Abstract
Background:
Affective-motivational and sensory-discriminative aspects of pain were investigated in patients with mild to moderate Alzheimer disease (AD) and healthy elderly controls using the cold pressor test tolerance and repetitive stimuli of warmth and heat stimuli, evaluating the stimulus-response function.
Methods:
A case-control design was applied examining 33 patients with mild to moderate AD dementia and 32 healthy controls with the cold pressor test (4°C). Warmth detection threshold (WDT) and heat pain threshold (HPT) were assessed using 5 stimulations. A stimulus-response function was estimated using 4 incrementally increasing suprathreshold heat stimuli.
Results:
Cold pressor tolerance was lower in patients with AD dementia than in controls (p = 0.027). There were no significant differences between groups regarding WDT and HPT. Significant successive increases in HPT assessments indicated habituation (p < 0.0001), which was similar in the 2 groups (p = 0.85). A mixed model for repeated measures demonstrated that pain rating of suprathreshold stimuli depended on HPT (p = 0.0004) and stimulus intensity (p < 0.0001). Patients with AD dementia had significantly lower increases in pain ratings than controls during suprathreshold stimulation (p = 0.0072).
Conclusion:
Our results indicate that AD dementia is not associated with a propensity toward development of sensitization or a lack of habituation, suggesting preservation of sensory-discriminative aspects of pain perception. The results further suggest that the attenuated cold pressor pain tolerance may relate to impairment of coping abilities. Paradoxically, we found an attenuated stimulus-response function, compared to controls, suggesting that AD dementia interferes with pain ratings over time, most likely due to memory impairment.
Clinical studies indicate that patients with Alzheimer disease (AD) dementia report less intense pain with an attenuated affective response.1 AD pathology includes involvement of areas processing affective-motivational aspects of pain, i.e., the amygdala, insula, and prefrontal cortex, while the sensory cortex is relatively well-preserved.2,3 These findings have led to the hypothesis that the affective-motivational aspects of pain perception are altered in AD dementia, where sensory-discriminative aspects may be intact,3 but experimental studies have not been able to validate this hypothesis consistently.4–6
In patients with AD dementia compared to controls, we recently observed lowered pain tolerance to mechanical stimuli, but not to a cold pressor test.7 Whether the reduced tolerance reflects increased sensitivity to pain, decrease in endogenous inhibitory capacity, deficient coping strategies, or anxiety associated with the experimental situation is not known. This may be examined by a repeated stimulation paradigm, leading either to decreasing (habituation)8 or increasing pain responses (sensitization).9 Abnormal pain processing has been shown in Parkinson disease with evidence of less habituation to repetitive pain stimuli.10 Therefore, in order to examine if sensory-discriminative or affective-motivational aspects of pain perception are altered in patients with AD dementia compared to controls, we investigated the stimulus-response function to suprathreshold painful heat stimuli. In order to evaluate patterns of habituation/sensitization, we investigated responses to repeated stimulation with nonpainful and painful warmth/heat stimuli. We also evaluated the affective-motivational aspects by assessing cold pressor pain tolerance.
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the Regional Committees on Health Research Ethics of the Capital Region of Denmark (protocol H-2-2011-122) and the Data Protection Agency (2007-58-0015). All participants gave informed consent to the study.
Participants and study design.
A case-control design was used including 33 patients and 32 healthy controls. Patients were recruited among outpatients from Memory Clinics at Rigshospitalet, University of Copenhagen, Denmark, and Roskilde Hospital, Denmark. All patients fulfilled ICD-10 and DSM-IV criteria for dementia and had a diagnosis of probable AD dementia according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.11 Patients were able to give informed consent and had a caregiver who was willing to participate. Patients had mild to moderate AD dementia, judged by a Mini-Mental State Examination (MMSE) score between 16 and 26 points and a Clinical Dementia Rating of 0.5–2, and were able to cooperate.
The controls were recruited from subjects who had previously participated in studies at the Memory Clinic, where they had been cognitively tested and found not to have cognitive impairment. Eighteen controls and 14 patients with AD dementia had participated in a previous study on pain perception, but as this was a separate study, they were reexamined. For all participants, exclusion criteria were significant psychiatric comorbidity, prior or present alcohol abuse, significant medical comorbidity, previous TIA or stroke, disorders that would interfere with pain perception such as diabetes, peripheral neuropathy, a chronic pain disorder, or current pain condition, or use of daily analgesics. At baseline, participants were examined neurologically and were excluded if they had symptoms or signs of neurologic or inflammatory disease that could interfere with pain perception.
Baseline characteristics.
The patient's cognitive status was evaluated using the Addenbrooke's Cognitive Examination test, which includes the MMSE, but expands on cognitive domains such as memory, language, and visuospatial functions, and includes test of verbal fluency. Activities of daily living (ADL) function was evaluated using the Functional Activities Questionnaire and the Instrumental ADL Scale,12 which evaluates 10 different instrumental ADL with a maximum score of 30 (dependent of help). Participants were screened for depression with the 15-item Geriatric Depression Scale.13 In the controls, an MMSE was applied as part of the inclusion criteria.
Reaction time was measured using http://getyourwebsitehere.com/jswb/rttest01.html, showing a red-green traffic light. Participants were instructed to press the button when the light changed from red to green (random intervals) (details described elsewhere7).
Experimental protocol.
The testing session consisted of 2 parts. The first part consisted of thermal sensitivity testing including determination of warmth detection threshold (WDT), heat pain threshold (HPT), and suprathreshold heat pain stimulation for assessment of stimulus-response function. The second part consisted of autonomic testing. Details and results are described elsewhere. All assessments were carried out by one examiner (C.J.-D.).
Threshold determination.
The protocol has been described in detail previously.7 Briefly, contact heat stimuli were given by a thermal stimulator (Medoc TSA-2001; Medoc, Ramat Yishai, Israel) with a Peltier element-based thermode (3.0 × 3.0 cm active surface area). Baseline temperature was 32°C, cutoff temperature 50°C, and rate of increase 0.5°C/s. Stimuli were applied to the volar side of the lower left arm with a random interval of 4–6 seconds. The participants were asked to press a switch when they first felt warmth (WDT), terminating the stimulation, returning the temperature to baseline. HPT was defined as the point at which the warmth sensation turned into pain. Five consecutive stimuli were applied for both WDT and HPT. Instructions were standardized.
Assessment of stimulus-response function.
After HPT assessments, 4 suprathreshold heat stimuli were applied. The temperatures of the stimuli (5 seconds) were based on individual HPTs (mean of the last 3 of 5 assessments) and were as follows: (1) HPT; (2) HPT + 20% × ΔHPT (ΔHPT = HPT − 32°C [baseline temperature]); (3) HPT + 40% × ΔHPT; and (4) HPT + 50% × ΔHPT. Rate of increase/decrease was 2°C/s and cutoff was 50°C. The interval between the stimulations was 2 minutes and successively increasing temperatures were used (starting with HPT and ending with HPT + 50% × ΔHPT). Participants were informed that 4 heat stimuli would be applied, but were not told if the temperature would be above or below pain threshold or if the stimulus would be warmer or less warm than the previous stimulus. After each stimulus the subject was asked to rate the pain on a horizontally held colored analog scale (CAS), which is the pain rating tool recommended in patients with mild to moderate dementia.14,15 The participants' ability to understand the scale had been tested at baseline and all were able to understand and explain the scale correctly.
Cold pressor test.
A recirculating water cooler (model 11371P [13 L]; VWR International, Radnor, PA) was used and water temperature maintained at 4°C (±0.25°C). The participant was asked to submerge the left hand in the water, covering the wrist, and to keep the hand in the water for 2 minutes. The subject was instructed to state when the cold stimulus became painful, indicating pain threshold. In case the participants withdrew the hand before the 2 minutes were completed, the time of withdrawal (seconds) was noted and represented pain tolerance. If the subject maintained the hand immersed for the entire 120 seconds, this represented their pain tolerance. Immediately after completion of the test, the participants were asked to rate the pain (CAS).
Statistical analysis.
Data distribution was tested by the Shapiro-Wilk test and by inspection of histograms. Values are given as mean (95% confidence interval) or median (25%–75% interquartile range) depending on the data distribution. Group differences were compared using Fisher exact test, t test, or the Mann-Whitney test, as appropriate. Differences between groups for WDT and HPT were compared by means for the last 3 stimuli as previously reported.7 Since sensory thresholds depend on the reaction time, corrective adjustments were made by subtracting ramp rate (0.5°C/s) × reaction time (s) from WDT and HPT. The 5 repeated assessments of WDT and HPT were compared using a mixed-model analysis for repeated measures for normally distributed data,16 since subsequent measurements were not independent of the previous measurements. Stimulus number was treated as a categorical variable. All models were fitted with an unstructured covariance matrix. Likewise, we used a similar model to investigate the stimulus-response function (CAS rating = percentage above threshold as a continuous variables). Scatterplots indicated that CAS rating and HPT were correlated and therefore HPT was controlled for in the model. For all models, reaction time was included as a covariate to evaluate influence of this parameter. A significance level of p < 0.05 was used. All data were analyzed using SAS 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
Clinical characteristics.
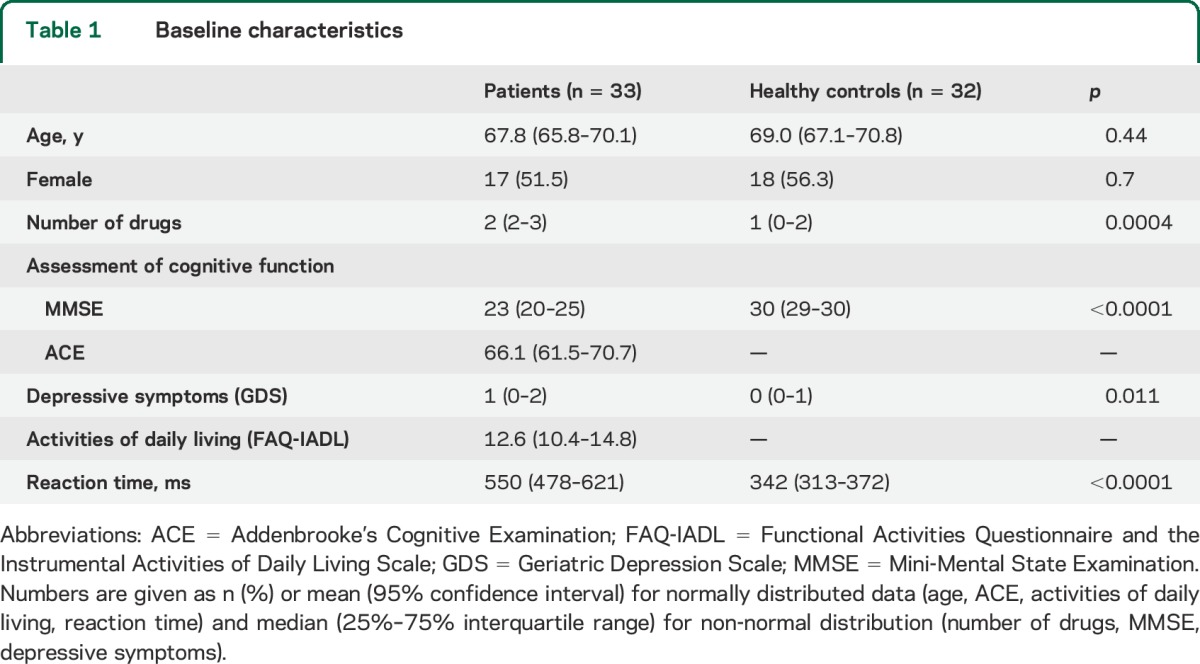
Baseline characteristics are shown in table 1. Patients used a higher number of medications than the controls (antidementia drugs and selective serotonin reuptake inhibitors). Patients had increased reaction time compared to controls.
Table 1.
Baseline characteristics
Thermal thresholds.
There was no overall difference between groups in regard to WDT or HPT (table 2). Values for WDT and HPT are presented in figure 1. The first of 5 WDT threshold values was larger than the subsequent values (p = 0.0074), while subsequent thresholds were of comparable magnitude. There was no difference between groups (p = 0.80), which did not change after adjustment for reaction time. For HPT, there was a successive, gradual increase between each stimulus (p < 0.001), indicating habituation, which did not differ between groups (p = 0.86). For both groups, HPT depended on reaction time (p = 0.0099), but there was no interaction between group and reaction time (p = 0.92).
Table 2.
Warmth detection and heat pain thresholds and results from the cold pressor test (pain threshold and tolerance and pain rating using the colored analog scale), stratified by patients and controls
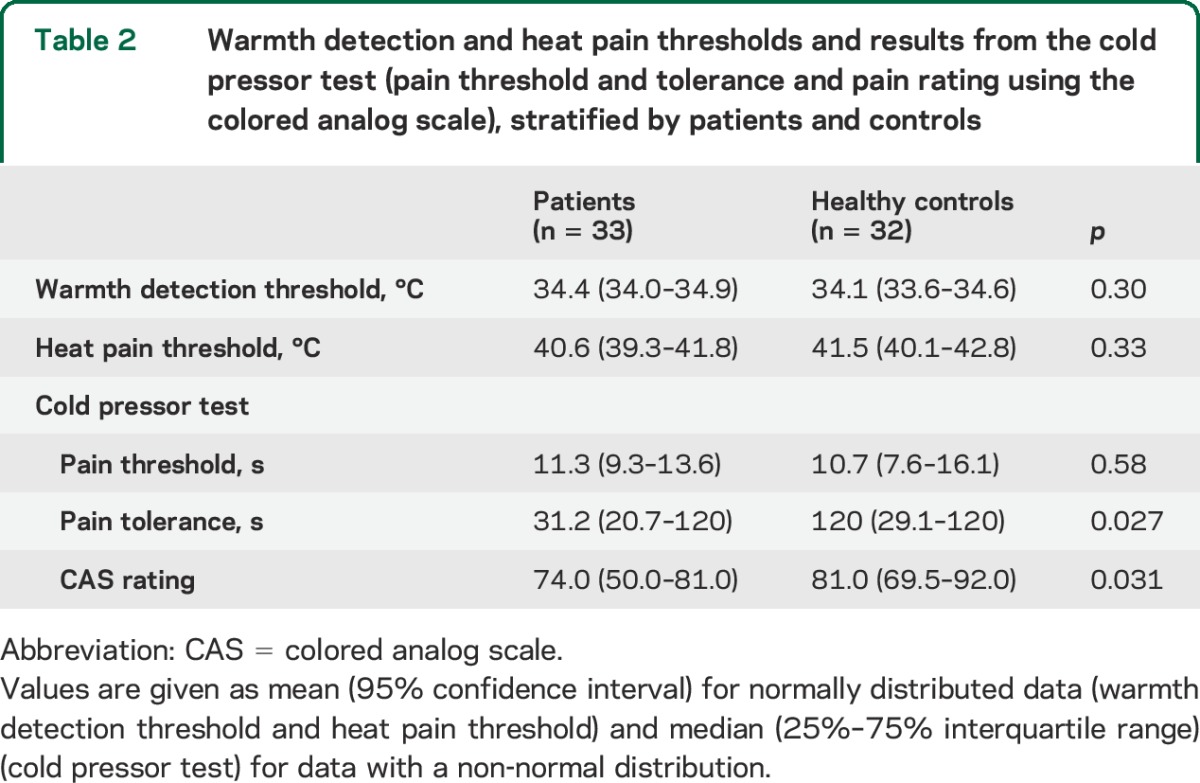
Figure 1. Warmth detection threshold and heat pain threshold examination.
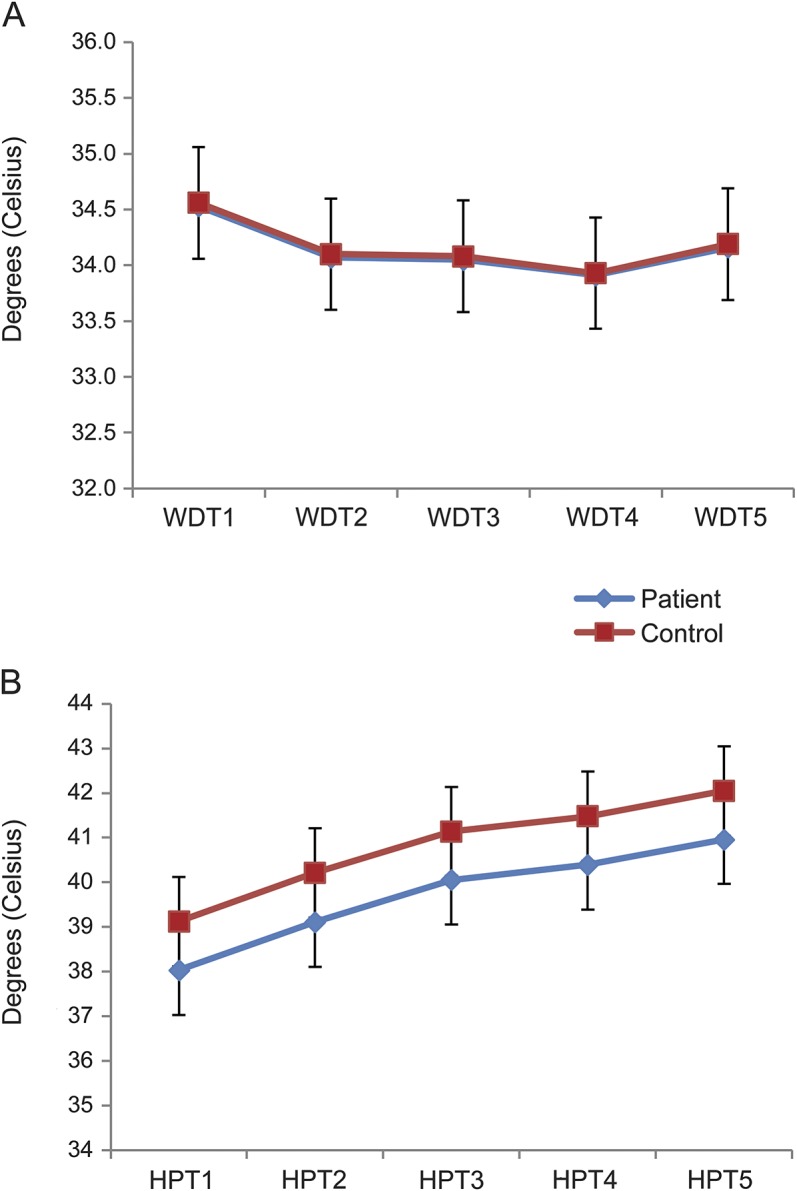
Mean values (95% confidence interval; °C) for warmth detection threshold (WDT) (A) and heat pain threshold (HPT) (B) for the 5 stimuli for patients (blue) and controls (red).
Stimulus-response function.
Figure 2 shows the magnitude of the heat stimulus (percentage above threshold) plotted against the CAS ratings for patients and controls. The analysis showed that the CAS rating was dependent on HPT (p = 0.0004) and percentage above HPT (p < 0.0001). There was no effect of reaction time (p = 0.39). There was a trend toward patients rating the pain more intense at threshold (patients vs controls: 32.6 vs 21.7, p = 0.058). Patients demonstrated a less steep rate of increase (ΔCAS rating/% ΔHPT) compared to controls (0.36 vs 0.61, p = 0.0072).
Figure 2. Stimulus-response curves for suprathreshold heat stimuli in patients and controls.
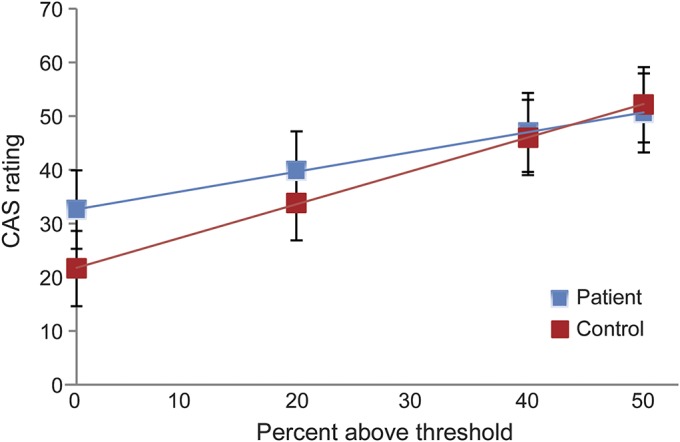
The horizontal axis indicates the percentage above the heat pain threshold (HPT), and the vertical axis the pain ratings (colored analog scale [CAS] [0–100 arbitrary units]; mean [95% confidence interval]) for patients (blue) and controls (red). Equations, describing the relations between stimulus and CAS ratings, are as follows: patients: CAS rating = 32.63 (standard error 4.27) + 0.36 (0.06) × percentage above threshold + 2.79 (0.74) × (HPT-40): controls: CAS rating = 21.66 (3.58) + 0.61 (0.07) × percentage above threshold + 2.79 (0.74) × (HPT-40).
Cold pressor test.
Figure 3 shows the percentage who tolerated the cold pressor test as a function of hand submersion time. Only 9 (27.3%) patients compared to 20 (62.5%) controls were able to tolerate the entire 120 seconds (p = 0.006). Patients and controls had comparable cold pressor pain thresholds (p = 0.58), but patients had a lower tolerance (table 2). Patients rated the pain experienced during the test lower than controls (table 2). There was no correlation between tolerance and CAS rating (Spearman rank correlation coefficient 0.071, p = 0.66).
Figure 3. Tolerance to the cold pressor test.
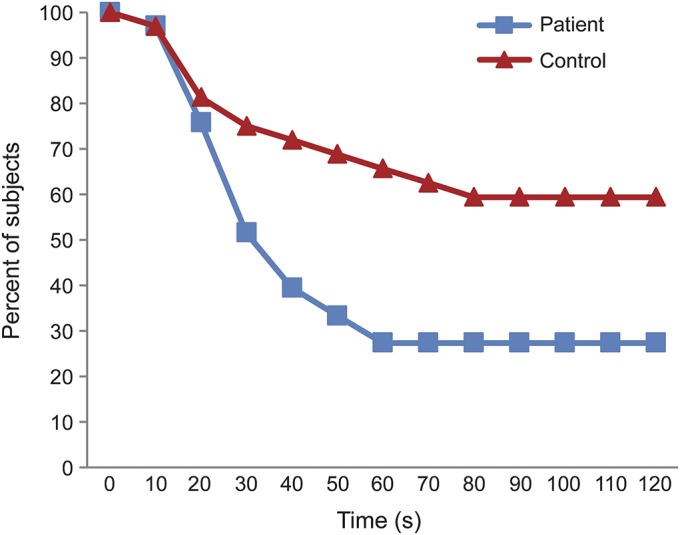
The percentage of patients and healthy controls maintaining the hand submersed in the cold water during the cold pressor test. The vertical axis indicates the duration (seconds) of immersion (maximum 120 seconds).
DISCUSSION
In this study we demonstrated a normal pattern of habituation to painful heat stimuli in patients with AD dementia compared to controls. By applying increasing intensities of suprathreshold heat stimuli, we found a trend toward an initial higher pain rating in patients compared to controls, but interestingly, patients demonstrated a less steep stimulus-response curve. At variance with the stimulus-response finding, we observed an attenuated cold pressor pain tolerance. Taken together, AD dementia seems to significantly impair affective-motivational aspects of pain depending on the modality and intensity of the stimulation.
Habituation is a reduction of the psychophysical response to a successively repeated stimulus.17 Both peripheral and central mechanisms are involved in pain habituation,18–20 and the normal pattern of habituation to heat pain in patients with AD dementia may actually reflect that spinal mechanisms are intact. Habituation to pain is thought to at least in part be mediated by the anterior cingulate cortex,18–20 which is thought to involve the antinociceptive system18,21 At early stages, AD involves the anterior cingulate cortex, and one may have expected to find evidence of central dysmodulation. The comparable responses in patients and controls provide evidence that AD dementia is not associated with a propensity toward development of sensitization or lack of habituation. In accordance with previous studies, we found no difference between patients and controls on sensory or pain thresholds,22–25 which together with a normal pattern of habituation point toward preservation of the sensory-discriminative aspects of pain perception.
Repeated heat stimulation was also investigated in relation to the suprathreshold stimulus-response function. Patients showed a trend toward higher pain ratings at pain threshold, but followed a less steep stimulus-response function compared to controls. In our previous study, we found a trend toward smaller inclines in patients during similar testing conditions with only 2 suprathreshold stimulations.7 A previous study found higher pain ratings of just noticeable pain, but similar ratings of weak and mild pain.4 One study found similar ratings at pain threshold, but lower ratings at an above threshold intensity in patients with AD dementia compared to controls.25 None of the studies evaluated stimulus-response functions, but their findings could be consistent with less steep stimulus-response curves. In the cognitively unimpaired, higher pain ratings and steeper stimulus-response curves indicate increased pain sensitivity,9 but this may not be the case in AD dementia. We found a similar pattern of habituation to repeated heat stimuli in patients and controls, indicating that the observed differences in stimulus-response function most likely are due to impairment of affective-motivational factors and not to differences in inherent sensory properties toward sensitization or habituation.
Dementia could influence pain ratings in a number of ways. Patients initially showed a trend towards higher pain ratings and when inspecting figure 1 the curve for the patients is above that of the controls, which could indicate increased fear/anxiety associated with the experimental situation. After the first suprathreshold stimuli, the patients seemed to adapt, and the subsequent pain ratings followed a less steep curve, a mechanism reported in chronic pain patients.26 Second, a less steep incline of slope may reflect memory impairment and an impaired ability to recall the previous intensity or prior ratings, which may impair discriminative ability27 and lead to a regression toward the mean effect. Finally, patients with early AD dementia exhibit a deficit in maintenance of vigilance over time under demanding tasks compared to elderly controls,28 which may result in impaired discriminative ability.
Pain tolerance in patients with AD dementia has been investigated in 2 previous studies.7,22 One study found an increased pain tolerance in patients with AD dementia using both ischemic and electric stimuli (n = 12).22 We recently reported lower tolerance using mechanical stimuli, but comparable tolerance to the cold pressor test in patients with AD dementia compared to controls (n = 29).7 In contrast, in the present study we found a lower tolerance of cold pressor pain in patients with AD dementia. One possible explanation for the different finding is that we used a more intense cold stimulus in the present study (4°C vs 10°C) and participants were instructed to endure 2 minutes, whereas in our previous study they were instructed to withdraw their hand when it became intolerable. Our results support a lower tolerance of experimental pain in patients with AD dementia, which is supported by increased responses to pressure-induced pain on fMRI4 and on recordings of facial expressions5 in patients with AD dementia.
A lower tolerance to cold pressor pain may seem at variance with a less steep stimulus-response function. One explanation could be related to differences in relation to stimulus modality or intensity. Alternatively, increased fear/anxiety due to the experimental situation could explain the attenuated tolerance and as mentioned the less steep stimulus-response function, but may also be the result of cognitive impairment. The cold pressor test is associated with severe pain, reaching its peak within the first 30–60 seconds. Impairment of executive function or coping abilities may impair the patient's ability to tolerate pain. It may also affect the patient's ability to cooperate with the test.
Among the strengths of our study are the inclusion of a well-characterized cohort of patients with AD dementia and implementation of standardized test methods, previously shown to have good reliability and reproducibility in this population.7 In addition, we used individualized suprathreshold heat stimuli in order to facilitate the evaluation of the stimulus-response pattern, thereby avoiding off-set analgesia (physiologic response where a small decrease in noxious heat stimulation results in a disproportionate large reduction in pain perception),29 reducing noise/signal ratio. Despite the fact that thermal stimulation was thoroughly examined during static and dynamic conditions, only one modality was included in the present testing paradigm. Extrapolation of our results to other stimulation modalities, e.g., electrical or mechanical stimuli, requires careful consideration and appropriate restraint. Furthermore, it may be discussed if the stimulus paradigm has the ability to discriminate between habituation and adaptation. While the response criterion remained fixed, the stimulus changed from barely noxious to clearly noxious, indicating that different nerve fiber systems likely are involved. Nevertheless, increasing stimulus intensities was required to reach the response criterion (HPT) both in AD dementia and controls. Another limitation is that the suprathreshold stimuli depended on pain threshold; a nonsignificant difference of 1°C between the 2 groups may slightly enhance the differences in slope values. However, HPT was adjusted for in our analysis. An additional limitation is that patients with concomitant diseases were excluded and results may therefore not be applicable to all patients with AD dementia, especially those with painful disorders. Further, some of the subjects had participated in a previous study. We controlled for this in the statistical analyses and no consistent effect on the results was demonstrated. Although the timelines for the studies were completely separated, a statistical association may exist between the studies due to recurrent inclusion of some subjects. Finally, only patients with mild to moderate AD dementia were examined, and our results cannot be translated to patients with more severe stages of AD dementia.
The overall question is whether patients with AD dementia experience pain to a similar degree as cognitively intact peers. We found a lower tolerance to cold pain, but using repeated stimuli we did not observe any evidence of increased sensitivity or lack of habituation to pain. Our findings suggest that patients with AD dementia perceive pain from experimental stimuli at least as well as healthy controls, but cognitive impairment may make patients with AD dementia less able to tolerate pain due to anxiety or due to an impaired ability to cope with pain. Paradoxically, we found an attenuated stimulus-response function compared to controls, indicating that AD dementia also affects pain ratings over time, most likely due to memory impairment. This represents an obvious challenge to clinical decisions based upon pain intensity assessments in this patient group. Future research should address whether our findings are specific to the experimental pain stimulation or even the stimulation modality, and whether the results can be translated to the clinical situation.
GLOSSARY
- AD
Alzheimer disease
- ADL
activities of daily living
- CAS
colored analog scale
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HPT
heat pain threshold
- ICD-10
International Classification of Diseases–10
- MMSE
Mini-Mental State Examination
- WDT
warmth detection threshold
AUTHOR CONTRIBUTIONS
C.J.-D., M.W., G.W., T.S.J., and M.B. designed the study. B.B.A., P.H., and C.J.-D. recruited the patients. C.J.-D. conducted the study. C.J.-D. conducted the statistical analysis under guidance from M.W. and a statistician. C.J.-D. wrote the first draft of the manuscript. All authors discussed the results, commented on the manuscript, and approved the final version of the manuscript.
STUDY FUNDING
The Danish Dementia Research Centre is supported by grants from the Danish Health Foundation and the Danish Ministry of Health.
DISCLOSURE
C. Jensen-Dahm reports no disclosures relevant to the manuscript. M. Werner has received unrestricted research grants (administered by the University Hospital administration) from Grünenthal GmbH, Germany, and Astellas Pharma A/S, Denmark. T. Jensen has received grant support and participated in advisory boards for the following: Pfizer, Astellas, and Grünenthal. M. Ballegaard, B. Andersen, and P. Høgh report no disclosures relevant to the manuscript. G. Waldemar is a board member of the Lundbeck Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Scherder E, Bouma A, Borkent M, Rahman O. Alzheimer patients report less pain intensity and pain affect than non-demented elderly. Psychiatry 1999;62:265–272. [DOI] [PubMed] [Google Scholar]
- 2.Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 1999;249(suppl 3):14–22. [DOI] [PubMed] [Google Scholar]
- 3.Scherder EJ, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Lancet Neurol 2003;2:677–686. [DOI] [PubMed] [Google Scholar]
- 4.Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer's disease. Brain 2006;129:2957–2965. [DOI] [PubMed] [Google Scholar]
- 5.Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain 2007;133:221–228. [DOI] [PubMed] [Google Scholar]
- 6.Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S. Influence of dementia on multiple components of pain. Eur J Pain 2009;13:317–325. [DOI] [PubMed] [Google Scholar]
- 7.Jensen-Dahm C, Werner MU, Dahl JB, et al. Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. Pain 2014;155:1439–1445. [DOI] [PubMed] [Google Scholar]
- 8.Leblanc J, Potvin P. Studies on habituation to cold pain. Can J Physiol Pharmacol 1966;44:287–293. [DOI] [PubMed] [Google Scholar]
- 9.Vierck CJ, Wong F, King CD, Mauderli AP, Schmidt S, Riley JL., III Characteristics of sensitization associated with chronic pain conditions. Clin J Pain 2014;30:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schestatsky P, Kumru H, Valls-Sole J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 2007;69:2162–2169. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988;24:709–711. [PubMed] [Google Scholar]
- 14.Scherder EJ, Bouma A. Visual analogue scales for pain assessment in Alzheimer's disease. Gerontology 2000;46:47–53. [DOI] [PubMed] [Google Scholar]
- 15.Hadjistavropoulos T, Fitzgerald TD, Marchildon GP. Practice guidelines for assessing pain in older persons with dementia residing in long-term care facilities. Physiother Can 2010;62:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West TB, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. London: Chapman & Hall/CRC; 2007. [Google Scholar]
- 17.Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 2009;92:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain 2007;131:21–30. [DOI] [PubMed] [Google Scholar]
- 19.Rennefeld C, Wiech K, Schoell ED, Lorenz J, Bingel U. Habituation to pain: further support for a central component. Pain 2010;148:503–508. [DOI] [PubMed] [Google Scholar]
- 20.Greffrath W, Baumgartner U, Treede RD. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain 2007;132:301–311. [DOI] [PubMed] [Google Scholar]
- 21.Doganci B, Breimhorst M, Hondrich M, Rodriguez-Raecke R, May A, Birklein F. Expectations modulate long-term heat pain habituation. Eur J Pain 2011;15:384–388. [DOI] [PubMed] [Google Scholar]
- 22.Benedetti F, Vighetti S, Ricco C, et al. Pain threshold and tolerance in Alzheimer's disease. Pain 1999;80:377–382. [DOI] [PubMed] [Google Scholar]
- 23.Benedetti F, Arduino C, Vighetti S, Asteggiano G, Tarenzi L, Rainero I. Pain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deterioration. Pain 2004;111:22–29. [DOI] [PubMed] [Google Scholar]
- 24.Gibson SJ, Voukelatos X, Ames D, Flicker L, Helme RD. An examination of pain perception and cerebral event-related potentials following carbon dioxide laser stimulation in patients with Alzheimer's disease and age-matched control volunteers. Pain Res Manag 2001;6:126–132. [DOI] [PubMed] [Google Scholar]
- 25.Rainero I, Vighetti S, Bergamasco B, Pinessi L, Benedetti F. Autonomic responses and pain perception in Alzheimer's disease. Eur J Pain 2000;4:267–274. [DOI] [PubMed] [Google Scholar]
- 26.Crombez G, Vervaet L, Baeyens F, Lysens R, Eelen P. Do pain expectancies cause pain in chronic low back patients? A clinical investigation. Behav Res Ther 1996;34:919–925. [DOI] [PubMed] [Google Scholar]
- 27.Rainville P, Doucet JC, Fortin MC, Duncan GH. Rapid deterioration of pain sensory-discriminative information in short-term memory. Pain 2004;110:605–615. [DOI] [PubMed] [Google Scholar]
- 28.Berardi AM, Parasuraman R, Haxby JV. Sustained attention in mild Alzheimer's disease. Dev Neuropsychol 2005;28:507–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–1071. [DOI] [PubMed] [Google Scholar]


