Abstract
Objective
Progranulin (PGRN) was previously isolated as an osteoarthritis (OA)-associated growth factor. Additionally, PGRN was found to play a therapeutic role in inflammatory arthritis mice models through antagonising tumour necrosis factor α (TNF-α). This study was aimed at investigating the role of PGRN in degradation of cartilage and progression of OA.
Methods
Progression of OA was analysed in both spontaneous and surgically induced OA models in wild type and PGRN-deficient mice. Cartilage degradation and OA were evaluated using Safranin O staining, immunohistochemistry and ELISA. Additionally, mRNA expression of degenerative factors and catabolic markers known to be involved in cartilage degeneration in OA were analysed. Furthermore, the anabolic effects and underlying mechanisms of PGRN were investigated by in vitro experiments with primary chondrocytes.
Results
Here, we found that deficiency of PGRN led to spontaneous OA-like phenotype in `aged' mice. Additionally, PGRN-deficient mice exhibited exaggerated breakdown of cartilage structure and OA progression, while local delivery of recombinant PGRN protein attenuated degradation of cartilage matrix and protected against OA development in surgically induced OA models. Furthermore, PGRN activated extracellular signal-regulated kinases (ERK) 1/2 signalling and elevated the levels of anabolic biomarkers in human chondrocyte, and the protective function of PGRN was mediated mainly through TNF receptor 2. Additionally, PGRN suppressed inflammatory action of TNF-α and inhibited the activation of β-Catenin signalling in cartilage and chondrocytes.
Conclusions
Collectively, this study provides new insight into the pathogenesis of OA, and also presents PGRN as a potential target for the treatment of joint degenerative diseases, including OA.
INTRODUCTION
Osteoarthritis (OA) is the most prevalent type of arthritis in the USA, characterised by synovitis, cartilage degeneration and osteophyte formation.1 As an age-related, progressive, degenerative joint disease, OA is the most common cause of disability in American adults and affects approximately 50 million adults, yet much remains to be elucidated for its inductive factors and underlying mechanisms, and there is no cure for OA.
Progranulin (PGRN) is a growth factor which has multiple functions. PGRN is expressed in various cells and plays a critical role in a number of physiological and disease processes including wound healing,2 bone regeneration,3 tumorigenesis4 and inflammation.5–10 Studies also found that insufficiency of PGRN caused degenerative disease of the nervous system in both humans and mouse models.11–13 We previously reported that PGRN was expressed in human articular cartilage, and its level was significantly elevated in cartilage of patients with OA and rheumatoid arthritis (RA).14 Additionally, PGRN also plays a crucial role in chondrocyte proliferation,15 differentiation and endochondral ossification of growth plate during development.16,17 Recently, we reported that PGRN antagonised tumour necrosis factor α (TNF-α) through binding to TNF receptors, and exhibited an anti-inflammatory function in inflammatory arthritis murine models.7,8,18,19 Microarray has identified PGRN as an OA-associated molecule. The role of PGRN in cartilage degradation and OA progression in vivo, however, remains unknown. Herein, we took advantage of several OA models as well as the chondrocytes isolated from humans and genetically modified mice to investigate the role of PGRN in the progression of OA, and to determine the underlying molecular mechanisms involved.
MATERIALS AND METHOD
Mice
All animal studies were performed in accordance with institutional guidelines, and approval by the Institutional Animal Care and Use Committee of New York University. Age-matched C57/BL6 male wild type (WT) mice, PGRN-deficient (PGRN−/−) mice, TNFR1-deficient (TNFR1−/−) mice and TNFR2-deficient (TNFR2−/−) mice were used for these experiments.
Ageing-associated and surgically induced OA models
All animals were provided with water and food ad libitum throughout these studies. For the ageing-associated model of OA, WT and PGRN−/− mice were kept up to the age of 10 months and were followed for spontaneous development of OA. For the surgically induced OA model, destabilisation of medial meniscus (DMM) surgery and anterior cruciate ligament transection (ACLT) surgery were performed in indicated mice. To induce OA models in rats, we performed ACLT and partial medial meniscectomy in age-matched rats.
Sandwich ELISA for COMP fragments
Serum concentration of cartilage oligomeric matrix protein (COMP) fragments was analysed by our new sandwich ELISA.20
Cartilage explant cultures
Cartilage explants from humans and mouse models were cultured as reported in our previous studies.17,21 Briefly, cartilage was dissected into tiny pieces and dispensed into tissue-culture flasks with Dulbecco's Modified Eagle's Medium (DMEM) supplemented with or without PGRN. After indicated incubating time, the conditioned medium was collected for glycosaminoglycan (GAG) synthesis analysis.
Statistical analysis
Results were expressed as mean values±SEM. Statistics were conducted as Student t test using SPSS software (SPSS, Chicago, Illinois, USA); p<0.05 was considered statistically significant.
Detailed protocols and other procedures, including generation of PGRN stable cell line, and purification of recombinant PGRN protein, micro-CT, histological analysis and immunostaining, histopathologic and quantificational evaluation of OA, primary chondrocytes culture, real-time PCR, safranin O staining and dimethylmethylene blue (DMMB) assay of GAG for cartilage explants, knockdown of TS7 in chondrocytes, luciferase reporter assay, transferase mediated dUTP nick end labelling (TUNEL) staining and western blotting, are provided in online supplementary data.
RESULTS
Deficiency of PGRN leads to OA-like phenotype in `ageing' mice
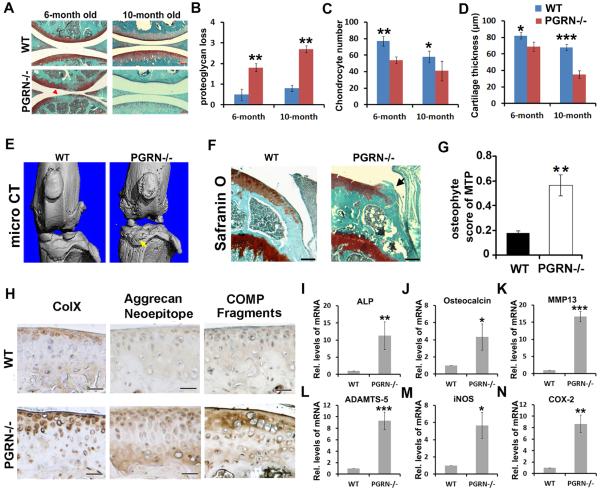
To determine whether loss of PGRN affects joint degeneration in ageing mice, we observed spontaneously developed OA in PGRN−/− mice with ageing. Safranin O staining for 3--month-old WT and PGRN−/− mice indicated no significant difference in articular cartilage (see online supplementary figure S1A–D), while 6-month-old and 10-month-old PGRN−/− mice displayed a remarkably greater loss of proteoglycan in articular cartilage compared with WT littermates (figure 1A). Quantification of the OA-like pathological changes revealed that loss of proteoglycan, the number of chondrocyte per defined cartilage area in the femur cartilage section, and articular cartilage thickness, were all significantly altered in PGRN−/− mice compared with WT controls (figure 1B–D). Reduced number of chondrocyte in cartilage of PGRN−/− mice was probably resulted from enhanced apoptosis of chondrocyte in PGRN−/− mice, assayed by TUNEL staining (see online supplementary figure S2A, B). Bone morphological assay was performed on the proximal tibia of 3-month-old WT and PGRN−/− mice, and no significant difference of bone quality was detected between the two genotypes (see online supplementary figure S3A, B). However, micro-CT (figure 1E), safranin O staining (figure 1F) and osteophyte score of medial tibial plateau (MTP) on the basis of histology (figure 1G) revealed significant osteophyte formation and ectopic subchondral sclerosis in 6-month-old PGRN−/− mice. Taken together, this set of experiments indicated disorganisation of bone metabolism in deficiency of PGRN, which may contribute to OA phenotype in knee joints. Moreover, cartilage samples from 6-month-old WT and PGRN−/− mice were analysed by immunohistochemistry for expression of Col X, the degradative neoepitope of Aggrecan and fragments of COMP. As shown in figure 1H, significant Col X (left panel), Aggrecan neoepitope (middle panel) and COMP fragments (right panel) were detected in cartilage of PGRN−/− mice compared with WT littermates. Total RNA extracts from cartilage of 6-month-old WT and PGRN−/− mice were collected and real-time PCR was performed. The result revealed that cartilage degeneration-associated factors, including alkaline phosphatase, osteocalcin, matrix metalloproteinase (MMP)13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, iNOS and COX-2 were significantly elevated in PGRN−/− mice (rI-N). ADAMTS-7 is known to associate with PGRN,16 and plays a detrimental role in OA development.21 Interestingly, knockdown of ADAMTS-7 attenuated the elevated chondrocyte catabolism seen in 6-month-old PGRN−/− mice (see online supplementary figure S4A, B).
Figure 1.
Progranulin (PGRN)−/− mice spontaneously developed an osteoarthritis (OA)-like phenotype with ageing. (A) Safranin O staining of knee joint articular cartilage. Significant proteoglycan loss in PGRN−/− mice was indicated (red arrow). Scale bar=100 μm. (B–D) Comparison of OA severity between wild type (WT) and PGRN−/− mice in ageing-associated model, as assessed by Safranin O staining loss score, chondrocyte number and articular cartilage layer thickness in the tibia. N=6 for each group. (E) Micro-CT images of knee joints from WT and PGRN−/− mice. Osteophyte formation was indicated by white arrows. (F) Safranin O staining of medial tibia plateu from WT and PGRN−/− mice. Osteophyte is indicated by the black arrow. Scale bar=100 μm. (G) Osteophyte score of WT and PGRN−/− mice, as measured through Safranin O result. (H) Immunostaining for Col X, Aggrecan degradation neoepitope and cartilage oligomeric matrix protein (COMP) fragments in cartilage of WT and PGRN−/− mice. Scale bar=50 μm. (I–N) Transcription levels of mature chondrocyte marker genes alkaline phosphatase (ALP), Osteocalcin, matrix metalloproteinase (MMP) 13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, iNOS and COX-2 from WT and PGRN−/− articular cartilage were measured by real-time PCR. The units are arbitrary, and the normalised values were calibrated against the WT control, here given the value of 1. Each real-time PCR was performed in triplicate. Values are the normalised mean±SEM; *p<0.05, **p<0.01 versus WT mice. Six mice were used in each group.
Deficiency of PGRN leads to exaggerated OA phenotype in surgically induced arthritis models
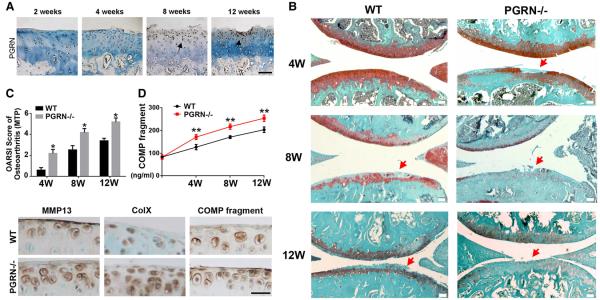
We have previously reported that PGRN is expressed in human articular cartilage, and its level is elevated in cartilage of patients with arthritis compared with normal controls.14 To determine whether the expression of this growth factor was altered in the course of OA progression, we used a surgical OA rat model, with ACLT and partial medial meniscectomy.21 Figure 2A shows that PGRN expression was elevated during OA development.
Figure 2.
Deficiency of progranulin (PGRN) led to exaggerated osteoarthritis (OA) progression in a surgically induced model. (A) Expression of PGRN during the OA development, assayed by immunohistochemistry. Femur cartilage sections were stained with anti-PGRN (brown) and counterstained with methyl green. Arrows indicate the signal. Scale bar=100 μm. (B) PGRN−/− mice exhibited accelerated degeneration of articular cartilage compared with wild type (WT) littermates, assayed by Safranin O staining. Red arrows indicated cartilage destruction. Scale bar=100 μm. (C) PGRN−/− mice displayed a significantly higher score of OA after destabilisation of medial meniscus (DMM) operation on the basis of Safranin O staining. (D) PGRN−/− mice presented a significantly higher level of cartilage oligomeric matrix protein (COMP) fragment in serum after induction of DMM model, assayed by ELISA. (E) Immunohistochemistry staining also showed stronger positive signals for Col X, matrix metalloproteinase (MMP)13 and COMP fragments in PGRN−/− group than WT group 8 weeks after DMM operation. Scale bar=50 μm. The values are mean±SEM of at least 3 independent experiments. Ten-week-old mice (n=7, respectively) were used in this experiment; *p<0.05, **p<0.01 versus WT mice.
To further study the role of PGRN in vivo, a surgically induced DMM OA model was established in both WT (n=7) and PGRN−/− (n=7) mice. Safranin O staining (figure 2B) revealed that more severe structure loss was presented in PGRN−/−- mice than in WT mice. Osteoarthritis Research Society International (OARSI) scoring of OA was performed, and figure 2C demonstrated that PGRN−/− mice had a significantly higher arthritic score than the WT group at all time points. Additionally, synovium inflammation and osteophyte formation of WT and PGRN−/− mice knee joint at 4 weeks after induction were assayed through histology, and PGRN−/− mice presented markedly more severe synovium inflammation as well as osteophyte formation compared with WT littermates (see online supplementary figure S5). COMP is known to be a critical extracellular matrix protein of cartilage. We have previously demonstrated that COMP fragmentation implies severity of cartilage degradation, and hace designed a novel ELISA for circulating COMP fragment level.20 In this study, sera were collected from both genotypes at indicated time points following DMM operation and assayed through COMP fragment-specific ELISA. The result revealed that deficiency of PGRN-exaggerated degradation of COMP after DMM operation (figure 2D). Immunohistochemistry staining also showed stronger positive signals for Col X, MMP13 and COMP fragments in PGRN−/− group than WT group at 8 weeks after DMM operation (figure 2E). It was previously reported that PGRN interacted with ADAMTS-7 and ADAMTS-12, and antagonised their metalloprotease function.14 In the present study, cartilage samples of WT and PGRN−/− mice were collected at 8 weeks after DMM operation, and immunohistochemistry was performed for ADAMTS-7 and ADAMTS-12. As revealed in online supplementary figure S6, PGRN deficiency led to increased expressions of ADAMTS-7 and ADAMTS-12 in OA models, which may also contribute to the susceptibility to surgically induced OA in PGRN-deficient mice.
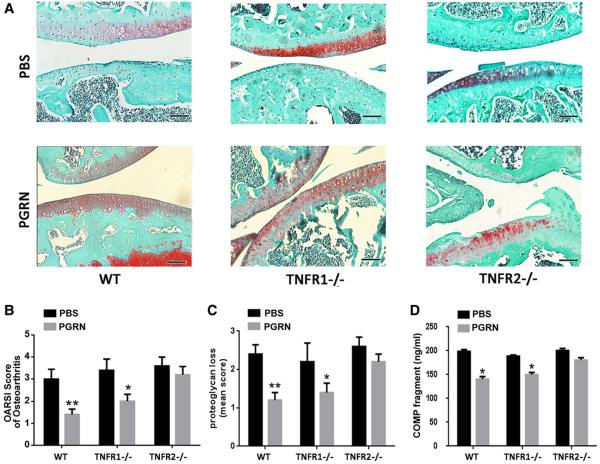
Recombinant PGRN protects against OA in a surgically induced model in vivo
To investigate whether recombinant PGRN has a protective effect against induction and development of OA, an ACLT model was established in WT, TNFR1−/− and TNFR2−/− mice (n=7 for each group), followed by intra-articular injection of phosphate buffered saline (PBS) or PGRN (6 μg) once a week for 4 weeks. As shown in figure 3A, surgically induced OA caused destruction of articular cartilage in all three genotypes of mice with intra-articular injection of PBS. By contrast, in WT mice, intra-articular injection of PGRN remarkably protected the structure of articular cartilage and maintained proteoglycan in the cartilage. Furthermore, PGRN treatment dramatically attenuated synovium inflammation observed in PBS-treated ACLT group of WT mice (see online supplementary figure S7). Moreover, recombinant PGRN treatment exhibited a similar effect in TNFR1−/− deficient group, while this protective effect of PGRN was largely lost when TNFR2 was deficient. Additionally, histological grading analysis showed significant improvement of OA score and attenuation of proteoglycan loss in cartilage with treatment of PGRN in WT and TNFR1−/− mice, but there was no significant change in TNFR2−/− mice (figure 3B, C). To further investigate OA development following treatment of PGRN, sera were collected from each group, and COMP fragment-specific ELISA was performed. As indicated in figure 3D, treatment of PGRN significantly diminished the levels of COMP-degradative fragments in WT and TNFR1−/− ACLT model, but not in TNFR2−/− mice. These results suggest that PGRN-mediated protection in OA is primarily through the TNFR2 pathway.
Figure 3.
Local delivery of recombinant progranulin (PGRN) attenuated osteoarthritis (OA) development in a surgically induced model. (A) Intra-articular injection of PGRN dramatically protected cartilage from degeneration following surgically induced OA model, assayed by Safranin O staining. (B) and (C) Osteoarthritis Research Society International (OARSI) score of OA and loss of Aggrecan based on the result of Safranin O staining. The day after anterior cruciate ligament transection (ACLT) operation, the mice were treated with either phosphate buffered saline (PBS) or PGRN once a week through intra-articular injection, and the samples were collected 4 weeks later. (D) Treatment of PGRN reduced serum level of cartilage oligomeric matrix protein (COMP) fragments in ACLT model, assayed by ELISA. The values are mean±SEM of at least 3 independent experiments; *p<0.05, **p<0.01 versus PBS-treated group. Scale bar, 100 μm. N=6 for each genotype.
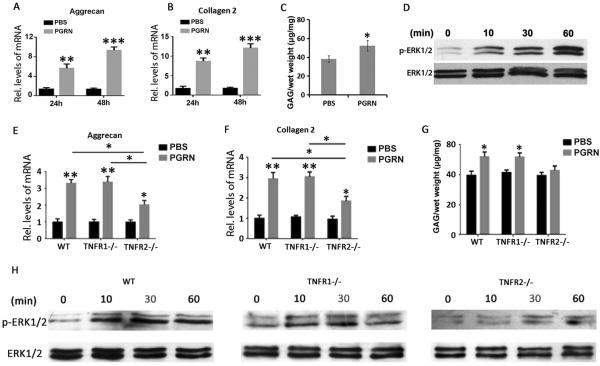
PGRN enhances anabolism of chondrocyte through activation of ERK signalling pathway in degenerative cartilage
It is known that PGRN stimulates anabolic biomarkers through activating the extracellular signal-regulated kinases (ERK) signalling pathway in a human chondrocyte cell line.17 To investigate whether PGRN can improve the anabolism of degenerative chondrocyte and help to reserve the disturbed balance of metabolism during degeneration, primary human chondrocytes were isolated from degenerated human cartilage, cultured in presence or absence of 200 ng/mL PGRN for 24 h and 48 h, and real-time PCR was performed. As shown in figure 4A, B, levels of anabolic markers, including Aggrecan and Col II, were significantly elevated in PGRN treatment group at both time points, indicating that PGRN may enhance anabolism of degenerative chondrocyte. Moreover, cartilage samples were dissected from patients with OA, and cultured in presence or absence of 200 ng/mL PGRN for 7 days. GAG synthesis assay was performed and the result indicated that GAG synthesis rate was significantly elevated in the PGRN treatment group when compared with the PBS group (figure 4C). It is well accepted that the ERK1/2 signalling pathway plays a crucial role in chondrocyte anabolism,22 together with previous findings that PGRN activates ERK1/2 signalling pathway in a stable chondrocyte cell line,17 prompted us to study whether this is also the truth for the role of PGRN in anabolism of primary human chondrocytes. The isolated chondrocytes were cultured in presence of 200 ng/mL PGRN for 0, 10, 30 and 60 min, then total protein was isolated, and western blot was performed for total and phosphorylated ERK1/2. As shown in figure 4D, recombinant PGRN dramatically activated ERK1/2, which was implied by phosphorylation of ERK1/2. As revealed in online supplementary figure S8, inhibition of ERK1/2 activity by mitogen/extracellular signal-regulated kinase (MEK) inhibitor remarkably repressed PGRN-induced anabolism in chondrocyte. Collectively, these findings suggested that PGRN treatment may promote anabolism of degenerative primary human chondrocytes through activation of ERK1/2 signalling.
Figure 4.
Recombinant Progranulin (PGRN) augmented extracellular signal-regulated kinases (ERK)1/2 signalling pathway and promoted anabolism of chondrocyte through tumour necrosis factor receptor 2 (TNFR2). (A), (B) Levels of collagen 2 and Aggrecan, as measured by real-time PCR. Primary chondrocytes were isolated from patients with osteoarthritis (OA), and cultured in presence or absence of 200 ng/mL PGRN for 24 and 48 h, respectively, followed by collection of total RNA and real-time PCR assay. (C) Glycosaminoglycan (GAG) synthesis of human cartilage with or without treatment of PGRN, detected by GAG synthesis assay. Specimens of human cartilage were isolated from patients with OA, and cultured in presence or absence of 200 ng/mL PGRN for 7 days, followed by GAG synthesis assay. (D) Activation of ERK1/2 signalling, detected by western blot. Primary chondrocytes were isolated from patients with OA and cultured with 200 ng/mL PGRN for indicated time points, then total protein was collected and western blot was performed for total and phosphorylated ERK1/2. (E), (F) mRNA levels of collagen 2 and Aggrecan. Primary chondrocytes were isolated from new-born wild type (WT), TNFR1−/− and TNFR2−/− mice, and cultured in presence or absence of 200 ng/mL PGRN for 48 h, followed by collection of total RNA and real-time PCR assay. (G) GAG synthesis of mouse femoral head cartilage with or without treatment of 200 ng/mL PGRN for 7 days, detected by GAG synthesis assay. (H) Activation of ERK1/2 signalling, detected by western blot. Primary chondrocytes were isolated from new-born WT, TNFR1−/− and TNFR2−/− mice and cultured with 200 ng/mL PGRN for indicated time points, then total protein was collected, and western blot was performed for total and phosphorylated ERK1/2. The values are mean±SEM of at least 3 independent experiments; *p<0.05, **p<0.01 versus phosphate buffered saline (PBS) treatment group.
PGRN-mediated anabolism of chondrocytes is, at least in part, through TNFR2
It is reported that TNF receptors are differently distributed in the cartilage of patients with OA.23 Together with our recent results that PGRN can competitively bind to TNFRs and mediate endochondral ossification mainly through TNFR2, we sought to investigate whether the anabolic effects of PGRN on chondrocytes depend on TNFRs. Articular chondrocytes from new-born WT, TNFR1−/− and TNFR2−/− mice were isolated and cultured for 1 week until confluent. Then the chondrocytes were treated with or without 200 ng/mL PGRN for 48 h before real-time PCR was performed. As shown in figure 4E, F, PGRN significantly elevated the levels of Aggrecan and Col II in WT, TNFR1−/− and TNFR2−/− chondrocytes, while the levels of anabolic factors were significantly higher in PGRN-treated WT and TNFR1−/− chondrocytes than in TNFR2−/− chondrocytes, which may indicate the anabolic effect of PGRN was significantly diminished when TNFR2 was deleted. Besides, we cultured cartilage explants from mouse femoral head in medium with/without 200 ng/mL PGRN for 7 days before GAG synthesis assay was performed. The result indicated that PGRN significantly enhanced the synthesis of GAG in WT and TNFR1−/− mouse cartilage explants, while this effect was lost in TNFR2−/− cartilage (figure 4G). Moreover, to test whether TNFRs were involved in the activation of ERK1/2 signalling by PGRN, we cultured chondrocytes from WT, TNFR1−/− and TNFR2−/− mice with 200 ng/mL PGRN for 0, 10, 30 and 60 min, then extracted the total protein, and performed western blot for total and phosphorylated ERK1/2. As shown in figure 4H, recombinant PGRN-induced phosphorylation of ERK1/2 in WT and TNFR1−/− chondrocytes, while delayed and weakened phosphorylation of ERK1/2 was observed in TNFR2−/− chondrocytes after treatment with PGRN, which may indicate that the activation of ERK1/2 signalling pathway by PGRN was greatly impaired in TNFR2-deficient chondrocytes. Collectively, these data indicated that PGRN-activated signalling and anabolic metabolism in chondrocytes is primarily mediated through TNFR pathway, which is also in accordance with the in vivo findings described above (figure 3).
PGRN protects against catabolism of chondrocyte through interacting with TNF-α and β-catenin signalling
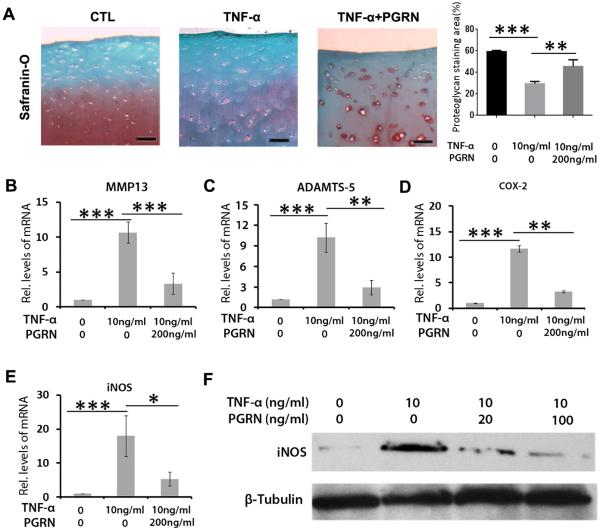
It is well established that inflammatory cytokine TNF-α plays an important role in cartilage degeneration in OA.24 The finding that PGRN prevented cartilage destruction in inflammatory arthritis mediated by TNF-α prompted us to investigate the potential association between PGRN and TNF-α in OA. Cartilage samples from patients with OA were isolated and cultured with 10 ng/mL TNF-α in presence or absence of 200 ng/mL PGRN for 48 h. As revealed in figure 5A, Safranin O staining of the cartilage samples indicated that TNF-α markedly enhanced loss of proteoglycan, while the additional use of PGRN largely rescued this effect of TNF-α. Moreover, total RNA was collected from each group, and real-time PCR was performed. As shown in figure 5B–E, levels of catabolic biomarkers including MMP13, ADAMTS-5, COX-2 and iNOS were significantly higher in the TNF-α-treated group. Additionally, chondrocytes were cultured with TNF-α and treated with indicated doses of PGRN for 72 h. Total protein was then collected, and western blot was performed for iNOS. As revealed in figure 5F, PGRN treatment inhibited TNF-α induction of iNOS in a dose-dependent manner. Although PGRN effectively inhibited TNF-α induction of chondrocyte catabolism, recombinant PGRN treatment only led to slight inhibition on the expressions of catabolic molecules in human OA chondrocytes (see online supplementary figure S9), suggesting that PGRN may mainly act as an antagonist of TNF-α, but not a direct inhibitor, in regulating chondrocyte catabolism.
Figure 5.
Progranulin (PGRN) antagonised TNF-α induced catabolic metabolism in chondrocyte. (A) PGRN significantly attenuated loss of proteoglycan in cartilage induced by TNF-α, assayed by Safranin O staining. Cartilage samples were isolated from patients with OA and cultured with 10 ng/mL TNF-α, in presence or absence of 200 ng/mL PGRN for 7 days. (B)–(E) Levels of degenerative biomarkers in chondrocyte, including matrix metalloproteinase (MMP)13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5, COX-2 and iNOS, as measured by real-time PCR. Primary chondrocytes were isolated from patients with OA and cultured with 10 ng/mL TNF-α for 48 h, in presence or absence of 200 ng/mL PGRN. (F) The protein level of iNOS in chondrocyte. Primary chondrocytes were treated as described in (B), and total protein was collected for western blot assay. The values are mean±SEM of at least 3 independent experiments. Scale bar=50 μm; *p<0.05, **p<0.01 versus phosphate buffered saline (PBS) treatment group.
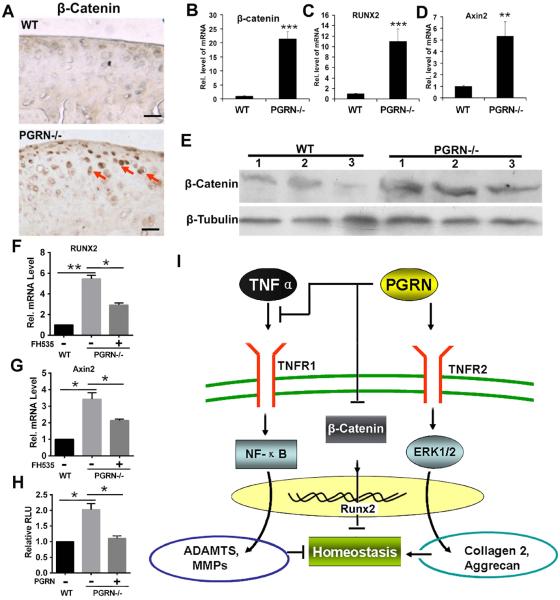
The fact that Wnt/β-catenin signalling pathway plays an important role in degeneration of cartilage structure,25,26 together with the recent report that loss of PGRN resulted in increased expressions of Wnt signalling molecules in a neural system,27,28 led us to examine whether Wnt/β-catenin signalling pathway might also be involved in the PGRN-deficiency-mediated cartilage degeneration. For this purpose, we first examined the effects of PGRN deletion on β-catenin expression in articular cartilage of 6-month-old mice. Immunohistochemistry of β-catenin was performed in cartilage of 6-month-old WT and PGRN−/− mice. As shown in figure 6A, β-catenin signal was stronger in cartilage tissue of PGRN−/− mice. Furthermore, cartilages from 6-month-old WT and PGRN−/− mice were harvested, and total RNA was extracted for real-time PCR assay. As shown in figure 6B, mRNA level of β-catenin was significantly higher in cartilage of PGRN−/− group. To further investigate Wnt/β-catenin signalling, expressions of downstream target genes, including Axin2 and RUNX2, in cartilage of 6-month-old WT and PGRN−/− mice were measured through real-time PCR. Axin2 and RUNX2 were both significantly upregulated in PGRN−/− articular cartilage (figure 6C, D), suggesting the enhanced activation of Wnt/β-catenin signalling pathway. Moreover, western blot result revealed that β-catenin protein level was also elevated in PGRN−/− mice when compared with WT groups (figure 6E). To further elucidate the importance of β-catenin signalling in mediating PGRN activity in chondrocytes and OA, primary chondrocytes were isolated from 6-month-old WT and PGRN−/− mice, and the effect of β-catenin inhibitor FH535 was examined. As indicated in figure 6F, G, PGRN−/− chondrocyte exhibited significantly higher levels of RUNX2 and Axin2, while this elevation was largely abolished with the addition of β-catenin inhibitor. Additionally, β-catenin-specific reporter gene assay was also measured. PGRN deficiency led to increased luciferase activity (figure 6H). However, recombinant PGRN completely reversed the enhanced reporter gene activity seen in PGRN−/− chondrocytes (figure 6H). Collectively, this set of assays suggested that Wnt/β-catenin signalling pathway may also be involved in the susceptibility of developing OA in PGRN-deficient mice.
Figure 6.
Deficiency of Progranulin (PGRN) augmented β-Catenin signalling pathway in vivo. (A) Detection of β-catenin in cartilage of 6-month-old wild type (WT) and PGRN−/− mice, assayed by immunohistochemistry. Scale bar=100 μm. (B)–(D) Levels of β-catenin and its downstream molecules including RUNX2 and Axin 2 in cartilage of 6-month-old WT and PGRN−/− mice, assayed by real-time PCR. (E) Protein level of β-catenin in cartilage of 6-month-old WT and PGRN−/− mice, detected by western blot. Total protein was collected from articular cartilage of 6-month-old WT and PGRN−/− mice, and western blot was performed. (F) and (G) Levels of RUNX2 and Axin2 in chondrocyte of 6-month-old WT and PGRN−/− mice in the presence or absence of β-catenin inhibitor, as measured by real-time PCR. (H) Additional treatment of PGRN improved exaggeration of β-catenin level in PGRN −/− chondrocyte, as detected by luciferase reporter gene assay. (I) A proposed model for the role of PGRN in osteoarthritis development. The values are mean±SEM of at least 3 independent experiments; *p<0.05, **p<0.01 versus WT mice. Six mice were used in each group for each experiment.
DISCUSSION
OA is characterised by the progressive breakdown of extracellular matrix proteins and subsequent loss of articular cartilage which is thought to be mediated by multiple factors. Ageing is a dominant trigger for degeneration.29,30 In this study, we observed spontaneously developed OA in `aged' PGRN−/− mice, characterised by exaggerated loss of proteoglycan, destruction of cartilage structure, and osteophyte formation. Furthermore, elevation of degenerative biomarkers, including ADAMTS-531 and MMP13,32,33 as well as enhancement of degradation products, which are mainly fragments of cartilage matrix, such as Aggrecan34 and COMP, are accepted parameters for OA pathogenesis. In the current study, the chondrocyte hypertrophic marker gene Col X, metalloproteinases known to be important for cartilage degradation ADAMTS-5 and MMP13, as well as the degradation of Aggrecan and COMP, were significantly higher in PGRN−/− mice compared with the WT group with ageing. PGRN was known to play a critical role in endochondral ossification during embryo development and to be expressed in osteoblasts.16,57 In the present study, we found osteophyte formation in the articular cartilage of PGRN−/− mice as early as 6 months old, which indicates disorder of bone metabolism in cartilage and subchondral bone of these mice. Collectively, these data imply that loss of PGRN may lead to accelerated OA though ageing.
Surgically induced DMM models in mice is well accepted in investigating the pathophysiology during the progression of OA.35 In this study, we generated a DMM model in WT and PGRN−/− mice, and PGRN−/− mice exhibited exaggerated progression of OA following induction of DMM. More severe loss of proteoglycan in Safranin O staining, significantly higher arthritis score based on histology, the elevated expression of Col X36 and MMP13,32,33 as well as more degraded COMP fragments20 were observed in the PGRN−/− group. An ACLT mice model37–40 was also chosen and treated by intra-articular injection with recombinant PGRN. Intra-articular injection was a widely used method for local delivery of molecules into the knee joint,41,42 and treatment with PGRN greatly alleviated the severity of OA in WT group, which was indicated by reduced synovium inflammation, less cartilage destruction, and significantly lower arthritic score.
It has been well accepted that chondroprotective molecules are potential approaches for therapy of OA.43 PGRN is known to promote the proliferation and differentiation of chondrocyte, and enhance cartilage regeneration process.17 In this study, we found that PGRN enhanced anabolism of degenerative chondrocytes. This implies that PGRN may play a protective role in degeneration of cartilage. Moreover, PGRN activation of ERK1/2 is involved in its regulation of numerous processes,17 and ERK1/2 is known to protect chondrocyte in OA.22 Here, we found that PGRN also activated ERK1/2 signalling in degenerative human chondrocytes, which may, at least in part, explain the mechanism underlying the PGRN-mediated chondroprotective effect.
Recently, PGRN was reported to promote endochondral ossi-fication by interacting with TNF/TNFR signalling, and PGRN-induced endochondral ossification depended on TNFR2.3 TNFRs have been reported to distribute differently in the cartilage from patients with OA.23 Thus, we explored whether TNFRs were involved in the protective effects of PGRN on chondrocytes by treating primary chondrocytes isolated from WT, TNFR1−/− and TNFR2−/− mice with PGRN. PGRN-induced anabolic effect and activation of ERK1/2 were dramatically impaired when TNFR2 was deficient; together with the results from in vivo study, these data indicated that the chondroprotective effects of PGRN may be mediated, at least in part, through TNFR2. To further investigate whether PGRN relies on ERK1/2 to improve anabolism in chondrocyte, we pre-treated the chondrocytes with an MEK inhibitor (U0126),44 and found that the proanabolic effects mediated by PGRN were significantly abolished, indicating that PGRN-mediated proanabolic effects depend on activating ERK1/2 signalling.
TNF-α is well established to play a critical role in cartilage destruction in arthritis, and anti-TNF agents have been extensively studied for therapy of inflammatory arthritis.45,46 TNF-α enhances expression of MMPs and ADAMTS,21 and these increased proteases, in turn, cause exaggerated degradation of cartilage matrix including Col II and Aggrecan, which promotes progression of OA. TNF-α-mediated activation of NF-κB signalling pathway is known to play an important role in the pathogenesis of OA.24 The finding that PGRN directly binds to TNFR1 and TNFR2, and prevents TNF-mediated inflammation in various conditions8,47,48 has been independently confirmed by several laboratories.8,9,48–54 Additionally, this finding was further confirmed and extended by the recent report that PGRN directly bound to TNFRSF25 (DR3) and inhibited TNF-like ligand 1A (TL1A) activity.55 PGRN binds to TNFR2 with higher binding affinity than to TNFR1,8,48 and TNFR2 has been demonstrated to be critical for PGRN activities. For instance, TNFR2 was reported to be important for PGRN-mediated anti-inflammatory and immunoregulatory activity.8 TNFR2 was found to be also required for PGRN-mediated protection of LPS-induced lung injury.56 Additionally, PGRN-stimulated bone formation and fracture healing also depended on TNFR2 signalling.3 Very recently, it was reported that PGRN regulation of ER stress depended on its interaction with TNFR2.54 Interestingly, PGRN antibodies were found to occur frequently in patients with RA,50 psoriatic arthritis,50 and inflammatory bowel diseases,51 and had significant neutralising effects on PGRN plasma levels. Our results that the PGRN-mediated chondroprotective role depends on TNFR2 also supported the concept that PGRN activities are primarily mediated by TNFR2.
Wnt/β-catenin signalling is extensively studied in OA development, and it is reported to promote cartilage degeneration and osteophyte formation in OA.25,26 Intriguingly, PGRN interacts with Wnt/β-catenin signalling in the neural system,27 which implied that the accelerated OA development in deficiency of PGRN may stem from the activated Wnt/β-catenin signalling in the mice chondrocytes. In the current study, we verified this point in the chondrocyte of PGRN−/− mice, which suggested that existence of PGRN may play a critical role in maintaining homeostasis of articular cartilage through associating with this signalling pathway.
On the basis of the present study and the various literature, a model was proposed for illustrating the role and regulation of PGRN in OA development (figure 6I). PGRN plays a protective role in the progression of OA through multiple pathways. First, PGRN activates the ERK1/2 signalling pathway and elevates levels of anabolic biomarkers, including collagen 2 and Aggrecan, in a TNFR2-dependent manner. This may restore the disturbed balance of chondrocyte metabolism during ageing and trauma processes. Second, PGRN interacts with TNF-α, which induces the expression of cartilage-degrading proteases and loss of cartilage. It is known that TNF-α can activate NF-κB signalling pathway and upregulate the levels of various MMPs and ADAMTS,24 thus participates in mediating cartilage degradation in OA. Additionally, PGRN associates with β-catenin pathway, which is also known to play a critical role in the development of OA. In summary, this study provides insights into the actions of the PGRN growth factor and its associated molecules, as well as their interactions in cartilage and degenerative arthritis, and may also lead to the development of novel therapeutic interventions for OA and other cartilage degenerative diseases.
Supplementary Material
Acknowledgements
This work was supported partly by NIH research grants R01AR062207, R01AR061484, R56AI100901, and a Disease Targeted Research Grant from Rheumatology Research Foundation (to C J Liu).
Ethics approval This study was approved by the NYU School of Medicine's Institutional Review Board (IRB).
Data sharing statement The investigators concur with the NIH mandate to share data generated from Federally Funded Research. They are committed to sharing their data, publishing the data, and in making available to the scientific community available molecular resources described in this publication. Patents have been filed by NYU that claim the chondroprotective role of PGRN in cartilage disorders (US08536126, 2013). Patentable data will be protected as per NYU guidelines.
Footnotes
Contributors All authors made substantial contributions to design, execution and reporting of the manuscript, and approved the final version.
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Herndon JH, Davidson SM, Apazidis A. Recent socioeconomic trends in orthopaedic practice. J Bone Joint Surg Am. 2001;83-A:1097–105. doi: 10.2106/00004623-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 2.He Z, Ong CH, Halper J, et al. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 3.Zhao YP, Tian QY, Frenkel S, et al. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–21. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–54. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- 5.Kessenbrock K, Frohlich L, Sixt M, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–47. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–28. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CJ. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585:3675–80. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–78. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587:1805–10. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 12.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 13.Wils H, Kleinberger G, Pereson S, et al. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol. 2012;228:67–76. doi: 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Lai Y, Tian Q, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–36. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu K, Zhang Y, Ilalov K, et al. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–55. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 16.Bai XH, Wang DW, Kong L, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–19. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng JQ, Guo FJ, Jiang BC, et al. Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J. 2010;24:1879–92. doi: 10.1096/fj.09-144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol Ther. 2012;133:124–32. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Siegel RM. Progranulin Resolves Inflammation. Science. 2011;332:427–8. doi: 10.1126/science.1205992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Y, Yu XP, Zhang Y, et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthritis Cartilage. 2012;20:854–62. doi: 10.1016/j.joca.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Y, Bai X, Zhao Y, et al. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Ann Rheum Dis. 2014;73:1575–84. doi: 10.1136/annrheumdis-2013-203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan D, Chen D, Hawse JR, et al. Bovine lactoferricin induces TIMP-3 via the ERK1/2-Sp1 axis in human articular chondrocytes. Gene. 2013;517:12–8. doi: 10.1016/j.gene.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb GR, Westacott CI, Elson CJ. Chondrocyte tumor necrosis factor receptors and focal loss of cartilage in osteoarthritis. Osteoarthritis Cartilage. 1997;5:427–37. doi: 10.1016/s1063-4584(97)80047-7. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Tang D, Shu B, et al. Conditional activation of beta-catenin signaling leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64:2611–23. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes KR, Pettit AR, Duan R, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. 2012;14:R253. doi: 10.1186/ar4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen EY, Wexler EM, Versano R, et al. Functional genomic analyses identify pathways dysregulated by progranulin deficiency, implicating Wnt signaling. Neuron. 2011;71:1030–42. doi: 10.1016/j.neuron.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korade Z, Mirnics K. Wnt signaling as a potential therapeutic target for frontotemporal dementia. Neuron. 2011;71:955–7. doi: 10.1016/j.neuron.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–8. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–87. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5:38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usmani SE, Pest MA, Kim G, et al. Transforming growth factor alpha controls the transition from hypertrophic cartilage to bone during endochondral bone growth. Bone. 2012;51:131–41. doi: 10.1016/j.bone.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Echtermeyer F, Bertrand J, Dreier R, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–6. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 35.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 36.von der Mark K, Kirsch T, Nerlich A, et al. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–11. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 37.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhen G, Wen C, Jia X, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh JL, Shen PC, Shiau AL, et al. Intraarticular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. J Orthop Res. 2010;28:1300–6. doi: 10.1002/jor.21134. [DOI] [PubMed] [Google Scholar]
- 40.Hayami T, Zhuo Y, Wesolowski GA, et al. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012;50:1250–9. doi: 10.1016/j.bone.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Willett NJ, Thote T, Lin AS, et al. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res Ther. 2014;16:R47. doi: 10.1186/ar4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jean YH, Wen ZH, Chang YC, et al. Intra-articular injection of the cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transected knee in rats: role of excitatory amino acids. Osteoarthritis Cartilage. 2007;15:638–45. doi: 10.1016/j.joca.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Horie M, Choi H, Lee RH, et al. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthritis Cartilage. 2012;20:1197–207. doi: 10.1016/j.joca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Ke J, Long X, et al. Insulin-like growth factor-1 boosts the developing process of condylar hyperplasia by stimulating chondrocytes proliferation. Osteoarthritis Cartilage. 2012;20:279–87. doi: 10.1016/j.joca.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Furst DE. Development of TNF inhibitor therapies for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(3 Suppl 59):S5–12. [PubMed] [Google Scholar]
- 46.Rasheed Z, Haqqi TM. Update on Targets of Biologic Therapies for Rheumatoid Arthritis. Curr Rheumatol Rev. 2008;4:246. doi: 10.2174/157339708786263915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jian J, Zhao S, Tian Q, et al. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587:3428–36. doi: 10.1016/j.febslet.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egashira Y, Suzuki Y, Azuma Y, et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J Neuroinflammation. 2013;10:105. doi: 10.1186/1742-2094-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurner L, Zaks M, Preuss KD, et al. Progranulin antibodies entertain a proinflammatory environment in a subgroup of patients with psoriatic arthritis. Arthritis Res Ther. 2013;15:R211. doi: 10.1186/ar4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurner L, Stoger E, Fadle N, et al. Proinflammatory Progranulin Antibodies in Inflammatory Bowel Diseases. Dig Dis Sci. 2014;59:1733–42. doi: 10.1007/s10620-014-3089-3. [DOI] [PubMed] [Google Scholar]
- 52.Kawase R, Ohama T, Matsuyama A, et al. Deletion of progranulin exacerbates atherosclerosis in ApoE knockout mice. Cardiovasc Res. 2013;100:125–33. doi: 10.1093/cvr/cvt178. [DOI] [PubMed] [Google Scholar]
- 53.Hwang HJ, Jung TW, Hong HC, et al. Progranulin protects vascular endothelium against atherosclerotic inflammatory reaction via Akt/eNOS and nuclear factor-kappaB pathways. PloS ONE. 2013;8:e76679. doi: 10.1371/journal.pone.0076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, Liu Y, Xia F, et al. Progranulin is required for proper ER stress response and inhibits ER stress-mediated apoptosis through TNFR2. Cell Signal. 2014;26:1539–48. doi: 10.1016/j.cellsig.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Liu C, Li XX, Gao W, et al. Progranulin-Derived Atsttrin Directly Binds to TNFRSF25 (DR3) and Inhibits TNF-Like Ligand 1A (TL1A) Activity. PloS ONE. 2014;9:e92743. doi: 10.1371/journal.pone.0092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Z, Li Q, Han Y, et al. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators Inflamm. 2012;2012:540794. doi: 10.1155/2012/540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao Z, Jiang B, Xie Y, et al. GEP, a local growth factor, is critical for odontogenesis and amelogenesis. Int J Biol Sci. 2010;6:719–29. doi: 10.7150/ijbs.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.