Abstract
Cholecystokinin (CCK) acts at the type 1 cholecystokinin receptor (CCK1R) to elicit satiety and is a well-established drug target for obesity. To date, small molecule agonists have been developed, but have failed to demonstrate adequate efficacy in clinical trials, and concerns about side effects and potential toxicity have limited further development of full agonists. The use of positive allosteric modulators (PAMs) without intrinsic agonist activity that are active only for a brief period of time after a meal might represent a safer alternative. Here, we propose a possible novel strategy to develop such compounds by modifying the agonist “trigger” of an existing small molecule agonist. We have studied analogues of the 1,5-benzodiazepine agonist, GI181771X, in which the N1-isopropyl agonist “trigger” was modified. While agonist activity was greatly reduced in these compounds, they acted as negative, rather than positive modulators. The parent drug was also found to exhibit no positive modulation of CCK action. Receptor structure-activity relationship studies demonstrated that the mode of docking these derivatives was distinct from that of the parent compound, perhaps explaining their action as negative allosteric modulators. We conclude that this outcome is likely characteristic of the parental agonist, and that this strategy may be more successfully utilized with a parental ago-PAM, possessing intrinsic positive modulatory activity.
Keywords: Cholecystokinin, CCK1R, G protein-coupled receptors, positive allosteric modulator, obesity
Cholecystokinin (CCK) is a gastrointestinal peptide hormone that is secreted from enteroendocrine I cells in the proximal small intestine in response to protein and fat in the diet. It plays important roles in nutritional homeostasis, acting through the type 1 CCK receptor (CCK1R) to stimulate gallbladder contraction and pancreatic exocrine secretion and to regulate gastrointestinal motility and transit. CCK is also known to act through the vagal afferent CCK1R to induce postcibal satiety1, an activity important for maintenance of normal body weight2–4. The activity of CCK to stimulate satiety has been demonstrated in various animal and human studies1,2,4–6, supporting a substantial effort to develop CCK1R agonists as a possible treatment for obesity. Indeed, several major pharmaceutical companies have developed potent agonists of this receptor, with candidate drugs advancing to clinical trials6–11. However, none of these drug development programs have apparently advanced beyond Phase II to reach regulatory approval for this application. The most advanced of these compounds, GI181771X synthesized at GlaxoSmithKline Research Laboratories (Research Triangle Park, NC), did not meet its primary weight loss efficacy endpoint in a 3 month trial in obese patients12. Another issue with using potent CCK1R agonists as drugs has been associated with on-target side effects of nausea, abdominal cramping, and/or diarrhea, and there is a theoretical concern that chronic dosing of full agonists might lead to the development and/or potentiation of pancreatic cancer13,14.
One possible strategy to take advantage of this drug target, while minimizing the aforementioned on-target side effects and possible toxicity, is to develop positive allosteric modulators (PAMs) without any intrinsic agonist activity. This strategy has been effectively utilized at other receptors, including the G protein-coupled calcium-sensing receptor and the GABA-A receptor, to yield highly effective and well tolerated drugs15,16. Such an agent would be expected to potentiate CCK1R pharmacology only during the limited and finite period of time when nutrients enter the duodenum to stimulate the release of CCK. This close mimicry of the natural effects of CCK might lead to body weight reduction while minimizing the side effects observed with CCK1R full agonists. Additionally, since the sites of action of allosteric drugs are distinct from those utilized by the natural orthosteric agonists, they are generally less well conserved evolutionarily and provide the possibility of a greater degree of specificity17. There is also the possibility of such drugs introducing ligand-directed bias to the signaling profile18,19. Given all the possible benefits of this type of drug over a full CCK1R agonist, however, no ligands of this receptor have been developed yet that demonstrate such a pharmacological profile.
In this study, we propose a new strategy to develop PAMs without intrinsic agonist activity that is directed at the CCK1R. This involves modification of small molecule agonist ligands to eliminate their intrinsic agonist activity, while retaining the molecular determinants for inducing conformational change in their target receptor toward its active state. We postulate that this could reduce the energy barrier to achieving the receptor conformation that stabilizes G protein association, without fully achieving that state. Furthermore, this would represent a conformation of the allosteric small molecule-binding pocket in the receptor helical bundle that is intermediate between the inactive and active states that can lower the threshold for CCK-induced activation, while not yet stabilizing G protein association on its own.
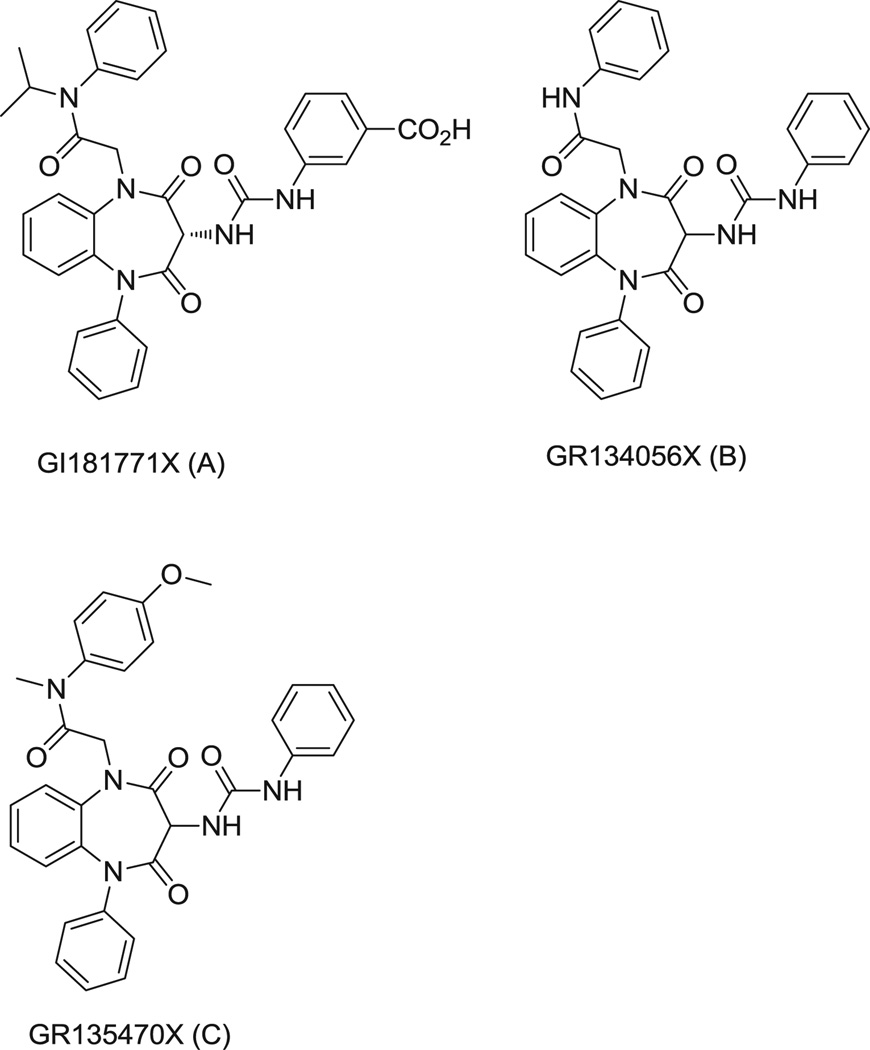
In order to test this approach, we have focused our attention on the 1,5-benzodiazepine full agonist of the CCK1R, GI181771X10 (Fig. 1A), which docks to an active conformation of the intramembranous small molecule ligand-binding site20. We have characterized this binding site as being allosteric, which is spatially distinct from that of the orthosteric CCK peptide ligand, using a series of experimental approaches21–23. Additionally, this intramembranous interhelical pocket has been shown to assume distinct conformations, with clear differences in its inactive state occupied by antagonist ligand24 from its active state occupied by agonist ligand20. Previous ligand structure-activity relationships have established that the N1-isopropyl substituent of the GI181771X represents its “trigger” region that is responsible for inducing CCK1R agonist activity of this compound10, and it has been shown that this group interacts with a specific residue (Leu 7.39, Ballesteros-Weinstein numbering system25) within TM7 of this receptor20.
FIGURE 1.
Structures of the 1,5-benzodiazepine ligands for the CCK1R used in the current study. Note the key differences in the N1-isopropyl group of the full agonist, GI181771X (A), that are present in the other two compounds, GR134056X that contains a hydrogen in this position (B) and GR135470X that contains a methyl group in this position (C).
We hypothesize that by modifying or eliminating the N1-isopropyl agonist “trigger”, the resultant derivative ligand will still be accommodated within the small-molecule binding pocket with a conformation that is close to its active conformation, while not inducing the conformational change associated with G protein activation. This could result in the desired pharmacological profile, with the absence of intrinsic activity and, hopefully, exhibit PAM activity. In order to study this hypothesis, we utilized two structural derivatives of GI181771X, GR134056X and GR135470X, also prepared at GlaxoSmithKline Research Laboratories that lacked the N1-isopropyl agonist “trigger” present in GI181771X (Fig. 1 B,C). Accordingly, GR134056X has a hydrogen (compound 11a10), and GR135470X possesses a methyl group (compound 11b10) replacing the N1-isopropyl group (Fig. 1).
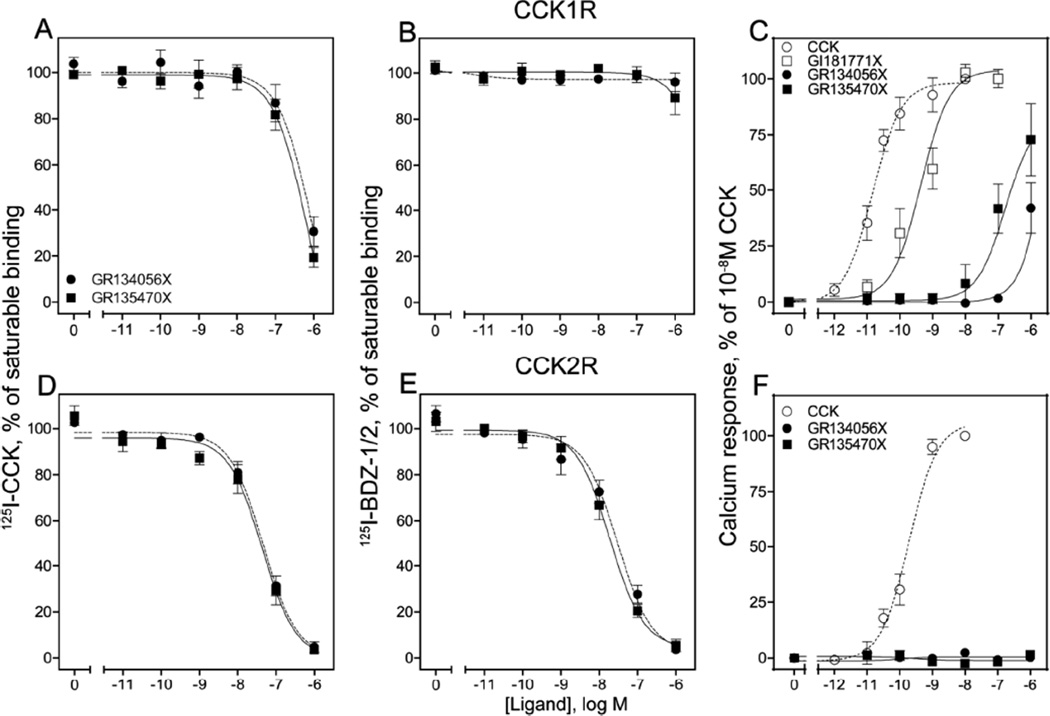
We studied the receptor binding characteristics of these compounds. We evaluated their abilities to inhibit the binding of the CCK-like radioligand, 125I-D-Tyr-Gly-[(Nle28,31)CCK-26–33]26 (125I-CCK), at the CCK1R or CCK2R expressed in membranes prepared from Chinese Hamster Ovary (CHO) cells stably expressing these receptors, as described previously24. The binding data were graphed and analyzed for IC50 values using the nonlinear least-squares curve-fitting routine in Prism (GraphPad, San Diego, CA). Both GR134056X and GR135470X bound to both CCK1R and CCK2R, reducing the binding of the orthosteric agonist radioligand, 125I-CCK, by ~80% and 100%, respectively (Fig. 2A, D). Unlabeled cholecystokinin octapeptide (CCK-26–33, based on the numbering of CCK-33; also known as CCK-8, Peninsula Laboratories) at 1µM concentration was used to determine non saturable binding. Of note, the IC50 values were significantly lower at the CCK2R by ~12-fold and ~9-fold, respectively, than at the CCK1R, indicating higher affinities for this receptor subtype (nM, 541 ± 139 vs. 46.3 ± 10.6 for GR134056X; 450 ± 104 vs. 48.6 ± 13.3 for GR135470X) (p< 0.05). The parent compound, GI181771X, had IC50 values for the binding of 125I-CCK of 105 ± 13nM at CCK1R and 5.6 ± 0.1nM at CCK2R20.
FIGURE 2.
Binding and biological activity of GR134056X and GR135470X at CCK1R and CCK2R. Shown are the data from competition-binding experiments with the orthosteric radioligand, 125I-CCK (A, D), or the allosteric radioligands, 125I-BDZ-1 or 2 (B, E) at the CCK1R (top panel) or the CCK2R (bottom panel) expressed on CHO membranes. Also shown are the intracellular calcium responses of these compounds at both subtypes of CCK receptors expressed on CHO-CCK1R and CHO-CCK2R cells (C, F). Data are represented as means ± S.E.M. from duplicate determinations from at least three independent experiments.
Since the intramembranous small molecule binding pockets within CCK1R and CCK2R represent the site of docking of benzodiazepine-based allosteric ligands20,24, the binding characteristics of GR134056X and GR135470X were also determined using previously characterized, highly selective allosteric antagonist radioligands of CCK1R (125I-BDZ-1, (S)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-enzo[e][1,4]diazepin-3-yl)urea27) and CCK2R (125I-BDZ-2, (R)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)urea27). Neither GR134056X nor GR135470X inhibited 125I-BDZ-1 binding at the CCK1R when used in concentrations as high 1µM, whereas they completely inhibited 125I-BDZ-2 binding at the CCK2R with IC50 (nM) values of 31.7 ± 8.6 and 20.3 ± 5.3, respectively (Fig. 2 B, E). Here, non-saturable binding was determined by using 1µM concentration of respective unlabeled BDZ compounds. Of note, GI181771X inhibited binding of 125I-BDZ-1 to CCK1R with IC50 value of 128 ± 49nM, and inhibited 125I-BDZ-2 binding to CCK2R with IC50 value of 2.7 ± 0.9nM. Evidently, the binding of GR134056X and GR135470X was greatly reduced when compared with GI181771X20, supporting an important role of the N1-isopropyl group to stabilize contact with functionally important residues in the receptor. Substitution of the isopropyl group by a hydrogen (GR134056X) or methyl group (GR135470X) would be expected to decrease the size and lipophilicity of this region of the ligand, possibly contributing to weaker binding. Also, GR134056X tended to bind with lower affinity than GR135470X, although this did not reach statistical significance. Any difference may reflect the size and/or lipophilicity of the isopropyl group relative to the hydrogen, and could also relate to differences in the populations of the amide rotamers in these compounds. Another potential explanation for possible weaker binding of GR134056X could be the conformational impact of the exposure of the hydrogen bond donor on the nitrogen (N1).
We also studied the abilities of GR134056X and GR135470X to stimulate intracellular calcium responses in CCK receptor-expressing cells. For this, we utilized intact receptor-bearing CHO cell lines, measuring intracellular calcium responses to the drug candidates using methods previously described20,28. Calcium responses are reported as percentages of maximal stimulation in response to 10nM CCK or 0.1mM ATP. Data were plotted using GraphPad Prism software, and peak responses and resulting EC50 values were identified. Although these compounds have higher affinities for the CCK2R than the CCK1R, they stimulated no intracellular calcium responses at the CCK2R-expressing cells (Fig. 2 F). Despite modification of the agonist “trigger”, some agonist activity (submaximal) was retained and observed in response to very high concentrations of these compounds (1µM) at the CCK1R (Fig. 2 C). It is not clear whether higher concentrations of these compounds may have demonstrated full efficacy, however the potencies for stimulating intracellular calcium responses were markedly reduced relative to the parent GI181771X, EC50 0.8 ± 0.2nM. The ligand with the methoxy substitution (GR135470X) retained more potent stimulatory activity (EC50 90 ± 40nM) than the compound with the hydrogen substitution (GR134056X) (EC50 >1µM).
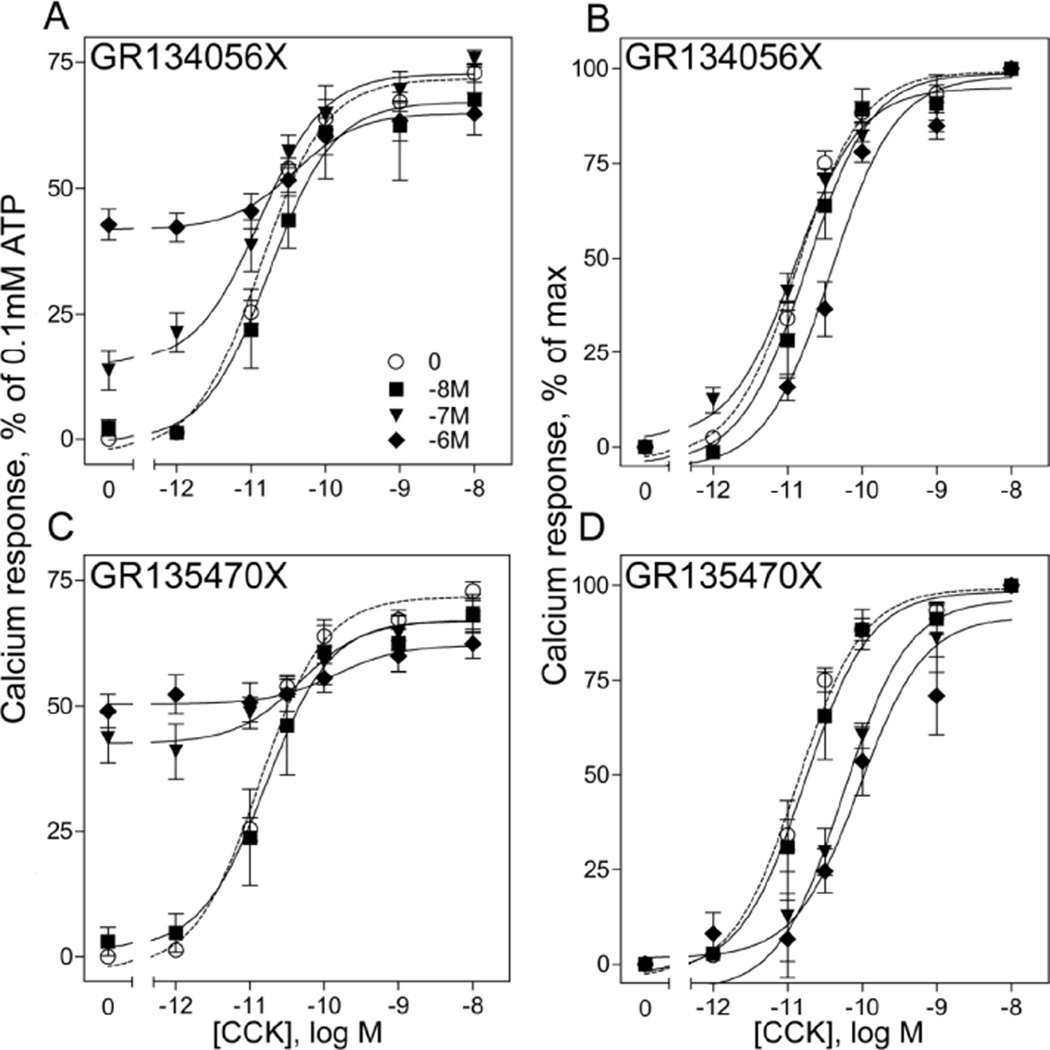
We also studied whether these GR134056X and GR135470X exhibited any PAM activity at the CCK1R. For this, the same cells and assay were utilized, studying full CCK dose-response curves in the presence of a series of concentrations of the potential modulatory allosteric ligands. It was observed that GR134056X exhibited a low, but significant intrinsic agonist response at very high concentrations (13% of the ATP positive control at 100nM, 42% at 1µM). It did not exhibit any significant changes in the CCK response curve up to a concentration of 1µM, and caused a shift to the right in this curve by 2.8-fold, along with a significant 11% decrease in the efficacy (p < 0.05) (Fig. 3 A, B, Table 1). In a similar protocol, GR135470X yielded a higher intrinsic response with increasing concentrations when compared with GR134056X (43% at 100nM and 48% at 1µM). It also induced a significant concentration-dependent shift to the right in the CCK dose-response curve (3.6-fold shift at 100nM, 8.5-fold shift at 1µM), and a corresponding significant reduction in efficacy of the CCK response by 14% in the presence of 1µM concentration (p < 0.05) (Fig. 3 C, D, Table 1). These effects are typical of negative allosteric modulation of the CCK responses, rather than the positive allosteric effects we desired.
FIGURE 3.
Effect of increasing concentrations of GR134056X and GR135470X on CCK responses at the CCK1R. Shown are CCK-stimulated intracellular calcium dose-response curves on CHO-CCK1R cells in the absence or presence of increasing concentrations of GR134056X (A, B), or GR135470X (C, D), with values expressed as percentages of the response to maximal stimulation achieved by 0.1mM ATP. Background responses were determined by addition of buffer only in the absence of any ligands, while maximal activation by 0.1mM ATP was used as a positive control. Curves shown in B and D represent values above the background stimulated by the agonist activity of the modulators. Data represent means ± S.E.M. of duplicate determinations from at least three to five independent experiments.
TABLE 1.
CCK-stimulated responses at CHO-CCK1R cells in the presence of increasing concentrations of GI181771X, GR134056X, and GR135470X.
| Concentration of compound (nM) |
+ GI181771X, CCK EC50 (pM) |
+ GR134056X, CCK EC50 (pM) |
+ GR135470X, CCK EC50 (pM) |
|---|---|---|---|
| 0 | 7.0 ± 1.0 | 14 ± 1.0 | 14 ± 1.0 |
| 0.01 | 13 ± 4.0 | ND | ND |
| 0.1 | 10 ± 3 | ND | ND |
| 0.316 | 2.4 ± 0.6 | ND | ND |
| 1 | 190 ± 120 | ND | ND |
| 10 | ND | 19 ± 5.0 | 20 ± 8.0 |
| 100 | ND | 14 ± 4.0 | 51 ± 7.0*** |
| 1000 | ND | 39 ± 4.0*** | 120 ± 35*** |
p < 0.0001, values compared with control; ND - not determined.
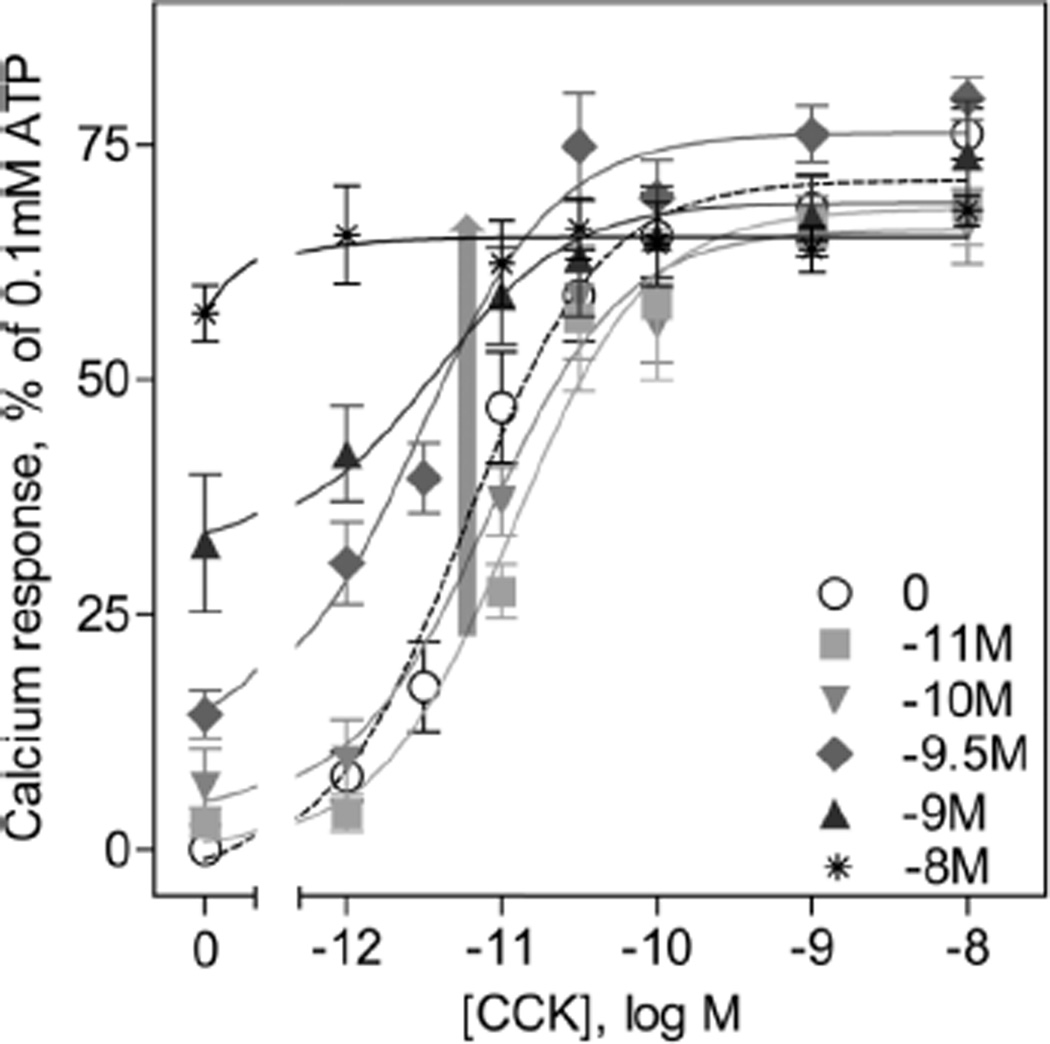
We also studied the parent agonist, GI181771X, to see if it exhibited any allosteric modulatory activity of CCK action. Increasing concentrations of the compound did not yield a significant left-shift or an increase in efficacy of the CCK-dose response curve, as expected for an ago-PAM (Fig. 4, Table 1). A significant dose-dependent increase in the agonist response to GI181771X was observed, making it difficult to determine an accurate EC50 value for the higher concentrations of compound.
FIGURE 4.
Effect of increasing concentrations of GI181771X on CCK responses at the CCK1R. Shown are CCK-stimulated intracellular calcium dose-response curves on CHO-CCK1R cells in the absence or presence of increasing concentrations of GI181771X, with values expresses as percentages of the response to maximum stimulation achieved by 0.1mM ATP. The gray arrow indicates no significant change in the EC50 values of the CCK response curves, indicating no PAM activity of GI181771X. Data represent means ± S.E.M. from duplicate determinations from at least four independent experiments.
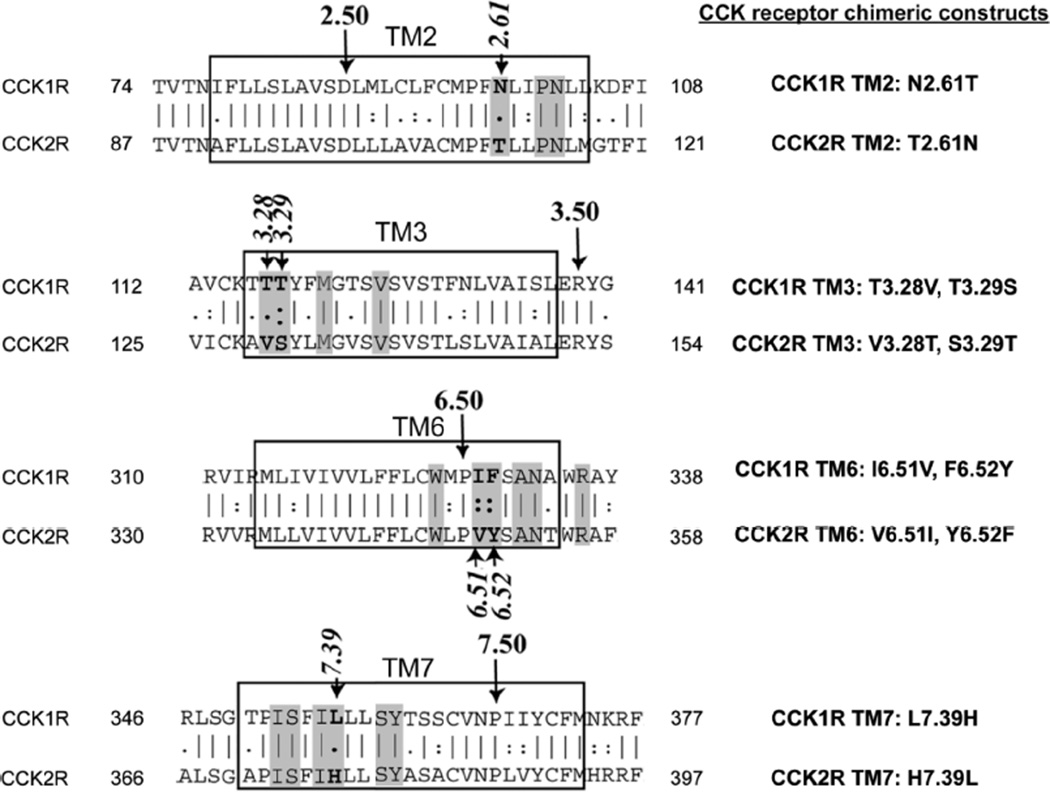
We further tested whether GR134056X and GR135470X might be docked differently than the GI181771X, suggesting the presence of a distinct conformation of the allosteric pocket, rather than one intermediate between the inactive and active conformations, as desired by our experimental strategy in order to exhibit PAM activity. For this, we utilized chimeric receptor constructs in which the residues within the helical bundle that line the allosteric pocket that are distinct in CCK1R and CCK2R were exchanged. Here, specific residues of individual TM2, TM3, TM6, and TM7 were exchanged with the corresponding residues of the other receptor subtype in binding assays (Fig. 5), thereby providing experimental data to help dock the ligands effectively. We have previously experimentally characterized the mode of docking of the allosteric antagonist, BDZ-124, and agonist, GI181771X20, by using the same constructs. We used tracers representing both of the allosteric antagonist radioligands, 125I-BDZ-1 or 125IBDZ-2, and the orthosteric agonist radioligand, 125I-CCK.
FIGURE 5.
Chimeric CCK receptor constructs used in this work. Shown is the alignment of primary sequences of transmembrane segments 2, 3, 6 and 7 of type 1 and type 2 CCK receptors, with those residues lining the intramembranous small molecule-binding pocket shaded. The most highly conserved residue in each segment is identified as number 50 according to the Ballesteros and Weinstein25 scheme. Those residues that line this pocket that are different in the two receptors were exchanged to create the noted chimeric CCK receptor constructs.
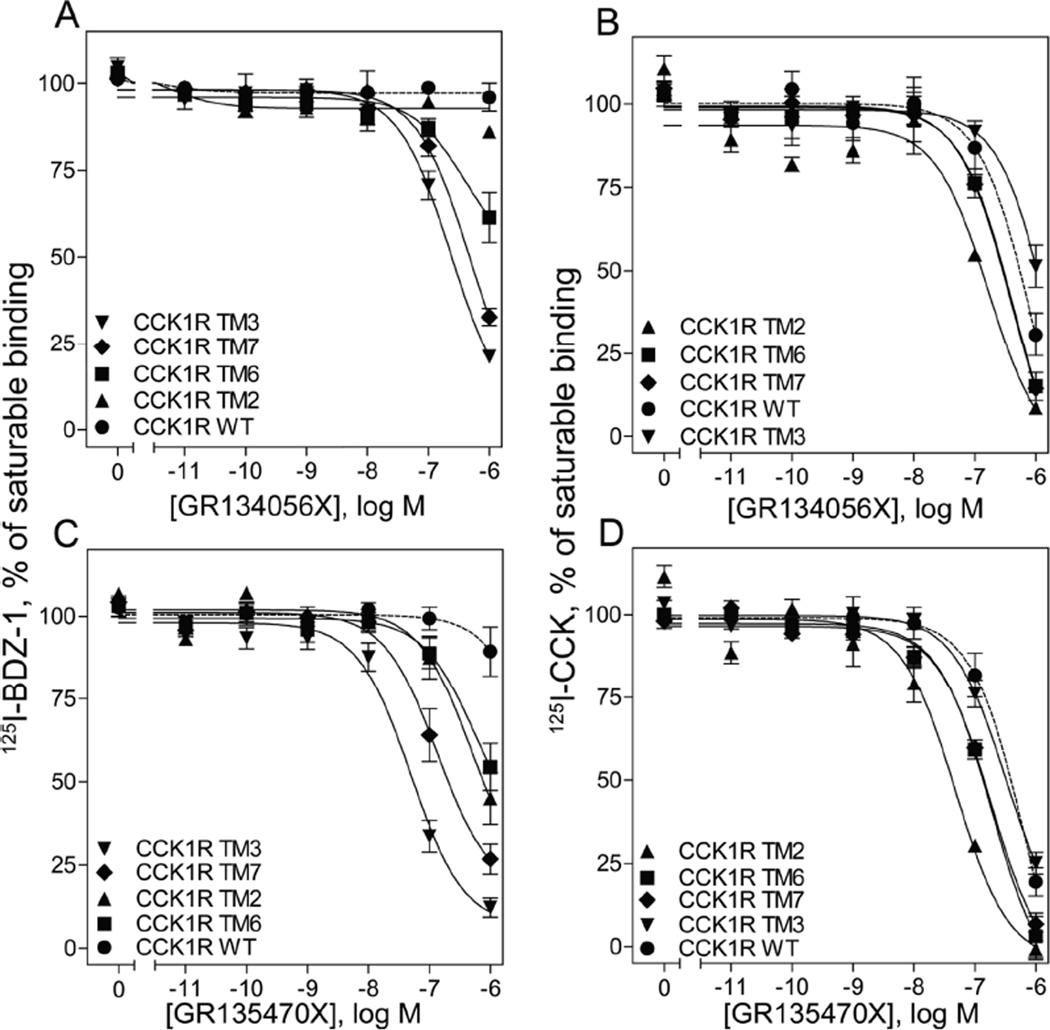
GR134056X and GR135470X were much less effective in competing for benzodiazepine radioligand binding than was GI181771X, with these competition-binding curves far to the right from what had been observed for the full agonist (Fig. 6 A, C, Table 2). These data also showed that the affinities of these compounds to bind to CCK1R were lower than CCK2R. We previously reported that the full agonist, GI181771X, displaced 125I-BDZ-1 almost completely at CCK1R TM mutant constructs, with a statistically significant increase in affinity only at the CCK1R TM3 mutant20. In contrast, GR134056X exhibited no competition for binding of 125I-BDZ-1 to CCK1R, with significant competition observed for the CCK1R TM3 and TM7 constructs (70% and 80%, respectively) (p < 0.05) and with CCK1R TM6 trending in this direction. GR135470X also exhibited little competition for binding of 125I-BDZ-1 to CCK1R, while it showed significant competition for the CCK1R TM3 and TM7 constructs (90% and 75%, respectively), and with CCK1R TM2 and TM6 trending in this direction.
FIGURE 6.
Competition-binding studies using CCK1R-based chimeric constructs. Shown are the competition-binding curves for GR134056X and GR135470X using the allosteric antagonist radiolabel, 125I-BDZ-1 (A, C), or the orthosteric CCK-like radiolabel (B, D) to CHO cell membranes expressing CCK1R TM chimeric constructs, where specific residues for individual TM segments were exchanged with the corresponding residue of CCK2R. Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Non saturable binding was determined by using 1µM unlabeled BDZ-1 or CCK. Data are expressed as means ± S.E.M. of duplicate determinations from at least three independent experiments.
TABLE 2.
Binding parameters of CCK1R-based chimeric constructs expressed in CHO cells.
| CCK1R-based chimeric receptors |
Receptor Abbreviations |
GR134056X, IC50 (nM) | GR135470X, IC50 (nM) | ||
|---|---|---|---|---|---|
| 125I-BDZ-1 | 125I-CCK | 125I-BDZ-1 | 125I-CCK | ||
| CCK1R WT | CCK1R | NDB | 541.0 ± 139.2 | NDB | 450.0 ± 104.1 |
| N2.61T | CCK1R TM2 | NDB | 128.0 ± 13.3* | 875.0 ± 296.2 | 40.3 ± 5.7* |
| T3.28V, T3.29S | CCK1R TM3 | 259.0 ± 60.0* | 1000 ± 115.5 | 50.2 ± 6.6* | 333.6 ± 33.1 |
| I6.51V, F6.52Y | CCK1R TM6 | >1000 | 316.7 ± 60.0 | >1000 | 151.0 ± 26.8* |
| L7.39H | CCK1R TM7 | 505.3 ± 97.4* | 303.3 ± 3.3 | 233.3 ± 66.6* | 153.3 ± 3.3* |
p < 0.05 values compared with wild type CCK1R; NDB - no detectable binding.
Using the 125I-CCK radiolabel, GR134056X was more efficient to inhibit binding at the TM2 mutant, exhibiting a significant 4.2-fold left shift, whereas GR135470X demonstrated an even greater increase in this effect, with an 11-fold left shift. GR135470X also exhibited a 3-fold shift for the TM6 construct and a 3-fold left shift with the TM7 construct (Fig. 6 B,D, Table 2).
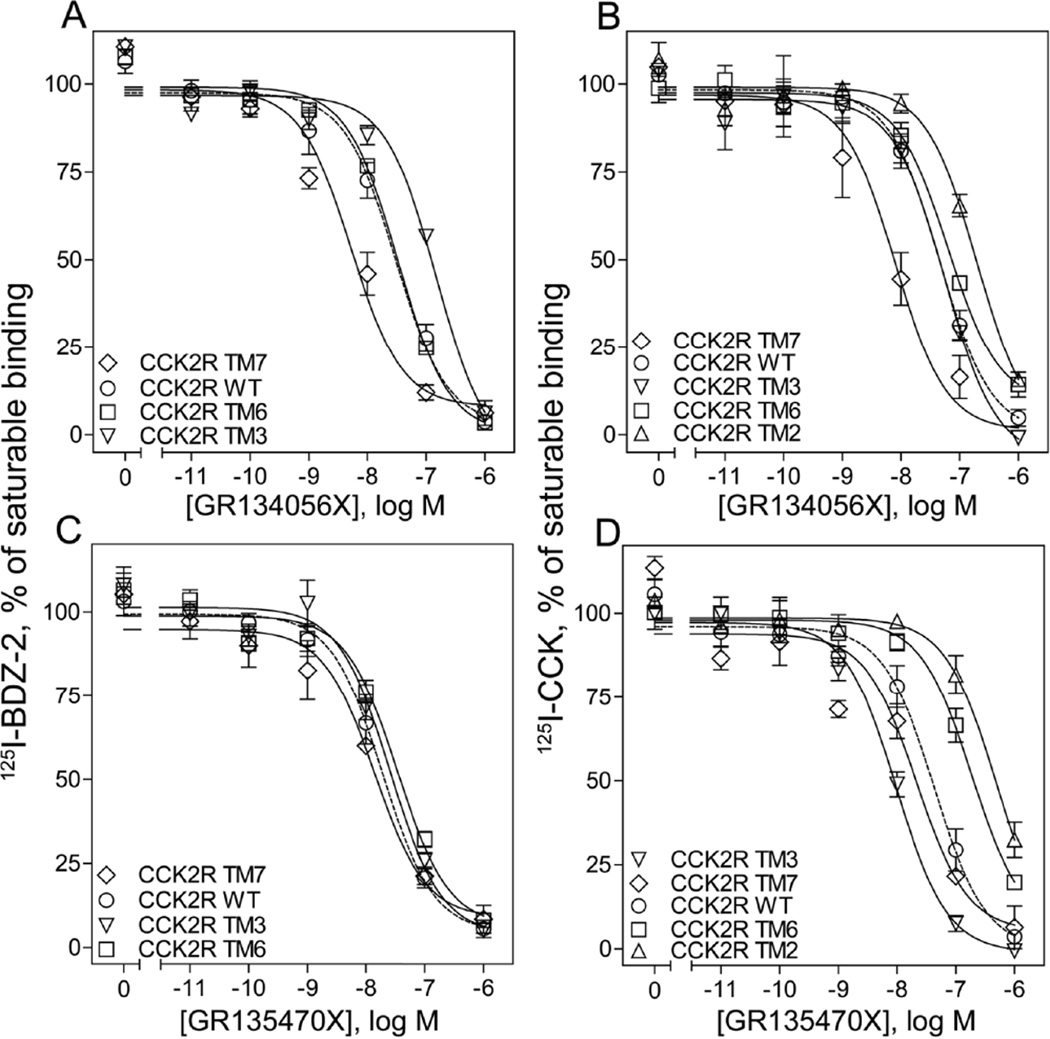
As previously observed, substitution of T2.61 present in the TM2 segment of CCK2R caused inhibition of BDZ-2 binding24, interfering with the usefulness of this construct in 125I-BDZ-2 binding studies. Substitution of TM3 of CCK2R with CCK1R residues gave a 4.2-fold right shift when compared to the wild type receptor, whereas the TM7 chimera gave a 5-fold left shift, when GR134056X was competed against 125I-BDZ-2 (Fig. 7 A, Table 3). In the case of GR135470X, none of the chimeras tested exhibited a change in binding affinities (Fig. 7 C, Table 3). Of note, GI181771X exhibited a significant decrease in binding affinity at both CCK2R TM3 and 7 mutants20. When using 125I-CCK radiolabel with GR134056X, the TM7 substitution yielded a 4-fold left shift, whereas the TM2 substitution resulted in a 4-fold right shift relative to wild type receptor (Fig. 7 B, Table 3). In the case of GR135470X, the TM3 substitution yielded a 5-fold left shift, while the TM2 and TM6 mutants exhibited significantly poorer affinities in 125I-CCK competition experiments (10-fold and 3.4-fold, respectively) (Fig. 7 D, Table 3).
FIGURE 7.
Competition-binding studies using CCK2R-based chimeric constructs. Shown are the competition-binding curves for GR134056X and GR135470X using the allosteric antagonist radiolabel, 125I-BDZ-2 (A, C), or the orthosteric CCK-like radiolabel (B, D) to CHO cell membranes expressing CCK2R TM chimeric constructs, where specific residues for individual TM segments were exchanged with the corresponding residue of CCK1R. Values represent percentages of saturable binding that were observed in the absence of competitor. Non-saturable binding was determined by using 1µM unlabeled BDZ-2 or CCK. Data are expressed as means ± S.E.M. of duplicate determinations from at least three independent experiments.
TABLE 3.
Binding parameters of CCK2R-based chimeric constructs expressed in CHO cells.
| CCK2R-based chimeric receptors |
Receptor abbreviations |
GR134056X, IC50 (nM) | GR135470X, IC50 (nM) | ||
|---|---|---|---|---|---|
| 125I-BDZ-2 | 125I-CCK | 125I-BDZ-2 | 125I-CCK | ||
| CCK2R WT | CCK2R | 31.7 ± 8.6 | 46.3 ± 10.6 | 20.3 ± 5.3 | 48.6 ± 13.3 |
| T2.61N | CCK2R TM2 | NDB | 188.0 ± 31.2* | NDB | 519.3 ± 138.2* |
| V3.28T, S3.29T | CCK2R TM3 | 135.0 ± 12.5** | 51.0 ± 2.8 | 26.2 ± 5.4 | 10.1 ± 1.6* |
| V6.51I, Y6.52F | CCK2R TM6 | 33.1 ± 2.6 | 68.3 ± 9.3 | 36.0 ± 5.5 | 164.7 ± 38.0* |
| H7.39L | CCK2R TM7 | 6.3 ± 2.0* | 11.5 ± 2.7* | 14.4 ± 1.8 | 24.6 ± 6.4 |
p < 0.05,
p < 0.001 values compared with wild type CCK2R using unpaired t-test; NDB - no detectable binding.
Overall, the benzodiazepine radioligand binding data at both series of CCK1R and CCK2R TM mutants supported the interpretation that both GR134056X and GR135470X clearly dock differently from the full agonist, GI181771X, and also exhibit differences from each other. Due to this, the compounds did not achieve the envisioned conformation of this allosteric ligand-binding pocket that was close to that of the active conformation, which likely explains the reason they failed to exhibit PAM activity.
In conclusion, this work relates to the possible role for a CCK1R PAM without intrinsic activity as a novel therapeutic for obesity. As an alternative to high-throughput screening to identify such compounds, we propose a new strategy based on modifying or removing the agonist “trigger” of an existing allosteric small molecule agonist. We tested this approach by utilizing the 1,5 benzodiazepine agonist, GI181771X. The advantage of using the 1,5-benzodiazepine, GI181771X, as a parent compound is that it has already been shown to represent a full agonist at the CCK1R10,20, its action has been demonstrated to be allosteric, with its site of docking distinct from that of CCK20, and its agonist “trigger” has already been defined10. We observed that agonist activity at the CCK1R was greatly reduced in two structurally-related 1,5-benzodiazepines without the N1-isopropyl group, GR134056X and GR135470X. However, these compounds did not enhance the CCK-mediated responses at this receptor, and, instead, acted as negative allosteric modulators, reducing the potency and efficacy of CCK activation of this receptor.
Using a receptor structure-activity relationship approach, we discovered that these compounds docked differently from the parent compound. Due to this, it is suggested that the conformation that is intermediate between the inactive and active states that was hoped for to lower the threshold for CCK-induced activation, while not yet stabilizing G protein association on its own, was not achieved. It is also notable that the parent compound, GI181771X, was found to exhibit no positive allosteric activity itself. This may explain why modification of the agonist trigger region did not result in PAM activity, while it did reduce agonist activity. This likely reflects these specific compounds, rather than a general property of this approach. This experimental strategy may be more successful when an agonist “trigger” can be modified in a ligand having a clear ago-PAM activity. Unfortunately, CCK1R ligands having that activity profile have not yet been identified.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health (DK032878) and by the Mayo Clinic. The authors would like to acknowledge the excellent technical assistance of M.L. Augustine and A.M. Ball.
ABBREVIATIONS
- BDZ
benzodiazepine
- CCK
cholecystokinin
- CCK1R
type 1 cholecystokinin receptor
- PAM
positive allosteric modulator
- TM
transmembrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Li Y, Owyang C. Gastroenterology. 1994;107:525. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 2.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. Am. J. Clin. Nutr. 1981;34:154. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 3.Smith GP, Gibbs J. Ann. N. Y. Acad. Sci. 1985;448:417. doi: 10.1111/j.1749-6632.1985.tb29936.x. [DOI] [PubMed] [Google Scholar]
- 4.Dockray GJ. Regul. Pept. 2009;155:6. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Annu. Rev. Physiol. 2008;70:239. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 6.Elliott RL, Cameron KO, Chin JE, Bartlett JA, Beretta EE, Chen Y, Jardine Pda S, Dubins JS, Gillaspy ML, Hargrove DM, Kalgutkar AS, LaFlamme JA, Lame ME, Martin KA, Maurer TS, Nardone NA, Oliver RM, Scott DO, Sun D, Swick AG, Trebino CE, Zhang Y. Bioorg. Med. Chem. Lett. 2010;20:6797. doi: 10.1016/j.bmcl.2010.08.115. [DOI] [PubMed] [Google Scholar]
- 7.Berger R, Zhu C, Hansen AR, Harper B, Chen Z, Holt TG, Hubert J, Lee SJ, Pan J, Qian S, Reitman ML, Strack AM, Weingarth DT, Wolff M, Macneil DJ, Weber AE, Edmondson SD. Bioorg. Med Chem. Lett. 2008;18:4833. doi: 10.1016/j.bmcl.2008.07.083. [DOI] [PubMed] [Google Scholar]
- 8.Sherrill RG, Berman JM, Birkemo L, Croom DK, Dezube M, Ervin GN, Grizzle MK, James MK, Johnson MF, Queen KL, Rimele TJ, Vanmiddlesworth F, Sugg EE. Bioorg. Med. Chem. Lett. 2001;11:1145. doi: 10.1016/s0960-894x(01)00164-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Hansen AR, Bateman T, Chen Z, Holt TG, Hubert JA, Karanam BV, Lee SJ, Pan J, Qian S, Reddy VB, Reitman ML, Strack AM, Tong V, Weingarth DT, Wolff MS, MacNeil DJ, Weber AE, Duffy JL, Edmondson SD. Bioorg. Med. Chem. Lett. 2008;18:4393. doi: 10.1016/j.bmcl.2008.06.057. [DOI] [PubMed] [Google Scholar]
- 10.Aquino CJ, Armour DR, Berman JM, Birkemo LS, Carr RA, Croom DK, Dezube M, Dougherty RW, Jr, Ervin GN, Grizzle MK, Head JE, Hirst GC, James MK, Johnson MF, Miller LJ, Queen KL, Rimele TJ, Smith DN, Sugg EE. J. Med. Chem. 1996;39:562. doi: 10.1021/jm950626d. [DOI] [PubMed] [Google Scholar]
- 11.Castillo EJ, Delgado-Aros S, Camilleri M, Burton D, Stephens D, O'Connor-Semmes R, Walker A, Shachoy-Clark A, Zinsmeister AR. Am. J. Physiol. Gastrointest. Liver. Physiol. 2004;287:G363. doi: 10.1152/ajpgi.00074.2004. [DOI] [PubMed] [Google Scholar]
- 12.Jordan J, Greenway FL, Leiter LA, Li Z, Jacobson P, Murphy K, Hill J, Kler L, Aftring RP. Clin. Pharmacol. Ther. 2008;83:281. doi: 10.1038/sj.clpt.6100272. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi H, Logsdon CD. Am. J. Physiol. 1993;265:G1177. doi: 10.1152/ajpgi.1993.265.6.G1177. [DOI] [PubMed] [Google Scholar]
- 14.Dawra R, Saluja A, Lerch MM, Saluja M, Logsdon C, Steer M. Biochem. Biophys. Res. Commun. 1993;193:814. doi: 10.1006/bbrc.1993.1698. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D. J. Pharmacol. Exp. Ther. 2004;308:627. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 16.Barker JL, Harrison NL, Mariani AP. Life Sci. 1986;39:1959. doi: 10.1016/0024-3205(86)90319-x. [DOI] [PubMed] [Google Scholar]
- 17.Wootten D, Christopoulos A, Sexton PM. Nat. Rev. Drug. Discov. 2013;12:630. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 18.Conn PJ, Christopoulos A, Lindsley CW. Nat. Rev. Drug. Discov. 2009;8:41. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey AE, Leach K, Valant C, Conigrave AD, Sexton PM, Christopoulos A. Endocrinology. 2012;153:1232. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 20.Harikumar KG, Cawston EE, Lam PC, Patil A, Orry A, Henke BR, Abagyan R, Christopoulos A, Sexton PM, Miller LJ. J. Biol. Chem. 2013;288:21082. doi: 10.1074/jbc.M113.480715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong M, Liu G, Pinon DI, Miller LJ. Biochemistry. 2005;44:6693. doi: 10.1021/bi050130q. [DOI] [PubMed] [Google Scholar]
- 22.Harikumar KG, Clain J, Pinon DI, Dong M, Miller LJ. J. Biol. Chem. 2005;280:1044. doi: 10.1074/jbc.M409480200. [DOI] [PubMed] [Google Scholar]
- 23.Harikumar KG, Pinon DI, Miller LJ. J. Biol. Chem. 2006;281:27072. doi: 10.1074/jbc.M605098200. [DOI] [PubMed] [Google Scholar]
- 24.Cawston EE, Lam PC, Harikumar KG, Dong M, Ball AM, Augustine ML, Akgun E, Portoghese PS, Orry A, Abagyan R, Sexton PM, Miller LJ. J. Biol. Chem. 2012;287:18618. doi: 10.1074/jbc.M111.335646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballesteros JA, Weinstein H. Biophys. J. 1992;62:107. doi: 10.1016/S0006-3495(92)81794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers SP, Pinon DI, Miller LJ. Int. J. Pept. Protein Res. 1988;31:429. doi: 10.1111/j.1399-3011.1988.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 27.Akgun E, Korner M, Gao F, Harikumar KG, Waser B, Reubi JC, Portoghese PS, Miller LJ. J. Med. Chem. 2009;52:2138. doi: 10.1021/jm801439x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai AJ, Harikumar KG, Miller LJ. J. Biol. Chem. 2014;289:18314. doi: 10.1074/jbc.M114.570200. [DOI] [PMC free article] [PubMed] [Google Scholar]