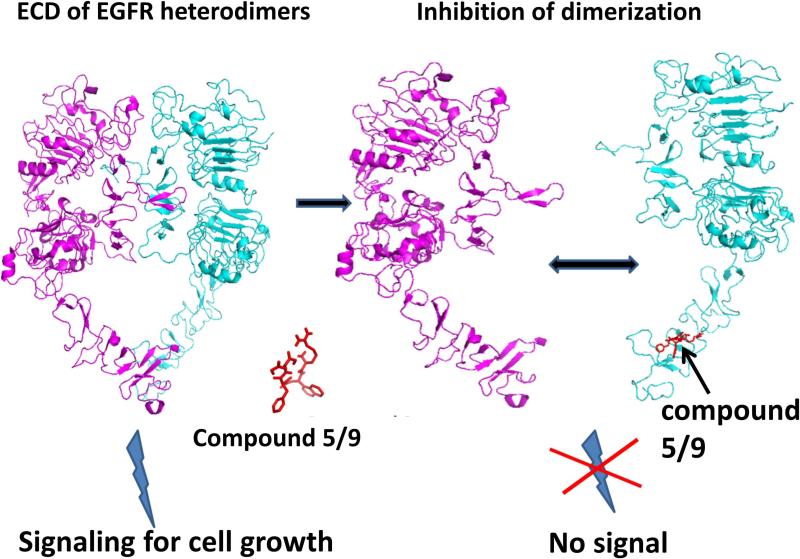
Human epidermal growth factor receptor-2 (HER2/ErbB2) is overexpressed in 25–30% of human breast cancer. The overexpression of HER2 is associated with more aggressive tumors and a poor prognosis (1-4). Furthermore, HER2 overexpression is found in both primary tumor and metastatic sites, suggesting that HER2-targeted therapy is useful in different stages of cancer (5). HER2 is intrinsically active, and is known to undergo heterodimerization with other EGFR receptors such as EGFR (HER1) and HER3. Although, in general, EGFRs undergo conformational change upon binding to EGF ligand, leading to dimerization, in HER2-overexpressed breast cancer the overexpression of receptors drives the heterodimerization in the absence of ligand. The extracellular region (ECD) of EGFRs has four domains (I to IV); among these, II and IV seem to be involved in stabilizing the dimers and making interfacial contact (6, 7). The crystal structure of homodimers of EGFR has been reported, and the protein-protein interaction (PPI) surface of the ECD of EGFRs is defined using this structure as a template (8, 9). The PPI of the ECD of EGFRs results in signaling through the transmembrane domain to the cytoplasmic tail, ultimately leading to transphosphorylation of the kinase domain. The exact details of how the signal is passed from the extracellular domain to the kinase domain are not yet clear. However, it is known that blocking the PPI of the ECD of EGFRs leads to blocking of signaling for the kinase domain (Figure 1) and, hence, blocking the PPI of the ECD of EGFR is therapeutically useful (10).
Figure 1.
Proposed mechanism of inhibition of EGFR heterodimerization by peptidomimetic compounds 5/9.
The current approach to treating HER2-overexpressed breast cancer is the use of monoclonal antibodies or a combination of antibodies with traditional chemotherapeutic agents or with kinase inhibitors (10-14). The antibody trastuzumab is known to bind to domain IV of a HER2 region that is not involved in receptor dimerization. The exact mechanism of how trastuzumab works is not known, and different possible mechanisms have been proposed (15, 16). Another antibody, pertuzumab, that binds to domain II of HER2 and inhibits HER2:EGFR interaction is available for cancer therapy (17). Although antibody therapy has been successful in treating HER2-positive breast cancer, there are limitations because of the size of the molecule, its shelf life, cardiotoxicity, and immunogenicity (18). Thus, there is a need for development of novel molecules that are not immunogenic and do not exhibit cardiotoxicity. Our approach is to target clinically validated HER2 domain IV with peptidomimetics and inhibit the protein-protein interactions of EGFRs (Figure 1). Unlike antibodies, peptidomimetics have advantages in terms of stability, modification, and molecular size (19-21). In our earlier reports we have described the design, synthesis, and structure-activity relationships of peptidomimetics that bind to HER2 domain IV, inhibit protein-protein interactions, and show antiproliferative activity in HER2-overexpressed breast cancer cell lines (22-26). The molecules we have developed are novel in the sense that they inhibit HER2 heterodimerization and affect the phosphorylation of the kinase domain of HER2. In this work, we have extended our studies on peptidomimetics compounds 5 and 9 (Figure 2), to show that these molecules inhibit PPI of HER2:HER3. These two compounds were chosen based on their potent antiproliferative activity (nanomolar IC50 values) specifically for HER2-overexpressing cancer cell lines. In an earlier report we have described the protein-protein interaction inhibition ability of compound 5 (24, 26). We have also evaluated these compounds for antitumor activity in a xenograft model of breast cancer. The results suggested that compounds 5 and 9 could suppress tumor growth in a xenograft mouse model. In addition, we have shown that these compounds inhibit PPI of HER2:HER3 and phosphorylation of HER2 better than control in tissue samples derived from in vivo studies. The stability of the compound was also investigated in mouse serum. The results indicated that compound 9 was detectable in mouse serum for up to 24 h, whereas compound 5 was detectable up to 48 h. These results suggest that peptidomimetics that inhibit PPI of EGFR:HER2 and HER2:HER3 could be useful therapeutic agents for breast cancer treatment.
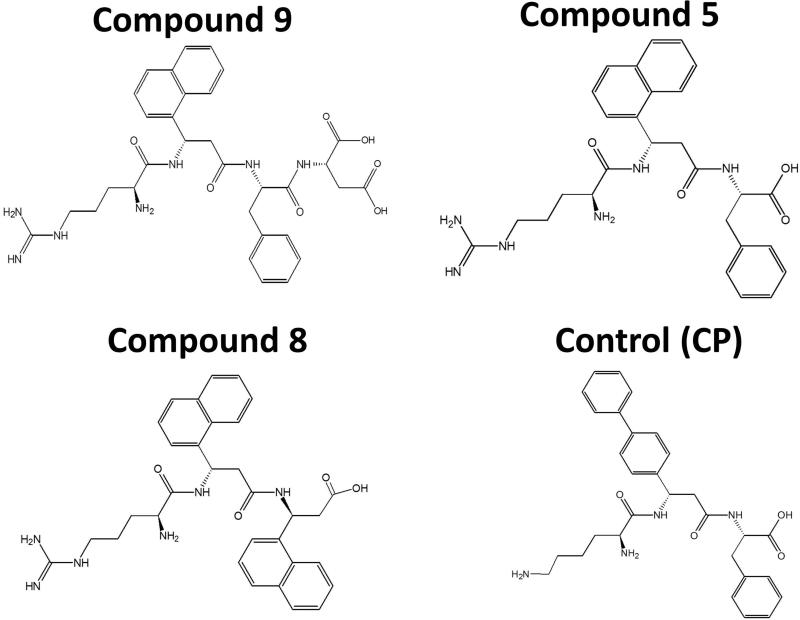
Figure 2.
Structures of compounds 9, 5, 8 and control (CP).
Materials and Methods
Materials
Compounds 5, 8, 9 and control were synthesized in our laboratory or obtained from custom synthesis (23, 25, 26). Lapatinib was from Selleckchem (Houston, TX). Cancer cell lines BT474, SKBR-3, Calu-3, MCF-7, SKOV-3, normal cell line MCF10A, and the media for cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Peptides were custom synthesized at LSU Agriculture Center, Biotechnology Laboratory (Baton Rouge, LA). Enzyme fragment complementation assay kit (PathHunter®) was from DiscoveRx Corp. (Fremont, CA) and PLA kit from Olink Bioscience (Uppsala, Sweden). Antibodies for immunoblot analysis were from Abcam, Inc. (Cambridge, MA) and Santa Cruz Biotechnology, Inc. (Dallas, TX). Novex® 4–20% tris-glycine gels and cell lysis buffer were obtained from Life Technologies (Grand Island, NY). Estrogen pellets used for in vivo studies were obtained from Innovative Research of America (Sarasota, FL), FITC-HER2 antibody for flow cytometry analysis was purchased from Abcam, Inc. (Cambridge, MA). CellTiter-Glo® reagent and TUNEL assay kit were from Promega (Madison, WI). Mouse serum was procured from Sigma-Aldrich (St. Louis, MO).
Cell Titer-Glo assay
Cell Titer-Glo® Luminescent assay (27) was performed to determine antiproliferative activity of compounds in the presence and absence of neuregulin (DiscoveRx Corp., Fremont, CA). The cells were coated in a 96-well plate and incubated overnight at 37°C and 5% CO2. Increasing concentrations of compounds made in serum-free medium were added to the wells with or without 0.3 μM neuregulin in triplicate. Negative and positive controls were cells treated with 1% sodium dodecyl sulfate (SDS) and 1% dimethyl sulfoxide (DMSO), respectively. After incubation for 72 h at 37°C and 5% CO2, CellTitre-Glo® detection reagent was added and luminescence readings were obtained from a plate reader. From the cell viability calculated, Prism® (GraphPad software, La Jolla, CA) was employed to develop a dose-response curve and IC50 values were determined.
Enzyme fragment complementation assay
U2OS cells provided with the PathHunter® assay kit were seeded in a 96-well plate at a density of 1 × 104 cells per well. After 24 h of incubation, compound 9 at various concentrations in the presence of HER3 ligand neuregulin (0.3 μM) was added to the cells and incubated for 3–4 h. Lapatinib and control compound (CP) (Figure 2) were used as positive and negative controls, respectively. Cells were washed, the detection reagent provided in the kit was added, and the luminescence was read using a microplate reader from Biotek (Winooski, VT). A concentration versus luminescence graph was plotted using Graphpad Prism. Relative intensity of luminescence compared to untreated cells corresponds to the amount of heterodimerization of HER2:HER3. The percentage of luminescence was calculated for the final presentation.
Proximity ligation assay (PLA)
PLA was performed using SKBR-3 cells as described previously (25). Briefly, 104 cells/well were incubated in an 8-well slide for 24 h at 37°C and 5% CO2. Compound 9 at 0.4 and 0.8 μM in serum-free medium was added to the wells and incubated for 36 h. The cells were fixed with cold methanol and blocked with 200 μL of 5% bovine serum albumin (BSA) for 1 h in a humidity chamber. Primary antibodies diluted in blocking solution were added and the slide was incubated at 4°C in a humidity chamber overnight. Then, cells were incubated with PLA probes at 1:5 dilution prepared in 2% BSA. Incubation with ligation mixture followed by amplification solution gives red florescence. The slide was counterstained with 4',6-diamidino-2-phenylindole (DAPI), and images at 40× were obtained using a Nikon Eclipse Ti-S (Melville, NY) or a confocal microscope (LSM 5 PASCAL).
Western blot analysis using SKBR-3, Calu-3, and SKOV3 cells
SKBR-3, Calu-3, and SKOV3 cells were treated with compounds (1 μM). Treatment with lapatinib (positive control at 0.07 μM) or control compound (negative control) was also performed. After 36 h, cell lysate was prepared using cell lysis buffer containing protease inhibitor and phosphatase inhibitor. Protein concentration was obtained using Bradford protein assay, and 40 μg of protein from each sample was loaded on Novex® 4–20% tris-glycine gels. Nitrocellulose membrane (GE Healthcare, Buckinghamshire, UK) was used to transfer the gels. After blocking with 2% BSA in TBST (Tris-buffered saline with 0.1% Tween 20), the membrane was incubated with primary and secondary antibodies diluted in blocking solution. Primary antibodies against total HER2 (T-HER2) and phosphorylated HER2 (p-HER2) were used at 1:300 dilution. Unbound antibodies were washed away with wash buffer and the membrane was incubated with peroxide substrate and enhancer solutions from a Super Signal-enhanced chemiluminescence kit (Pierce, Rockford, IL). A C-Digit Blot Scanner (LI-COR Biotechnology, Lincoln, NE) was employed to expose the membrane and obtain images. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control.
TUNEL assay
Lab-Tek® slides were seeded with SKBR-3 cells at 104 cells/well and incubated overnight at 37°C and 5% CO2. Compound 9 was added to the wells at 10 μM concentration and incubated for 48 h at 37°C. Cells were fixed using 10% buffered formalin and permeabilized by adding 0.2% Triton X-100 in PBS at room temperature for 5 min. 100 μL of rTdT reaction mixture containing biotinylated nucleotide was added to each well and incubated for 60 min at 37°C in a humidity chamber. The SSC solution (20× provided with the kit) diluted to 2× was added to the slide to terminate the reaction. The unincorporated biotinylated nucleotides on the slide were washed off three times with PBS. The blockade of endogenous peroxidases in the cells was done using 0.3% hydrogen peroxide in PBS at room temperature. After two PBS washes, diluted streptavidin HRP solution (1:500 in PBS) was added to each well and incubated for 30 min at room temperature. Diaminobenzidine (DAB) components of the kit were combined as specified and added to the slide at 100 μL/well. After drying, DAPI was used to counterstain the slide for nuclei, and images were obtained using a Nikon Eclipse TS100. The cells that had undergone apoptosis were counted and quantified.
HER2 expression monitored by flow cytometry
BT-474 cells were treated with compounds 5 or 9 at 5 μM concentration as well as lapatinib at 0.07 μM, and were incubated for 48 h at 37°C. After trypsinization, the cells were washed twice with PBS and resuspended at a density of 1 × 106 cells/100 μL PBS in Eppendorf tubes. FITC-labeled anti-HER2 was added at 5 μg/mL and incubated for 90 min. Cells were washed and spun down at 400 × g for 10 min and resuspended in 2 mL PBS. The solution was then transferred into flow cytometry tubes on ice and analyzed using a BD FACS flow cytometer. The shift in the number of cells with or without FITC was observed, and the effect on expression of HER2 after treating with compounds was evaluated compared to that of cells without any treatment.
Stability of compound in mouse serum
100 μL of stock solutions (6.66 mg/mL) of compounds 5 or 9 was incubated with 900 μL of mouse serum (Sigma-Aldrich). A sample of 100 μL was collected for each time point and 500 μL of chilled acetonitrile was added to each to precipitate the serum proteins (28). This sample was vortexed and incubated on ice, followed by centrifugation at 5000 rpm for 10 min. The supernatant, which contained compound 5 or 9 was purified by passing through a SEP-PAK C18 column. The samples were injected to the mass spectrometer along with a freshly prepared solution of compound 8 with known concentration. Compound 8, an analog of compounds 5 and 9 (Figure 2), was used as an internal standard against which the sample peaks were compared to obtain relative intensities. Compound 8 has a molecular weight of 569 (23), which resembles that of the sample under investigation; hence, it was logical to use it as an internal standard.
In vivo studies
All animals were handled in strict accordance with good animal practice as defined by NIH guidelines, and all animal work was approved by the IACUC, University of Louisiana at Monroe (ULM). Athymic nude mice (Foxn1nu/Foxn1+, female, 4–5 weeks) were purchased from Harlan Laboratories (Indianapolis, IN) and maintained in the ULM College of Pharmacy vivarium. Estrogen (17β-estradiol) pellets (0.72 mg, 90-day release) were implanted subcutaneously in all the mice under anesthesia. BT-474 cells suspended in serum-free RPMI-1640 medium were injected into the second left mammary gland, orthotopically (nearly 2 million cells per mouse) as described by our collaborator in an earlier study (29). Animals were monitored daily, and body weight was measured. Fourteen days following the injections, visible tumors were developed. Tumor size was assessed twice weekly by measuring tumor diameter with calipers; tumor volume was calculated using the formula V = [(length × width2)/2] (30). Once the tumors reached 3 mm in diameter, mice were randomly divided into four groups of six and labeled as negative control, positive control, treatment group 1, and treatment group 2. The treatment period for each group was 19 days. Treatment groups received compounds 5 or 9 dissolved in saline. The intratumor injections of peptidomimetics were given at a dose of 4 mg/kg twice a week. This dose was based on our pilot studies as well as studies reported in the literature (31, 32). A pilot study was conducted using small number of animals (N=3) and treating the xenograft tumor in mice with compound 5 at three different doses. Considering the fact that the compounds 5 and 9 are peptidomimetics and have N-and C-termini free, and are susceptible to enzymatic degradation, a dosing of 4mg/kg was proposed for the in vivo studies. Control groups received intratumor saline injections twice weekly, and lapatinib was administered at 10 mg/kg once a week by intraperitonial injection (33, 34). Mice were sacrificed when tumors reached 10 mm diameter in size. The marker to end the experiments was based on tumor doubling time rather than tumor volume. Since tumor doubling time was 4 times in 19 days, experiments were stopped at the 19th day (35). Tumor growth curves for all the groups were constructed and compared using statistical analysis. Three tumors per group were removed surgically and maintained at –80°C for immunoblot analysis while the remaining three tumors were fixed and maintained in paraffin blocks. Microsections of tumors (5 μm) were performed; microsections were stained with haemotoxylin and eosin (H & E) and analyzed for necrosis by a pathologist. All data are presented as the mean ± SE. Statistical differences were evaluated by Mann Whitney U test as well as one-way ANOVA analysis of data from different groups, and the criterion for statistical significance was p < 0.05.
Immunoblot analysis of tumors
Frozen tumors were homogenized in lysis buffer, and the tumor lysates were collected. Protein concentration was analyzed using a Bradford protein assay kit. Immunoblot analysis was performed by loading equal amounts of protein from the tumor lysates of three groups in a manner similar to the immonoblot analysis performed using cell lysates of SKBR-3 cells. Primary and secondary antibodies were used to evaluate the levels of total HER2 and phosphorylated HER2.
Immunohistochemistry studies on tumor tissue sections
Tumor sections were deparaffinized using xylene and rehydrated with decreasing concentrations of ethanol. Antigen retrieval on the microsections of the breast tumors was done in a steaming sodium citrate buffer (10 mM, 0.05% Tween-20, pH 6.0) for 5 min. The tumor sections were then used to analyze inhibition of HER2:HER3 dimerization using proximity ligation assay as described above.
Results
Antiproliferative activity
We reported the synthesis, analytical characterization, and antiproliferative activity of compounds 5 and 9 (Figure 2) in BT-474 cell lines in our earlier studies (22-24, 26). Here we have extended the studies to other cancer cell lines such as SKOV-3, an ovarian cancer cell line, Calu-3, a lung cancer cell line that overexpresses HER2, and MCF10A, a normal breast cell line. In addition, we wanted to investigate whether these antiproliferative activities will be affected by the presence of HER2:HER3 dimerization inducer neuregulin, since we have used neuregulin in some of the dimerization studies. The results indicated that compound 9 exhibited antiproliferative activity with IC50 values in the nanomolar range in HER2-overxpressed cancer cell lines such as BT-474, SKBR-3, SKOV-3, and Calu-3 (Supporting Information Table S1). However, in MCF-7 and MCF10A cell lines the antiproliferative activity was in the higher micromolar range. In the presence of neuregulin, the antiproliferative activity was not significantly changed.
Inhibition of PPI of HER2:HER3 by compounds 5 and 9
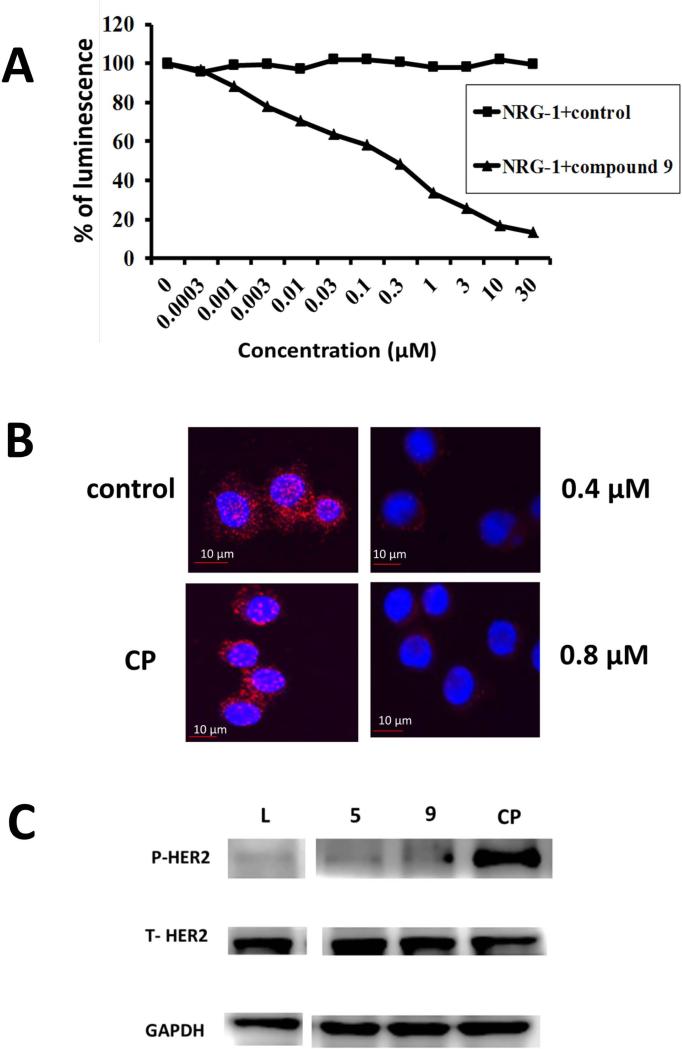
Our peptidomimetics were designed to inhibit protein-protein interactions of EGFRs, in particular, the interaction of HER2 with HER3. Inhibition of extracellular domain HER2 heterodimerization inhibits the intracellular activation of signaling cascades, leading to controlled cell proliferation. In our previous studies, we have shown that compounds 5 and 9 exhibit antiproliferative activities in the nanomolar range and compound 5 inhibited HER2 heterodimerization in vitro (23, 24). The ability of compound 9 to inhibit the PPI of HER2:HER3 was evaluated using an enzyme fragment complementation assay (36). Dimerization of HER2:HER3 was induced by NRG1, a ligand for HER3. Upon treatment with compound 9 in the presence of NRG1, a dose-dependent decrease in the luminescence of U2OS cells was detected in a β-galactosidase-based assay, suggesting that HER2:HER3 dimerization was inhibited (Figure 3A). The dose-response curve obtained was compared to that of a control, and the results clearly indicated that HER2:HER3 heterodimerization induced by NRG1 in U2OS cell lines was inhibited by compound 9. The IC50 value for inhibition by compound 9 was 0.3 μM. To further verify that compound 9 inhibits HER2:HER3 heterodimerization, a PLA was performed (37). In PLA, if two proteins are in proximity (≤ 16 Å), they can be detected by antibodies tagged with a DNA fragment. SKBR-3 cells that overexpress HER2 were incubated with specifies-specific antibodies for HER2 and HER3 and PLA reagents, and detection was done with a red fluorescent probe using a fluorescence microscope. There were number of red dots (Figure 3B), suggesting HER2:HER3 heterodimerization. When a control compound (Figure 3B) was incubated with SKBR-3 cells and PLA detection was done, red dots were still present, suggesting that the control compound did not inhibit HER2:HER3 heterodimerization. On the other hand, when compound 9 was incubated at two different concentrations (0.4 and 0.8 μM), there was a significant decrease in the number of red dots, clearly indicating the inhibition of heterodimerization of HER2:HER3.
Figure 3.
A) Inhibition of heterodimerization of HER2:HER3 in HER2, HER3 transfected U2OS cells by compound 9 at different concentrations using enzyme fragment complementation assay (DiscoveRx). Dose-response curve for inhibition of HER2:HER3 heterodimerization by 9 in the presence of 0.3 μM NRG1 (triangles). Control compound (CP) in the presence of 0.3 μM NRG-1 (filled squares).
B) HER2:HER3 heterodimerization and its inhibition by compound 9 observed by PLA. Concentration-dependent inhibition of HER2:HER3 heterodimers was observed. SKBR-3 cells showing only HER2:HER3 heterodimerization as red spots. A control compound (CP) did not inhibit HER2:HER3 heterodimerization. At a sub-optimum dose of compound 9 (0.4 μM), HER2:HER3 heterodimerization was inhibited to a lesser extent. At optimum dose of compound 9 (0.8 μM), HER2:HER3 heterodimerization was significantly inhibited.
C) Effect of compound 9 on phosphorylation of HER2 kinase domain. SKBR-3 cells that overexpress HER2 protein upon treatment with compound 9 (1 μM). Compound 5 and lapatinib (L) (0.07 μM) and control compound (CP) are shown for comparison. The visualization of GAPDH was used to ensure equal sample loading in each lane. Phosphorylation was detected using p-HER2 antibody. Total HER2 protein is also shown.
Effect on phosphorylation of kinase domain
It is well known that heterodimerization of HER2:HER3 leads to phosphorylation of the kinase domain of HER2 (6) and leads to downstream signaling. To evaluate whether compound 9 inhibits the phosphorylation of the kinase domain by inhibiting ECD of HER2:HER3, immunoblot analysis was performed. SKBR-3 cells that overexpress HER2 protein were treated with compound 9, and phosphorylation was detected using anti-phospho HER2. Compared to the controls, compound 9 inhibited the phosphorylation significantly (Figure 3C). Similar results were obtained when the compounds were incubated with lung cancer cell lines (Calu-3) that overexpress HER2 protein. However, in the case of ovarian cancer cell lines that overexpress HER2, compound 9 showed inhibition of phosphorylation while compound 5 did not (Supporting Information Figure S1 A&B).
Effect of compounds on the expression level of HER2 on cell surface and apoptosis
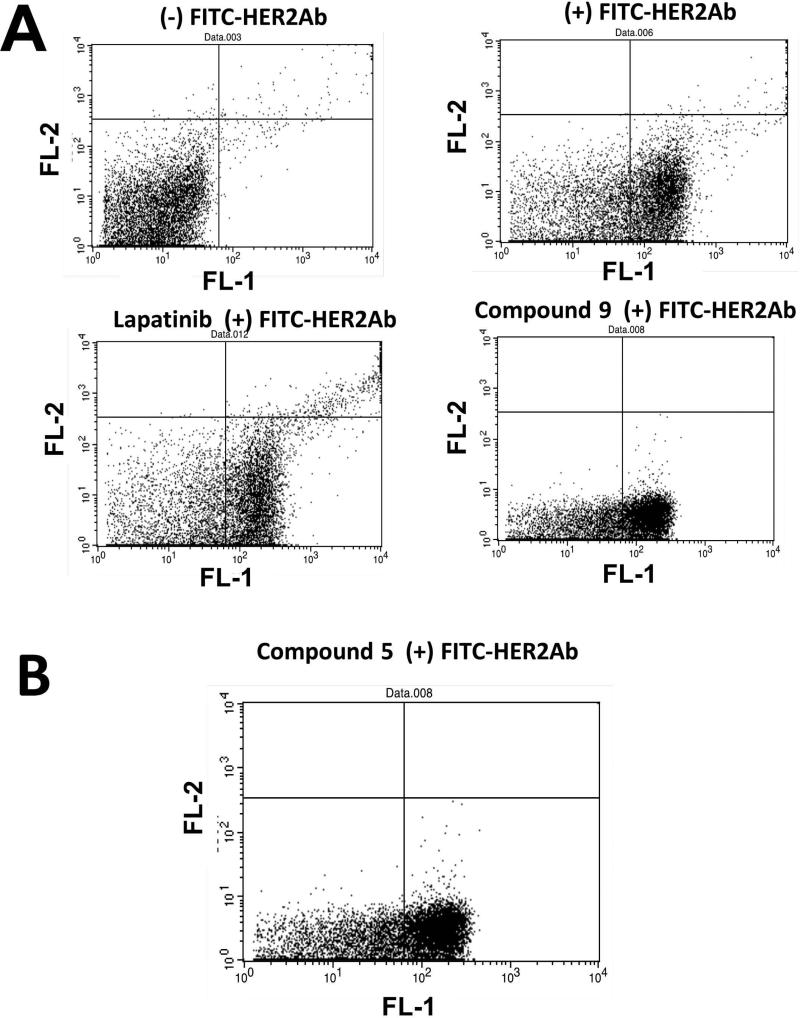
It is known that trastuzumab, which binds to domain IV of HER2, downregulates HER2 expression on the cell surface (38). Trastuzumab is known to bind to domain IV of HER2 (39). Since our compounds are proposed to bind to domain IV of HER2, we wanted to investigate whether compounds 5 and 9 would have any effect on HER2 expression on the cell surface. A fluorescence-labeled antibody to HER2 ECD was used to monitor the HER2 expression on the surface. Flow cytometry data suggested that there was no shift of data to the left quadrant in the presence of compounds at different intervals of time. These results suggest that the total HER2 protein levels in treatment samples were not affected by compounds 5 and 9 (Figures 4A and B). Lapatinib, a well-known kinase inhibitor, was also used for comparison. Since the surface expression of HER2 was not affected, we wanted to evaluate whether the effect of compound 9 is simply inhibition of heterodimerization and phosphorylation or does it lead to any other downstream signaling effect such as apoptosis.
Figure 4.
A) Effect of compounds on HER2 expression studied by flow cytometry. BT-474 cells without any FITC-labeled antibody to HER2, BT474 cells with FITC-antiHER2, cells treated with lapatinib and labeled with FITC-anti-HER2, cells treated with compound 9 with FITC-anti-HER2. B) Cell treated with compound 5.
To evaluate the effect of compound 9 on apoptosis, compound 9 was incubated with SKBR-3 cells for 36 h, after which the cells were evaluated for death by apoptosis. Compound 9 was able to induce apoptosis in cancer cell line SKBR-3 compared to positive and negative controls (Supporting Information Figures S2 & S3). It is known that lapatinib is a dual kinase inhibitor of EGFR and HER2 and does not bind to the extracellular domain. However, we used lapatinib as a control for enzyme fragment complementation assays and in vivo studies.
Effect of compounds on tumor growth in a xenograft model of breast cancer
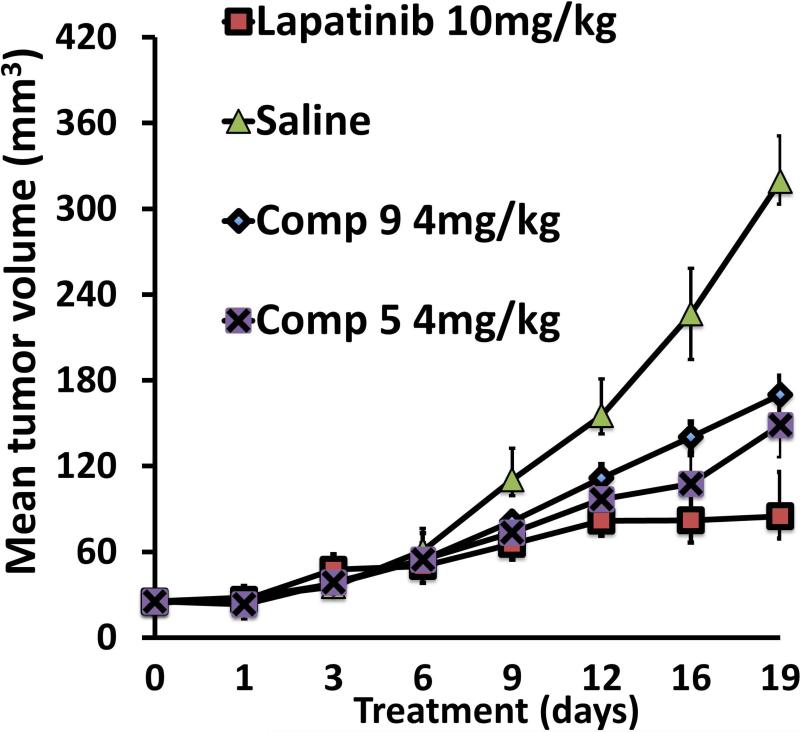
To evaluate whether the designed peptidomimetic compounds 5 and 9 could inhibit breast tumor growth, xenograft breast tumors induced by injecting BT-474 cells were treated with compounds 5 or 9 at 4 mg/kg twice a week via intratumor injection just below the breasts. Based on our hypothesis, compounds 5 and 9 were expected to inhibit HER2 heterodimerization in vivo, thus leading to delayed tumor growth. During the course of the experiment, the tumor size in the control xenograft group without any treatment continued to increase, reaching a diameter of 10 mm in 19 days. On the other hand, compounds 5 and 9 produced a delay in tumor growth, which was significant compared to that of control (Figure 5). Using one-way ANOVA analysis, the statistical difference between control and treatment groups was found to have a significance of p=0.03 (p<0.05). By using Mann Whitney U test, the Z-score is calculated to be 2.3 (U=1) and the treatment group was significantly different from vehicle control with a p-value less than 0.05.
Figure 5.
Antitumorigenic activity of compounds 9 (diamonds) and 5 (crosses). Compounds 5 and 9 (4 mg/Kg) delayed the tumor growth in athymic nude mice significantly compared to the control group (filled triangles) without any treatment (standard error plotted). For comparison, tumor suppression by lapatinib is also shown (squares). It is very clear that there is a significant difference between the control and treatment groups.
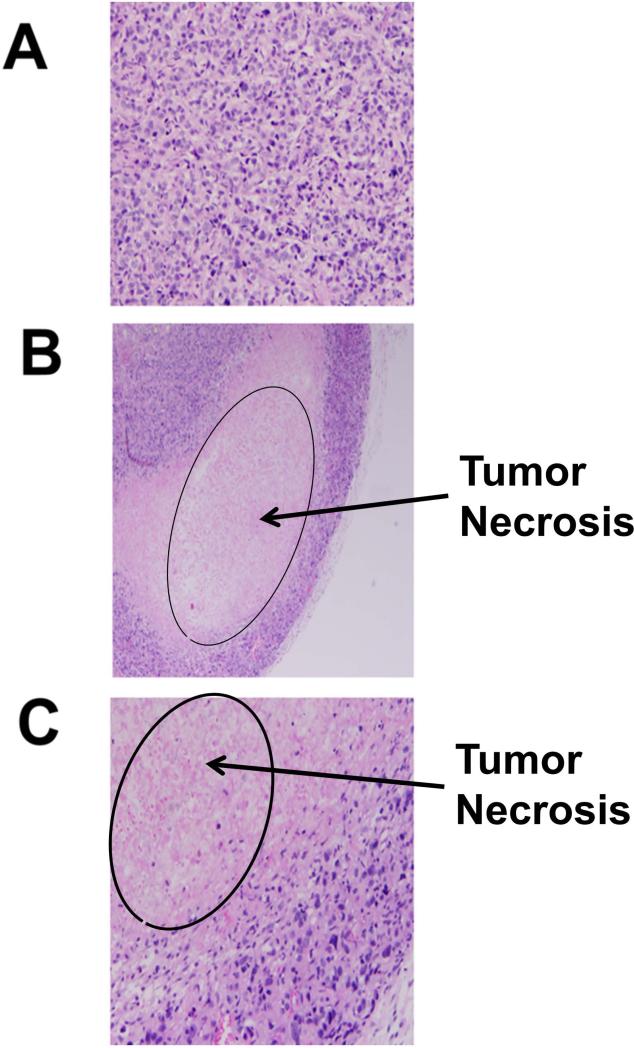
Tumors were removed at the end of the experiment period, fixed in formalin, and cut into 5 μ slices for slides. H&E staining of the tumor microsections revealed tumor necrosis caused by compounds 5 and 9 (Figures 6A–C).
Figure 6.
H and E staining of tumor sections of mouse with and without compound treatments. A) Tumor tissue without any compound treatment, B) compound 5 treatment, C) compound 9 treatment. Sections from treated tumors (B, C) showed areas of necrosis that presented as eosinophilic debris with no cellular structures and nuclei.
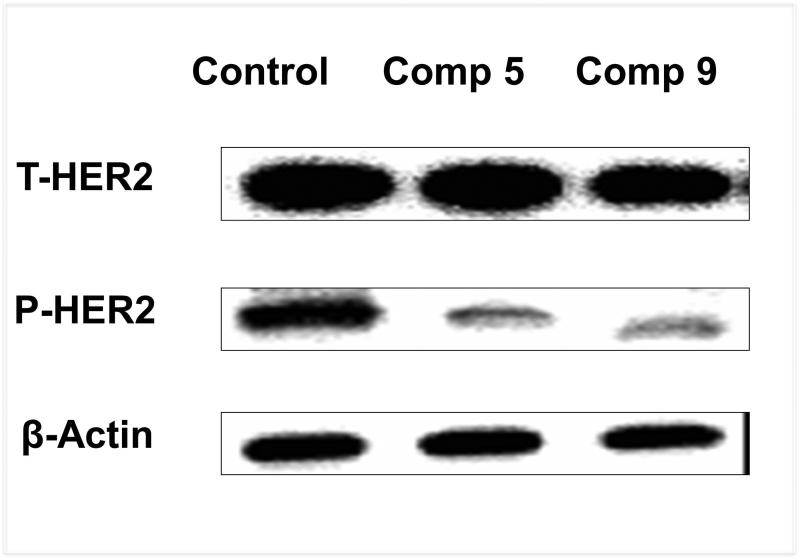
In addition, previous findings suggested that inhibition of HER2 heterodimerization with either EGFR or HER3 should inhibit intracellular transphosphorylation of HER2. To evaluate the effect of phosphorylation, proteins were extracted from tumor sections and phosphorylation of HER2 protein was analyzed. Results from Western blot analysis of the tumor samples showed a significant inhibition of HER2 phosphorylation by peptidomimetic compounds 5 and 9 (Figure 7).
Figure 7.
Western blot analysis of the HER2 phosphorylation level of treatment and control groups. Compounds 5 and 9 significantly inhibited HER2 phosphorylation compared to the untreated control group.
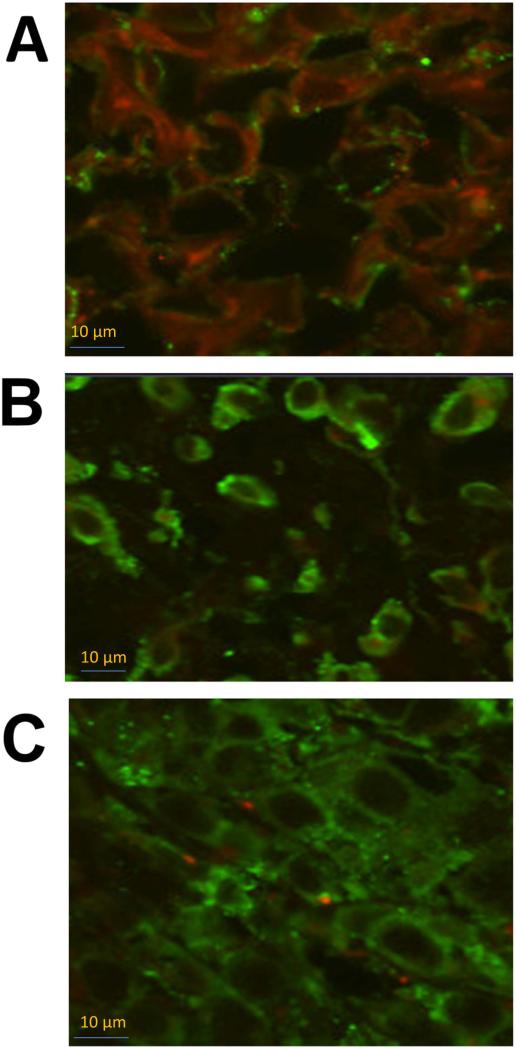
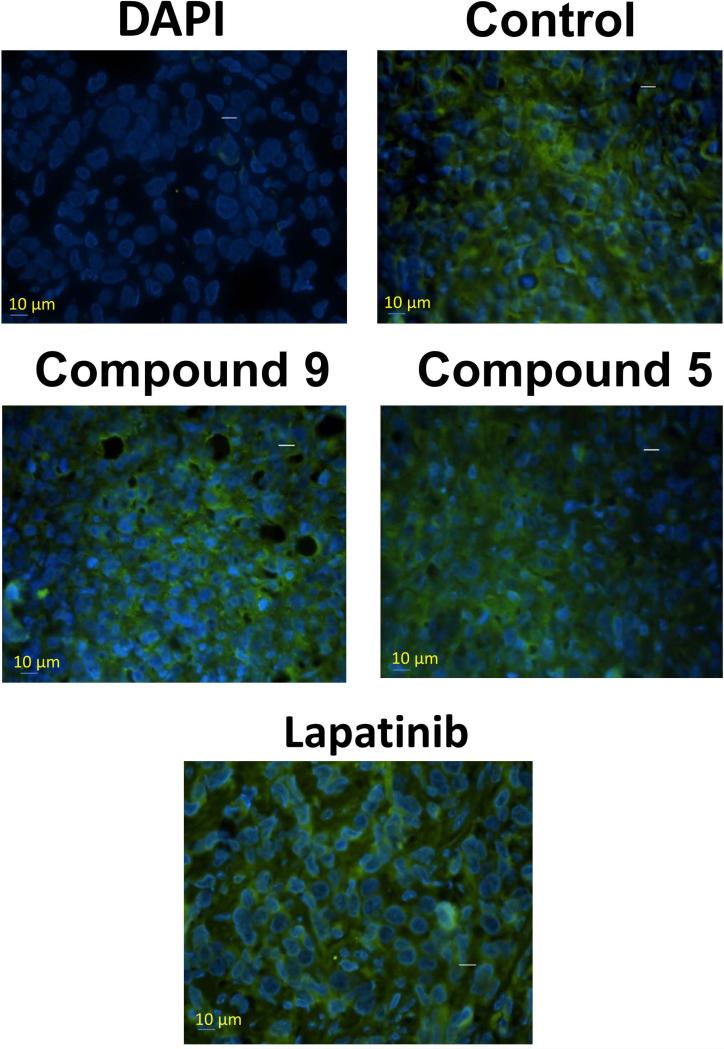
Fixed tumor sections were evaluated by PLA for heterodimerization of HER2:HER3 and its inhibition by compounds 5 and 9. Slides from PLA were viewed using a confocal microscope for detection of HER2:HER3 dimerization. Samples of untreated tumor sections of mice exhibited red color, suggesting heterodimerization of proteins. Samples from tumors treated with compounds 5 and 9 exhibited markedly diminished signals from dimerization as indicated by a significant decrease in red color compared to untreated samples, indicating a decrease in the heterodimerization of HER2:HER3 in vivo (Figure 8). Tumor sections were also evaluated for HER2 expression using FITC-labeled HER2 antibody. Figure 9 shows the expression of HER2 in tumor sections treated with compounds 5 and 9 and lapatinib, as well as tumor sections from the control group. From this figure it is clear that treatment with compound 9 did not decrease the HER2 expression on the cell surface in tumors.
Figure 8.
Confocal images of PLA on breast tumor sections. A) PLA on control mouse without any treatment. Red color fluorescence indicate HER2:HER3 heterodimerization. PLA on mouse treated with 4 mg/kg of B) compounds 9 and C) 5. Compounds specifically inhibited HER2:HER3 heterodimerization significantly compared to the control without any treatment as indicated by the small number of red dots.
Figure 9.
HER2 expression in tumor sections of mice evaluated by fluorescently labeled anti-HER2 antibody (green fluorescence). Tissue section without any antibody treatment and labeled with nuclear stain DAPI. Tissue section without any compound treatment, compounds 9, 5, and lapatinib treatment are shown. Notice the HER2 expression in all the tumors suggesting that compounds do not affect the expression of HER2 protein.
Stability of compounds 5 and 9 in mouse serum
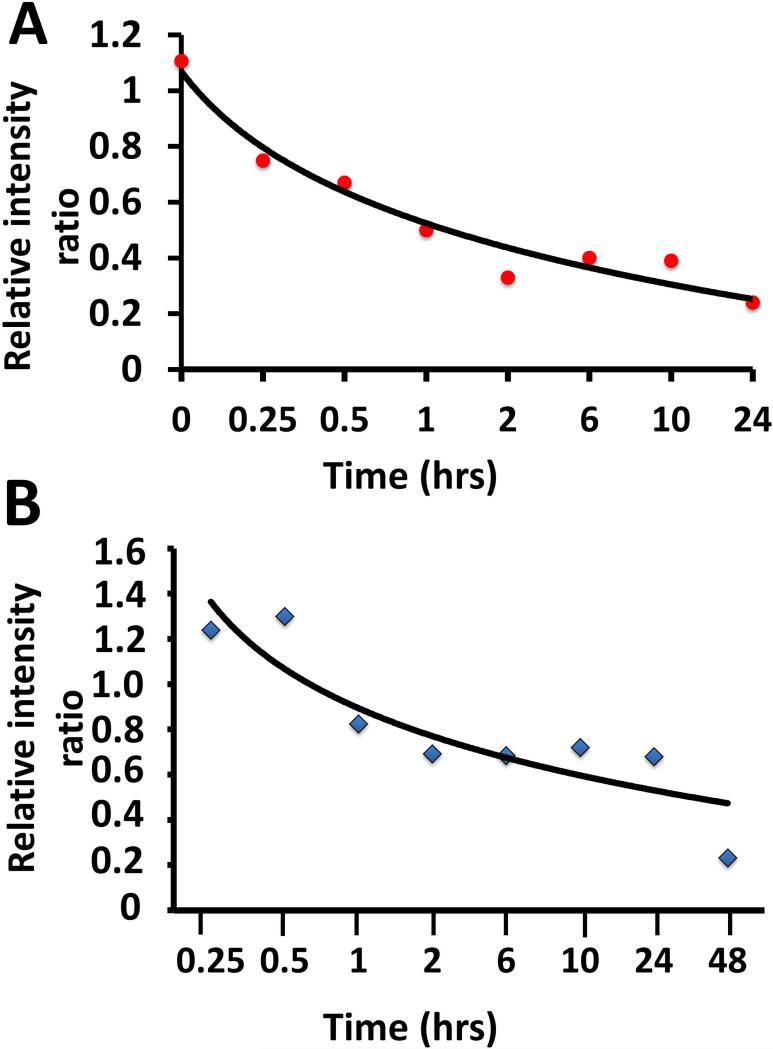
Compounds 5 and 9 are peptidomimetics with N- and C-termini susceptible to enzymatic degradation in vivo. To prevent enzymatic peptide degradation, several strategies are available (40-42). Before we proceed with intravenous administration of the compound in future studies, understanding the stability of the compound in serum is important. The stability of the compounds was evaluated by incubating in mouse serum. It is more relevant to perform this study in mouse serum as the in vivo effect of the compound was assessed in a mouse model. Compound 8 (Figure 2) was used as control compound to calculate relative intensity using mass spectrometry. The serum stability study showed that the compound is detectable in serum even after 24 h (Figure 10). The half-life obtained from the relative intensities is about 1 h for compound 9 and 3 h for compound 5.
Figure 10.
Stability of compounds 9 and 5 incubated in mouse serum and analyzed at different intervals of time. The amount of remaining intact peptide was detected by mass spectrometry using freshly prepared compound 8 an analog of compounds 9 and 5 as internal standard. A) compound 9, B) compound 5. Compound 5 was more stable compared to compound 9 in serum as seen from the graph.
Discussion
HER2 protein is deregulated in ~20% of primary breast carcinomas and is associated with a poor survival rate (10). HER2 is a preferred dimerization partner for other EGFRs for signaling since it is known to be in an open conformation state (12). Antibodies targeted to the HER2 extracellular domain inhibit the signaling for cancer cell growth have been therapeutically useful (43, 44). Here, we used peptidomimetics 5 and 9 that target the HER2 extracellular domain, in particular domain IV, to inhibit the interaction between HER2 and other EGFRs. In HER2-positive breast cancer, HER2:HER3 heterodimerization seems to be important compared to that of other EGFR heterodimers (12). Using PLA and PathHunter assays, we have shown that compound 9 inhibits HER2:HER3 dimerization (Figure 3). Thus, the designed compounds inhibit protein-protein interactions of HER2 and HER3 extracellular domains. Flow cytometry analysis indicated that the expression of HER2 in cells treated with these compounds was unaltered. Tumor sections from in vivo samples were analyzed for HER2 expression. Tumor sections that were untreated or treated with these compounds exhibited HER2 expression, suggesting that there was no decrease in HER2 expression following this treatment (Figures 9). This ruled out the possibility that the activity of our compounds was due to a change in total protein levels. Moreover, the compounds inhibited heterodimerization of HER2 with other EGFRs and, thereby, phosphorylation of HER2, which is evident from the immunoblot analysis of SKBR3 cells (Figure 3C). This clearly suggests that, although the compounds bind to the HER2 extracellular domain, in particular to domain IV, the mechanism of action of these compounds is different than that of the antibody trastuzumab.
In the xenograft model of breast cancer, compounds 5 and 9 clearly produced a significant reduction in tumor growth compared to the negative control and a positive control, lapatinib. Consistent with the in vitro results, immunoblots obtained with in vivo samples showed a decrease in phosphorylated HER2 levels in the compound treatment groups compared to control (Figure 7). PLA carried out on tumor sections clearly suggested that compounds 5 and 9 inhibit the HER2:HER3 heterodimerization under in vivo conditions. Taken together, these studies suggest that inhibition of HER2 heterodimerization with other EGFRs by compounds 5 and 9 hinders intracellular trans-phosphorylation, leading to controlled cell proliferation and tumor growth.
The involvement of extracellular domains of EGFR proteins in dimerization and signaling has been studied. However, at present, no crystal structures of heterodimers have been reported. Domains II and IV of ECD of EGFR and HER2 are known to be important in PPI (45, 46). Domain II interactions have been studied in EGFR (HER1) and are known to be involved in heterodimerization with other EGFRs. The domain IV C-terminal is near the transmembrane domain, and the details of its participation in dimerization have not been elucidated. While blocking of domain IV by the antibody trastuzumab is known to be clinically significant in breast cancer (4), the exact mechanism by which the binding of antibodies and a small molecule designed to bind to the HER2 domain IV alters HER2-mediated signaling is not well understood. Mutation in domain IV of EGFR is known to impair phosphorylation (46). It is also known that alteration or truncation of domain IV changes the heteromeric signaling of EGFR (47). Domain IV is very flexible, and the nature of the interaction of domain IV in EGFR-HER2/HER3-HER2 proteins is dynamic (8). There are controversial reports about the involvement of the C-terminal portion of domain IV in the dimerization process (48-50). Although the kinase domains of EGFRs are targeted for therapeutic effects in cancer, they develop resistance because of mutation. Our approach provides a new way of targeting HER2-overexpressed cancer cells. In the present study, the compounds were administered directly near the tumor and, thus, the stability of these compounds in circulation is not known. However, the studies described here will provide information for design of targeted therapeutic agents using PPI inhibition strategy. The compounds we have designed are targeted to domain IV of HER2 to inhibit HER2:HER3 interaction. In HER2-positive breast cancer, HER2:HER3 heterodimerization has proven to be more significant than EGFR:HER2 heterodimerization and, hence, these compounds have significance in HER2-positive cancer therapy.
Conclusion
Based on the in vitro and in vivo data presented here, we can conclude that peptidomimetics can be targeted to domain IV of HER2 and that this can result in inhibition of dimerization, hindered phosphorylation and, ultimately, inhibition of signaling for cell growth. Overall, our findings suggest that our designed peptidomimetic compounds 5 and 9 are efficient in inhibiting extracellular domain heterodimerization of EGFR, in particular, HER2:HER3. Inhibition of ECD of HER2:HER3 resulted in inhibition of phosphorylation of the kinase domain of HER2, thereby abrogating the downstream signaling in breast cancer cells. Compounds 5 and 9 delayed tumor growth in a xenograft model of breast cancer; this serves as preliminary evidence for the activity of these compounds in vivo.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8P20GM103424. The authors would also like to thank Dr. Briski for providing the confocal microscopy facility.
Footnotes
Conflict of Interest
There is no conflict of interest with any of the authors of the manuscript.
Supplementary data
Supplementary data related to the article can be found in TableS1 & FigureS1-S4
References
- 1.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 2.Slamon DJ. Proto-oncogenes and human cancers. N Engl J Med. 1987;317:955–7. doi: 10.1056/NEJM198710083171509. [DOI] [PubMed] [Google Scholar]
- 3.Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13:663–73. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- 4.Chang JC. HER2 inhibition: from discovery to clinical practice. Clinical Cancer Res. 2007;13:1–3. doi: 10.1158/1078-0432.CCR-06-2405. [DOI] [PubMed] [Google Scholar]
- 5.Niehans GA, Singleton TP, Dykoski D, Kiang DT. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993;85:1230–5. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- 6.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol cell. 2003;12:541–52. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 8.Lu C, Mi LZ, Grey MJ, Zhu J, Graef E, Yokoyama S, Springer TA. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol Cell Biol. 2010;30:5432–43. doi: 10.1128/MCB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–87. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 10.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 11.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–7. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 13.Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug saf. 2013;36:413–26. doi: 10.1007/s40264-013-0050-x. [DOI] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–14. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 16.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–38. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Esserman LJ, DeMichele A. Accelerated Approval for Pertuzumab in the Neoadjuvant Setting: Winds of Change? Clin Cancer Res. 2014;20:3632–3636. doi: 10.1158/1078-0432.CCR-13-3131. [DOI] [PubMed] [Google Scholar]
- 18.Sathish JG, Sethu S, Bielsky MC, de Haan L, French NS, Govindappa K, Green J, Griffiths CE, Holgate S, Jones D, Kimber I, Moggs J, Naisbitt DJ, Pirmohamed M, Reichmann G, Sims J, Subramanyam M, Todd MD, Van Der Laan JW, Weaver RJ, Park BK. Challenges and approaches for the development of safer immunomodulatory biologics. Nat Rev Drug Discov. 2013;12:306–24. doi: 10.1038/nrd3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1:847–58. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 20.Hruby VJ, Cai M, Nyberg J, Muthu D. Approaches to the rational design of selective melanocortin receptor antagonists. Expert Opin Drug Discov. 2011;6:543–57. doi: 10.1517/17460441.2011.565743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschutz MK, Zauner W, Mattner F, Otava A, Buschle M, Bernkop-Schnurch A. Improvement of the enzymatic stability of a cytotoxic T-lymphocyte-epitope model peptide for its oral administration. Peptides. 2002;23:1727–33. doi: 10.1016/s0196-9781(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 22.Banappagari S, Ronald S, Satyanarayanajois SD. A conformationally constrained peptidomimetic binds to the extracellular region of HER2 protein. J Biomol Struct Dyn. 2010;28:289–308. doi: 10.1080/07391102.2010.10507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banappagari S, Ronald S, Satyanarayanajois SD. Structure-activity relationship of conformationally constrained peptidomimetics for antiproliferative activity in HER2-overexpressing breast cancer cell lines. MedChemComm. 2011;2:752–9. doi: 10.1039/C1MD00126D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banappagari S, Corti M, Pincus S, Satyanarayanajois S. Inhibition of protein-protein interaction of HER2-EGFR and HER2-HER3 by a rationally designed peptidomimetic. J Biomol Struct Dyn. 2012;30:594–606. doi: 10.1080/07391102.2012.687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanthala S, Gauthier T, Satyanarayanajois S. Structure-activity relationships of peptidomimetics that inhibit PPI of HER2-HER3. Biopolymers. 2013;101:693–702. doi: 10.1002/bip.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banappagari S, McCall A, Fontenot K, Vicente MG, Gujar A, Satyanarayanajois S. Design, synthesis and characterization of peptidomimetic conjugate of BODIPY targeting HER2 protein extracellular domain. Eur J Med Chem. 2013;65C:60–9. doi: 10.1016/j.ejmech.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolliday N. High-throughput assessment of Mammalian cell viability by determination of adenosine triphosphate levels. Curr Protoc Chem Biol. 2010;2:153–61. doi: 10.1002/9780470559277.ch100045. [DOI] [PubMed] [Google Scholar]
- 28.Majumdar S, Anderson ME, Xu CR, Yakovleva TV, Gu LC, Malefyt TR, Siahaan TJ. Methotrexate (MTX)-cIBR conjugate for targeting MTX to leukocytes: conjugate stability and in vivo efficacy in suppressing rheumatoid arthritis. J Pharm Sci. 2012;101:3275–91. doi: 10.1002/jps.23164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhinge KN, Gupta V, Hosain SB, Satyanarayanajois SD, Meyer SA, Blaylock B, Zhang QJ, Liu YY. The opposite effects of doxorubicin on bone marrow stem cells versus breast cancer stem cells depend on glucosylceramide synthase. Int J Biochem Cell Biol. 2012;44:1770–8. doi: 10.1016/j.biocel.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patwardhan GA, Zhang QJ, Yin D, Gupta V, Bao J, Senkal CE, Ogretmen B, Cabot MC, Shah GV, Sylvester PW, Jazwinski SM, Liu YY. A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PloS One. 2009;4:e6938. doi: 10.1371/journal.pone.0006938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao Y, Wang C, Cao B, Hirsch BM, Song J, Markowitz SD, Ewing RM, Sedwick D, Liu L, Zheng W, Wang Z. Gain of interaction with IRS1 by p110alpha-helical domain mutants is crucial for their oncogenic functions. Cancer cell. 2013;23:583–93. doi: 10.1016/j.ccr.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PT. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J Immunol. 2013;191:217–27. doi: 10.4049/jimmunol.1300231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–19. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 34.Formisano L, Nappi L, Rosa R, Marciano R, D'Amato C, D'Amato V, Damiano V, Raimondo L, Iommelli F, Scorziello A, Troncone G, Veneziani B, Parsons SJ, De Placido S, Bianco R. Epidermal growth factor-receptor activation modulates Src-dependent resistance to lapatinib in breast cancer models. Breast Cancer Res. 2014;16:R45. doi: 10.1186/bcr3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace J. Humane endpoints and cancer research. Ilar J. 2000;41:87–93. doi: 10.1093/ilar.41.2.87. [DOI] [PubMed] [Google Scholar]
- 36.Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–38. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 2002;20:473–7. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 38.Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–900. [PubMed] [Google Scholar]
- 39.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr., Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 40.Hess S, Ovadia O, Shalev DE, Senderovich H, Qadri B, Yehezkel T, Salitra Y, Sheynis T, Jelinek R, Gilon C, Hoffman A. Effect of structural and conformation modifications, including backbone cyclization, of hydrophilic hexapeptides on their intestinal permeability and enzymatic stability. J Med Chem. 2007;50:6201–11. doi: 10.1021/jm070836d. [DOI] [PubMed] [Google Scholar]
- 41.Hunter HN, Jing W, Schibli DJ, Trinh T, Park IY, Kim SC, Vogel HJ. The interactions of antimicrobial peptides derived from lysozyme with model membrane systems. Biochim Biophys Acta. 2005;1668:175–89. doi: 10.1016/j.bbamem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Tugyi R, Uray K, Ivan D, Fellinger E, Perkins A, Hudecz F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc Natl Acad Sci USA. 2005;102:413–8. doi: 10.1073/pnas.0407677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landgraf R. HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks. Breast Cancer Res. 2007;9:202. doi: 10.1186/bcr1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roskoski R., Jr The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2013;79C:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Berezov A, Chen J, Liu Q, Zhang HT, Greene MI, Murali R. Disabling receptor ensembles with rationally designed interface peptidomimetics. J Biol Chem. 2002;277:28330–9. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 46.Saxon ML, Lee DC. Mutagenesis reveals a role for epidermal growth factor receptor extracellular subdomain IV in ligand binding. J Biol Chem. 1999;274:28356–62. doi: 10.1074/jbc.274.40.28356. [DOI] [PubMed] [Google Scholar]
- 47.Kumagai T, Katsumata M, Hasegawa A, Furuuchi K, Funakoshi T, Kawase I, Greene MI. Role of extracellular subdomains of p185c-neu and the epidermal growth factor receptor in ligand-independent association and transactivation. Proc Natl Acad Sci USA. 2003;100:9220–5. doi: 10.1073/pnas.1633546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys. 2008;37:353–73. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan V, Lax I, Schlessinger J. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130:323–34. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 50.Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol. 2005;25:7734–42. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.