Abstract
Weight-loss dieting often leads to loss of control, rebound weight gain, and is a risk factor for binge pathology. Based on findings that food restriction (FR) upregulates sucrose-induced trafficking of glutamatergic AMPA receptors to the nucleus accumbens (NAc) postsynaptic density (PSD), this study was an initial test of the hypothesis that episodic “breakthrough” intake of forbidden food during dieting interacts with upregulated mechanisms of synaptic plasticity to increase reward-driven feeding. Ad libitum (AL) fed and FR subjects consumed a limited amount of 10% sucrose, or had access to water, every other day for ten occasions. Beginning three weeks after return of FR rats to AL feeding, when 24-hour chow intake and rate of body weight gain had normalized, subjects with a history of sucrose intake during FR consumed more sucrose during a four week intermittent access protocol than the two AL groups and the group that had access to water during FR. In an experiment that substituted noncontingent administration of d-amphetamine for sucrose, FR subjects displayed an enhanced locomotor response during active FR but a blunted response, relative to AL subjects, during recovery from FR. This result suggests that the enduring increase in sucrose consumption is unlikely to be explained by residual enhancing effects of FR on dopamine signaling. In a biochemical experiment which paralleled the sucrose behavioral experiment, rats with a history of sucrose intake during FR displayed increased abundance of pSer845-GluA1, GluA2, and GluA3 in the NAc PSD relative to rats with a history of FR without sucrose access and rats that had been AL throughout, whether they had a history of episodic sucrose intake or not. A history of FR, with or without a history of sucrose intake, was associated with increased abundance of GluA1. A terminal 15-min bout of sucrose intake produced a further increase in pSer845-GluA1 and GluA2 in subjects with a history of sucrose intake during FR. Generally, neither a history of sucrose intake nor a terminal bout of sucrose intake affected AMPA receptor abundance in the NAc PSD of AL subjects. Together, these results are consistent with the hypothesis, but the functional contribution of increased synaptic incorporation of AMPA receptors remains to be established.
Keywords: Sucrose, Nucleus Accumbens, Food Restriction, AMPA Receptors, Postsynaptic Density
INTRODUCTION
Weight-loss dieting often leads to loss of control, poor food choices, and the regain or surpassing of baseline body weight (Rogers and Hill, 1989; Vitousek et al., 2004a; Vitousek et al., 2004b; Polivy and Herman, 2006; Polivy et al., 2008). In fact, a history of weight-loss dieting predicts future weight gain (e.g., Lowe et al., 2006) and may contribute to obesity (Polivy and Herman, 2006). In addition, dieting periods are common in the history of binge eaters (Stice et al., 2008), and cycling between food restriction (FR) and free feeding is a strong predictor of overeating palatable food in response to stress (Wardle et al., 2000).
Previously, it was demonstrated that FR induces neuroadaptations that increase intracellular signaling and gene expression downstream of D1 dopamine (DA) receptor stimulation in nucleus accumbens (NAc; Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005; Carr et al., 2010). Among the behavioral concomitants are increased rewarding effects of D1 receptor agonists and psychostimulant drugs, and resistance to extinction of a cocaine conditioned place preference acquired during a prior ad libitum fed (AL) state (Cabeza de Vaca and Carr, 1998; Zheng et al., 2013). In light of evidence that drug addiction represents a “hijacking” of the neurocircuitry that mediates appetitively motivated behavior (Kelley and Berridge, 2002; Cardinal and Everitt, 2004; Di Chiara, 2005; Volkow et al., 2008; Davis and Carter, 2009; Frascella et al., 2010), the enhanced responsiveness to drugs and associated cues during FR likely reflect exploitation of neuroadaptations that normally promote foraging, reward-related learning, and ingestive behavior during periods of food scarcity. Recent developments in Western societies, including prevalent dieting and an abundance of supranormally rewarding energy-dense food, present a set of conditions with potential to ingrain another type of maladaptive behavior, namely, excessive reward-driven feeding. Evidence in support of a lasting alteration of CNS responsiveness to palatable food among historical dieters was recently provided by a fMRI study demonstrating that, in the fed state, historical dieters show increased activation of reward-related brain regions in response to highly palatable food when compared to nondieters and current dieters (Ely et al., 2013).
The mesoaccumbens DA pathway is involved in normal and abnormal eating behavior (Bassareo and Di Chiara, 1999a,b; Palmiter, 2007; Kenny, 2011), and mediates the reinforcing effects of most drugs of abuse (Wise and Bozarth, 1985; Pontieri et al., 1995; Feltenstein and See, 2008). Addiction research indicates that enduring changes in NAc neuronal circuitry, resulting from repeated strong activation of convergent DA- and glutamate-coded inputs, plays an important role in drug craving and compulsive use (Hyman et al., 2006). Electrophysiological studies support a view of NAc organization in which distinct neuronal ensembles encode behavior associated with different rewards, with reward type-selectivity and number of dedicated neurons subject to change as a result of experience (Pennartz et al., 1994; Carelli and Ijames, 2001; Peoples and Cavanaugh, 2003; Deadwyler et al., 2004; Cameron and Carelli, 2012). A common molecular mechanism of activity-dependent synaptic plasticity in the CNS involves trafficking of GluA1-containing AMPA receptors (Hollmann and Heinemann, 1994; Malinow, 2003; Kessels and Malinow, 2009). AMPARs are co-expressed with DA receptors in striatal neurons (Bernard et al., 2007; Glass et al., 2008), and most NAc AMPARs are either GluA1/GluA2 or GluA2/GluA3 heteromers (Reimers et al., 2011). GluA2/GluA3 traffic constitutively to synapses, while trafficking of GluA1-containing receptors is dependent on synaptic activity (Barry and Ziff, 2002; Greger et al., 2007). AMPAR trafficking in NAc has been implicated in sensitization, craving, and relapse to cocaine seeking (Cornish et al., 1999; Cornish and Kalivas, 2000; Boudreau and Wolf, 2005; Conrad et al., 2008; Famous et al., 2008; Wolf and Ferrario, 2010; 65-71; Xie et al., 2011), and Ca2+-permeable AMPARs in NAc have been implicated in the enhanced rewarding effects of amphetamine and D1 receptor stimulation in FR rats (Carr et al., 2010; Peng et al., 2014).
Phosphorylation of GluA1 on Ser845 by the D1 receptor-regulated cAMP pathway (Roche et al., 1996; Greger and Ziff, 2002; Esteban et al., 2003; Boehm et al., 2006; Oh et al., 2006; Ehlers et al., 2007; Man et al., 2007; He et al., 2009; Lee, 2012), or the NMDA receptor-regulated cGKII pathway (Serulle et al., 2007) increases neuronal excitability and serves as the first of two steps whereby cytoplasmic AMPARs are trafficked to the synapse as the mechanistic underpinning of experience-dependent behavioral plasticity (Shi et al., 2001; Barry and Ziff, 2002; Derkach et al., 2007; Kessels and Malinow, 2009). Activation of this mechanism in D1 DA receptor-expressing NAc medium spiny neurons (MSNs) would be expected to increase reward-directed behavior (Lobo et al., 2010; Lobo and Nestler, 2011). It is therefore of interest that brief intake of 10% sucrose was shown to increase NAc phosphorylation of GluA1 on Ser845 in FR but not AL rats (Carr et al., 2010). Further, seven consecutive daily 5-min episodes of sucrose intake increased GluA1 abundance in the NAc postsynaptic density (PSD), and quantitative electron microscopy revealed an increased intraspinous GluA1 population (Tukey et al., 2013). Comparing AL and FR rats, it was found that sucrose intake increased GluA1 and GluA2 abundance in the PSD with a greater effect in FR rats (Peng et al., 2011). Given the role of AMPARs in synaptic strengthening (Malinow, 2003; Whitlock et al., 2006; Greger et al., 2007) and behavior modification (Kessels and Malinow, 2009; Whitlock et al., 2006), the FR-induced upregulation of sucrose-induced AMPAR trafficking may cause episodes of loss of control during severe dieting to increase vulnerability to excessive sucrose consumption after free feeding conditions have resumed.
To test predictions of this hypothesis, subjects in the present study had episodic limited access to sucrose or tap water during FR or AL diet conditions. FR subjects were then returned to AL feeding and experimental testing was initiated only after daily chow intake had normalized. Testing consisted either of measuring 1-h intake of 10% sucrose three times per week for four weeks, or obtaining nucleus accumbens tissue samples for biochemical assay following 15-min access to sucrose or water, or removal of subjects from the home cage without fluid access. Two predictions were tested: 1. Rats with a history of episodic sucrose intake during FR would consume more sucrose than both (i) rats with an identical history of FR but without sucrose intake, and (ii) rats with an identical history of sucrose intake but without FR; 2. Rats with a history of episodic sucrose intake during FR would display increased AMPAR abundance in the NAc PSD relative to both (i) rats with an identical history of FR but without sucrose, and (ii) rats with an identical history of sucrose intake but without FR.
EXPERIMENTAL PROCEDURES
Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Experiment 1
Subjects and feeding regimens
Subjects were 40 mature, male Sprague Dawley rats (Taconic Farms, Germantown, NY) weighing between 350-450g at the start of the experiment. They were individually housed in plastic cages with bedding, had ad libitum access to tap water, and were maintained on a 12:12 h light:dark cycle with lights on at 0700 h. Half of the subjects had ad libitum access to LabDiet 5001 (LabDiet, St. Louis, MO). The remaining half were FR, initially receiving 10-12 g provided as a single daily meal at 1800 h. Once body weight of the FR subjects had decreased by 20% (~2 weeks) from the initial value, daily feeding was titrated (8-16 g) to clamp body weight for the remainder of the FR phase of the experiment. A treatment consisting of episodic limited access to sucrose or tap water (see below) was initiated for both AL and FR groups once the FR group had been stabilized for one week at the target body weight (i.e., ~3 weeks after implementation of FR). The treatment period continued for 21 days. This was followed by a return of all FR rats to AL feeding conditions.
Treatment with episodic limited sucrose access
On Monday, Wednesday and Friday of each week, subjects had access to a graduated drinking tube placed in the home cage for one hour. The tube, inserted at 1200 h, contained 26 ml of either 10% sucrose (w/v; Sigma-Aldrich, St. Louis, MO) or tap water. Bottle weights were recorded before and after each session. No spillage was observed during intermittent observation of animals, and no additional method of detecting or correcting for spillage was implemented. The volume and period of availability were chosen based on previous work indicating that AL and FR subjects would consume ~20 ml, which was the maximum volume that could be obtained based on shape and angle of the drinking tube. Neither diet group consumed appreciable amounts of tap water. Each subject had a total of ten sessions of fluid access.
Despite the above-described strategy for matching sucrose consumption between diet groups during treatment, AL rats drank slightly less (~10%), on average, than FR rats. This difference is deemed unlikely to account for outcomes of the study based on previous and present results. For example, when AL and FR rats consumed identical volumes of 10% sucrose, FR rats displayed a rapid increase in Ser845-GluA1 phosphorylation not seen in AL rats (Carr et al., 2010), as well as markedly greater synaptic insertion of GluA1 and GluA2 (Peng et al., 2011). More importantly, despite repeated consumption of large volumes of sucrose by AL rats in the present study, they showed no changes in future sucrose consumption or AMPA receptor abundance relative to controls (see Results).
Testing for sucrose intake with intermittent unlimited access
Following restoration of AL feeding in FR rats, 24-h chow intake and body weight were measured in all subjects daily during the first two weeks and every 2-3 days thereafter. When daily food intake of the previously FR subjects did not differ from AL subjects for three consecutive measurements, confirmed by mixed 2-way ANOVA, testing was initiated. This criterion was satisfied by the 22nd day after the previously FR subjects had been returned to AL feeding. In the test phase, all rats had 1-h access to 10% sucrose on Monday, Wednesday and Friday of each week for four consecutive weeks as in the protocol of Corwin and colleagues (Wojnicki et al., 2001; Corwin and Wojnicki, 2013). Drinking tubes containing 200 ml of 10% sucrose were placed in home cages at 1800 h. Bottles were weighed before and after each test session and sucrose consumption was recorded.
The volume of sucrose solution consumed by each rat on each test day was averaged for each test week. These results were analyzed by three-way mixed ANOVA (2×2×4) with previous diet and previous fluid as between-subjects factors and test week as the within-subject factor. Significant interaction effects were followed by lower order ANOVAs. Additional comparisons of interest were performed by protected t-test using the pooled error term from the appropriate ANOVA in the denominator of the t-statistic.
Experiment 2
Subjects and feeding regimens
Subjects and feeding regimens were as in Experiment 1, with the exception that a total of ninety rats were evenly divided between the AL and FR diet regimen. Initial treatment with episodic sucrose access was also as in Experiment 1, with the exception that 30 of the 45 subjects in each diet group had access to 10% sucrose and 15 had access to tap water.
Testing for AMPAR phosphorylation and abundance in the NAc PSD
Once 24-h chow intake of the previously FR groups had normalized (confirmed as above), the terminal day of the experiment was scheduled. As terminal treatment, one half of the subjects in each diet group with a history of sucrose intake (i.e., 15 subjects) were given 15-min access to 10% sucrose immediately prior to sacrifice. The remaining half with a history of sucrose intake were taken directly from home cage for sacrifice. The subjects from each diet group with a history of access to tap water (i.e., 15 subjects) were given 15-min access to water immediately prior to sacrifice. Fifteen subjects were sacrificed per day between 1030 h and 1200 h. For sacrifice, subjects were briefly exposed to CO2 and decapitated by guillotine. Brains were rapidly extracted and NAc was dissected on ice. Bilateral NAc samples from three rats per treatment condition were pooled for fractionation, yielding a total of five tubes for each of the six terminal treatment conditions. Protease inhibitor cocktail and phenylmethanesulfonyl fluoride (PMSF) were added to 0.32M sucrose solution containing 1 mM NaHCO3, 1 mM MgCl2, and 0.5 mM CaCl2 (Solution A). Brain tissue was rinsed, homogenized, and subsequently diluted to 10% weight/volume in Solution A. After being well mixed, 50 μl of the whole cell homogenates were stored at −80°C until use as described in (Peng et al., 2011).
Subcellular fractionation and Western analyses
The whole cell homogenate was centrifuged at 2000g for 10 minutes, after which intact cells and nuclei formed a pellet at the bottom of the tube. The supernatant was saved, and the pellet was resuspended in Solution A. The homogenate was again centrifuged at 1400g for 10 minutes. The supernatant was collected and combined with the previously collected supernatant. They were centrifuged together at 1400g for 10 minutes and then at 13,800g for 30 minutes. The pellet was collected and homogenized in 0.32 M sucrose solution containing 1 mM of NaHCO3, protease inhibitor cocktail, and PMSF (Solution B). This homogenate was placed on a sucrose gradient and centrifuged for 2-hr at 82,500g. The synaptosomal layer was collected from the interface of the 1 M and 1.2 M sucrose layers. The sample was then resuspended in Solution B and centrifuged at 82,500g for 45 minutes. After centrifugation, the upper liquid was discarded, and the pellet was resuspended in a solution of 25 mM Tris, at pH 7.4.
To isolate the PSD fraction, an equal volume solution containing 1% Titron X-100, 0.32 M sucrose, and 12 mM Tris, at pH 8.1 was added to the resuspended sample. The mixture was then rocked at 4°C for 15 minutes, followed by centrifugation at 13,800g for 30 minutes. After centrifugation, the upper liquid was discarded, and the pellet was resuspended in a solution of 25 mM Tris, at pH 7.4 with 2% SDS and stored at −80°C until use (Peng et al., 2011).
Western blotting
Proteins were separated by electrophoresis on precast 4%-12% sodium dodecyl sulfate polyacrylamide gels (Lonza, Rockland, ME). Dual-colored protein standard molecular weight markers (Bio-Rad, Hercules, CA) were loaded to assure complete electrophoretic transfer and estimate the size of bands of interest. Proteins were electrophoretically transferred to nitrocellulose membranes and blocked for 60 minutes with 5% nonfat milk in phosphate buffered saline with 0.05% Tween-20 (PBST) with shaking at room temperature. The blots were cut at the 70kDa mark. The upper halves of the blots were incubated with primary antibodies for target proteins, and the lower halves of the blots were incubated with primary antibody for the protein loading control, α-tubulin by shaking overnight at 4°C (in 3% nonfat milk/PBST).
Primary antibodies included rabbit polyclonal anti-phospho-Ser845-GluA1 (1:1,500; AB5849, Millipore, Temecula, CA), mouse monoclonal anti-GluA1 (1:1500; MAB2263, Millipore, Temecula, CA), rabbit polyclonal anti-GluA2 (1:1000; PA1-4659, Thermo Scientific, Rockford, IL), mouse monoclonal anti-GluA3 (1:500; MAB5416, Millipore), rabbit polyclonal anti-PSD95 (1:1000; AB9708, Millipore, Temecula, CA) and mouse monoclonal anti-α-tubulin (1: 5000; T6199, Sigma-Aldrich, St. Louis, MO). Blots were washed 3 × 8 minutes in PBST, incubated with the appropriate secondary antibodies (1: 15,000) for 1 hour at room temperature (in 3% nonfat milk/PBST), washed 2 × 8 minutes in PBST, and 1 × 8 minutes in PBS, then treated with West Pico enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL) and exposed to film to visualize bands of interest.
Data were based on at least triplicate immunoblots analyzed using NIH Image J software. Following densitometry, intensities of bands corresponding to GluA1, GluA2 and GluA3 for each sample were divided by intensities of the corresponding α-tubulin bands. Intensities of bands corresponding to pSer845-GluA1 were divided by intensities of the corresponding total GluA1 protein. Results were expressed in comparison to the normalized control, defined as the group that had been AL throughout and had a history of access to tap water. Results were analyzed by 2-way ANOVA, with significant interaction effects followed by planned comparisons, based on predictions, or post-hoc comparison of cell means using Tukey’s HSD test.
Experiment 3
Following observation in Experiment 1 that rats engaging in episodic sucrose intake during FR displayed persistently elevated sucrose intake despite six weeks of restored AL food access (see Results below), an experiment was conducted to determine whether a parallel phenomenon occurs in subjects treated and then challenged with d-amphetamine.
As with effects on food motivation and reward, rewarding and stimulant effects of amphetamine are enhanced by FR (Deroche et al., 1995; Cabeza de Vaca and Carr, 1998). In addition, behavioral cross-sensitization occurs between sucrose and amphetamine, such that intermittent exposure to one augments the locomotor-activating effect of the other (Avena and Hoebel, 2003a, 2003b), providing additional evidence of overlapping mechanisms of their incentive effects. It was reasoned that if enhanced sucrose intake in Experiment 1 were mediated either by incomplete recovery of CNS or metabolic effects of FR despite restoration of free feeding, or by neuroplastic changes induced by sucrose during FR which are similarly induced by amphetamine, locomotor-activating effects of amphetamine would be augmented both during active FR and during recovery. If, on the other hand, responsiveness to amphetamine were to normalize following return to AL feeding, a role of sucrose-specific neuroplastic changes induced during FR, enduring beyond the conditions that prevailed during induction, would be supported.
Subjects and feeding regimens
Subjects were 32 mature, male Sprague Dawley rats (Taconic Farms, Germantown, NY) weighing between 350-450g at the start of the experiment. They were individually housed in plastic cages with bedding, had ad libitum access to water, and were maintained on a 12:12 h light:dark cycle with lights on at 0700 h. Half of the subjects had ad libitum access to LabDiet 5001. The remaining half were maintained FR as in Experiment 1. A treatment consisting of intermittent administration of d-amphetamine or saline vehicle was initiated for both AL and FR groups once the FR group had been stabilized for one week at the target body weight (i.e., ~3 weeks after implementation of FR). The treatment period continued for 10 days and consisted of 5 daily injections given at 48-72 h intervals. This was followed by a return of all FR rats to AL feeding conditions.
Treatment with d-amphetamine
On six occasions prior to the initiation of treatment, rats were habituated to transport to the laboratory from the vivarium and were handled in the test room where treatment was to be administered in the first and fifth treatment sessions. Subsequently, every 48-72 hours, 8 rats in each diet group were injected with d-amphetamine (Sigma-Aldrich; 1.0 mg/kg, i.p.) and the other 8 with saline vehicle (1.0 ml/kg, i.p.). Each rat received a total of 5 injections during this ~10 day period. Prior to the first and fifth injections, rats were placed in a locomotor activity test chamber where horizontal and vertical activity were measured for 1 h using the VersaMax System (Accuscan, Columbus, OH, USA) which monitored animal activity via a grid of 16×16 infrared light beams that traverse the chamber (42×42×30 cm) front to back and left to right. Information about beam status, scanned at a rate of 100 times per second, was stored to disk. Rats were then injected and activity measured for another 1 h period. The second, third, and fourth injections were administered to rats in their home cages. Following the fifth session, all FR rats were returned to AL feeding. Body weights and 24 h chow intake of all animals were measured regularly. When 24 h intake and rate of body weight gain of the previously FR rats had stabilized for one week and did not differ from the AL group, the test phase was initiated.
Testing for d-amphetamine-induced hyperactivity
Once per week for 3 consecutive weeks each rat was placed in a locomotor test chamber and activity was measured for a 1 h habituation period. Rats were then injected with d-amphetamine (1.0 mg/kg, i.p.), returned to the test chamber, and activity was measured for an additional 1 h.
RESULTS
Experiment 1
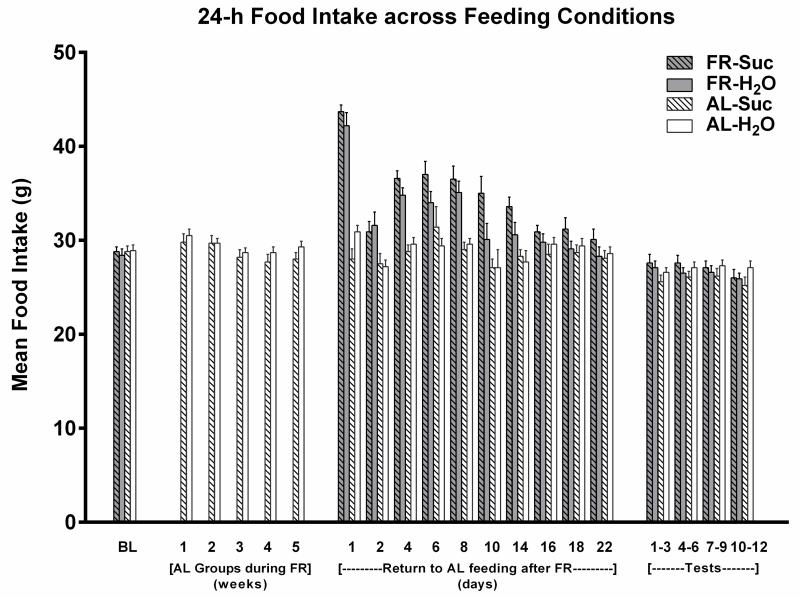
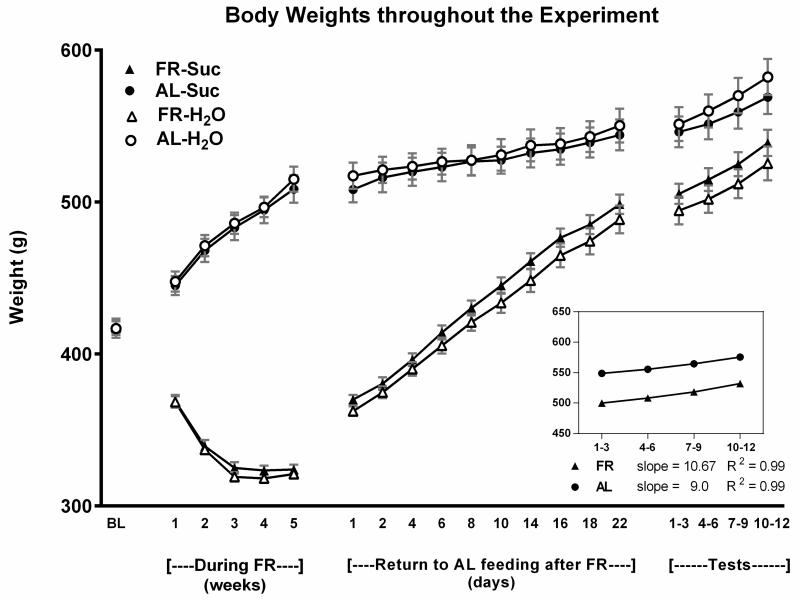
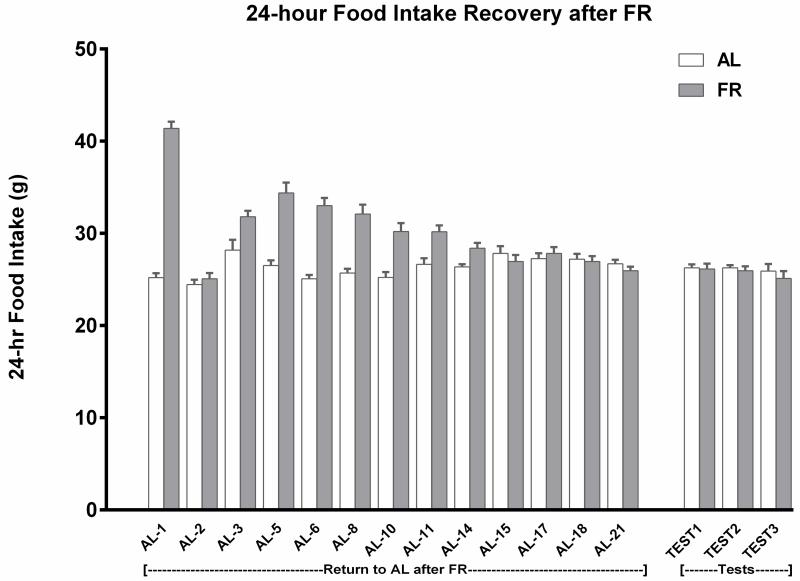
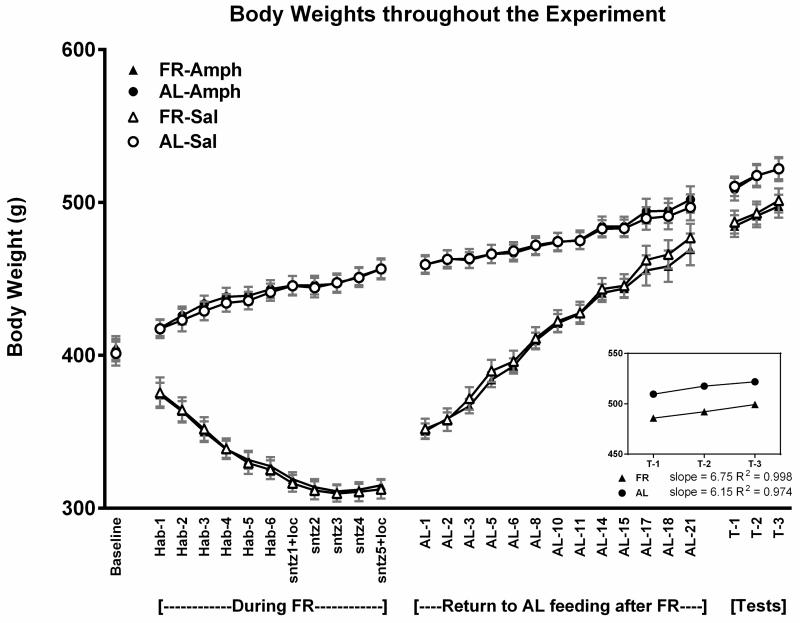
Across the ten treatment sessions of 1-h access to 10% sucrose, AL rats consumed an average of 17.4 ± 0.31 and FR rats consumed an average of 19.1 ± 0.28 ml per session. Following the return of FR rats to AL feeding conditions, daily monitoring suggested that 24-h intake of chow normalized during the third week (Fig 1). This was confirmed by absence of difference between groups across the final three measurement days (Fdiet;1,38 = 1.35, p>.10; Fdiet × days; 2,76 = 0.13). During the 22 days of intake normalization, body weights of the previously FR group increased from 323 ± 3.0 to 494 ± 7.8 g. During the same period, body weights of the AL group increased from 512 ± 8.5 to 547 ± 10.6 g (Fig 2). The rate of body weight gain from day 22 through the end of the testing phase, four weeks later, did not differ appreciably between groups; slope of the regression line describing body weight gain over test days in previously FR rats was 10.67, and the slope in AL rats was 8.97 (Fig 2). Consequently, in the fourth week of testing body weights of the previously FR and AL groups were 532 ± 7.0 and 575 ± 8.1 g, respectively. In order to assess whether the previously FR subjects were likely to have normalized metabolically at this time point, blood was sampled from two additional groups (n=8), one of which underwent six weeks of FR followed by six weeks of recovery and the other of which remained AL throughout. Plasma leptin levels, determined by ELISA (kit #ab100773; Abcam, Cambridge MA) did not differ between these groups (AL: 1387 ± 249 pg/ml, former FR: 1098 ± 131 pg/ml; t(14)=1.03).
Figure 1.
Mean (± s.e.m) 24 hour intake of LabDiet 5001 for each treatment group (n=10) during each phase of Experiment 1. During weeks 1-5, two groups were food-restricted (FR) and intake data are omitted. Following the restoration of ad libitum (AL) feeding, the two FR groups displayed a compensatory increase in 24 h intake. Their intakes normalized during the third week, in which there was no difference across the final three measurements between subjects that did and did not have a history of FR.
Figure 2.
Mean body weights (± s.e.m) of the four treatment groups (n=10) during each of the four phases of Experiment 1: (i) at baseline, before diet manipulation and treatment, (ii) when two groups were food-restricted (FR) and all groups received treatment with episodic limited intake of 10% sucrose or water, (iii) during restoration of ad libitum (AL) feeding in the FR groups, and (iv) during the four week intermittent testing of sucrose intake. The rates of body weight gain during the four weeks of testing were similar for rats with and without a history of FR.
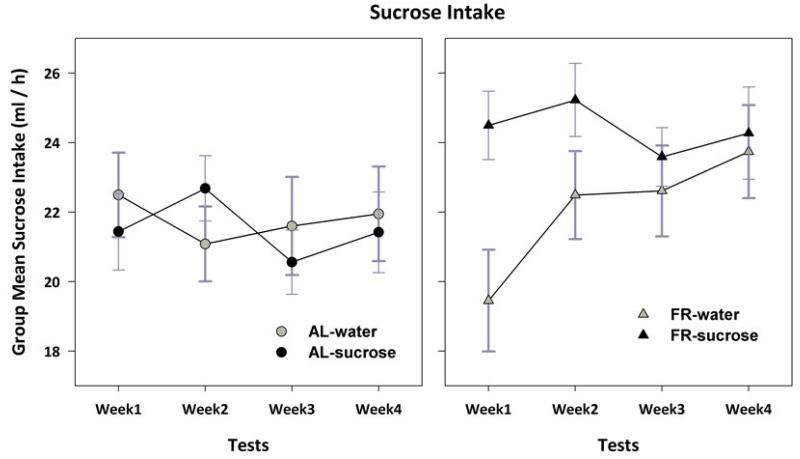
Analysis of sucrose intake across the four weeks of testing indicated a 3-way interaction between previous feeding condition, previous fluid, and week (F3,108 = 3.6, p<.025), and 2-way interactions between previous feeding condition and week (F3,108 = 2.7, p<.05), and previous fluid and week (F3,108 = 3.5, p<.025; Fig 3). Subsequent analysis of the AL groups indicated no main effects of week (F3,54 = 0.8) or previous fluid (F1,18 = .02), and no interaction (F3,54 = 2.0, p>.10). Additional analyses therefore focused on the previously FR groups. Two-way ANOVA indicated interaction between previous fluid and week (F3,54 = 4.95, p<.01), and an effect of test week (F3,27 = 10.2, p<.001). These effects were characterized by a linear trend of increasing sucrose intake (F1,9 = 16.6, p<.01) in the group with previous access to tap water (F1,9 = 16.6, p<.01). Comparison of the previously FR group with exposure to sucrose to the two AL groups combined, indicated that the former consumed more sucrose throughout the four weeks of testing (F1,28 = 5.4, p<.05). In addition, the FR groups combined consumed more sucrose than AL groups during weeks 3 (t(114)=2.28, p<.01) and 4 (t(114)=2.62, p<.01).
Figure 3.
Mean (± s.e.m) 1-h intake of 10% sucrose during each week in which tests were conducted Monday, Wednesday and Friday. Results for ad libitum fed (AL) and previously food-restricted (FR) subjects with and without a history of episodic sucrose intake are displayed in the left and right panels, respectively. The group with a history of sucrose intake during FR (filled triangles in right panel) consumed more sucrose during test weeks than AL groups and the group with a history of water access during FR (see text for details).
Finally, in the fourth week of testing there was no correlation between body weight and sucrose consumption in either the previously FR group (r=0.10) or the AL group (r=0.06).
Experiment 2
Across the ten treatment sessions of 1-h access to 10% sucrose, AL rats consumed an average of 17.0 ± 0.4 and FR rats consumed an average of 19.1 ± 0.8 ml per session. During the three weeks of intake normalization, body weights of the previously FR group increased from 303 ± 3.0 to 473.8 ± 5.2 g. During the same period, body weights of the AL group increased from 506 ± 11.1 to 541.4 ± 12.4 g. On the terminal day, the previously FR rats that were given access to sucrose prior to sacrifice consumed 15.3 ± 1.1 ml, and the AL rats consumed 13.3 ± 1.1 ml.
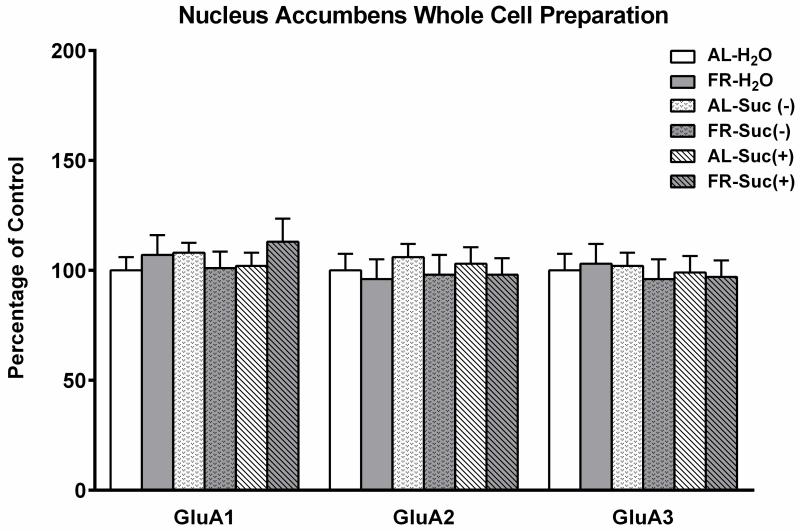
In whole cell homogenates prepared from NAc, no differences were observed in levels of total GluA1, GluA2, or GluA3 as a function of previous diet, fluid treatment, or their interaction (Fig 4). This suggests that treatments had no long term effects on synthesis and/or degradation of AMPARs.
Figure 4.
Effects of a history of food restriction (FR), with and without episodic intake of 10% sucrose, on AMPA receptor GluA1, GluA2 and GluA3 abundance in nucleus accumbens whole cell homogenate. Previously FR and control ad libitum fed (AL) subjects were either taken from the home cage, or had 15-min access to 10% sucrose or tap water prior to brain harvesting. Following densitometry, intensities of bands corresponding to the target AMPA receptor protein for each sample were divided by the intensities of the corresponding α-tubulin bands. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the AL group with a history of episodic access to tap water and a terminal treatment of 15-min access to tap water. n=15 per group, with 3 NAc pooled per tube for fractionation.
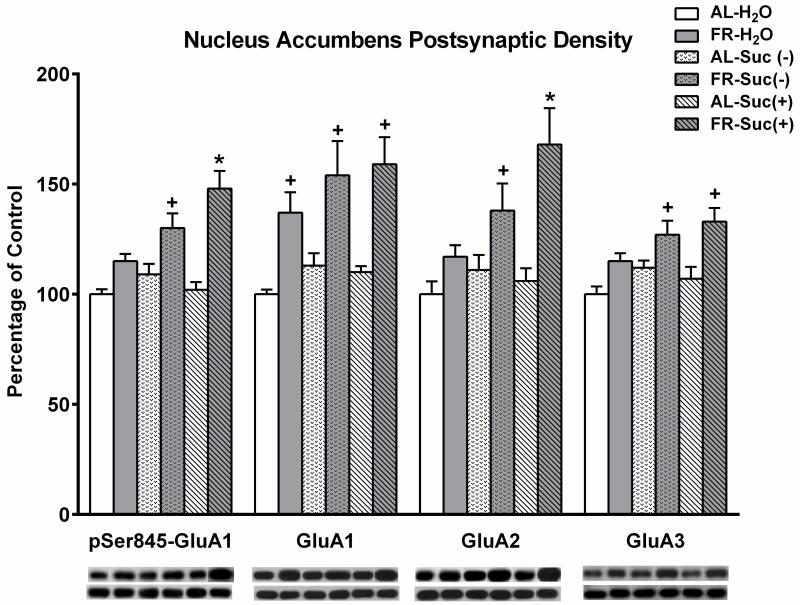
In the PSD fraction (Fig 5), levels of pSer845-GluA1 were higher in previously FR rats that consumed sucrose prior to sacrifice relative to all other groups except for the previously FR group with a history of sucrose intake but no sucrose prior to sacrifice (Fdiet × fluid; 2,24 = 4.88, p<.025; Tukey comparisons at least, p<.05). The previously FR group with a history of sucrose intake but no sucrose on the day of sacrifice showed higher levels of pSer845-GluA1 than each of the three AL groups (all at least, p<.05).
Figure 5.
Effects of a history of food restriction (FR), with and without episodic intake of 10% sucrose, on abundance of pSer845-GluA1, GluA1, GluA2, and GluA3 in nucleus accumbens postsynaptic density. Following densitometry, intensities of bands corresponding to the target AMPA receptor protein for each sample were divided by the intensities of the corresponding α-tubulin bands. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the AL group with a history of episodic access to tap water and a terminal treatment of 15-min access to tap water.
Representative immunoblots are included in sequence corresponding to bars immediately above. n=15 per group, with 3 NAc pooled per tube for fractionation. * greater than all AL groups and the previously FR group with access to tap water, p at least <.05; + greater than all AL groups, p at least <.05.
Levels of total GluA1 in the PSD were higher in previously FR groups relative to AL groups (Fdiet,1,24 = 30.3, p<.0001). While there were trends suggesting that previously FR rats with a history of sucrose intake had higher levels of GluA1 than those with a history of water access, these effects were not significant.
Levels of total GluA2 were higher in previously FR relative to AL rats (F diet; 1,24 = 20.0, p<.001), and planned comparisons attributed this difference to the FR group with a history of sucrose access and sucrose prior to sacrifice, which had higher levels than all other groups (p at least <.05), and the FR group with a history of sucrose access that did not have sucrose prior to sacrifice, which had higher levels than all AL groups (p at least <.05).
Levels of total GluA3 were also higher in previously FR groups relative to AL groups (F diet; 1,24 = 21.5, p<.0001), and planned comparisons attributed this difference to the two FR groups that had a history of sucrose intake whose levels were higher than all AL groups (p at least <.05).
Levels of the control synaptic protein, PSD95, did not differ among groups.
Experiment 3
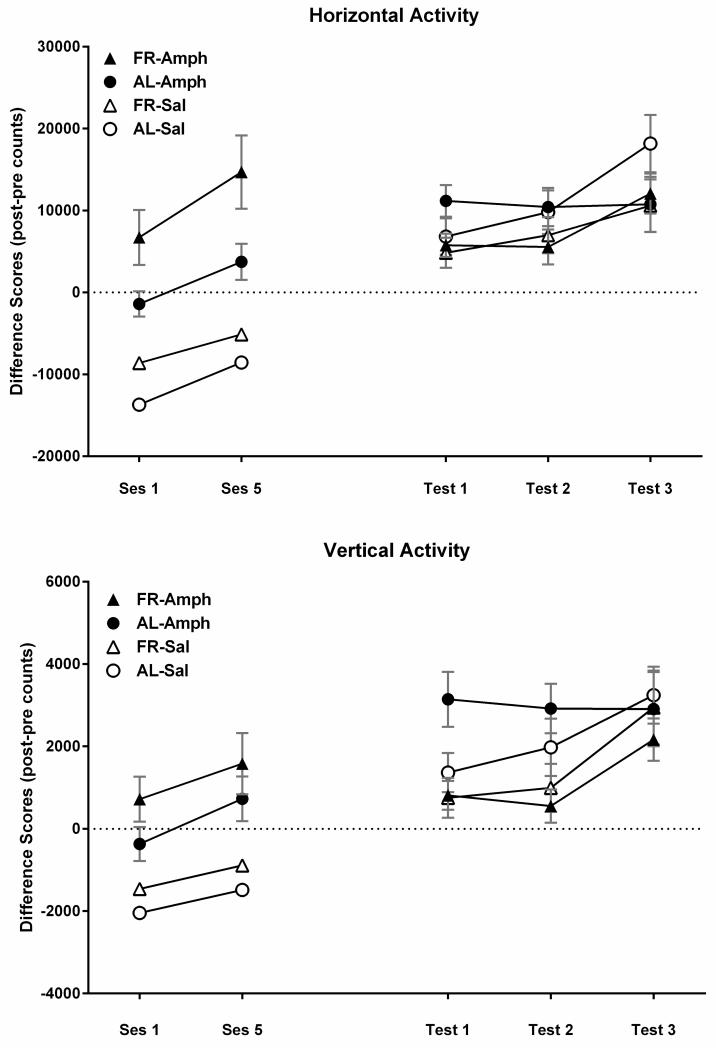
In the two locomotor activity test sessions, which bracketed three home-cage injection treatments, amphetamine increased horizontal (F1,44 = 61.2, p<.001) and vertical activity (F1,44=33.8, p<.001), and FR rats displayed greater activity than did AL rats on both measures (horizontal: F1,44=13.1, p<.001; vertical: F1,44=4.52, p<.05). In addition, both feeding groups displayed an increase in amphetamine-induced activity between the first and fifth injection, indicating sensitization (Fig 6; horizontal: F1,44=21.6, p<.001; vertical: F1,44=15.6, p<.001).
Figure 6.
Mean (± s.e.m) difference scores (post-injection minus pre-injection) for horizontal (top) and vertical activity (bottom) induced by d-amphetamine (1.0 mg/kg, i.p.) or saline vehicle during the treatment (left) and challenge (right) phases of d-amphetamine-induced hyperactivity testing. During treatment, separate groups of ad libitum fed (AL) and food-restricted (FR) rats (n=8) were injected with d-amphetamine or saline every 48-72 hours. Activity was recorded in response to the first and fifth injections, with intervening injections administered in the home cage. The test phase was initiated 3 weeks after all FR rats had been returned to AL feeding. D-amphetamine was injected once per week for 3 consecutive weeks and activity measures were taken in the two former FR groups and the two groups that had been AL throughout (see text for statistical analyses).
Following the return of FR rats to AL feeding conditions, daily monitoring suggested that 24-h intake of chow normalized by the end of the second week (Fig 7). This was confirmed by absence of difference between groups across the final four measurement days of the third week (Fdiet;1,44 =0.25; Fdiet × days; 3,138 =1.0). During the 21 days of intake normalization, body weights of the previously FR group increased from 296 ± 4.1 to 473 ± 9.7 g. During the same period, body weights of the AL group increased from 455 ± 7.7 to 499 ± 8.6 g (Fig 8). The rate of body weight gain from day 22 through the end of the testing phase, three weeks later, did not differ between groups; slope of the regression line describing body weight gain over test days in previously FR rats was 6.75, and the slope in AL rats was 6.15.
Figure 7.
Mean (± s.e.m) 24 hour intake of LabDiet 5001 for each treatment group (n=8) in Experiment 3. Following the restoration of ad libitum (AL) feeding, the two FR groups displayed a compensatory increase in 24 hr intake. Their intakes normalized during the third week, in which there was no difference across the final four measurements between subjects that did and did not have a history of FR.
Figure 8.
Mean body weights (± s.e.m) of the four treatment groups (n=8) during each phase of Experiment 3: (i) at baseline, before diet manipulation and treatment, (ii) when two groups were food-restricted (FR) and all groups were then habituated to the activity chamber on 6 occasions followed by sensitizing (sntz) injections of d-amphetamine (1.0 mg/kg, i.p.) or saline vehicle on five occasions, (iii) during restoration of ad libitum (AL) feeding in the FR groups, and (iv) during the three weekly d-amphetamine challenge test sessions. The rates of body weight gain during the four weeks of testing were similar for rats with and without a history of FR.
In the first follow-up amphetamine challenge test session, the two diet groups previously treated with amphetamine responded differently in horizontal (Fdiet × test;1,22 = 14.99, p<.001) and vertical activity (Fdiet × test;1,22 = 16.09, p<.001) relative to their 5th treatment session three weeks earlier (Fig 6);. A three-way ANOVA over the three weeks of amphetamine challenge testing, showed that vertical activity was overall greater in the AL groups than in the groups that had previously been FR (Fdiet;1,44=5.47, p<.025) with a similar, but not significant, trend in horizontal activity (Fdiet;1,44=3.3, p=.076).
DISCUSSION
In the behavioral component of the present study, half of the subjects in each diet group had a history of ten 1-h sessions in which they consumed a limited amount of sucrose. The rationale for this treatment was based on previous biochemical observations. It had been observed that when AL and FR rats consumed a relatively small and equal volume of 10% sucrose solution, FR but not AL rats displayed increased NAc phosphorylation of GluA1 on Ser845 (Carr et al., 2010). This effect was likely a consequence of increased sucrose-induced DA release, as demonstrated by Avena and coworkers in underweight rats (Avena et al., 2008b), and upregulated signaling downstream of the D1 DA receptor and/or NMDA receptor, as previously demonstrated using the present FR protocol (Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005; Carr et al., 2010). In addition, when AL and FR diet groups consumed sucrose in a series of daily brief access periods, both displayed increased synaptic abundance of GluA1 and GluA2 immediately after the final episode of intake, with a greater effect in FR than AL rats (Peng et al., 2011). Thus, based on the role of GluA1-containing AMPA receptor phosphorylation and trafficking in synaptic plasticity and behavior modification, it was hypothesized that following the restoration of AL feeding and normalization of daily chow intake, rats with a history of sucrose intake during FR would continue to display greater abundance of AMPARs in the NAc PSD, relative to rats with a history of sucrose intake without FR, and rats with a history of FR without sucrose, as well as an overall greater intake of sucrose during intermittent 1-h access periods. The overarching hypothesis, of which this study was an initial test, is that episodic intake of highly palatable food during periods of severe dieting interact with upregulated mechanisms of synaptic plasticity to produce a long-lasting increase in reward-driven feeding.
Results indicated that subjects with a history of sucrose intake during FR maintained a high level of sucrose consumption throughout the four weeks of testing, which was greater than both of the groups that had been AL. This behavior was not likely a result of prior FR alone because the pattern of sucrose consumption in rats that had been FR without prior sucrose intake was significantly different. This difference is one finding suggesting that the sustained increase in sucrose intake is not simply residual homeostatic overconsumption. Additional findings supporting this conclusion are: (1) 24-h chow intake did not differ between groups after the second week of restored AL feeding, (2) the rate of body weight gain in the AL groups and former FR groups were similar across the four weeks of sucrose intake testing, (3) there was no correlation between sucrose intake and body weight in either of the feeding groups, (4) circulating leptin levels at a time-point corresponding to the 4th week of sucrose testing did not differ between former FR and AL groups, and (5) former FR subjects were less, rather than more, responsive to the locomotor-activating effect of amphetamine than subjects which had been AL throughout. This latter finding is of particular interest in as much as it suggests that the increased responsiveness to both sucrose (Domingos et al., 2011) and amphetamine (Deroche et al., 1995; Cabeza de Vaca and Carr, 1998) during active FR do not carry over together into recovery. This would seem further evidence that the CNS underpinnings of enhanced incentive effects during FR are no longer present, or at least no longer predominant, after 3-7 weeks of recovery. In fact, the divergence of sucrose and amphetamine responsiveness during recovery from FR is similar to the divergence seen in AL subjects maintained on a palatable high-energy diet; rewarding effects of sucrose escalate (La Fleur et al., 2007) while rewarding effects of psychostimulants diminish (Wellman et al., 2007; Davis et al., 2008). The present divergence is the first evidence, to our knowledge, that recovery from underfeeding is accompanied by diminished responsiveness to a psychostimulant drug of abuse. The amphetamine protocol used in this study is one that has been shown to facilitate future acquisition and progressive ratio responding for i.v. self-administration of d-amphetamine (Vezina, 2004). Thus, it may be the case that rebound hyperphagia and weight gain, which are common in abstinent psychostimulant addicts (Edge and Gold, 2011), provide some degree of buffering against relapse.
The biochemical results indicate that a history of sucrose intake during FR produces changes in synaptic abundance of AMPARs in NAc that are evident ~4 weeks after subjects have been returned to AL feeding. The subset of these subjects taken directly from the home cage displayed higher levels of GluA2, GluA3, and pSer845-GluA1 in the PSD relative to the FR group with no history of sucrose intake and all groups that had been AL fed throughout the study. A speculative interpretation of these findings is that the increased synaptic abundance of GluA1 and GluA2 observed in FR rats immediately after a bout of sucrose intake (Peng et al., 2011) were replaced by constituitively cycled GluA2/GluA3, which have been shown to preserve synaptic strength following a transient activity-dependent synaptic incorporation of GluA1-containing AMPA receptors (Shi et al., 2001). The elevated level of pSer845-GluA1 in this group suggests that even several weeks out from FR, GluA1-containing AMPARs are being stabilized in the membrane and primed for synaptic delivery. Phosphorylation on Ser845 prevents lysosomal degradation of surface GluA1-containing AMPARs (He et al., 2009) and facilitates trafficking to the extrasynaptic membrane (Oh et al., 2006). Synaptic incorporation may depend on presentation of an adequate stimulus and resultant phosphorylation on Ser818 by protein kinase C (Boehm et al., 2006). Recently, the two-step mechanism of synaptic incorporation, involving D1 DA receptor mediated phosphorylation on Ser845 and PKC-dependent synaptic incorporation triggered by Ca2+ has been demonstrated in striatal MSNs in culture (Tukey and Ziff, 2013). Moreover, in AL fed rats that engaged in daily brief bouts of sucrose intake, electron microscopy revealed that a terminal bout of sucrose intake was the adequate stimulus for rapid synaptic incorporation (Tukey et al., 2013).
Interestingly, rats with a history of FR, irrespective of sucrose intake, displayed elevated levels of GluA1 in the NAc PSD. This increase was previously seen in subjects during active FR (Peng et al., 2011; Peng et al., 2014). Because GluA1 is the only AMPAR subunit with increased abundance in the PSD of “unstimulated” FR rats it was hypothesized that FR increases synaptic incorporation of GluA2-lacking, Ca2+-permeable AMPARs. This hypothesis was supported by findings that NASPM, a selective antagonist of Ca2+-permeable AMPARs, microinjected in NAc, reversed the enhancing effect of FR on rewarding effects of amphetamine (Peng et al., 2014) and a D1 DA receptor agonist (Carr et al., 2010). The apparent continuation of increased GluA1 in the NAc PSD after restoration of AL feeding may contribute to the sustained increase in sucrose consumption of rats with a history of sucrose intake during FR, and the escalation of sucrose consumption over weeks in rats with a history of FR without sucrose. The continued elevation of GluA1 raises the question of why rats with a history of FR did not display an enhanced response to amphetamine after restoration of AL feeding. One possibility is that the separable ventral striatal neuronal populations that mediate reward and locomotor stimulation are differentially subject to the synaptic incorporation of GluA1 or its continuation after restored AL feeding (Sellings and Clarke, 2003). A second possibility concerns the involvement of ventral tegmental area and NAc in the induction and expression, respectively, of locomotor sensitization (Perugini and Vezina, 1994; Cador et al., 1995); recovery from FR may alter the dynamic between these two brain regions in such a way that sensitization is reversed and/or the mechanism accounting for “incubation” of sensitization during the 3-wk hiatus between treatment and the first challenge is impeded. It is also possible that unidentified CNS concomitants of recovery from FR supersede the contribution of increased GluA1 in relation to amphetamine- but not sucrose-induced behavior. Finally, a more complete understanding of biochemical findings as they relate to both sucrose and amphetamine behavioral effects will be enabled by differential evaluation of D1- vs D2-expressing medium spiny neurons (MSNs) in NAc, in as much as excitation of D1-expressing MSNs facilitates reward and locomotor activity while excitation of D2-expressing MSNs inhibits both (Lobo et al., 2010; Lobo and Nestler, 2011; Kravitz et al., 2012). Methods of the present study do not enable this differentiation.
Overall, the present study provides evidence suggestive of both synaptic strengthening and priming for further activity-dependent synaptic plasticity in rats with a history of sucrose intake during FR. Among the many key questions to be addressed going forward are how long these changes continue beyond the return to AL feeding, and whether a causal relationship between AMPAR trafficking/synaptic abundance and sucrose seeking and consumption can be demonstrated. The present finding that rats with a history of sucrose intake during FR consume more sucrose than rats that have always been AL, with or without a history of sucrose intake, is consistent with this possibility. In addition, the finding that a terminal bout of sucrose intake in rats with a history of FR and sucrose showed the highest levels of pSer845-GluA1, GluA2, and a trend toward higher GluA1, in the PSD would seem consistent with the idea that a history of FR and sucrose primes activity-dependent trafficking of AMPARs.
Excessive reward-driven feeding is a significant public health problem. The oftentimes counterproductive, or “boomerang”, effect of dieting on future food intake and body weight, and the role of severe dieting as a risk factor for binge pathology are well established. Similarly, episodes of loss of control during periods of severe dieting are common. The present study supports the possibility that in mimicking an adaptive response to food scarcity, severe dieting upregulates mechanisms of synaptic plasticity in NAc, such that episodic intake of highly palatable energy-dense food ingrains that behavior in a manner that leads to excessive intake after free feeding conditions have been restored.
Highlights.
Episodic sucrose intake during food restriction increases future sucrose intake;
Recovery from food restriction decreases sensitivity to amphetamine;
A history of food restriction with episodic sucrose intake increases pSer845-GluA1, GluA2, and GluA3 in the accumbens PSD
ACKNOWLEDGEMENTS
This research was supported by the Klarman Family Foundation Grants Program in Eating Disorders Research, DA003956, DA036784, and T32 DA007254 from NIDA/NIH.
Abbreviations
- NAc
nucleus accumbens
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PSD
postsynaptic density
- FR
food restriction
- AL
ad libitum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or financial ties to disclose.
REFERENCES
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003a;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neurosci. 2003b;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neurosci. 2008;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MR, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neurosci. 1999a;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999b;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates of the induction and expression of behavioral sensitization to amphetamine. Neurosci. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de, Vaca, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neurosci. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Corwin RLW, Wojnicki FHE. Binge-type eating induced by limited access to optional foods. In: Avena NM, editor. Animal Models of Eating Disorders. Springer; New York: 2013. pp. 51–68. [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hayashizaki S, Cheer J, Hampson RE. Reward, memory and substance abuse: functional neuronal circuits in the nucleus accumbens. Neurosci Biobehav Rev. 2004;27:703–711. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MO, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids: I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: A case of homology? Physiol Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge PJ, Gold MS. Drug withdrawal and hyperphagia. Lessons from tobacco and other drugs. Curr Pharmaceut Design. 2011;17:1173–1179. doi: 10.2174/138161211795656738. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely AV, Childress AR, Jagannathan K. Differential reward response to palatable food cues in past and current dieters: An fMRI study. Obesity. 2013 doi: 10.1002/oby.20599. Epub: 20 September 2013, DOI: 10.1002/oby.20599. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann NY Acad Sci. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Lane DA, Colago EEO, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210:750–761. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Haberny S, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes. 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5:1–11. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:1–11. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, Maille C, McKinney S, Stice E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. 2006;47:83–90. doi: 10.1016/j.appet.2006.03.160. [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Peng X-X, Cabeza de Vaca S, Ziff E, Carr KD. Involvement of nucleus accumbens AMPA receptor trafficking in augmentation of d-amphetamine reward in food-restricted rats. Psychopharmacol. 2014;231:3055–3063. doi: 10.1007/s00213-014-3476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-X, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011;65:1024–1031. doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Cavanaugh D. Differential changes in signal and background firing of accumbal neurons during cocaine self-administration. J Neurophysiol. 2003;90:993–1010. doi: 10.1152/jn.00849.2002. [DOI] [PubMed] [Google Scholar]
- Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994;270:690–696. [PubMed] [Google Scholar]
- Polivy J, Herman PC. An evolutionary perspective on dieting. Appetite. 2006;7:30–35. doi: 10.1016/j.appet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman PC, Coelho JS. Caloric restriction in the presence of attractive food cues: External cues, eating, and weight. Physiol Behav. 2008;94:729–733. doi: 10.1016/j.physbeh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci (USA) 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav. 1989;14:387–397. doi: 10.1016/0306-4603(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor Stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–62303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff E. A novel GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:270–288. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller NP, Marti NC. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J Abnorm Psychol. 2008;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey D, Ferreira J, Antoine S, D’Amour J, Ninan I, Cabeza de Vaca S, Incontro S, Horwitz J, Hartner D, Guarini C, Khatri L, Goffer Y, Xu D, Titcombe R, Khatri M, Marzan D, Mahajan S, Wang J, Froemke R, Carr KD, Aoki C, Ziff E. Sucrose ingestion induces rapid AMPA receptor trafficking. J Neurosci. 2013;33:6123–6132. doi: 10.1523/JNEUROSCI.4806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey DS, Ziff EB. Ca2+-permeable AMPA (-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid) receptors and dopamine D1 receptors regulate GluA1 trafficking in striatal neurons. J Biol Chem. 2013;288:35297–35306. doi: 10.1074/jbc.M113.516690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vitousek KM, Gray JA, Grubbs KM. Caloric restriction for longevity: I. Paradigm, protocols and physiological findings in animal research. Eur Eating Disorders Rev. 2004a;12:279–299. [Google Scholar]
- Vitousek KM, Manke FP, Gray JA, Vitousek MN. Caloric restriction for longevity: II – The systematic neglect of behavioural and psychological outcomes in animal research. Eur Eating Disorders Rev. 2004b;12:338–360. [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiat Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–574. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinforcement of cocaine-seeking behavior in rats. Addict Biol. 2011;18:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liu S, Cabeza de, Vaca S, Carr KD. Effects of time of feeding on psychostimulant reward,conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacol. 2013;227:307–320. doi: 10.1007/s00213-013-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]