Abstract
Objective:
Seizures can cause vestibular symptoms, even without obvious epileptic features. We sought to characterize epileptic vertigo or dizziness (EVD) to improve differentiation from nonepileptic causes, particularly when vestibular symptoms are the sole manifestation.
Methods:
We conducted a systematic review with electronic (Medline) and manual search for English-language studies (1955–2014). Two independent reviewers selected studies. Study/patient characteristics were abstracted. We defined 3 study population types: (1) seizures, some experiencing vertigo/dizziness (disease cohort); (2) vertigo/dizziness, some due to seizures (symptom cohort); (3) vertigo/dizziness due to seizures in all patients (EVD-only cohort).
Results:
We identified 84 studies describing 11,354 patients (disease cohort = 8,129; symptom cohort = 2,965; EVD-only cohort = 260). Among 1,055 EVD patients in whom a distinction could be made, non-isolated EVD was present in 8.5%, isolated EVD in 0.8%. Thorough diagnostic workups (ictal EEG, vestibular testing, and brain MRI to exclude other causes) were rare (<0.1%). Ictal EEG was reported in 487 (4.3%), formal neuro-otologic assessment in 1,107 (9.7%). Localized EEG abnormalities (n = 350) were most frequently temporal (79.8%) and uncommonly parietal (11.8%). Duration of episodic vestibular symptoms varied, but was very brief (<30 seconds) in 69.6% of isolated EVD and 6.9% of non-isolated EVD.
Conclusions:
Non-isolated EVD is much more prevalent than isolated EVD, which appears to be rare. Diagnostic evaluations for EVD are often incomplete. EVD is primarily associated with temporal lobe seizures; whether this reflects greater epidemiologic prevalence of temporal lobe seizures or a tighter association with dizziness/vertigo presentations than with other brain regions remains unknown. Consistent with clinical wisdom, isolated EVD spells often last just seconds, although many patients experience longer spells.
Vertigo (defined as a sensation of self-motion when no self-motion is occurring or as the sensation of distorted self-motion during an otherwise normal head movement)1 or dizziness (defined as a sensation of disturbed or impaired spatial orientation without a false/distorted sense of motion)1 are common symptoms, often reported by patients known to have epileptic seizures. These vestibular symptoms may be related to the seizure itself (i.e., representing an aura symptom), to side effects of antiepileptic drug (AED) therapy, or may be linked to a second, comorbid nonepileptic disorder (e.g., vestibular migraine). Episodic vestibular symptoms deemed to result directly from focal, intermittent epileptic discharges have been variously known as epileptic vertigo,2 vestibular epilepsy,3 vestibular seizures,4 vertiginous seizures,4–6 or epilepsia tornado.7 In this article, we use the more inclusive term epileptic vertigo or dizziness (EVD).
Seminal work by Penfield and Jasper8 established the scientific basis for understanding the pathophysiology of EVD. By means of surface electrical stimulation, they elicited illusionary transient sensations of rotation, translation, or undirected motion in their patients, mapping the extent of the vestibular cortex and demonstrating that seizures cause vertigo/dizziness because they lead to hyperactivity in vestibular cortical areas. They described EVD during both temporal and parietal lobe stimulation. These cortical areas were confirmed by Kahane et al.,9 reporting vestibular sensations after electrical stimulation centered around a lateral cortical temporo-parietal area, known as the temporo-peri-Sylvian vestibular cortex. Less frequently, Kahane et al. found vestibular sensations by stimulating within the occipital, frontal, and insular cortex. Congruent but somewhat more expansive than these experimentally derived results, epileptic discharges on ictal EEG in symptomatic EVD were individually localized to frontal,2 frontotemporal,10 temporal,11,12 temporo-parieto-occipital,13 and parietal14 areas, suggesting either processing of vestibular-related input across large cortical regions15 or spread of excitation to or from nearby regions. Lesion studies in humans provide additional insights into the extent of cortical network processing of vestibular/graviceptive inputs. Insular and parietal lesions dominate the literature on vertigo/dizziness from stroke,16 including the seminal study of Brandt et al.,17 reporting shifts in perceived verticality in lesions affecting the posterior insula.
The differential diagnosis for isolated episodic vertigo/dizziness is broad and cuts across medical specialties, including neurology (vestibular migraine, TIA, seizures), otolaryngology (Meniere disease, vestibular paroxysmia), internal medicine (hypoglycemia), cardiology (arrhythmia), and psychiatry (panic attacks), so knowing when (or when not) to consider EVD would be helpful. When obvious seizure-related symptoms or signs (e.g., a generalized convulsion) accompany vestibular symptoms, EVD is readily recognized. EVD causing isolated vestibular symptoms was reported,2,18,19 but there is limited information about prevalence and clinical characteristics (e.g., accompanying symptoms, duration) that might help distinguish it from nonepileptic causes.
We performed a systematic review of studies reporting on (1) vestibular symptoms in seizure patients (disease cohort), (2) seizures in vestibular patients (symptom cohort), and (3) clinical details in reports of patients with EVD (EVD-only cohort). The aim of this work was to better characterize the prevalence of non-isolated EVD (ni-EVD) and isolated EVD (i-EVD) in adults and children, seizure localization, and episode duration.
METHODS
A MEDLINE search was performed (April 2014) to identify articles reporting on EVD using terms related to seizures/epilepsy and vertigo/dizziness. Both retrospective and prospective case series and single case reports were eligible. Studies sought for reported on the differential diagnosis of dizzy patients or on aura symptoms in patients with a given type of epilepsy. Specific search terms and inclusion/exclusion criteria can be found in appendix e-1 on the Neurology® Web site at Neurology.org. We identified 1,137 electronic citations for screening and included 84 studies (appendix e-1; flow chart). When extracting the data from the selected studies, we assessed the type of the study, whether the vestibular symptoms were isolated or accompanied by other seizure symptoms, the duration of EVD episodes, and EEG findings. In all EVD patients, we rated the quality of EEG and vestibular assessment and the quality of the association between seizures and vertigo/dizziness (table 1).
Table 1.
Rating of EEG and vestibular assessment and strength of association between seizures and vertigo/dizziness

A single expert epileptologist (P.W.K.) assessed all published EEG recordings from the included studies for quality to establish a definitive diagnosis of EVD. Seizure location and laterality was retrieved where EEG recordings were available and were coded as temporal, parietal, occipital, or frontal in origin. If the reported seizure location was linked to more than one of these areas (e.g., frontotemporal), a count was given to each lobe for calculating the relative frequency of discharges in different lobes.
RESULTS
Demographic information.
Eighty-four studies reporting on 11,354 patients matched the inclusion criteria (appendix e-2, table e-1, and table e-2). The majority of these 84 studies were retrospective case series (n = 52, 61.9%) or single cases (n = 26, 31.0%) (table e-1). The remaining 6 studies were prospective (n = 5, 6.0%) or ambispective (n = 1, 1.2%) case series. Retrospective case series were the most frequent study type both in the disease (81.1%, study size = 1–1,563 patients) and the symptom (83.3%, study size = 8–907 patients) cohort, while prospective case series were rare in either cohort (disease cohort: 8.1%, study size = 3–1,897; symptom cohort: 11.1%, study size = 70–142). Overall, 71.6% of patients were in the disease cohort, while 26.1% were in the symptom cohort. The remaining 2.3% fulfilled criteria for the EVD-only cohort (table 1). The distribution of adult and pediatric patients among the cohorts was distinct: 69% of all adults belonged to the disease cohort; 87% of all children were part of the symptom cohort. Various other diagnoses were reported in 2,499 patients (table 2).
Table 2.
Characterization of study sample
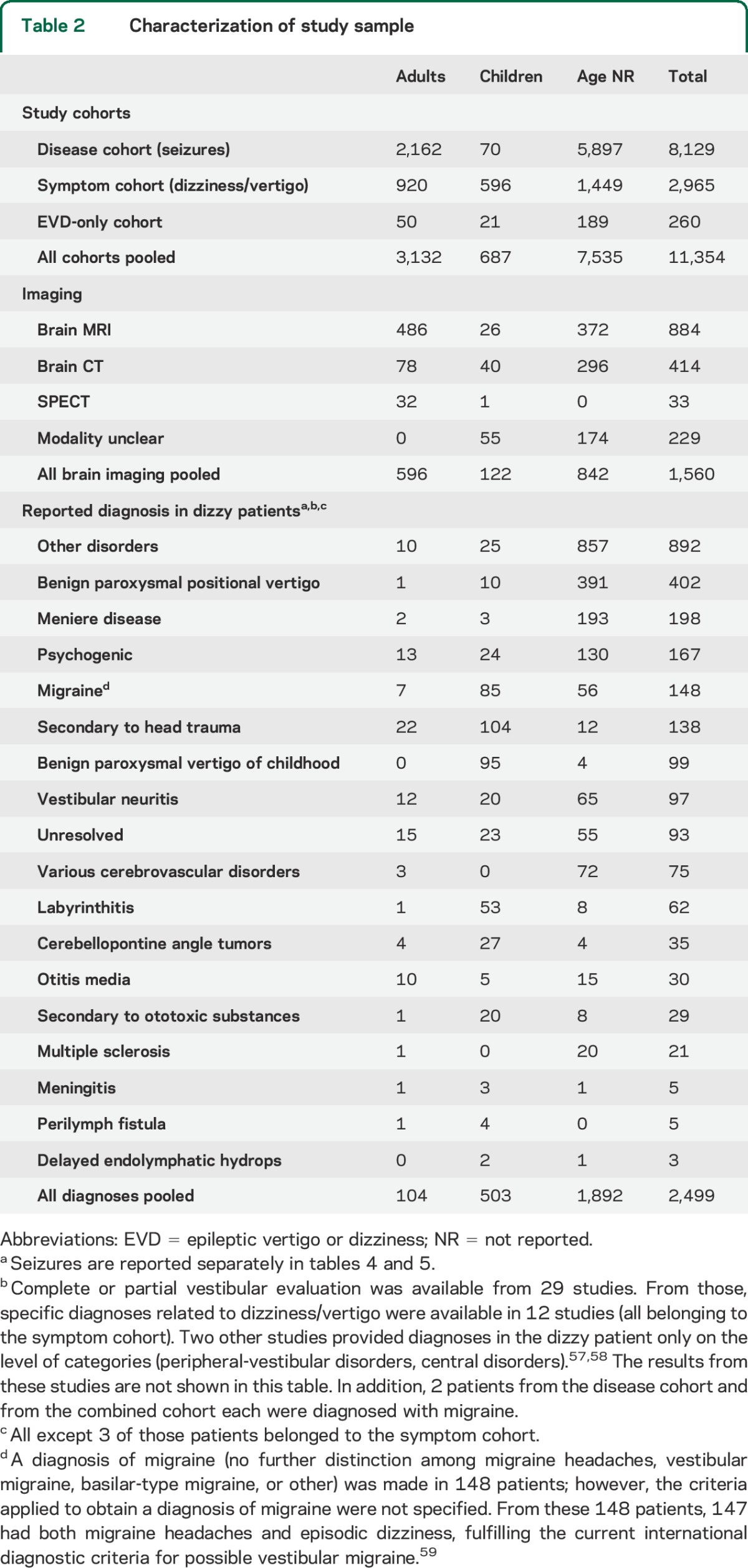
Diagnostic testing.
EEG findings were reported in 50.9% of patients. Surface recordings were available in 50.1%, intracranial recordings in 2.7%. We judged the quality of EEG assessment based on the presence/absence of EEG-documented seizures and clinical findings during these seizures. A high-quality (ictal) EEG assessment was found in 4.3%, a medium-quality (interictal) EEG assessment in 46.6%. In 49.1%, the electrographic assessment was considered low quality, and the diagnosis of seizures was made on the clinical history alone (table 3).
Table 3.
Summary of vestibular and EEG assessment
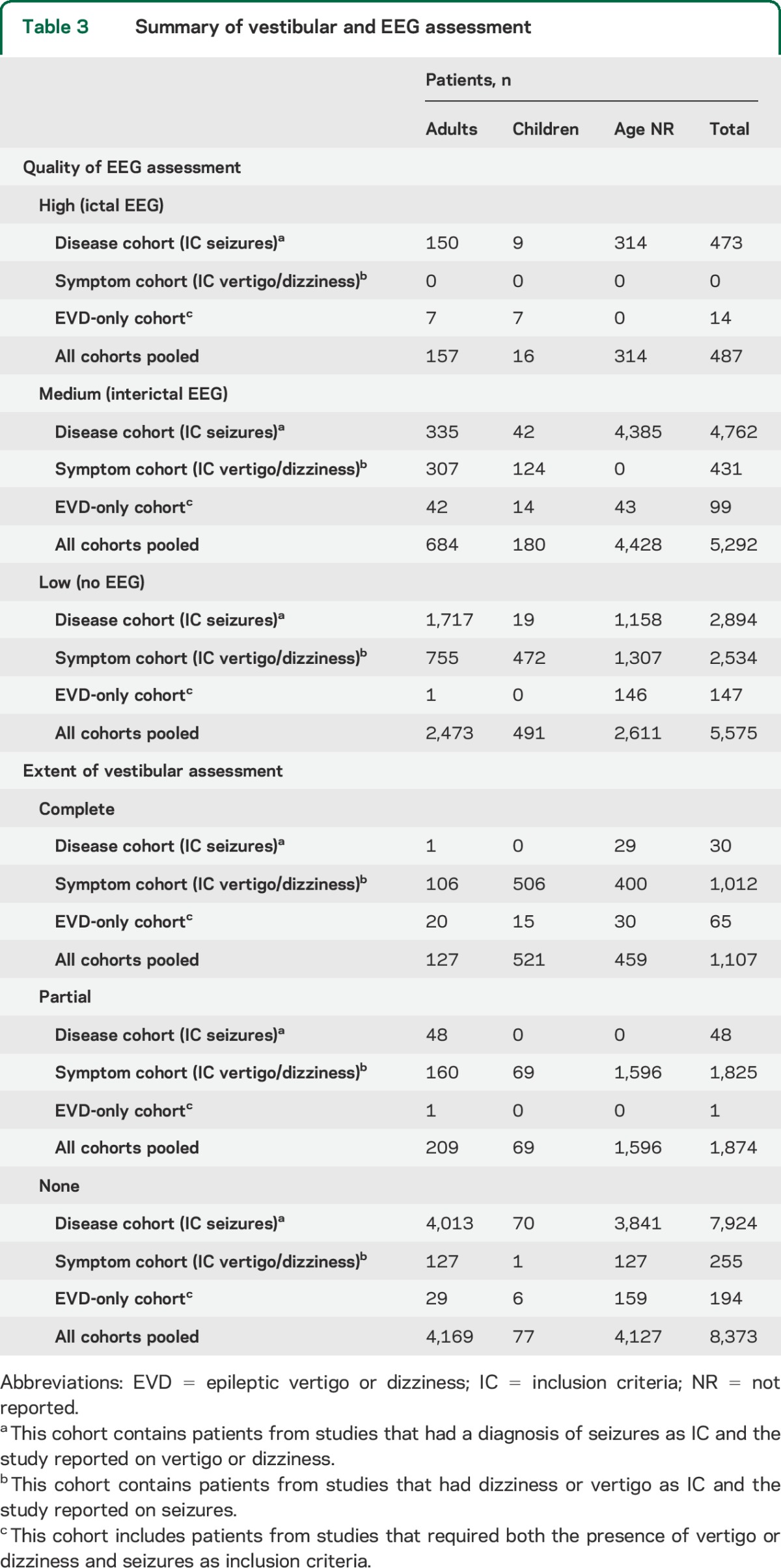
Neuroimaging was available in 13.7%, vestibular testing in 26.3% (table 3). In many of these patients, results from vestibular testing were not reported and only in 4 patients (0.04%) was complete vestibular testing accompanied by ictal EEG and MRI.
Fraction of patients with ni-EVD and i-EVD.
From 11,354 patients included (disease cohort = 8,129; symptom cohort = 2,965; EVD-only cohort = 260), 1,055 patients (9.3%) had EVD, and were coded as ni-EVD (n = 967, 8.5% of total) or i-EVD (n = 88, 0.8% of total) (table 4). Of all EVD patients, 64.7% were in the disease cohort, 10.7% were in the symptom cohort. The frequency for diagnosing i-EVD or ni-EVD was about 2-fold greater for patients in the disease cohort (683/8,129, 8.4%) relative to the symptom cohort (113/2,965, 3.8%).
Table 4.
Distribution of patients with EVD with regards to strength of association between EVD and EEG
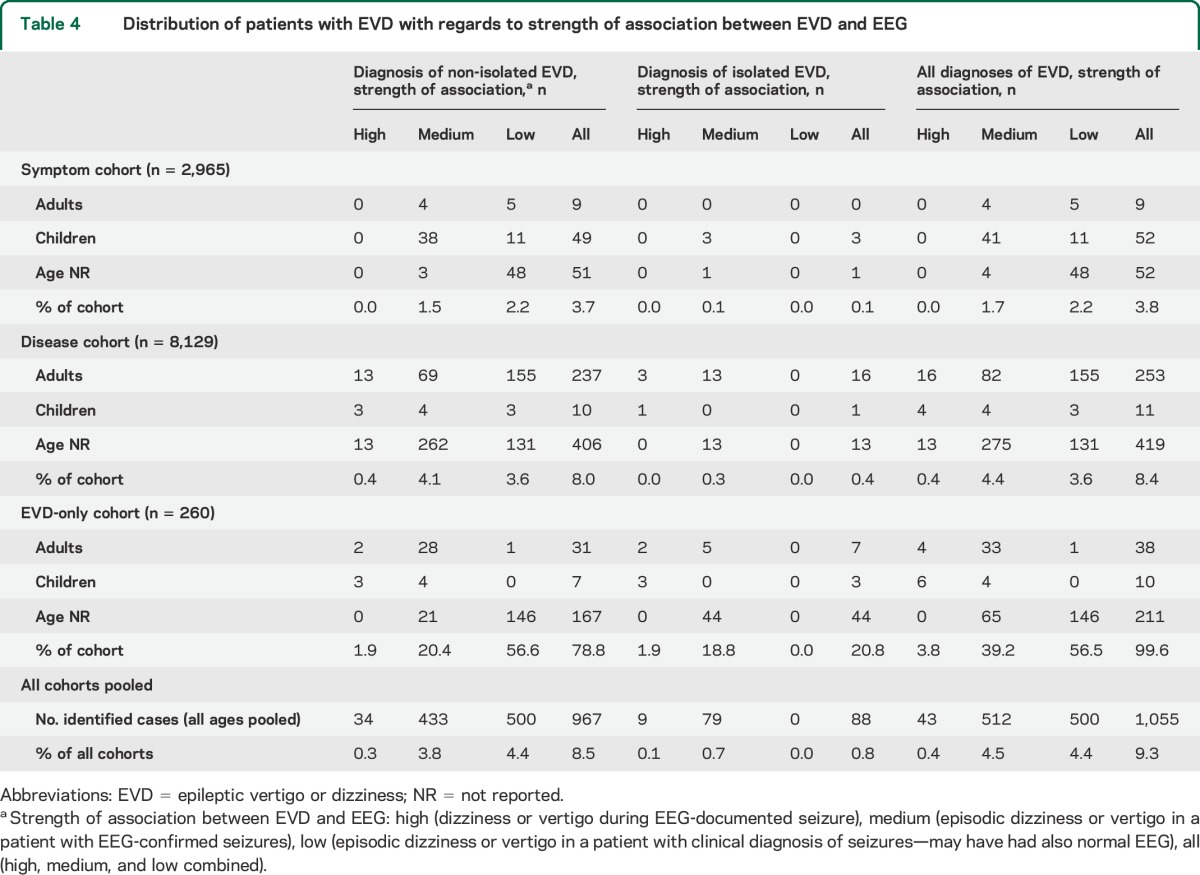
In the study sample, the overall fraction of EVD in children (73/687, 10.6%) and adults (300/3,132, 9.6%) was similar, but the distribution was heterogeneous across study cohorts. In the symptom cohort, EVD was diagnosed nearly 9-fold more often in children (52/596, 8.7%) than in adults (9/920, 1.0%); by contrast, in the disease cohort, EVD was diagnosed just 1.3-fold more often in children (11/70, 15.7%) than adults (253/2,162, 11.7%).
Quality of the association between epilepsy and vertigo/dizziness.
From all EVD patients, a high-quality association was found in 4.1% (43/1,055), while medium (512/1,055, 48.5%) or low (500/1,055, 47.4%) quality was noted in the remaining 95.9%. Published EEG traces from EVD cases (n = 33) were reviewed. From the 14 EEG recordings originally rated as ictal, this was confirmed in 11, while in 2 cases19,20 the EEG data presented was only probably consistent with an ongoing seizure and in one case21 no seizure was depicted. Diagnosis in the remaining 19 cases (interictal EEG changes) was confirmed.
Seizure location.
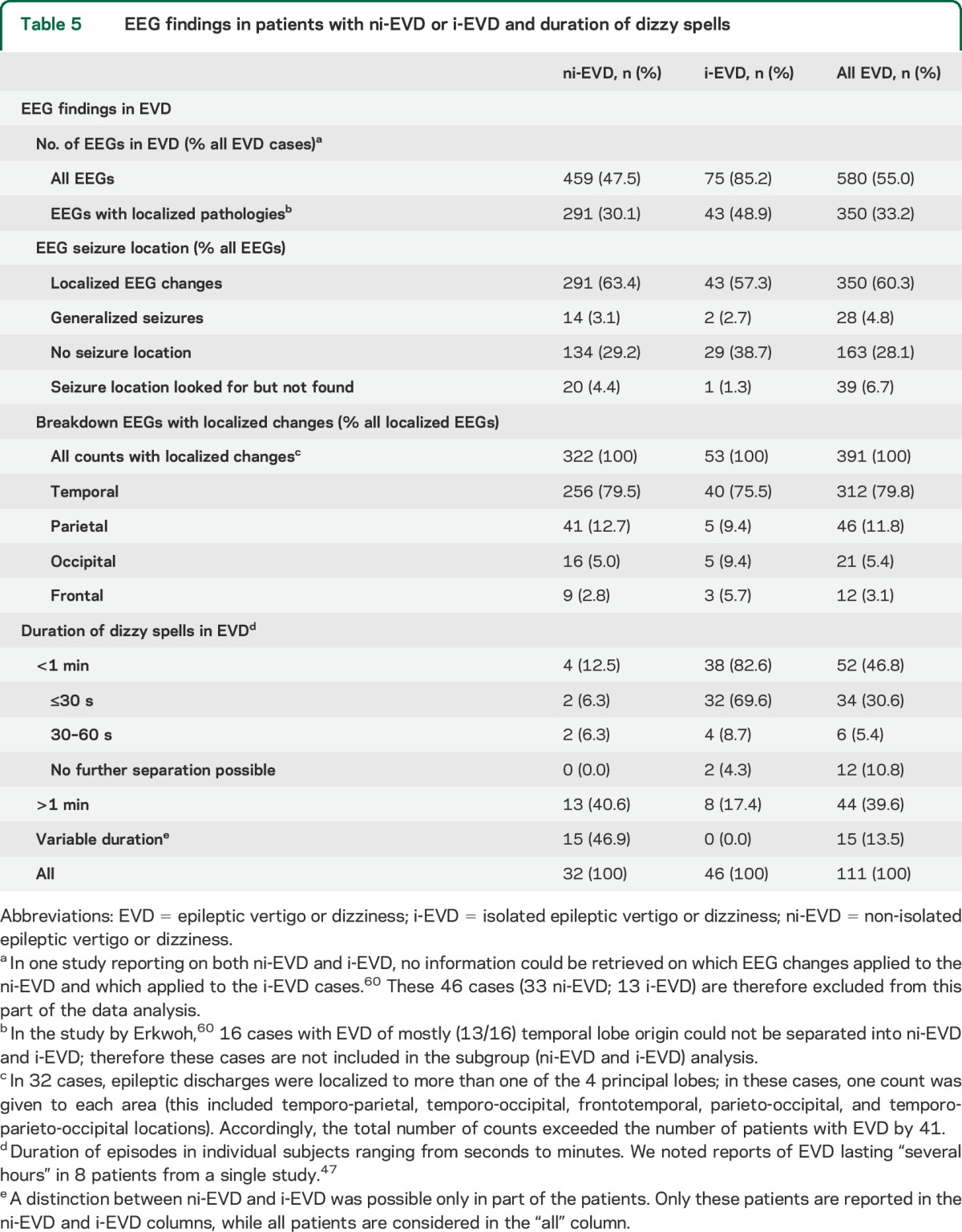
Results from ictal/interictal EEG recordings were available in 580 EVD patients (table 5). EEG seizure locations (n = 350) were assigned as described in the Methods. Since more than one location was reported in 73 patients, the total count (n = 391) exceeded the number of EVD patients with localized EEG changes. The most frequent location was temporal (79.8%); parietal (11.8%), occipital (5.4%), and frontal (3.1%) seizures were found in small fractions.
Table 5.
EEG findings in patients with ni-EVD or i-EVD and duration of dizzy spells
Lateralization of focal EEG changes.
From the 350 EVD cases with localized EEG changes, the side of the EEG focus was reported in 126 (36.0%). Right-hemisphere (n = 51) and left-hemisphere (n = 57) seizure foci occurred with similar frequency (40.5% vs 45.2%, Fisher exact p = 0.50). In 18 cases, a bihemispheric focus was observed. In ni-EVD (n = 86; 10 bilateral foci), right- and left-hemisphere foci had identical frequency (n = 38, 44.2% each). For i-EVD (n = 40; 8 bilateral foci), left-hemispheric foci were slightly more frequent than right-hemispheric foci (19 vs 13; 47.5% vs 32.5%, p = 0.21).
For cases with information on sidedness of focal EEG changes (n = 126), we assessed laterality by seizure location (total of 163 locations recorded). There were clinically meaningful but not statistically significant differences in sidedness based on the seizure location (right % [n] vs left % [n]): (1) temporal (37.8% [37] vs 44.9% [44], p = 0.35); (2) parietal (59.0% [23] vs 35.9% [14], p = 0.06); (3) occipital (62.5% [10] vs 25% [4], p = 0.06); and (4) frontal (30.0% [3] vs 70.0% [7], p = 0.18).
Duration of EVD and effect of treatment.
Specific numbers on EVD duration were available in 108 patients (table 5). Comparing i-EVD and ni-EVD cases, brief spell duration (≤30 seconds) was noted in a much larger fraction in i-EVD cases than ni-EVD cases (69.6% vs 6.9%, p < 0.001).
Effect of treatment with various AEDs was reported in 110 patients (10.4%), with 90.0% considered responders. Among responders with details on drug treatment (n = 54), phenytoin (n = 16), carbamazepine (n = 12), and valproate (n = 5) were applied most often (table e-2).
Type of vestibular and accompanying seizure symptoms.
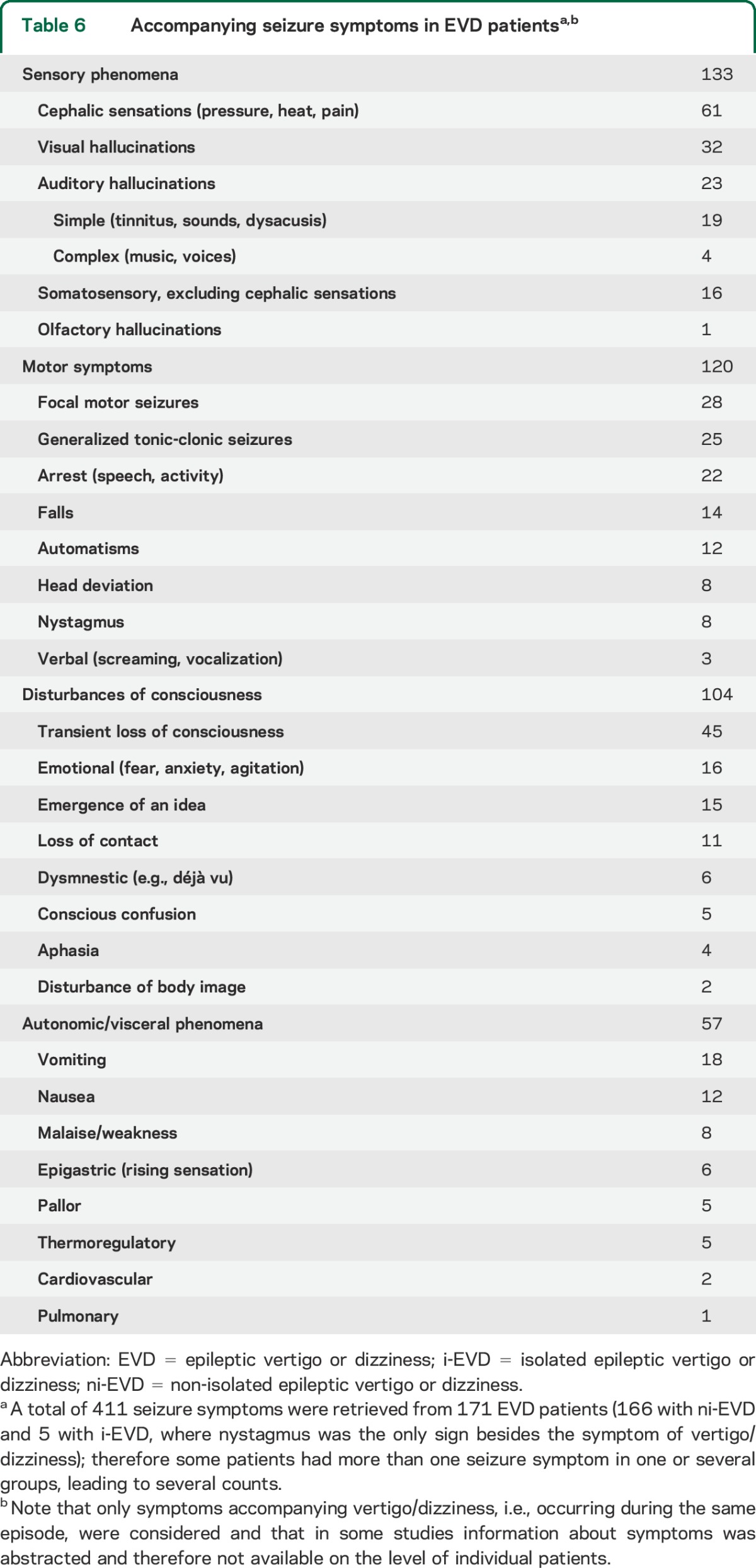
A majority of studies did not provide definitions of the terms used to describe the vestibular spells (9,506 patients, 83.7%), including vertigo or vertiginous (6,302, 55.5%), dizziness (93, 0.8%), and vertigo or dizziness (3,114, 27.4%). Definitions for vertigo and dizziness were provided in 9 studies (vertigo = 304 patients [2.7%], dizziness = 2 patients [0.2%]) (appendix e-1). In 3 studies (1,539, 13.6%), the 4 categories brought forward by Drachman and Hart22 (dizziness vs vertigo vs lightheadedness vs faintness) were used. From the 1,055 EVD patients, definitions for vertigo (n = 135, 12.8%) and dizziness (n = 2, 0.2%) were provided in 137. Information about other seizure symptoms/signs occurring in the same epileptic attack could be retrieved from 171 EVD patients (table 6).
Table 6.
Accompanying seizure symptoms in EVD patientsa,b
DISCUSSION
Our systematic review of EVD in the medical literature offers quantitative confirmation for the clinical intuition that EVD is uncommon, and isolated EVD is rare (although it is probably more prevalent in children presenting vestibular symptoms than in adults). It further confirms that, when EVD is isolated, episodes are most often very brief (lasting seconds), although our results also suggest that longer episodes (lasting minutes) are not uncommon. This study suggests 2 other findings that are somewhat unexpected: (1) epileptic foci causing EVD are much more likely to be temporal than parietal, and (2) they are equally likely to originate in the right and left hemispheres. These results may assist clinicians in determining whether their patients presenting vertigo or dizziness should be evaluated for seizures and whether those with epilepsy require further vestibular assessment.
EVD prevalence in adults and children.
We cannot draw strong inferences about the incidence or prevalence of EVD in the general population, but our data offer some insights into the epidemiology of EVD. First, ni-EVD appears to be uncommon in both symptom (3.7%) and disease (8.0%) cohorts; since some of these might have actually been missed comorbid vestibular disorders (see below), and studies not reporting vertigo/dizziness in epilepsy or epilepsy in dizziness (i.e., with lower prevalence) would not have been captured by our search, these numbers are probably plausible upper limits for the frequency of EVD in dizziness and in epilepsy. The higher prevalence of ni-EVD in the disease cohort makes intuitive sense (i.e., known seizure patients are more likely to have epileptic dizziness than patients evaluated for dizziness).
Second, i-EVD is probably rare in both dizziness (0.1%) and in epilepsy (0.4%). It is not surprising that i-EVD would be much less common than ni-EVD. Although this difference could be attributed to diagnostic ascertainment bias,23 it seems plausible that i-EVD is, indeed, substantially less common in both symptom and disease cohorts. Again, because our search strategy was skewed toward studies seeking EVD in dizziness, these estimates probably represent plausible upper bounds, although we cannot be sure that more rigorous diagnostic ascertainment would not have identified some missed cases.
Third, as suggested previously, EVD may be more common in children than in adults.24 This claim is supported by our finding of a higher frequency of EVD in children relative to adults in our symptom cohort (ratio children:adults, 8.7:1), which suggests that EVD probably accounts for a greater fraction of vertigo/dizziness presentations in children than adults (or at least that EVD is a more frequent focus of diagnostic inquiry and publication in pediatric populations). For the disease cohort, EVD was diagnosed with roughly equal frequency (ratio children:adults, 1.1:1), indicating that seizures, when present, are about equally likely to produce vertigo/dizziness in children and adults (although this finding must be viewed cautiously given the small number of children relative to adults in this cohort: 70 vs 2,162). These findings suggest that it is not a biological interaction with age (e.g., seizures in a young brain are more likely to produce dizziness), but an epidemiologic one. In other words, the proportion of dizziness cases due to EVD is probably higher in children than adults based on different population distributions of etiologies for dizziness in children vs adults. This could be because seizures are more common in children, other causes (e.g., benign paroxysmal positional vertigo, stroke) are more common in adults, or a combination of both.
Seizure location and lateralization.
A critical role of temporal areas in self-motion perception has been previously proposed based on functional MRI studies during caloric irrigation,25 case reports with focal lesions within the temporal gyrus,26,27 and transcranial magnetic stimulation over the superior temporal gyrus.28 Illusionary sensations of self-motion can be elicited by cortical electrical stimulation within a fairly large area including temporal and parietal lobes, but also, less frequently, occipital and frontal lobes,8 and may include sensations of rotation in different planes, translations, and indefinable body motion.9 The high frequency of temporal over parietal lobe seizure location in our review does not reflect the distribution noted in these electrical stimulation studies and current expert opinion. Potentially, this discrepancy is a result of the overall higher prevalence of temporal lobe seizures compared to parietal lobe seizures in epileptic patients29 and as a result, EVD is more often associated with a temporal than a parietal location on epidemiologic rather than biological grounds.
Regardless, based on the distribution of seizure locations observed here, symptoms and signs of temporal lobe origin (e.g., rising epigastric sensations, auditory hallucinations, emotional/dysmnestic phenomena) should be sought in patients with suspected EVD. However, temporal lobe seizures may be difficult to diagnose clinically and by standard surface EEG,30,31 with invasive recordings offering improved spatial resolution and increased sensitivity.32,33 From our 580 EVD patients with EEG available, only a small fraction underwent intracranial EEG recordings. This probably suggests that, clinically, some patients with temporal lobe EVD remain undiagnosed because of a false-negative surface EEG.
Previously, a right-hemisphere dominance for EVD had been proposed,34 which is a sensible hypothesis, given the right hemisphere's predominant role in spatial perception. In our dataset, however, no statistically significant differences were found. Left-hemisphere foci were slightly more common overall in this sample. There was, however, some heterogeneity based on the focus of seizure activity: a slight left-sided predominance was noted for temporal lobe seizures, but a relatively large (and borderline statistically significant) right-sided predominance was noted in the subgroups with parietal and occipital lobe seizures.
Duration of EVD: From few seconds to several minutes.
Knowledge about the duration of vertigo/dizziness is important to narrow the differential diagnosis.35 Traditionally, EVD has been considered to last a few seconds only,18 and consequently, the clinician may have dismissed a diagnosis of EVD if spells exceeded this duration. In this review, we found brief spells (≤1 minute) in 46.3% of cases. The remaining patients presented with spells lasting several minutes (a duration typically associated with vestibular migraine,36 Meniere disease,37 or episodic ataxia38) or had spells of variable duration. Based on our data, dizzy spells exceeding a few seconds duration and lasting up to several minutes should still be considered as potentially epileptic. For the i-EVD cases, the dictum that epileptogenic vertigo/dizziness lasts a few seconds held true with a duration of ≤30 seconds in 69.6%, suggesting that the clinical presentation of seizures (isolated vertigo/dizziness vs with other seizure-like symptoms) may be linked to the duration of the dizzy spells. It is biologically plausible that short bursts of epileptic activity would be more likely to produce short-lived, isolated symptoms, while longer runs of epileptic activity would be more likely to be sustained and spread, causing longer, nonisolated episodes of EVD.
Interestingly, almost all cases with EVD localized to the temporal lobe (in which spell duration was provided) were in the range of few seconds to less than 1 minute, while the few EVD cases with nontemporal location lasted longer. This could indicate that EVD in temporal lobe seizures is associated with especially brief dizzy spells. Spontaneous spells lasting seconds are also typical in vestibular paroxysmia,39 a condition known to be exquisitely carbamazepine-responsive,40 so some such patients might readily be confused for i-EVD or vice versa, including in our series, where the quality of vestibular assessments was generally low. Whether a high frequency of attacks per day (as seen in some patients with vestibular paroxysmia)40 excludes i-EVD is unknown; the reports we reviewed described patients with daily or more frequent spells in only 2 cases.10,41 Patients with suspected vestibular paroxysmia should probably be assessed by EEG to exclude the possibility of i-EVD as an alternative cause for brief paroxysms before treatment with carbamazepine.
High rate of AED treatment response.
We found response rates to AED treatment in EVD of 90%. Some have suggested this be used as a diagnostic test to point to an ictal etiology in responders.18,34 However, treatment response information was available in only 10% and, for example, successful treatment strategies for vestibular paroxysmia and vestibular migraine include AEDs such as carbamazepine, valproate, and topiramate.40,42 Thus, response to AED treatment in patients with episodic vertigo/dizziness does not prove an epileptic origin of the spells.
Quality of EEG and vestibular and imaging assessment.
A thorough diagnostic workup (brain MRI plus complete vestibular testing plus ictal EEG) was rarely performed. In the disease cohort, only 9/34 case series placed emphasis on vestibular symptoms in study design,3,6,34,43–48 which may explain why vestibular testing was infrequent in this cohort. Likewise, in the symptom cohort, seizures were just one of many differential diagnostic considerations, explaining a low rate of obtaining EEG.
Incomplete workups in the disease cohort might have led to overdiagnosis of EVD (misattribution of dizziness from another cause to known epilepsy causing other symptoms); likewise, incomplete workups in the symptom cohort might have led to underdiagnosis of EVD. Of note, the lifetime prevalence of migraine headaches is about 2.4 times greater in patients with diagnosed epilepsy than in their relatives without epilepsy,49 and the lifetime prevalence of migraine in the epileptic population is 8%–24%.49–51 Likewise, in migraine headache patients, the lifetime prevalence of epilepsy is 1%–17%,50 which is clearly higher than that of epilepsy in the general population (0.6%–1.5%).52 This nonchance association may have led to diagnostic misclassification of vestibular migraine as EVD or vice versa.
Epileptic nystagmus.
From all EVD patients identified, only 1% presented with both EVD and epileptic nystagmus. This rate may be falsely low as, in most EVD patients, seizures were not observed by a physician and eyewitness reports of nystagmus are usually not available. The cortical areas associated with seizure activity leading to epileptic nystagmus spread across temporal, parietal, and occipital lobes and are presumably related to the control of smooth-pursuit or saccadic eye movements.53 These seizure locations partially overlap with the mostly temporal locations for EVD in our systematic review. However, reviews in the literature collecting cases of epileptic nystagmus report rates of associated episodic vertigo/dizziness of only 5%–7%.54–56 This dissociation suggests that the cortical areas eliciting epileptic nystagmus and EVD are activated at the same time by an epileptic attack in only a minority of cases. If the clinician therefore witnesses a seizure presenting with EVD, it is unlikely that he or she will detect nystagmus and vice versa.
EVD is primarily associated with temporal lobe seizures and we found ni-EVD is more common than i-EVD; i-EVD appears to be rare. Consistent with clinical wisdom, i-EVD spells often last just seconds, although many patients experience longer spells. Epileptic nystagmus is an infrequent accompaniment, despite its apparent similarity. Prospective diagnostic studies in unselected symptom or disease cohorts would be required to confirm and refine these estimates further, which are based largely on retrospective case series data. In the meantime, clinicians should consider EVD when assessing patients with dizziness/vertigo, particularly children with very brief episodes or when other suspicious symptoms are present.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Ali Saber Tehrani for support in obtaining the original publications of the studies included here.
GLOSSARY
- AED
antiepileptic drug
- EVD
epileptic vertigo or dizziness
- i-EVD
isolated epileptic vertigo or dizziness
- ni-EVD
non-isolated epileptic vertigo or dizziness
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Tarnutzer conceived of the study, performed the literature search, reviewed identified manuscripts and selected matching inclusion criteria, performed the statistical analysis, drafted and critically reviewed the manuscript, and approved the final version of the manuscript. Dr. Lee reviewed identified manuscripts and selected matching inclusion criteria, critically reviewed and edited the manuscript, and approved the final version of the manuscript. Dr. Robinson performed the literature search, critically reviewed and edited the manuscript, and approved the final version of the manuscript. Dr. Kaplan reviewed identified EEG traces, critically reviewed and edited the manuscript, and approved the final version of the manuscript. Dr. Newman-Toker conceived of the study, critically reviewed and edited the manuscript, and approved the final version of the manuscript.
STUDY FUNDING
Dr. Tarnutzer was supported by the Swiss National Science Foundation, the Betty and David Koetser Foundation for Brain Research, and the Zurich Center for Integrative Human Physiology, Switzerland.
DISCLOSURE
A. Tarnutzer, S. Lee, and K. Robinson report no disclosures relevant to the manuscript. P. Kaplan reports grants from Qatar National Research Foundation (EEG in the ICU for nonconvulsive status epilepticus); personal fees from royalties from Wiley, Demos for books on epilepsy, EEG; honoraria for international congresses and grand rounds from Europe and North America; travel to give lectures from Europe and North America; consulting fee for EEG reading from Eisai Pharma; expert testimony on qEEG in the courtroom; and nonfinancial support as a nonpaid board member of ACNS and ABCN. D. Newman-Toker reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1–13. [DOI] [PubMed] [Google Scholar]
- 2.Kluge M, Beyenburg S, Fernandez G, Elger CE. Epileptic vertigo: evidence for vestibular representation in human frontal cortex. Neurology 2000;55:1906–1908. [DOI] [PubMed] [Google Scholar]
- 3.Behrman S. Vestibular epilepsy. Brain 1955;78:471–486. [DOI] [PubMed] [Google Scholar]
- 4.Alpers BJ. Vertiginous epilepsy. Laryngoscope 1960;70:631–637. [DOI] [PubMed] [Google Scholar]
- 5.Eviatar L. Dizziness in children. Otolaryngol Clin North Am 1994;27:557–571. [PubMed] [Google Scholar]
- 6.Barac B. Vertiginous epileptic attacks and so-called vestibulogenic seizures. Epilepsia 1968;9:137–144. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed I. Epilepsia tornado. J Kans Med Soc 1980;81:466–467. [PubMed] [Google Scholar]
- 8.Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little, Brown; 1954. [Google Scholar]
- 9.Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann Neurol 2003;54:615–624. [DOI] [PubMed] [Google Scholar]
- 10.Nicita F, Papetti L, Spalice A, et al. Epileptic nystagmus: description of a pediatric case with EEG correlation and SPECT findings. J Neurol Sci 2010;298:127–131. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Herrero D, Fernandez-Torre JL, Barrasa J, Calleja J, Pascual J. Abdominal epilepsy in an adolescent with bilateral perisylvian polymicrogyria. Epilepsia 1998;39:1370–1374. [DOI] [PubMed] [Google Scholar]
- 12.Bartolomei F, Regis J, Donnet A, Gastaut JL. Development of focal chronic epilepsy following focal status epilepticus in adult patients. Neurophysiol Clin 1999;29:271–276. [DOI] [PubMed] [Google Scholar]
- 13.Erbayat Altay E, Serdaroglu A, Gucuyener K, Bilir E, Karabacak NI, Thio LL. Rotational vestibular epilepsy from the temporo-parieto-occipital junction. Neurology 2005;65:1675–1676. [DOI] [PubMed] [Google Scholar]
- 14.Beun AM, Beintema DJ, Binnie CD, Debets RM, Overweg J, Van Heycop ten Ham MW. Epileptic nystagmus. Epilepsia 1984;25:609–614. [DOI] [PubMed] [Google Scholar]
- 15.Hewett R, Bartolomei F. Epilepsy and the cortical vestibular system: tales of dizziness and recent concepts. Front Integr Neurosci 2013;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Lee SH, Mantokoudis G, et al. Vertigo and dizziness in anterior circulation cerebrovascular disease: a systematic review. Neurology 2014;82(suppl):P3.092. [Google Scholar]
- 17.Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol 1994;35:403–412. [DOI] [PubMed] [Google Scholar]
- 18.Kogeorgos J, Scott DF, Swash M. Epileptic dizziness. Br Med J 1981;282:687–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiest G, Zimprich F, Prayer D, Czech T, Serles W, Baumgartner C. Vestibular processing in human paramedian precuneus as shown by electrical cortical stimulation. Neurology 2004;62:473–475. [DOI] [PubMed] [Google Scholar]
- 20.Schneider RC, Calhoun HD, Crosby EC. Vertigo and rotational movement in cortical and subcortical lesions. J Neurol Sci 1968;6:493–516. [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Kim YH, Hwang Y, Kang B, Kim DH, Kwon YS. Epileptic nystagmus and vertigo associated with bilateral temporal and frontal lobe epilepsy. Clin Exp Otorhinolaryngol 2013;6:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323–334. [DOI] [PubMed] [Google Scholar]
- 23.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978;299:926–930. [DOI] [PubMed] [Google Scholar]
- 24.Eviatar L, Eviatar A. Vertigo in children: differential diagnosis and treatment. Pediatrics 1977;59:833–838. [PubMed] [Google Scholar]
- 25.Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain 2008;131:2538–2552. [DOI] [PubMed] [Google Scholar]
- 26.Hegemann S, Fitzek S, Fitzek C, Fetter M. Cortical vestibular representation in the superior temporal gyrus. J Vestib Res 2004;14:33–35. [PubMed] [Google Scholar]
- 27.Paduch T, Baborie A, Krauss JK. Bifocal temporal ganglioglioma. Neurosurg Rev 1999;22:112–116. [DOI] [PubMed] [Google Scholar]
- 28.Tarnutzer AA, Lasker AG, Zee DS. Continuous theta-burst stimulation of the right superior temporal gyrus impairs self-motion perception. Exp Brain Res 2013;230:359–370. [DOI] [PubMed] [Google Scholar]
- 29.van Ness PC. Frontal and parietal lobe epilepsy. In: Wyllie E, ed. The Treatment of Epilepsy: Principles and Practice. Philadelphia: Lea & Febiger; 1993:525–532. [Google Scholar]
- 30.Lieb JP, Walsh GO, Babb TL, Walter RD, Crandall PH. A comparison of EEG seizure patterns recorded with surface and depth electrodes in patients with temporal lobe epilepsy. Epilepsia 1976;17:137–160. [DOI] [PubMed] [Google Scholar]
- 31.Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia 1997;38:642–654. [DOI] [PubMed] [Google Scholar]
- 32.Dubeau F, McLachlan RS. Invasive electrographic recording techniques in temporal lobe epilepsy. Can J Neurol Sci 2000;27(suppl 1):S29–S34; discussion S50–S52. [DOI] [PubMed] [Google Scholar]
- 33.Jan MM, Sadler M, Rahey SR. Electroencephalographic features of temporal lobe epilepsy. Can J Neurol Sci 2010;37:439–448. [DOI] [PubMed] [Google Scholar]
- 34.Hewett R, Guye M, Gavaret M, Bartolomei F. Benign temporo-parieto-occipital junction epilepsy with vestibular disturbance: an underrecognized form of epilepsy? Epilepsy Behav 2011;21:412–416. [DOI] [PubMed] [Google Scholar]
- 35.Newman-Toker DE. Symptoms and signs of neuro-otologic disorders. Continuum 2012;18:1016–1040. [DOI] [PubMed] [Google Scholar]
- 36.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–892. [DOI] [PubMed] [Google Scholar]
- 37.Sajjadi H, Paparella MM. Meniere's disease. Lancet 2008;372:406–414. [DOI] [PubMed] [Google Scholar]
- 38.Jen JC, Graves TD, Hess EJ, Hanna MG, Griggs RC, Baloh RW. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain 2007;130:2484–2493. [DOI] [PubMed] [Google Scholar]
- 39.Best C, Gawehn J, Kramer HH, et al. MRI and neurophysiology in vestibular paroxysmia: contradiction and correlation. J Neurol Neurosurg Psychiatry 2013;84:1349–1356. [DOI] [PubMed] [Google Scholar]
- 40.Hufner K, Barresi D, Glaser M, et al. Vestibular paroxysmia: diagnostic features and medical treatment. Neurology 2008;71:1006–1014. [DOI] [PubMed] [Google Scholar]
- 41.Furman JM, Crumrine PK, Reinmuth OM. Epileptic nystagmus. Ann Neurol 1990;27:686–688. [DOI] [PubMed] [Google Scholar]
- 42.Fotuhi M, Glaun B, Quan SY, Sofare T. Vestibular migraine: a critical review of treatment trials. J Neurol 2009;256:711–716. [DOI] [PubMed] [Google Scholar]
- 43.Tartara A, Manni R, Mira E, Mevio E. Polygraphic study of vestibular stimulation in epileptic patients. Rev Electroencephalogr Neurophysiol Clin 1984;14:227–234. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JR, Drachman DA. Dizziness, epilepsy and the EEG. Dis Nerv Syst 1977;38:431–435. [PubMed] [Google Scholar]
- 45.Weintraub MI, Smith BH. Vertigo: epileptic manifestation of vertebral-basilar artery insufficiency. NY State J Med 1969;69:1441–1446. [PubMed] [Google Scholar]
- 46.Niedermeyer E, Hinchcliffe R. Vertigo and the electroencephalogram. Electroencephalogr Clin Neurophysiol 1965;18:78–81. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen E, Jepsen O. Epileptic vertigo. Acta Psychiatr Neurol Scand Suppl 1956;108:301–310. [DOI] [PubMed] [Google Scholar]
- 48.Smith BH. Vestibular disturbances in epilepsy. Neurology 1960;10:465–469. [DOI] [PubMed] [Google Scholar]
- 49.Ottman R, Lipton RB. Comorbidity of migraine and epilepsy. Neurology 1994;44:2105–2110. [DOI] [PubMed] [Google Scholar]
- 50.Andermann E, Andermann F. Migraine–epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E, eds. Migraine and Epilepsy. Boston: Butterworths; 1987:281–291. [Google Scholar]
- 51.Marks DA, Ehrenberg BL. Migraine-related seizures in adults with epilepsy, with EEG correlation. Neurology 1993;43:2476–2483. [DOI] [PubMed] [Google Scholar]
- 52.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010;51:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan PW, Tusa RJ. Neurophysiologic and clinical correlations of epileptic nystagmus. Neurology 1993;43:2508–2514. [DOI] [PubMed] [Google Scholar]
- 54.Weber YG, Roesche J, Lerche H. Epileptic nystagmus: two case reports, clinical and pathophysiological review of the literature. J Neurol 2006;253:767–771. [DOI] [PubMed] [Google Scholar]
- 55.Stolz SE, Chatrian GE, Spence AM. Epileptic nystagmus. Epilepsia 1991;32:910–918. [DOI] [PubMed] [Google Scholar]
- 56.White JC. Epileptic nystagmus. Epilepsia 1971;12:157–164. [DOI] [PubMed] [Google Scholar]
- 57.Herr RD, Zun L, Mathews JJ. A directed approach to the dizzy patient. Ann Emerg Med 1989;18:664–672. [DOI] [PubMed] [Google Scholar]
- 58.Ojala M, Vaheri E, Larsen TA, Matikainen E, Juntunen J. Diagnostic value of electroencephalography and brainstem auditory evoked potentials in dizziness. Acta Neurol Scand 1988;78:518–523. [DOI] [PubMed] [Google Scholar]
- 59.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167–172. [DOI] [PubMed] [Google Scholar]
- 60.Erkwoh R. Psychopathology of vestibular aurae. Psychopathology 1990;23:129–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


