Key Points
Delivery of ZFNs and donor templates results in high levels of gene correction in human CD34+ cells from multiple sources, including SCD BM.
Modified CD34+ cells are capable of engrafting immunocompromised NSG mice and produce cells from multiple lineages.
Abstract
Sickle cell disease (SCD) is characterized by a single point mutation in the seventh codon of the β-globin gene. Site-specific correction of the sickle mutation in hematopoietic stem cells would allow for permanent production of normal red blood cells. Using zinc-finger nucleases (ZFNs) designed to flank the sickle mutation, we demonstrate efficient targeted cleavage at the β-globin locus with minimal off-target modification. By codelivering a homologous donor template (either an integrase-defective lentiviral vector or a DNA oligonucleotide), high levels of gene modification were achieved in CD34+ hematopoietic stem and progenitor cells. Modified cells maintained their ability to engraft NOD/SCID/IL2rγnull mice and to produce cells from multiple lineages, although with a reduction in the modification levels relative to the in vitro samples. Importantly, ZFN-driven gene correction in CD34+ cells from the bone marrow of patients with SCD resulted in the production of wild-type hemoglobin tetramers.
Introduction
Sickle cell disease (SCD) is one of the most common monogenic diseases in the world, with >250 000 new patients each year.1 Caused by a single point mutation in the seventh codon of the β-globin gene, the disease is characterized by anemia and severe acute painful crises with frequent hospitalizations, limiting the average lifespan to just 36 to 40 years of age.2,3 The only currently available cure for SCD is an allogeneic hematopoietic stem cell transplant; however, very few patients have a fully matched donor available, and those receiving mismatched transplants may suffer from immune complications such as graft rejection or graft-versus-host disease. Patients with SCD are candidates for autologous gene therapy: correction of the patient’s own hematopoietic stem cells (HSCs), followed by reinfusion of those modified cells with the goal of having the treated patient produce functioning erythrocytes throughout life. Several groups have performed nontargeted gene therapy for hemoglobinopathies using lentiviral vectors, and although these approaches show promise, they carry risks of insertional oncogenesis from semirandom vector integration.4-6 An ideal approach to gene therapy for SCD would be to correct the canonical sickle mutation in the DNA of a patient’s hematopoietic stem cells such that those cells differentiate into erythroid cells that permanently produce wild-type (WT) adult β-globin under the regulation of the endogenous transcriptional control elements.
Zinc-finger nucleases (ZFNs) offer the ability to target gene modification to specific genomic sites in cells. These chimeric endonucleases are able, on dimerization, to create a double-strand break (DSB) in the DNA. Two major cellular DNA repair mechanisms correct DSBs: nonhomologous end joining (NHEJ) and homology-directed repair (HDR). NHEJ repair can lead to the introduction of errors at the break site, knocking out gene function (as is the goal with therapies for HIV which target chemokine receptor type 5 [CCR5]).7,8 However, in the presence of a homologous donor template, cells may perform HDR using the donor template (typically the sister chromatid) to repair the break.9,10 In this article, we refer to errant repair via NHEJ as “indels” and DNA repair via HDR as “gene correction” (when the result is the correction of the sickle mutation) or “gene modification” (when the result is a change to the gene other than correction of the sickle mutation to the WT base). In the case of gene correction for SCD, this would involve codelivery of a normal segment of the β-globin gene spanning the sickle mutation site in conjunction with the targeted nuclease, leading to HDR-mediated incorporation of the WT base in place of the sickle mutation, thereby permanently correcting the cell’s genome. Several groups have investigated the use of targeted nucleases for gene correction or targeted insertion at the β-globin locus in cell lines, as well as induced pluripotent stem cells.11-15 However, to date, none of these targeted nuclease-based approaches for SCD have been successful in a clinically relevant cell source of hematopoietic stem and progenitor cells (HSPCs).
Here, we show that engineered ZFNs can specifically target the β-globin gene and induce high levels of DNA cleavage in CD34+ HSPCs. When the ZFNs were delivered along with either an integrase-defective lentiviral vector (IDLV) or an oligonucleotide (oligo) donor, they efficiently effected gene correction at the β-globin locus in HSPCs. Genome-wide evaluation of ZFN specificity revealed off-target cleavage only in the highly homologous and functionally dispensable δ-globin gene. We demonstrate that ZFN and donor-modified CD34+ cells are able to differentiate into erythroid, myeloid, and lymphoid cell types, both in vitro and in vivo, when engrafted into immune-deficient NOD.Cg-PrkdSCIDIl2rgtm1Wjil/SzJ (NSG) mice. Finally, we achieved targeted gene correction of the sickle mutation in bone marrow (BM) CD34+ cells from SCD patients leading to the production of WT hemoglobin (HbA). This work demonstrates site-specific gene correction using ZFNs in HSPCs and provides the groundwork for a potential therapy to treat SCD.
Materials and methods
All human BM samples from volunteer donors with SCD have been used following University of California–Los Angeles (UCLA) institutional review board protocol 10-001399 with written informed consent, in accordance with the Declaration of Helsinki. Use of umbilical cord blood collected at normal births was deemed exempt from Institutional Review Board review because it was anonymous medical waste. All work with mice was done under protocols approved by the UCLA Animal Care Committee. See supplemental Materials available on the Blood Web site for additional methods.
Electroporation and transduction
CD34+ cells were thawed at 37°C, washed in Iscove’s modified Dulbecco’s medium (Life Technologies) supplemented with 20% fetal bovine serum (Gemini Bio-products) and 1× glutamine, penicillin, and streptomycin (Gemini Bio-products) and prestimulated for 48 hours in X-VIVO15 medium (Lonza) containing glutamine, penicillin, streptomycin, 50 ng/mL stem cell factor, 50 ng/mL fms-related tyrosine kinase 3 ligand (Flt3-L), and 50 ng/mL thrombopoietin (Peprotech). For electroporation, 200 000 cells per reaction were spun at 90g for 15 minutes, resuspended in 100 μL of BTXpress buffer (Harvard Apparatus), mixed with indicated amounts of ZFN mRNA and/or oligonucleotide as applicable, and pulsed once at 250 V for 5 milliseconds in the BTX ECM 830 Square Wave Electroporator (Harvard Apparatus). Following electroporation, cells rested for 10 minutes at room temperature before the addition of culture medium and transfer to plates in a total of 500 μL. The donor IDLV was present in the final culture medium following electroporation at the concentrations described for appropriate samples and washed out the following day.
Gene modification and Surveyor Nuclease assay
The Surveyor Nuclease assay (Cel-1) was used to determine ZFN-induced site-specific allelic disruption. A 410-bp region surrounding the ZFN binding site was polymerase chain reaction (PCR) amplified from 200 ng of genomic DNA using Cel1Fwd (5′-gacaggtacggctgtcatca-3′) and Cel1Rev (5′-cagcctaagggtgggaaaat-3′) using Accuprime Taq Hi-Fi (Life Technologies). Denaturation, reannealing, digestion, and electrophoretic and densitometry analysis were completed as previously described.16
Site-specific gene modification was detected by restriction fragment length polymorphism (RFLP). A 1.1-kb region outside of the homologous donor template region was PCR amplified (primers described in supplemental Materials and Methods). To quantify gene modification at HhaI, a quantitative PCR-based assay was used. A set of 2 PCR reactions was performed using the 1.1-kb PCR product described above as a template. The unpurified PCR template was diluted 1:5000, of which 1 μL was used in each of the following 25-μL reactions. The first PCR was performed to amplify modified genomes, using primers HhaIFwd (5′- gaagtctgccgttactgcg-3′) and HhaIRev (5′-cccagtttctattggtctcc-3′). The second PCR was performed to normalize the input template using primers ExonIIFwd (5′-ctcggtgcctttagtgatgg-3′) and ExonIIRev (5′-gactcaccctgaagttctc-3′). Both of these PCRs were made quantitative using Power SYBR Green PCR Master Mix (Life Technologies) and acquired on ViiA7 (Life Technologies). Frequency of gene modification was determined using the Ct (cycles to threshold) difference between the 2 reactions and a plasmid standard curve.
Globin paralogs were PCR amplified and deep-sequenced on an Illumina MiSeq machine. The primers used for PCR are as follows: HBB, 5′-acacgacgctcttccgatctnnnngggctgggcataaaagtcag-3′ and 5′-gacgtgtgctcttccgatcttccacatgcccagtttctatt-3′; HBD, 5′-acacgacgctcttccgatctnnnntaaaaggcagggcagagtcga-3′ and 5′-gacgtgtgctcttccgatctacatgcccagtttccatttgc-3′; HBE1, 5′-acacgacgctcttccgatctnnnnctgcttccgacacagctgcaa-3′ and 5′-gacgtgtgctcttccgatcttcacccttcattcccatgcat-3′; HBG1 and HBG2, 5′-acacgacgctcttccgatctnnnnggaacgtctgaggttatcaat-3′ and 5′-gacgtgtgctcttcc gatcttccttccctcccttgtcc-3′. HBG1 and HBG2 were coamplified, and sequence reads were assigned to either HBG1 or HBG2 using locus-specific single nucleotide polymorphisms within the amplicon. Mixed bases within the forward primers allow for cluster deconvolution during sequencing.
Transplantation of cord blood and mobilized peripheral blood CD34+ cells into NSG mice
To evaluate the IDLV donor approach, fresh cord blood (CB) CD34+ cells from multiple healthy individuals were prestimulated for 2 days before being electroporated with ZFN mRNA and transduced with donor IDLV (2 × 107 TU/mL; multiplicity of infection [MOI] = 50). The prestimulation medium consisted of X-VIVO15 media (Lonza) containing glutamine, penicillin, streptomycin, 50 ng/mL stem cell factor, 50 ng/mL Flt-3L, and 50 ng/mL TPO (Peprotech). Following 1 day of recovery, 1 × 106 viable cells in PBS (Corning) with 0.1% bovine serum albumin (Sigma) were transplanted by tail-vein injection into 6- to 8-week-old NSG mice (The Jackson Laboratory) after 250-cGy total body irradiation. Control samples consisted of CD34+ cells that were cultured in parallel but not exposed to mRNA, electroporation, or IDLV (mock-treated). Small aliquots were cultured in vitro for multiple analyses. Mobilized peripheral blood (mPB) samples were frozen 1 day following electroporation, thawed at a later date, and allowed 1 day of recovery before transplantation as above by tail-vein injection into 6- to 8-week-old NSG mice. The number of mice transplanted for each experiment was determined based on available source material (cells in each arm) at time of injection. Final tissue analysis for gene modification rates (high-throughput sequencing) was completed in a blinded manner.
Results
ZFNs efficiently cleave the β-globin locus
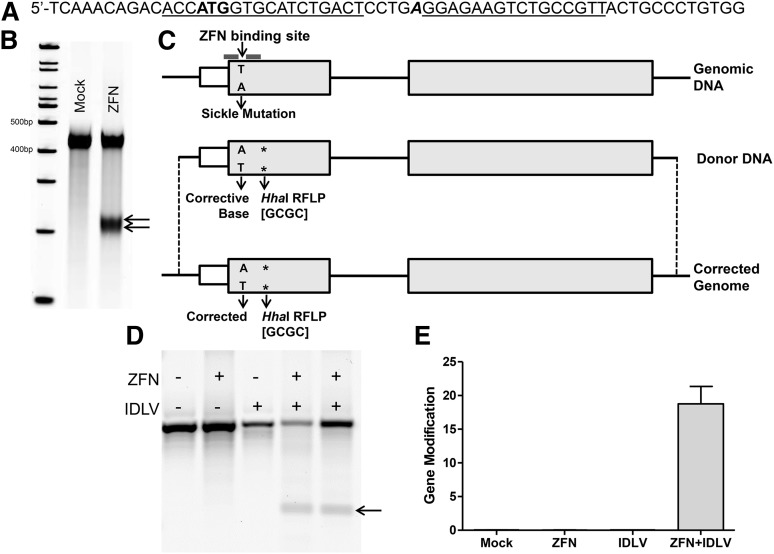
ZFNs were designed to target the sickle mutation of the human β-globin locus (Figure 1A; supplemental Table 1). Electroporation of in vitro transcribed mRNA encoding the ZFNs into CD34+ HSPCs isolated from both human umbilical CB and mPB resulted in effective cleavage of the target locus, as determined by the Surveyor Nuclease assay (Figure 1B). The ZFNs were able to induce 35% to 65% allelic disruption at the β-globin locus depending on the cell type (supplemental Table 2).
Figure 1.
Cleavage and correction at the β-globin locus in CD34+ cells using an IDLV donor. (A) A portion of exon I of β-globin showing the ZFN target site (underlined) atop the start codon (bold) and the location of the sickle mutation (bold, italic). (B) Representative gel showing targeted cleavage at the β-globin locus in CB CD34+ cells. Cells were analyzed 3 days after electroporation with in vitro transcribed mRNA encoding the ZFNs. Mock represents untreated CD34+ cells. Arrows indicate cut bands following PCR amplification and digestion with Surveyor Nuclease. (C) Schematic of site-specific gene correction at the sickle mutation. Details of the donor construct and resulting genomic DNA on cleavage by ZFNs and repair by HDR. Location of sickle mutation and HhaI RFLP (asterisk) is indicated. Translated regions of exons to scale, introns, and 5′ untranslated region not to scale. (D) Representative RFLP gel for targeted gene modification of β-globin. CB CD34+ cells were electroporated with in vitro transcribed ZFN mRNA and transduced with donor IDLV. Cells were harvested 4 days after treatment, PCR amplified from outside the donor region, digested with HhaI enzyme, and resolved on an agarose gel. Arrow shows cleaved product, indicating incorporation of the RFLP into the genome at the target site. (E) Gene modification percentages in CD34+ cells. CB CD34+ cells were electroporated with in vitro transcribed ZFN mRNA (10 μg/mL) and transduced with donor IDLV (2 × 107 TU/mL). Cells were harvested 3 days after treatment and PCR amplified from outside the donor region, and qPCR was completed with primers designed to specifically detect the incorporation of the silent base change generating the HhaI RFLP and normalized to primers binding in exon II of the β-globin locus in the amplicon (n = 4 for all conditions). Error bars, mean ± standard deviation.
ZFNs drive high levels of gene modification at the β-globin locus
Following successful cleavage at the targeted β-globin locus, we sought to determine whether correction of the sickle base at this site was possible using a homologous donor template. To this end, 2 types of gene correction templates were designed and tested in parallel: a short DNA oligo and an IDLV. The 1.1-kb human β-globin gene fragment donor template cloned into the IDLV was designed to include the corrective change at the sickle mutation, as well as a silent RFLP to create a HhaI restriction site for surrogate analysis of homologous recombination (Figure 1C). The donor template was delivered by IDLV, allowing for efficient transduction of CD34+ HSPCs with minimal cytotoxicity, transiently producing high template copy numbers with minimal genomic integration17 (supplemental Figure 1). Gene modification levels in the CD34+ cells treated with the ZFN plus the IDLV donor were initially determined by the HhaI RFLP digestion (Figure 1D) and a quantitative PCR-based (qPCR) assay in an average of 18.0 ± 2.2% of alleles (Figure 1E). Optimizations of ZFN mRNA and IDLV donor concentrations were performed (supplemental Figures 2 and 3) and demonstrated the importance of titrating the ZFN reagents in the cell type of interest to achieve high-level modification while maintaining cell numbers and viability.
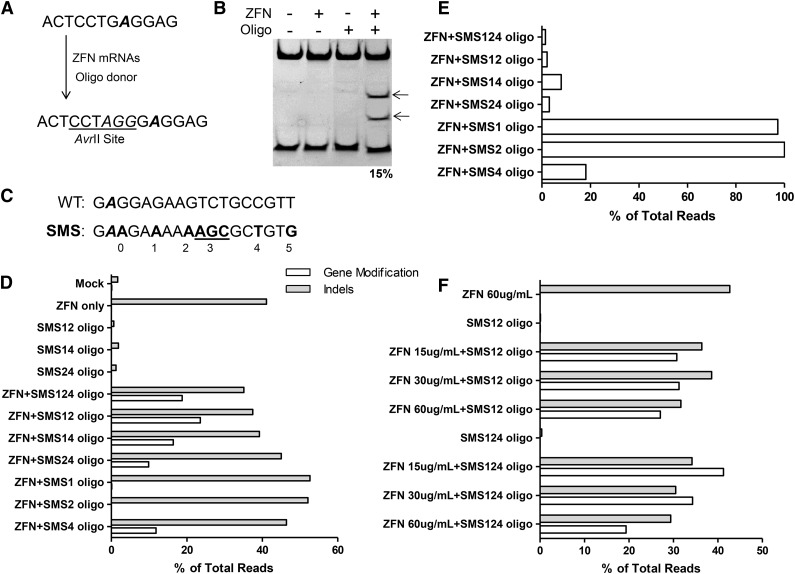
Use of an oligonucleotide as a gene modification/correction template would have advantages of speed, cost, reproducibility, and ease of experimentation.18 Initial experiments using an oligonucelotide donor template in HSPCs from mPB introduced a 3-bp sequence designed to create an AvrII RFLP in the β-globin gene (HBB) and yielded 15% gene modification (Figure 2A-B). To refine the use of oligo donors, a panel of oligonucleotides was tested (supplemental Table 3) corresponding to one or the other strand and of symmetrically increasing length centered on the ZFN cleavage site. Use of longer reverse-strand oligonucleotides gave the highest level of gene modification (supplemental Figure 4). Because gene modification or correction at the SCD mutation site will not change the sequence or spacing of the ZFN binding sites, silent mutations were designed into the donor template and cointroduced into the chromosome to prevent or reduce ZFN binding and recleavage of modified alleles. Use of reverse-strand donors is compatible with introduction of silent mutations at the 3′ ZFN binding site (Figure 2C). Various combinations of silent mutations were introduced into the donor oligo, and the frequency of gene modification was assayed. The combination of silent mutation sites (SMSs) 1 and 2 (SMS12) yielded the highest level of gene modification and was used in all subsequent experiments (Figure 2D). The inverse relationship between gene-modified alleles and modified-then-recleaved alleles suggests that the SMS donors indeed serve to block ZFN recleavage (Figure 2E). Examination of HBB transcripts in HSPC pools and in burst-forming unit, erythroid colonies found no evidence for alteration of splicing of mRNA from any SMS allele (data not shown). Comprehensive analysis of molecular outcomes at β-globin after treatment with ZFNs and the SMS124 donor revealed only minor levels of unexpected DNA repair events such as those from a combination of homology-directed and NHEJ-based gene modification (1.5%) or from NHEJ-based capture of the oligonucleotide (0.4%; Table 1). Finally, the overabundance of partial homology-directed gene modification events (SMS12 vs SMS24) lends strong support to the idea that new DNA synthesis during oligonucleotide-templated gene modification occurs using the left-hand 3′ single-stranded end of the resected DSB (supplemental Figure 5A-B).19 Optimal combinations of ZFNs, oligonucleotide, and HSPC donor allowed gene modification of 30% to 40% of alleles (Figure 2F).
Figure 2.
Correction at the β-globin locus in CD34+ cells using an oligonucleotide donor. (A) Schematic of oligonucleotide-directed gene modification. The top sequence is the genomic DNA of the β-globin locus with the site of the sickle mutation in bold and italicized. The sequence of the modified locus is shown on the bottom with the inserted bases shown in italics. (B) Gene modification in mPB CD34+ cells with an oligonucleotide donor. PAGE of an AvrII-digested PCR amplicon of the β-globin locus. The fragment contains a native AvrII site, cleavage of which serves as an internal control for AvrII digestion (the lower band on the gel). Arrows indicate AvrII cleavage products. (C) Six possible sites of silent mutation in the SBS 33501 ZFN binding site. Sickle mutation italicized in bold, and all possible silent mutation sites are in bold (including those not discussed). (D) Silent mutations increase gene modification at β-globin. mPB CD34+ cells were transfected with ZFNs (30 μg/mL) and the indicated donor oligonucleotide (3 μM). Introduction of the relevant silent mutation was assayed via high-throughput sequencing. White bars indicate gene modification; gray bars indicate indels. (E) Silent mutations block ZFN recleavage. Alleles with indels were examined for evidence of homology-mediated modification. Shown are the percentages of alleles with gene modification that also have evidence of NEHJ-driven indels. (F) Optimization of ZFN concentration and donor type. NHEJ-driven indels (gray bars) and gene modification (white bars) were assayed by high-throughput DNA sequencing. Given the depth of high-throughput DNA sequencing, measurement error is expected to be very low.
Table 1.
Molecular outcomes from treatment of HSPCs with ZFNs and an oligonucleotide donor
| Outcome | Frequency (%) |
|---|---|
| No change | 46.1 |
| NHEJ-mediated deletions | 30.5 |
| Oligonucleotide-templated gene modification | 18.8 |
| NHEJ-mediated insertions | 2.8 |
| Half gene modification, half NHEJ | 1.5 |
| Capture of the donor oligonucleotide | 0.4 |
Thus, ZFNs are able to induce both high levels of targeted DSBs as well as high levels of site-specific gene modification in primary HSPCs when delivered alongside a homologous donor template.
Comprehensive ZFN specificity assessment
Because β-globin has high homology to other globin genes (δ-, ε-, Aγ-, Gγ-, and pseudo-β-globin), the ZFNs were designed to avoid cleavage in these regions (supplemental Figure 6A). Cleavage rates at homologous globin genes were assayed using the Surveyor Nuclease assay and by high-throughput DNA sequencing. Analysis of each of these regions revealed off-target modification at only the highly homologous δ-globin gene in mPB, CB, and SCD BM CD34+ cells (supplemental Figure 6B-C; supplemental Table 2).
We complemented these direct tests of off-target ZFN cleavage with a more comprehensive, unbiased genome-wide approach to identify off-target sites. This assay is based on the propensity of a nonhomologous IDLV to be captured at the sites of DSBs and uses downstream clustered integration site analysis (CLIS) of the integrations in K562 cells, selected for their permissiveness to IDLV capture.20 As predicted, the CLIS analysis revealed trapping at β-globin and δ-globin (supplemental Figure 7; supplemental Table 4). No other putative off-target sites in the CLIS analysis that had significant homology to the ZFN binding sites were found. These results demonstrate the high level of specificity of this pair of ZFNs to their intended target site on a genome-wide scale.
In vitro differentiation of modified HSCs
To ensure that gene-modified HSPCs were capable of a normal broad spectrum of erythroid and myeloid differentiation, treated cells were assayed both as single cells and in bulk culture. Single cells were monitored for colony-forming potential in methylcellulose medium containing cytokines that promote HSPC differentiation. HSPCs treated with either ZFNs alone or treated with ZFNs and an oligonucleotide donor generated similar numbers and patterns of erythroid and myeloid clones (supplemental Figure 8). Genotyping of individual erythroid colonies confirmed the presence of the intended modifications at the expected frequency (data not shown). In addition, HSPCs were induced to differentiate to red blood cells in bulk culture.21 Cells were sampled throughout red blood cell differentiation, and the frequency of modified cells was measured by high-throughput DNA sequencing. Consistent with the single cell analysis of erythroid colonies grown in methylcellulose, essentially no change in the frequency of modified cells was found during differentiation to red blood cells in bulk culture regardless of the starting level of gene modification in the pool (supplemental Figure 9A). In a parallel experiment, we converted 14% of β-globin alleles to the sickle form (HbS) and assayed the globin chains present after 18 days of erythroid differentiation by high-performance liquid chromatography (HPLC). Approximately 18% of the hemoglobin produced by these cells was HbS, demonstrating the impact of the gene modification event at the protein level (supplemental Figure 9B).
Engraftment in NSG mice
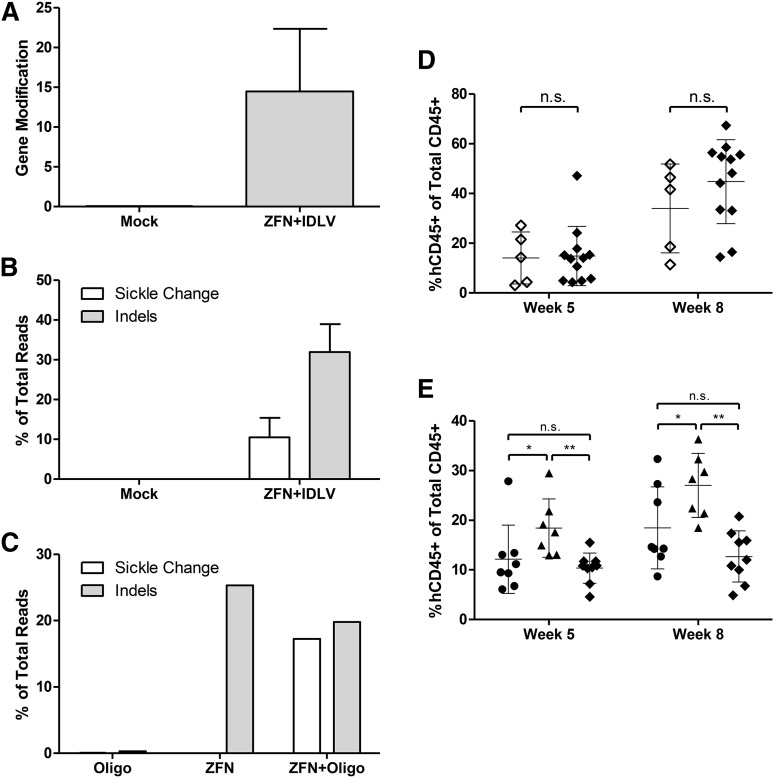
To determine whether the ZFN-modified cells maintain their hematopoietic repopulating capacity, ZFN- and IDLV donor-treated or mock-treated HSPCs were xenografted into immune-deficient NSG mice. As with the in vitro studies described thus far, WT cells are modified using donor templates containing the sickle mutation as a reverse model to allow for tracking of modified cells. Parallel culture of the cells in vitro showed that gene modification rates ranged from 5% to 20% by qPCR RFLP analysis, with a mean value of 14.5 ± 6.4% (Figure 3A). High-throughput sequencing revealed 10.5 ± 4.0% of alleles contained the exchanged base at the sickle location with insertions or deletions (indels) seen in 32.0 ± 9.9% (Figure 3B). In addition, the hematopoietic potential of these cells was evaluated in an in vitro colony-forming assay; the cells maintained their broad spectrum colony-forming ability relative to untreated mock samples in all analyzable lineages (supplemental Figure 10A).
Figure 3.
Transplantation of ZFN and donor-treated cells into NSG mice. (A) Gene modification rates of bulk transplanted cells treated with ZFN+IDLV and cultured in vitro as determined by qPCR for the RFLP at 7 days after electroporation. Mock cells are untreated (n = 3 independent experiments). (B) Modification at the sickle base evaluated by high-throughput sequencing for ZFN+IDLV-modified CD34+ cells. Results of sequencing of the β-globin locus showing percentage of total aligned reads containing the changed wild-type to sickle base (T), as well as insertions and deletions (indels) at the cut site. Same samples as in A. Changed base, white; indels, gray. (C) CD34+ cells were electroporated with Oligo, ZFN, or ZFN+Oligo and cultured in vitro before transplantation into NSG mice. Modification rates at the sickle base and indels are shows as in B (n = 1 experiment). (D) Engraftment in the peripheral blood of transplanted mice at 5 and 8 weeks after transplant. Human engraftment determined as a percentage of hCD45+ cells out of the total hCD45+ and mCD45+ cells by flow cytometry of cells from mice receiving either mock- or ZFN+IDLV-treated cells. Mock, open diamonds; ZFN+IDLV, closed diamonds. (n = 3 independent experiments; mock, n = 5; ZFN+IDLV, n = 12; unpaired t test). (E) Engraftment in the peripheral blood as in D of cells from mice receiving either Oligo-, ZFN-, or ZFN+Oligo-treated cells. Oligo, circles; ZFN, triangles; ZFN+Oligo, diamonds (Oligo, n = 8; ZFN, n = 7; ZFN+Oligo, n = 9); 1-way analysis of variance. n.s., not significant. *P < .05, **P < .01. Error bars, mean ± standard deviation.
To evaluate HSPCs modified with the oligonucleotide donor (SMS12s as in supplemental Table 3), mPB CD34+ cells were electroporated with mRNA encoding the ZFNs and the donor template oligonucleotide. Control samples consisted of cells treated only with oligonucleotide or only with ZFNs. Analyses of samples cultured in vitro revealed 17% gene modification and 20% indels before transplant (Figure 3C); parallel samples plated in methylcellulose medium demonstrated that cells treated with both ZFNs and a donor oligonucleotide maintained their colony-forming potential (supplemental Figure 10B).
At 5 and 8 weeks after injection, the transplanted mice were evaluated for engraftment of human HSPCs measured as the percentage of human CD45+ cells of total CD45+ cells, both human and murine. Engraftment levels of ZFN and IDLV-treated HSPCs were comparable to those of untreated controls with an average of 14.8 ± 11.4% at week 5 and increased to 45% at week 8 (Figure 3D). Lineage analysis (CD3 for T cells, CD33 for myeloid cells, CD34 for HSPCs, CD19 for B cells, and CD56 for natural killer cells) of the peripheral blood of the mice was as expected in this model22 (supplemental Figure 11A). Analysis of the human cells in mice receiving cells treated with both ZFN and an oligonucleotide donor revealed an HSPC differentiation spectrum similar to that of the ZFN and IDLV-treated HSPCs, although with lower engraftment levels in mice receiving cells treated with the oligonucleotide or ZFN and oligonucleotide (Figure 3E; supplemental Figure 11B).
Mice were euthanized at 16 weeks after transplant to allow for evaluation of human cell engraftment and gene modification levels. For the mice receiving ZFN- and IDLV-treated cells, evaluation of the PB revealed high levels of engraftment and expected lineage distribution (supplemental Figure 12A-B). Analysis of the BM compartment was similar (supplemental Figure 13A-B). Likewise, in mice that received cells treated with both ZFNs and oligonucleotide, engraftment levels and lineage distribution were similar to those seen in the cohort of mice receiving ZFN and IDLV-treated HSPCs in the PB and BM (supplemental Figures 12C-D and 13C-D).
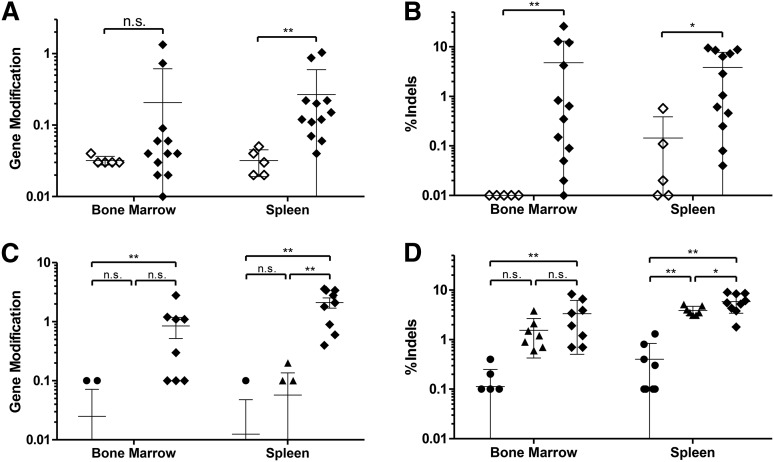
To determine whether the human cells present in the mice were modified by the ZFN reagents, genomic DNA was isolated from BM and spleen tissues of each mouse, and the human β-globin gene was interrogated. Sequence analysis of the tissues of mice receiving ZFN- and IDLV-treated cells revealed the presence of gene modification in these mice, although at a lower frequency (0.21 ± 0.39% and 0.27 ± 0.31% for BM and spleen, respectively) than the 10.5% gene modification levels observed for input cells (Figure 4A). Analysis of the indels caused by NHEJ revealed higher levels of changes than by HDR, with 4.8 ± 7.8% of all sequence reads from the BM-containing indels and 3.8 ± 3.7% from the spleens (Figure 4B).
Figure 4.
Gene-modified cells persist in NSG mice. (A) Gene modification rates in the bone marrow and spleen of transplanted mice at 16 weeks in cells from mice receiving either mock- or ZFN+IDLV-treated cells. High-throughput sequencing of the β-globin locus showing percentage of total aligned reads containing the modified base at the sickle mutation. Mock, open diamonds; ZFN+IDLV, closed diamonds; unpaired t test. (B) Sequencing of the β-globin locus showing insertions and deletions (indels) at the cut site as a percentage of total aligned reads. Mock, open diamonds; ZFN+IDLV, closed diamonds (n = 3 independent experiments; mock, n = 5; ZFN+IDLV, n = 12); unpaired t test. (C) Modification at the sickle base in cells from mice receiving either Oligo-, ZFN-, or ZFN+Oligo-treated cells as described in A. (D) Sequencing results for indels of cells from mice receiving either Oligo-, ZFN-, or ZFN+Oligo-treated cells as in B. Oligo, circles; ZFN, triangles; ZFN+Oligo, diamonds (Oligo, n = 8; ZFN, n = 7; ZFN+Oligo, n = 9); 1-way analysis of variance. n.s., not significant; asterisk indicates significance: *P < .05, **P < .01. Error bars, mean ± standard deviation. Values of zero cannot be plotted on a log scale but were used to calculate the error bars.
Genomic analysis of the BM and spleens of mice receiving ZFN- and oligonucleotide-treated cells bearing 17.3% gene modification at the sickle base and 19.8% indels in the bulk population prior to administration also demonstrated maintenance of gene modification. DNA from these tissues was subjected to high-throughput sequencing, revealing 0.85 ± 0.81% and 2.11 ± 1.19% targeted gene modification in the BM and spleen, respectively (Figure 4C). Assessment of the indels in these tissues showed levels of 3.34 ± 2.65% in the BM and 5.86 ± 2.30% in the spleen (Figure 4D). These results demonstrate that CD34+ cells that have undergone site-specific DNA cleavage by ZFNs and homology-directed gene modification are capable of engrafting and undergoing multilineage differentiation.
Gene correction in sickle BM
Because patients with SCD are not candidates for stem cell mobilization with granulocyte colony-stimulating factor,23 we obtained CD34+ HSPCs from BM aspirates of these patients. Site-specific gene correction was performed using ZFN mRNA and the IDLV donor. Cells were placed in an erythroid expansion medium and subsequently differentiated using an established method24,25 (supplemental Figure 14A). In addition, a portion of the cells were evaluated for their colony-forming potential. The cells treated with ZFN and IDLV showed modestly lower (35%) colony-forming ability compared with mock, nonelectroporated control samples (supplemental Figure 14B-C).
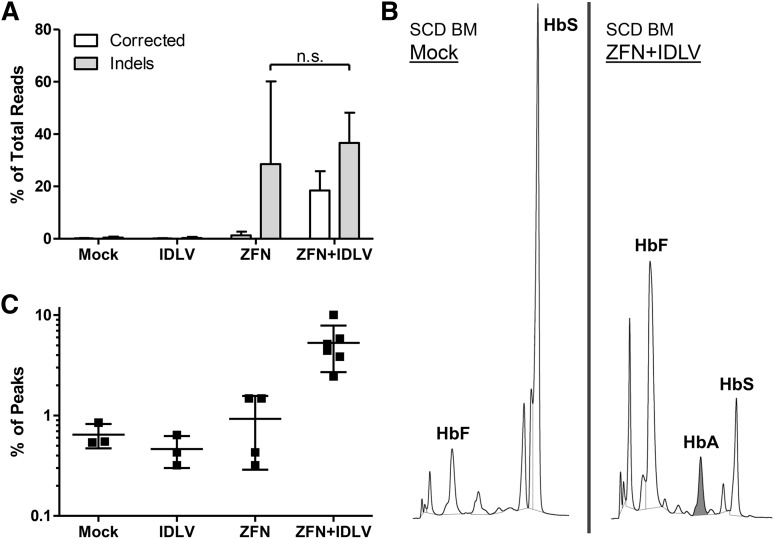
Following the initial erythroid expansion (but before enucleation), cells were harvested for genomic analysis. Deep sequencing showed correction of the SCD mutation in 18.4 ± 6.7% of the reads (Figure 5A). Additionally, sequencing confirmed that most alleles containing the correction of the SCD mutation also contained a base change at the HhaI location, indicating that the majority of HDR-driven events encompass at least the 22-bp distance between those 2 bases. Following the conclusion of the erythroid culture, samples were collected for analysis of the globin tetramers by HPLC (Figure 5B). The presence of an HbA peak in erythroid cells derived from the ZFN- and IDLV-treated samples demonstrates that the gene correction led to functional conversion of the βS allele to a βA allele. No such peak was seen in erythrocytes derived from mock-treated cells. The HbF peak showed a relative increase due to the decrease in the HbS peak (as a result of ZFN-mediated allelic disruption) in ZFN-treated samples. The relative induction of HbA in ZFN and IDLV-treated samples averaged 5.3 ± 0.02%, with protein correction levels up to 10.0% (Figure 5C). These results demonstrate the ability of the ZFNs in combination with an IDLV donor to functionally correct CD34+ cells from the BM of patients with SCD.
Figure 5.
Functional correction of sickle bone marrow CD34+ cells. Bone marrow CD34+ cells from patients with SCD were electroporated with in vitro-transcribed ZFN mRNA and transduced with donor IDLV carrying the WT base at the sickle location and grown under erythroid conditions. (A) Correction at the sickle mutation evaluated by high-throughput sequencing. Results of sequencing of the β-globin locus showing percentage of total aligned reads containing the corrected WT base (A) at the sickle mutation, as well as insertions and deletions (indels) at the cut site. Corrected base, white; indels, gray. (B) HPLC of differentiated erythroid cells at the termination of culture. Cells were pelleted and lysed, and supernatant was analyzed by HPLC. (Left) SCD mock sample. (Right) SCD ZFN+IDLV sample. Shading indicates HbA:WT adult hemoglobin peak. (C) Quantification of the percent of HbA out of the total area under the curve represented by the main peaks. HbA, WT adult hemoglobin; HbF, fetal hemoglobin; HbS, sickle hemoglobin; n.s., not significant (n = 2 independent experiments; mock, n = 3; ZFN only, n = 4; IDLV only, n = 3; ZFN+IDLV, n = 6).
Discussion
The results shown here demonstrate high levels of gene correction of the SCD mutation in human HSPCs using ZFNs in vitro. In the present study, we designed a pair of ZFNs to cleave the β-globin locus. In combination with a homologous donor template (delivered as either an oligonucleotide or via an integrase-defective lentiviral vector), these ZFNs are able to induce HDR at high levels in progenitor cells.
Analysis of the ZFN cleavage sites revealed that the large majority of nuclease activity took place at the β-globin locus target site, with a smaller fraction of cleavage at the homologous δ-globin gene. The impact of the cleavage of δ-globin in a therapeutic setting has yet to be determined. Due to the low level of δ-globin expression in adult erythroid cells (<3.5% of all globin), its loss in a subset of cells is unlikely to be detrimental. An unbiased, genome-wide evaluation of ZFN off-target cleavage sites in K562 cells using IDLV end capture demonstrated the high specificity of this ZFN pair, with only background integration into naturally fragile sites seen. Although no unbiased, comprehensive genome-wide technique to capture the complete repertoire of DSBs induced by targeted nucleases exists, these end capture experiments provide one of the most thorough analyses available.
When the ZFN- and donor-treated cells were transplanted into mice, engraftment and the lineage distribution of the cells were equivalent between mock-treated and ZFN- and donor-treated samples. However, despite average levels of gene modification of 10% to 20% in the in vitro samples before transplantation, the gene correction levels in the human cells in the spleens and BM of the mice after 16 weeks of engraftment were markedly lower. These findings are consistent with recently published work in the field26 and imply that more mature progenitor cells are corrected more efficiently than the earlier, more primitive, HSCs, which are the ultimate target required to achieve sustained clinical benefit.
The reduction in the efficiency of homology-directed gene correction in more primitive HSCs stands in contrast to strategies using only site-specific gene disruption (CCR5).7 The process of gene correction is fundamentally different from and more complex than gene disruption, as a donor template containing the corrective bases must be codelivered and cellular DNA damage repair pathways must resolve the DSB using HDR rather than NHEJ. As NHEJ is favored in quiescent, primitive HSCs,27 a bias in repair pathway choice might limit gene correction in primitive HSCs. Thus, it is possible that the ZFNs are acting with similar efficiency in the mature and primitive cell populations, but the active repair pathway in each cell type differs such that HDR is more active in more mature cells and less so in the primitive HSCs. This hypothesis is supported by the fact that the cells transplanted into mice maintained their input levels of indels to a greater extent than they did for gene modification (7.4- and 4.3-fold change in indels compared with 43.9- and 11.7-fold change in gene modification for IDLV and oligo experiments, respectively).
Experiments in the BM CD34+ cells from patients with SCD provide promise for clinical translation. Levels of correction of the canonical sickle mutation (at least in erythroid progenitor cells) averaged 18% for these experiments, and, following differentiation, these cells produced corrected WT hemoglobin (HbA). Based on data from allogeneic HSC transplants for SCD, donor chimerism of 10% to 30% can result in significant clinical improvement as a result of the selective advantage the normal donor-derived red blood cells.28,29 In addition, heterozygotes for the SCD mutation normally do not experience symptoms of the disease.30 Thus, correcting only 1 allele in each HSC may prove sufficient to alleviate a large portion of the symptoms associated with SCD.
Despite recent advances in lentiviral-based gene therapy for hemoglobinopathies, and specifically SCD,24,31 potential complications remain due to the need for long-lasting and appropriately regulated expression of the therapeutic transgene. Site-specific correction using targeted nucleases of the canonical A to T disease-causing sickle transversion in HSCs offers the unique ability to maintain expression of β-globin under its endogenous promoter and locus control region. Further, the genome correction reagents only require a 1-time, transient ex vivo treatment of the cells to result in permanent correction. Together these data support the continued development of genome correction in HSPCs as a potential treatment of SCD.
Acknowledgments
The authors thank Rebecca Chan for processing the CB units and Kismet Baldwin for technical assistance with the murine work. The UCLA Eli & Edythe Broad Center for Regenerative Medicine & Stem Cell Research cores for Flow Cytometry and DNA Sequencing were essential to the studies.
This work was supported by grants from the Hina Patel Foundation, the National Institutes of Health (NIH) Heart, Lung, and Blood Institute (2P01 HL073104), the Interdisciplinary Training In Virology and Gene Therapy award (5 T32 AI060567 to M.D.H.), the Ruth L. Kirschstein National Research Service Award (GM007185 to A.R.C.), the Initiative to Maximize Student Diversity (NIH, National Institute of General Medical Sciences GM55052 to D.L.), the Doris Duke Charitable Foundation (Innovations in Clinical Research), and the California Institute for Regenerative Medicine (CIRM TR4-06823).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.D.H. and G.J.C. designed, performed, and analyzed experiments and wrote the manuscript; M.C.M. and Z.R. designed, performed, and analyzed experiments; M.L.K., M.H., D.L., D.G., G.R.L., F.U., S.S., A.Z., P.-Q.L., and A.R. designed and performed experiments; A.V.J., A.R.C., and A.W. designed experiments and analyzed data; D.E.P., L.Z., and E.J.R. designed the ZFNs and experiments; X.W. performed all statistical analyses; and M.C.H., P.D.G., R.P.H., and D.B.K. advised the project.
Conflict-of-interest disclosure: The following authors are full-time employees of Sangamo BioSciences and might own Sangamo stock or derivatives: G.J.C., M.C.M., A.Z., P.-Q.L., D.E.P., L.Z., E.J.R., A.R., M.C.H., and P.D.G. All other authors declare no competing financial interests.
Correspondence: Donald B. Kohn, 3163 Terasaki Life Sciences Bldg, 610 Charles E. Young Dr East, Los Angeles, CA 90095; e-mail: dkohn1@mednet.ucla.edu.
References
- 1.Modell B, Darlison M. Global Epidemiology of Haemoglobin Disorders and Derived Service Indicators. Bull World Health Organ. 2008;86(6):480-487. [DOI] [PMC free article] [PubMed]
- 2.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 3.Delea TE, Hagiwara M, Thomas SK, Baladi JF, Phatak PD, Coates TD. Outcomes, utilization, and costs among thalassemia and sickle cell disease patients receiving deferoxamine therapy in the United States. Am J Hematol. 2008;83(4):263–270. doi: 10.1002/ajh.21049. [DOI] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavazza A, Moiani A, Mavilio F. Mechanisms of retroviral integration and mutagenesis. Hum Gene Ther. 2013;24(2):119–131. doi: 10.1089/hum.2012.203. [DOI] [PubMed] [Google Scholar]
- 6.Boulad F, Wang X, Qu J, et al. Safe mobilization of CD34+ cells in adults with β-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014;123(10):1483–1486. doi: 10.1182/blood-2013-06-507178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14(12):8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urnov FD, Miller JC, Lee YL, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 11.Sun N, Zhao H. Seamless correction of the sickle cell disease mutation of the HBB gene in human induced pluripotent stem cells using TALENs. Biotechnol Bioeng. 2014;111(5):1048–1053. doi: 10.1002/bit.25018. [DOI] [PubMed] [Google Scholar]
- 12.Voit RA, Hendel A, Pruett-Miller SM, Porteus MH. Nuclease-mediated gene editing by homologous recombination of the human globin locus. Nucleic Acids Res. 2014;42(2):1365–1378. doi: 10.1093/nar/gkt947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncz KK, Prokopishyn NL, Abdolmohammadi A, et al. Small fragment homologous replacement-mediated modification of genomic beta-globin sequences in human hematopoietic stem/progenitor cells. Oligonucleotides. 2006;16(3):213–224. doi: 10.1089/oli.2006.16.213. [DOI] [PubMed] [Google Scholar]
- 14.Sebastiano V, Maeder ML, Angstman JF, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29(11):1717–1726. doi: 10.1002/stem.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou J, Mali P, Huang X, Dowey SN, Cheng L. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood. 2011;118(17):4599–4608. doi: 10.1182/blood-2011-02-335554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joglekar AV, Hollis RP, Kuftinec G, Senadheera S, Chan R, Kohn DB. Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol Ther. 2013;21(9):1705–1717. doi: 10.1038/mt.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nightingale SJ, Hollis RP, Pepper KA, et al. Transient gene expression by nonintegrating lentiviral vectors. Mol Ther. 2006;13(6):1121–1132. doi: 10.1016/j.ymthe.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Pruett-Miller SM, Huang Y, et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat Methods. 2011;8(9):753–755. doi: 10.1038/nmeth.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storici F, Durham CL, Gordenin DA, Resnick MA. Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast. Proc Natl Acad Sci USA. 2003;100(25):14994–14999. doi: 10.1073/pnas.2036296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel R, Lombardo A, Arens A, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol. 2011;29(9):816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- 21.Giarratana MC, Rouard H, Dumont A, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118(19):5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;81:15.21.1-15.21.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blau CA. Adverse effects of G-CSF in sickle cell syndromes. Blood. 2001;97(12):3682–3682. [Google Scholar]
- 24.Romero Z, Urbinati F, Geiger S, et al. β-globin gene transfer to human bone marrow for sickle cell disease. J Clin Invest. 2013;123(8):3317–3330. doi: 10.1172/JCI67930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 26.Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510(7504):235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreani M, Hsieh MM, Testi M, et al. In mixed hematopoietic chimerism, the donor red cells win. Haematologica. 2010;96(1):13–15. doi: 10.3324/haematol.2010.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters MC, Patience M, Leisenring W, et al. Multicenter Investigation of Bone Marrow Transplantation for Sickle Cell Disease. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7(12):665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 30.Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med. 2013;3(10):a011783–a011783. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrakasan S, Malik P. Gene therapy for hemoglobinopathies: the state of the field and the future. Hematol Oncol Clin North Am. 2014;28(2):199–216. doi: 10.1016/j.hoc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]