Abstract
Objective:
To assess long-term safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods:
Patients completing FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS) were eligible for this dose-blinded, parallel-group extension study, continuing fingolimod 0.5 mg/day or 1.25 mg/day, or switching from placebo to either dose, randomized 1:1. Efficacy variables included annualized relapse rate (ARR), brain volume loss (BVL), and confirmed disability progression (CDP). Between-group analyses were conducted in the intent-to-treat (ITT) population from FREEDOMS baseline to end of study. Within-group analyses compared years 0–2 (FREEDOMS) and years 2–4 (extension) in the extension ITT population.
Results:
Of 1,272 patients (FREEDOMS ITT population), 1,033 were eligible, and 920 enrolled in the extension study (continuous-fingolimod: 0.5 mg [n = 331], 1.25 mg [n = 289]; placebo–fingolimod: 0.5 mg [n = 155], 1.25 mg [n = 145]); 916 formed the extension ITT population (n = 330; n = 287; n = 154; n = 145) and 773 (84%) completed. In the continuous-fingolimod groups, ARR was lower (p < 0.0001), BVL was reduced (p < 0.05), and proportionately more patients were free from 3-month CDP (p < 0.05) than in a group comprising all placebo–fingolimod patients. Within each placebo–fingolimod group, ARR was lower (p < 0.001, both) and BVL was reduced after switching (p < 0.01, placebo–fingolimod 0.5 mg). Rates and types of adverse events were similar across groups; no new safety issues were reported.
Conclusion:
Efficacy benefits of fingolimod during FREEDOMS were sustained during the extension; ARR and BVL were reduced after switching.
Classification of evidence:
This study provides Class IV evidence that long-term fingolimod treatment is well-tolerated and reduces relapse rates, disability progression, and MRI effects in patients with RRMS.
Fingolimod (FTY720), a sphingosine 1-phosphate receptor modulator, is the first oral disease-modifying therapy approved for the treatment of relapsing multiple sclerosis (MS).1,2 Clinical efficacy was investigated in 3 double-blind, randomized, phase 3 trials in patients with relapsing-remitting MS (RRMS): Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting MS (TRANSFORMS), FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS), and FREEDOMS II.3–5
In TRANSFORMS, fingolimod reduced annualized relapse rate (ARR) at 1 year by 52% compared with IM interferon-β-1a, and showed significant benefits on MRI outcomes, including brain atrophy.3 In the 24-month placebo-controlled FREEDOMS trial, fingolimod 0.5 mg significantly reduced ARR (0.18 vs 0.40 on placebo; p < 0.001), disability progression (hazard ratio 0.70; p = 0.02), MRI lesion activity (number of new or enlarged lesions on T2-weighted images, gadolinium [Gd]-enhancing lesions; p < 0.001 for all), and brain atrophy (brain volume loss [BVL] at 2 years, −0.84% vs −1.31% on placebo; p < 0.001).4 Fingolimod 0.5 mg also significantly reduced ARR, MRI measures, and brain atrophy over 2 years in the FREEDOMS II trial.5 The FREEDOMS II trial was similar in design and objectives to FREEDOMS, except it included additional measures (e.g., Holter monitoring) at the request of the Food and Drug Administration.5 We report results from the FREEDOMS trial extension, the objective of which was to evaluate the long-term efficacy, safety, and tolerability of fingolimod in patients with RRMS.
METHODS
Study oversight and design.
Study oversight and steering committee members have been reported previously.4 This extension study consisted of a dose-blinded, parallel-group phase and an open-label phase; the study was to continue until drug approval and availability. A protocol amendment stopping use of fingolimod 1.25 mg in all MS clinical studies was made in November 2009, when unblinding of the FREEDOMS trial revealed higher discontinuation rates following an adverse event (AE) and little efficacy benefit associated with the 1.25-mg dose compared with the 0.5-mg dose.4 Following this amendment, all patients began to transfer to the open-label phase, receiving fingolimod 0.5 mg/day. Between June 2010 and June 2011, patients who had participated in the phase 26,7 and phase 33–5 clinical trials could migrate from the respective extension studies to continue on fingolimod 0.5 mg in a separate open-label study (LONGTERMS [ClinicalTrials.gov number NCT01281657]).
Standard protocol approvals, registrations, and patient consents.
All patients who completed the 24-month FREEDOMS trial were eligible for the extension (ClinicalTrials.gov number NCT00662649); locations and eligibility criteria for FREEDOMS have been described.4 Exclusion criteria included discontinuation of study drug due to an AE or onset of chronic immune system disease requiring immunosuppressive treatment during FREEDOMS. All patients gave written informed consent. The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and with the Declaration of Helsinki.8,9 The protocol and all amendments were approved by each site's institutional review board/independent ethics committee.
Randomization and masking.
The randomization procedure used in FREEDOMS has been described.4 Patients who received fingolimod in FREEDOMS continued on the same blinded dose in the extension. Patients who received placebo in FREEDOMS were re-randomized (1:1) to oral fingolimod 0.5 mg or 1.25 mg once daily in the extension; a separate medication randomization list was produced by the study sponsor, using a validated system that automated the random assignment of medication numbers to medication packs containing the study drug. Patients, investigators, site personnel, independent evaluating physicians, and first dose administrators remained blinded to the treatment dose until the implementation of the protocol amendment. During the dose-blinded phase, study drug was packaged in a blinded fashion and was dispensed by the investigator according to patients' randomization numbers. Thereafter, all patients received open-label fingolimod 0.5 mg once daily, but remained blinded to their treatment assignment during FREEDOMS.
Procedures and assessments.
In order to maintain blinding of drug assignment during FREEDOMS, all patients were monitored by an independent physician following their first dose of drug in the extension, which was taken the day after the last dose during FREEDOMS. Definitions for ARR and disability progression have been reported.4 Relapse and safety assessments were scheduled at months 24.5, 25, 26, and 27, and then every 3 months. Expanded Disability Status Scale (EDSS) score was assessed every 3 months by a specially trained and certified independent physician not involved in the patients' care.10 Standardized MRI scans were obtained every 12 months and processed centrally at the MS MRI Evaluation Center (Basel, Switzerland). Relapse, safety, EDSS assessments, and MRI scans were also obtained at the end of the extension and at follow-up visits. Safety was overseen by an independent data and safety monitoring board. Details of clinical and MRI assessments are given in the supplemental data on the Neurology® Web site at Neurology.org.
Statistical analysis.
Sample size was based on the number of patients who entered the extension rather than statistical power calculation. Between-group comparisons of the effects of continuous vs delayed initiation of fingolimod therapy were evaluated in the FREEDOMS intent-to-treat (ITT) population (all patients randomized in FREEDOMS who received at least one dose of study drug, including patients who did not enter the extension study). Comparisons were made for outcomes assessed from FREEDOMS baseline (month 0) to end of study (EoS), between continuously treated patients (continuous fingolimod 0.5 mg or 1.25 mg groups) and all patients who switched from placebo to fingolimod (combined switch group). Within-group comparison of treatment effects between months 0 and 24 (during FREEDOMS) and months 24 and 48 (during the extension) was made in the extension ITT population (all randomized patients who received at least one dose of extension study drug) and in the subgroup of patients within the extension ITT population who completed 48 months of therapy (48-month completer population). Baseline characteristics and safety outcomes were assessed using descriptive statistics. Extension-phase efficacy analyses were exploratory and 2-sided (significance level, 0.05), with no adjustment for multiple analyses. Details of statistical tests used for between-group and within-group analyses are given in the supplemental data.
Classification of evidence.
Given that safety and efficacy were demonstrated in FREEDOMS, the extension phase was designed to determine if treatment effects are sustained beyond 2 years, if switching from control to active therapy replicates this efficacy, and whether late-onset safety events occur. This study provides Class IV evidence that daily fingolimod 0.5 mg is well-tolerated in the long term, with no late-onset safety events. Patients switching to fingolimod experienced fewer relapses, Gd-enhancing lesions, and T2 lesions (all p < 0.001) after switching. When compared over the whole observation period with those initially randomized to placebo, patients initially randomized to fingolimod retained the benefits of earlier treatment: lower ARR (p < 0.0001), proportionately fewer patients with confirmed disability progression (p < 0.05), fewer Gd-enhancing and T2 lesions (p < 0.0001), and less BVL (p < 0.05).
RESULTS
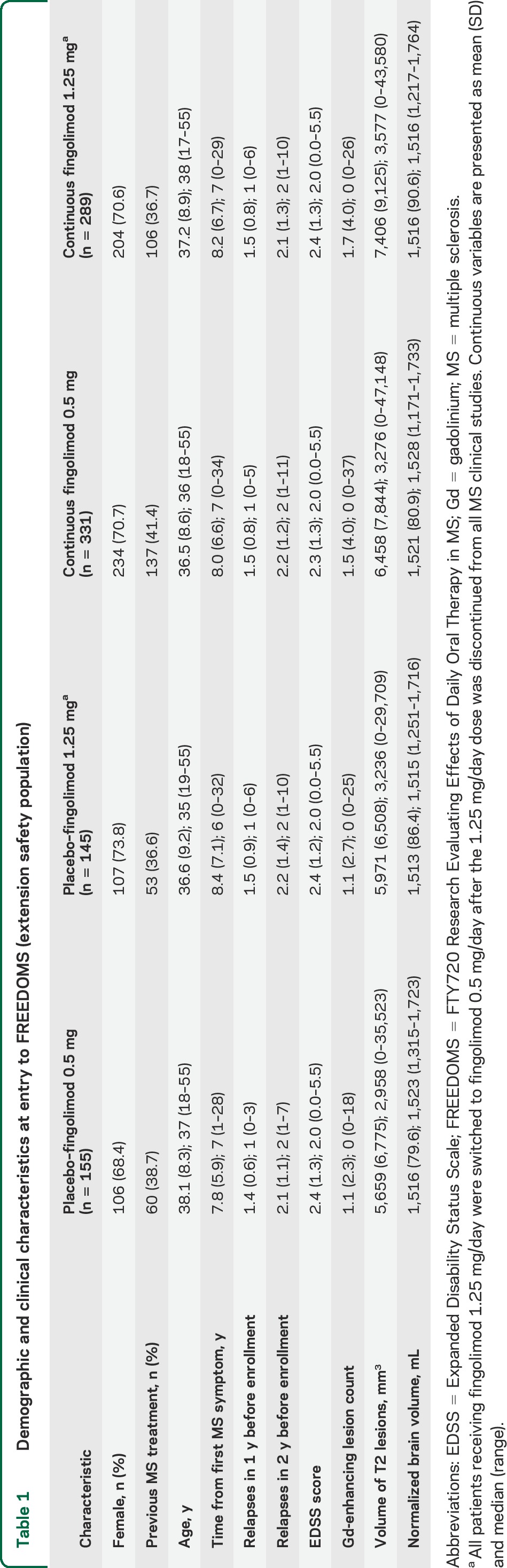
Of 1,272 patients randomized (FREEDOMS ITT population), 1,033 (81%) completed FREEDOMS and were eligible for the extension; 920 patients (89%) entered the extension (extension safety population), 916 (99.6%) of whom formed the extension ITT population, and 773 (84%) completed. Patient flow by treatment group and reasons for discontinuation are shown in figure 1; a data summary of patients who chose not to enroll is included in the supplemental data. At FREEDOMS baseline, disease and patient characteristics in the extension ITT population were similar across treatment groups, although the mean number of Gd-enhancing T1 lesions and T2 lesion volumes were slightly higher in the continuous fingolimod than in the switch groups (table 1). Baseline characteristics of the 48-month completer population (table e-1) and of the extension ITT population were similar.
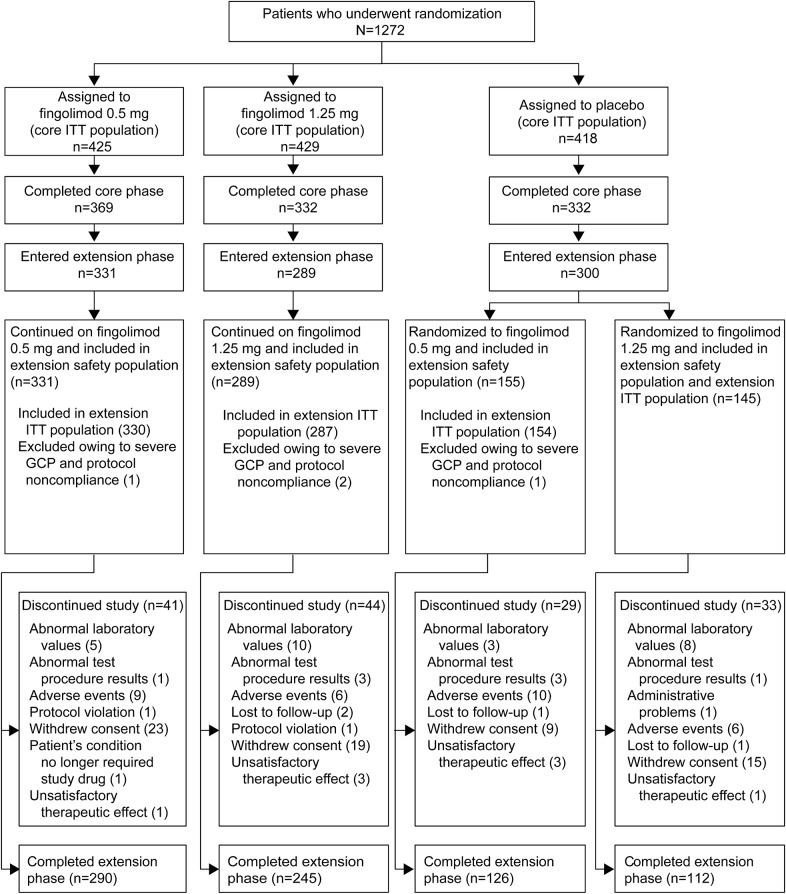
Figure 1. Patient disposition.
Reasons for discontinuation from FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS) were reported previously.4 Only those patients who completed FREEDOMS were eligible to enter the extension phase; 38 patients in the fingolimod 0.5 mg group, 43 in the fingolimod 1.25 mg group, and 32 in the placebo group decided not to participate in the extension. GCP = good clinical practice. ITT = intent to treat.
Table 1.
Demographic and clinical characteristics at entry to FREEDOMS (extension safety population)
The FREEDOMS study ran from 2005 to 2009 and the extension ran from February 2008 to June 2011. Time spent in the extension depended on the time of enrollment and when migration to LONGTERMS commenced at each study site. Over 90% of patients (856/920) completed 12 months of treatment in the extension (i.e., attended the month 36 visit), 88% (811/920) reached month 42, 44% (402/920) reached month 48, and 9% (87/920) reached month 54. The respective mean (SD) duration of exposure to fingolimod in the extension safety population was 1,394 (208) and 1,372 (225) days in the continuous fingolimod 0.5 mg and 1.25 mg groups, and 669 (206) and 626 (248) days in the placebo–fingolimod 0.5 mg and 1.25 mg switch groups.
Efficacy.
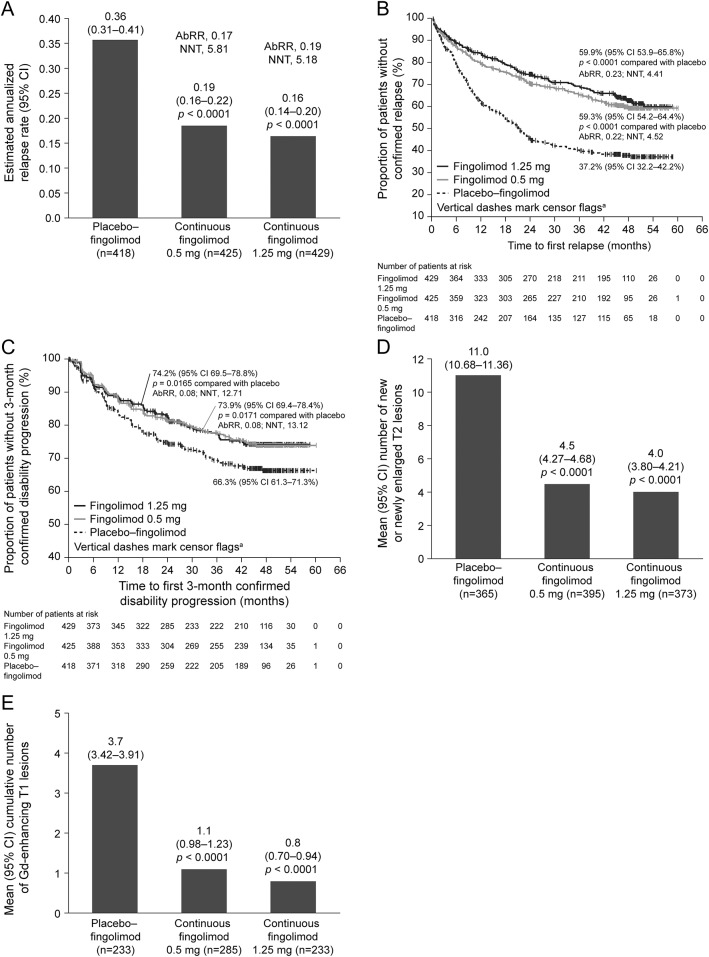
From month 0 to EoS, ARR in the continuous fingolimod groups was lower than in the combined switch group (figure 2A), corresponding to reductions of 48% (ARR ratio 0.52 [95% confidence interval (CI) 0.42–0.64]) and 54% (ARR ratio 0.46 [95% CI 0.37–0.57]) for the fingolimod 0.5 mg and 1.25 mg doses, respectively (both p < 0.0001). Similar advantages favoring the continuous fingolimod groups over the combined switch group were seen for the proportion of relapse-free patients at EoS and for the risk of relapse from month 0 to EoS (figure 2B). Comparing months 24–48 with months 0–24 within group in the extension ITT population, ARRs were significantly reduced in both switch groups, and remained low in the continuous fingolimod groups (table e-2). When the same within-group comparison was made in the 48-month completer population, the reduction in ARR was significant in the placebo–fingolimod 0.5 mg switch group (p < 0.0001), and showed a trend toward reduction in the placebo–fingolimod 1.25 mg switch group (p = 0.0643).
Figure 2. Between-group comparisons (month 0 to end of study, FREEDOMS ITT population).
(A) Annualized relapse rate (ARR) estimated from a negative binomial model adjusted for treatment, pooled country, number of relapses in the 2 years before enrollment, and FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS) baseline Expanded Disability Status Scale score; p values are for the ARR ratio between active treatment ARR and placebo ARR. (B) Time to first confirmed relapse with Kaplan-Meier estimate of patients free from relapse at end of study (EoS). aCensor flags indicate the time in study for patients with no confirmed relapse during the time interval, patients for whom follow-up ended before a confirmed relapse occurred, and patients who dropped out prior to a relapse. (C) Time to 3-month confirmed disability progression based on EDSS score with Kaplan-Meier estimate of patients free from progression at EoS. (D) Cumulative number of new or newly enlarged T2 lesions compared using a negative binomial model adjusted for treatment, FREEDOMS baseline volume of T2 lesions, and pooled country. (E) Cumulative number of gadolinium (Gd)-enhancing T1 lesions from month 0 to EoS, including patients with all assessments during that time interval; p values are for comparisons with the placebo–fingolimod group. AbRR = absolute risk reduction; CI = confidence interval; NNT = number needed to treat.
At EoS, the proportions (95% CI) of patients free from 3-month (figure 2C) and 6-month confirmed disability progression in the continuous fingolimod groups were 74% (69%–78%) and 80% (76%–84%) in the 0.5 mg group, 74% (70%–79%) and 79% (75%–84%) in the 1.25 mg group, and 66% (61%–71%) and 73% (68%–77%) in the combined switch group. Compared with the combined switch group, the respective risk of disability progression confirmed after 3 months and 6 months was reduced by 27% (hazard ratio [HR] 0.73 [95% CI 0.56–0.95]; p = 0.0189) and 31% (HR 0.69 [95% CI 0.51–0.93]; p = 0.0140) in the continuous fingolimod 0.5 mg group and by 29% (HR 0.71 [95% CI 0.55–0.93]; p = 0.0138) and 30% (HR 0.70 [95% CI 0.52–0.95]; p = 0.0211) in the continuous fingolimod 1.25 mg group.
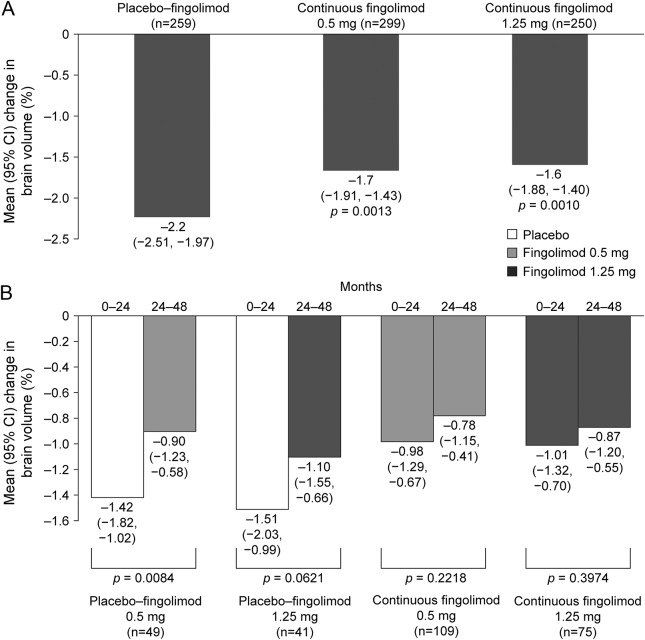
From day 0 to EoS, BVL was significantly lower in the continuous fingolimod 0.5 mg and 1.25 mg groups than in the combined switch group (figure 3A). For analysis of BVL, evaluable patients in the extension ITT population coincided with those evaluable in the 48-month completer population. In this group, the rate of BVL during months 24–48 was lower after switching to fingolimod 0.5 mg, and showed a trend toward reduction after switching to 1.25 mg compared with months 0–24 on placebo. During months 24–48, there were no differences among the 4 treatment groups in rates of brain volume reduction (figure 3B).
Figure 3. Percentage brain volume change.
(A) Between-group comparisons of changes in brain volume from month 0 to end of study in the FTY720 Research Evaluating Effects of Daily Oral Therapy in MS (FREEDOMS) intent-to-treat (ITT) population. Percentage brain volume change was compared using a rank analysis of covariance adjusted by treatment, normalized brain volume at FREEDOMS baseline, and country. (B) Within-group comparisons (months 24–48 vs months 0–24) in the extension ITT population and 48-month completer subgroup. Comparisons were made with the Wilcoxon signed-rank test. All patients receiving fingolimod 1.25 mg/day were switched to fingolimod 0.5 mg/day after the 1.25 mg/day dose was discontinued from all multiple sclerosis clinical studies. In this analysis, the evaluable individuals in the extension ITT population coincided with those evaluable in the 48-month completer subgroup; therefore the findings shown represent those for both groups. n = number of patients with brain volume change data for both time periods. CI = confidence interval.
The mean number of new or newly enlarged T2 lesions from month 0 to EoS was significantly lower in both continuous treatment groups than in the combined switch group (figure 2D). The cumulative mean number of Gd-enhancing T1 lesions from month 0 to EoS was also significantly lower in the continuous fingolimod groups than in the combined switch group (figure 2E). In each switch group in the extension ITT population, there was a significant reduction in the mean number of Gd-enhancing T1 lesions and of new or newly enlarged T2 lesions, and a significant increase in the proportion of patients free from Gd-enhancing T1 lesions or new or newly enlarged T2 lesions during months 24–48 compared with months 0–24 (table e-2). The clinical and MRI outcomes of the 48-month completers (table e-3) were similar to those of the FREEDOMS ITT population during the FREEDOMS study.4
Safety during the extension study.
The proportions of patients experiencing any AE, infections/infestations, cardiac disorders, or serious AEs were broadly similar across all groups (table 2). The most frequently reported AEs were nasopharyngitis, upper respiratory tract infection, lymphopenia, headache, and influenza (table 2). Abnormal hepatic enzyme levels were most common among patients switching to fingolimod 1.25 mg, and were more common in the switch groups than in the continuous fingolimod groups. Among AEs of special interest, there were 3 instances of macular edema, none of which was classified as serious. AEs leading to discontinuation of study drug occurred less frequently in the long-term continuous fingolimod groups than in the switch groups, and the most frequent events included lymphopenia, increased alanine aminotransferase, basal cell carcinoma, and dyspnea (each occurring in <1.4% of patients in any treatment group); there were no deaths.
Table 2.
Adverse events (extension safety population)
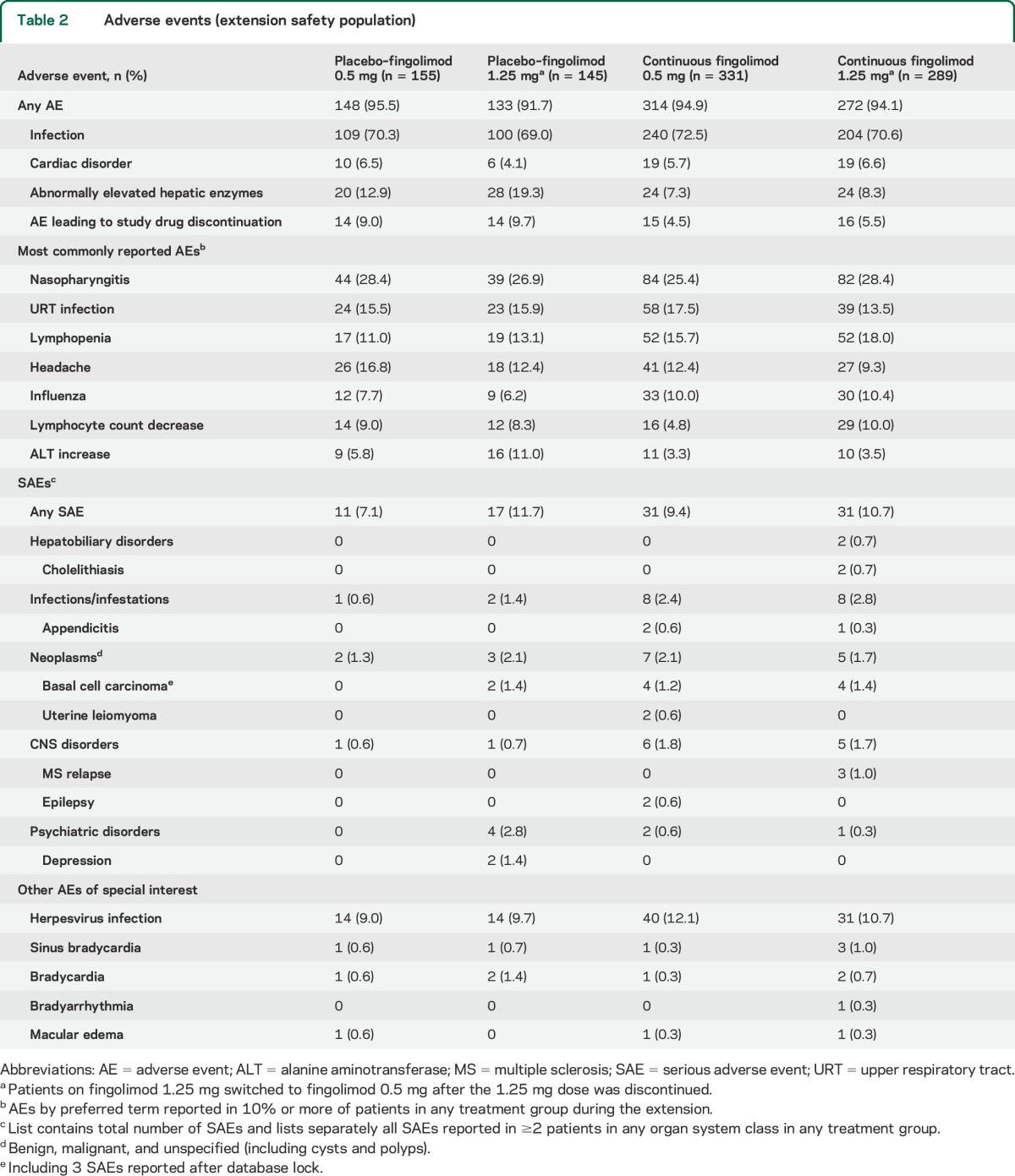
Small increases in blood pressure were observed in patients in the switch groups, while blood pressure in patients in the long-term continuous treatment groups remained stable over time. Consistent with first-dose effects seen in FREEDOMS and with other previous clinical experiences,4,11 a transient decrease in heart rate and a delay in atrioventricular conduction were observed in patients in the switch groups upon fingolimod initiation. Symptomatic first-dose bradycardia was seen in 2 patients, one with symptoms of severe dizziness and one with a mild feeling of cold. A transient episode of second-degree atrioventricular block on day 1 of therapy was reported in one patient who was asymptomatic and completed the extension study.
Five pregnancies were reported; 2 patients had normal, full-term pregnancies and delivered healthy babies. One patient had a therapeutic abortion when an ultrasound revealed that the fetus had tetralogy of Fallot. Another patient had a therapeutic abortion after an ultrasound revealed fetal death. One patient had an elective abortion.
DISCUSSION
This extension of the pivotal FREEDOMS study provides robust evidence that the low level of disease activity seen with fingolimod during years 1 and 2 in FREEDOMS was sustained during years 3 and 4, suggesting persistence of the treatment effect. Overall, this study confirmed there was no relevant difference between the 2 fingolimod doses regarding clinical and MRI-related outcomes. Patients who started fingolimod during the extension experienced significant improvements in clinical and MRI measures, essentially replicating, in this within-group comparison, the findings from the between-group comparison in FREEDOMS. However, patients who were initially randomized to fingolimod and continued therapy for a mean period of approximately 46 months still retained an advantage based on clinical and paraclinical measures at EoS, compared with those who delayed starting treatment until the extension study. This observation both supports the evolving position in the MS community for early treatment and provides evidence for a continued effect of fingolimod for up to 4 years.
Fingolimod was the first MS treatment to demonstrate a beneficial effect on BVL in phase 3 studies compared with placebo,4,5 and with IM interferon-β-1a.3 The comparably low rates of BVL across all groups during this extension study are consistent with the assumption that the effect of fingolimod on this structural outcome is continuous and not confined to the treatment initiation phase. Further analyses and long-term observations must clarify the biological and functional implications of this effect.
The lack of a placebo-control group in our study limits conclusions regarding efficacy. Participants knew that the placebo arm had terminated, but their treatment assignment during FREEDOMS remained blinded, as did their dose, until all participants received fingolimod 0.5 mg. Personnel at the MRI evaluation center remain blinded to treatment assignment, with no access to individuals' clinical data. Bias could result from differential drop-out of patients experiencing a lack of efficacy or AEs during FREEDOMS, but notably, baseline characteristics among patients completing 48 months were comparable to those in the FREEDOMS ITT population. Similarly, bias could arise because approximately 11% of eligible patients chose not to enroll in the trial extension. Their reasons were not recorded, but an exploratory analysis comparing enrollers and nonenrollers is described in the supplemental data. Finally, the study was terminated before all patients reached month 48 on study medication. Therefore, periods for within-group comparisons varied, but this variation was similar across treatment groups.
No new safety findings were observed in this extension compared with the 2-year controlled trial. As expected, AEs associated with treatment initiation were increased in the switch group; however, the incidence of AEs, serious AEs, and AEs related to fingolimod's mode of action were similar across groups. The overall frequency of AEs was also similar across treatment groups in FREEDOMS, but AEs associated with discontinuation of treatment (primarily liver enzyme elevations) were more common in the fingolimod 1.25 mg group than in other groups.4,12 This was not particularly evident in the extension; increased alanine aminotransferase was among the AEs that led to discontinuation, but this was recorded in all groups.
The lower percentage of patients who discontinued fingolimod owing to AEs in the continuous groups vs the switch groups may partly reflect selective drop-out of patients who experienced these AEs with fingolimod during FREEDOMS (cardiac AEs, macular edema, hepatic enzyme elevation),4 but this also suggests good tolerability of long-term treatment, with no late-occurring, unexpected safety findings. This is supported by a recent safety analysis of several clinical studies of fingolimod in RRMS that included patients with over 7 years of exposure to the drug.12 Cardiac effects associated with initiation of fingolimod were transient and have been reviewed extensively elsewhere.13 Blood pressure increased slightly in patients initiating fingolimod during the extension, but remained stable in the continuous-treatment groups, suggesting that this effect occurs early with fingolimod and then plateaus after the first few months of treatment.12,13
In this extension study, we found continuously low disease activity among patients initially randomized to fingolimod, and significant improvements in clinical and MRI outcomes after patients switched to fingolimod from placebo. The fact that patients starting early on fingolimod retained the advantage gained in their first 2 years of treatment compared with those initially randomized to placebo accentuates the importance of early treatment and implies a continuous benefit of fingolimod on both clinical measures and BVL. In conjunction with the absence of new safety or tolerability issues, these findings support the value of fingolimod in the long-term treatment of RRMS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and investigators who took part in this study; Peter Calabresi for participating in the steering committee; and Oxford PharmaGenesis Ltd. for editorial assistance, collating the comments of authors, and editing the paper for submission.
GLOSSARY
- AE
adverse event
- ARR
annualized relapse rate
- BVL
brain volume loss
- CI
confidence interval
- EDSS
Expanded Disability Status Scale
- EoS
end of study
- FREEDOMS
FTY720 Research Evaluating Effects of Daily Oral Therapy in MS
- Gd
gadolinium
- HR
hazard ratio
- ITT
intent-to-treat
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- TRANSFORMS
Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing–Remitting MS
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Professor Kappos was involved in the design of the study, collection, analysis, and interpretation of the data, and drafting and revising the manuscript. Professor O'Connor was involved in the design of the study, collection, analysis, and interpretation of the data, and revising the manuscript. Professor Radue was involved in the design of the study, collection, analysis, and interpretation of the data, figure design, and revising the manuscript. Professor Polman was involved in the design of the study, collection and interpretation of the data, and revising the manuscript. Professor Hohlfeld was involved in the design of the study, interpretation of the data, and revising the manuscript. Professor Selmaj was involved in the design of the study, collection and interpretation of the data, and revising the manuscript. Dr. Ritter was the statistician responsible for analysis of the data and was involved in their interpretation, as well as drafting and revising the manuscript. Dr. Schlosshauer was responsible for operational aspects of the study, including collection of data and ensuring the study was conducted as mandated by the protocol, and was involved in analysis and interpretation of the data, and revising the manuscript. Dr. von Rosenstiel was involved in the design of the analysis plan, analysis and interpretation of the data, and revising the manuscript. Dr. Zhang-Auberson was involved in the design of the analysis plan, analysis and interpretation of the data, and writing and revising the manuscript. Dr Francis was involved in the design of the analysis plan, analysis and interpretation of the data, as well as planning, writing, and review of the manuscript.
STUDY FUNDING
The study was sponsored by Novartis Pharma AG, Basel, Switzerland.
DISCLOSURE
L. Kappos: institution (University Hospital Basel) received in the last 3 years and used exclusively for research support: steering committee, advisory board, and consultancy fees from Actelion, Addex, Bayer Health Care, Biogen, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi-Aventis, Santhera, Siemens, Teva, UCB, and Xenoport; speaker fees from Bayer Health Care, Biogen, Merck, Novartis, Sanofi-Aventis, and Teva; support of educational activities from Bayer Health Care, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; royalties from Neurostatus Systems GmbH; and grants from Bayer Health Care, Biogen, Merck, Novartis, Roche, Swiss MS Society, the Swiss National Research Foundation, the European Union, and Roche Research Foundations. P. O'Connor: Abbott Labs, Actelion, Bayer, Biogen Idec, Celgene, EMD Merck Serono, Genentech, Genzyme, Lilly, Novartis, Roche, Sanofi-Aventis, and Teva Pharmaceuticals. Dr. O'Connor receives consultation fees from the MS Society of Canada. E.W. Radue: Actelion, Basilea Pharmaceutica Ltd., Biogen Idec, Merck Serono, and Novartis. C. Polman: Actelion, Biogen Idec, Bayer Schering, GlaxoSmithKline, Merck Serono, Novartis, Roche, Teva, and UCB. R. Hohlfeld: Bayer, Biogen-Idec, Merck-Serono, Novartis, Sanofi-Aventis, and Teva. K. Selmaj: Novartis, Biogen Idec, Merck Serono, Roche, ONO Pharmaceuticals, and Genzyme. S. Ritter is an employee of Novartis Pharmaceuticals Corporation. R. Schlosshauer is an employee of Novartis Pharma AG. P. von Rosenstiel is an employee of Novartis Pharma AG. L. Zhang-Auberson is an employee of Novartis Pharma AG. G. Francis is an employee of Novartis Pharmaceuticals Corporation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002;277:21453–21457. [DOI] [PubMed] [Google Scholar]
- 2.Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med 2011;12:213–228. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415. [DOI] [PubMed] [Google Scholar]
- 4.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 5.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545–556. [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124–1140. [DOI] [PubMed] [Google Scholar]
- 7.Comi G, O'Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010;16:197–207. [DOI] [PubMed] [Google Scholar]
- 8.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed November 21, 2014. [Google Scholar]
- 9.World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed November 21, 2014. [Google Scholar]
- 10.Neurostatus. Neurostatus training and documentation DVD for a standardized neurological examination and assessment of Kurtzke's functional systems and Expanded Disability Status Scale for MS patients. Available at: http://www.neurostatus.net. Accessed November 21, 2014.
- 11.European Medicines Agency. Gilenya EU summary of product characteristics (updated September 5, 2014). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed November 21, 2014.
- 12.Kappos L, Cohen J, Collins W, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Mult Scler Relat Disord 2014;3:494–504. [DOI] [PubMed] [Google Scholar]
- 13.Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J 2014;168:632–644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.