Abstract
The hematopoietic stem cell (HSC) niche commonly refers to the pairing of hematopoietic and mesenchymal cell populations that regulate HSC self-renewal, differentiation, and proliferation. Anatomic localization of the niche is a dynamic unit from the developmental stage that allows proliferating HSCs to expand before they reach the bone marrow where they adopt a quiescent phenotype that protects their integrity and functions. Recent studies have sought to clarify the complexity behind the HSC niche by assessing the contributions of specific cell populations to HSC maintenance. In particular, perivascular microenvironments in the bone marrow confer distinct vascular niches that regulate HSC quiescence and the supply of lineage-committed progenitors. Here, we review recent data on the cellular constituents and molecular mechanisms involved in the communication between HSCs and putative niches.
The origins of hematopoiesis
The hematopoietic system supplies our body with >100 billion mature blood cells every day that carry out functions such as oxygen transport, immunity, and tissue remodeling. Hematopoietic stem cells (HSCs), located at the top of the hematopoietic hierarchy, are responsible for replenishing our pool of blood cells throughout life. Early work by James Till and Ernest McCulloch provided evidence that single bone marrow cells could give rise to multilineage progenitors1,2 and could undergo at least short-term self-renewal.3 These studies paved the way to the conceptual hierarchy in HSC differentiation and the role of HSCs in the maintenance of hematopoietic homeostasis. Whether and how HSCs could autonomously modulate their function or be influenced by extrinsic factors, however, has remained poorly understood until recently. In the adult stage, most HSCs are found in a quiescent state that protects them from genotoxic insults and ensures their long-term repopulating ability.4-6 The state and function of HSCs must be finely tuned to protect their self-renewal capacity and prevent their exhaustion, which is crucial for blood system homeostasis. Differences in spatial localization of colony-forming unit, spleen, within rodent long bones is associated with a discrete proliferative state, which suggests that specific microenvironments within the bone marrow can regulate the state and function of hematopoietic stem and progenitor cells (HSPCs).7 Bone marrow stromal cells promote ex vivo proliferation and differentiation of HSPCs in long-term cultures, supporting the notion that microenvironmental cues may influence the fate of HSCs and modulate hematopoiesis.8 This idea is crystalized by the “niche hypothesis,” in which the niche forms a regulatory unit that limits the entry of HSCs into the cell cycle, thereby protecting them from exhaustion or from errors in DNA replication.9 Therefore, identification of molecular cues that regulate the fate of HSCs will improve our knowledge of the regulation of hematopoiesis in health and disease.
During development, HSCs traffic between niches in order to establish hematopoiesis. Primitive hematopoiesis takes place in the yolk sac approximately on embryonic day 7.0 (E7.0) when immature precursors give rise to erythrocytes that will supply oxygen to the developing embryo.10 The presence of the first definitive HSC known to be able to fully reconstitute the hematopoietic system upon transplantation is found in the aorta-gonad-mesonephros in mice and humans.11,12 However, some studies have suggested that yolk sac cells from E9.0 to E10.0 can mature into definitive HSCs when transplanted into a newborn rather than an adult mouse.13,14 In addition, the placenta represents a significant reservoir of HSCs during development.15,16 Once the vasculature is developed, HSCs migrate to the fetal liver on or near E12.0 where they expand and differentiate.10 Fetal liver HSCs are actively cycling in contrast to their bone marrow counterparts and can also outcompete adult bone marrow HSCs when transplanted into irradiated recipients.17 During HSC expansion in the fetal liver, chondrocytes and osteoblasts are produced within mesenchymal condensations to create cartilage and bone.10 Skeletal remodeling is associated with bone vascularization, which allows homing of HSCs and colonization of the fetal bone marrow by E17.5.10 This process is mediated through CXCL12 production by bone marrow stromal cells, which attracts HSCs expressing CXCR418 and specific adhesion molecules expressed on bone marrow endothelium.19,20
A shelter between blood and bone
Knowledge of the identities and functions of HSC niches has markedly improved in the past few years (Figure 1). Although the association of progenitor activity with the endosteum has been acknowledged for several decades,7 a direct role for osteoblasts in HSC maintenance has been suggested by experiments showing that cultured osteoblasts are capable of expanding hematopoietic progenitors in vitro,21,22 which led to studies revealing that the genetic or pharmacologic manipulation of osteoblast numbers correlates with HSC counts in the bone marrow.23,24 In addition, imaging of the transplanted lineage-negative progenitor fraction of bone marrow cells shows that progenitors are preferentially distributed along the endosteal region.25 Osteoblasts have been proposed to support HSC function by forming direct interactions via N-cadherin–mediated adhesion,24 although this idea has been highly controversial. Functional studies using conditional knockout of N-cadherin (Cdh2) in hematopoietic and stromal cells,26 osteoprogenitors,27 and osteoblasts28 have not revealed any change in HSC numbers, although overexpression of N-cadherin has been reported to alter HSC numbers.29 Activated osteoblasts can produce osteopontin, which limits HSC expansion,30 as well as angiopoietin-1 and thrombopoietin, which bind the Tie2 and MPL receptors, respectively, and contribute to HSC quiescence.6,31,32 Bone resorption and the subsequent release of calcium by osteoclasts also promote HSC maintenance and localization to the endosteal region.33,34 By contrast, the signaling lymphocytic activation molecule cell surface markers CD150 and CD48 have identified HSCs in proximity to sinusoidal blood vessels.35 Using a knockin mouse strain expressing green fluorescent protein (GFP) driven in the Cxcl12 locus, a chemokine critical for the maintenance and quiescence of HSCs, perivascular cells known as CXCL12-abundant reticular (CAR) cells are reported to contact HSCs mainly near sinusoids in endosteal and nonendosteal marrow.36 In vivo imaging of the bone marrow in the calvarium reveals that specific sinusoidal domains expressing both CXCL12 and E-selectin are targeted for homing and engraftment of normal and leukemic HSCs.37 Innervation by the sympathetic nervous system regulates HSC mobilization through circadian release of noradrenaline, which modulates CXCL12 expression in the bone marrow.38,39 In addition, sympathetic nerves play a critical role in bone marrow regeneration in which ablation of adrenergic innervation in the bone marrow impairs HSC recovery after chemotherapy.40 Physical association between nerves and bone marrow vasculature also supports the importance of a vascular niche for HSCs. Recent imaging studies of the bone marrow did not reveal a significant association between osteoblasts and HSCs.41,42 These data and the selective deletion of critical niche factors in osteoblasts (see “Perivascular niches dictate HSC fate”), argue that osteoblasts do not directly contribute to HSC maintenance.
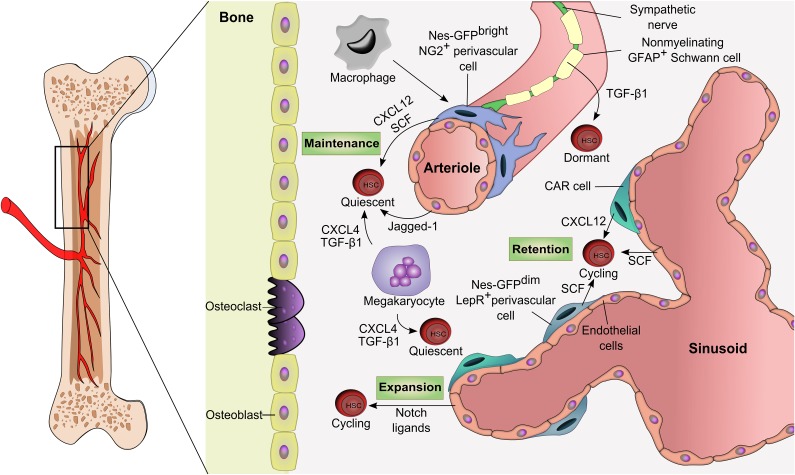
Figure 1.
The adult bone marrow HSC niche. The vasculature has emerged as a key structure for the maintenance of HSCs in the bone marrow. Dormant HSCs are found around arterioles where factors such as CXCL12 and SCF secreted by perivascular, endothelial, Schwann, and sympathetic neuronal cells promote their maintenance. Less quiescent or activated HSCs are located near sinusoidal niches which are likely diverse in their influence for self-renewal, proliferation, and differentiation. Hematopoietic cells such as macrophages or megakaryocytes are examples of HSC-derived progeny that can feed back to the niche to influence HSC migration or proliferation. GFAP, glial fibrillary acidic protein; TGF-β1, transforming growth factor beta-1.
Although it has been suggested that the endosteal region is enriched in HSCs, its boundary may not be as narrow as initially suggested.43 The endosteum is highly vascularized by the presence of sinusoids with CAR cells36 and also with arterioles that often run along the endosteal area,42 both of which are associated with HSCs. After transplantation in irradiated mice, HSCs preferentially home to the endosteal surfaces of the trabecular bone region but randomly distribute in nonirradiated recipients.44,45 HSCs undergo expansion after bone marrow damage in the endosteal region where osteoblasts and blood vessels are in close proximity.44,45 Lethal irradiation is known to disrupt the sinusoidal network, which may account for the relocalization of HSCs to the endosteum.46 Since the endosteum is vascularized with arteriolar vessels that are more resistant to genotoxic insults, it is likely that vascular niches differentially contribute to bone marrow regeneration.
Perivascular niches dictate HSC fate
By tracking the origins of HSCs, studies have linked HSC activity to vascular development.15 The endothelial layers of the dorsal aorta along with the vitelline and umbilical arteries have been proposed as primary sites for the emergence of definitive HSCs.11,47 Defective angiogenesis in AML1-deficient mice that lack definitive hematopoiesis can be rescued by the presence of HSCs, which suggests that they can play role in shaping the vascular network during development.48 By using Runx1-lacZ and CD41 markers to mark HSCs, it has been shown that HSCs are generated in the placental vasculature of mouse embryos prior to liver colonization.49 Stromal cell lines derived from human placenta can support the expansion of HSPCs and express pericytic markers.50 Transcriptome analyses have revealed that organ-specific molecular signatures are involved in the HSPC supportive activity of stromal cells.51 The associations among HSCs and perivascular and endothelial cells suggests that these cell populations can indeed coexist in an environment that promotes their maintenance and expansion. Mesenchymal stem and progenitor cell (MSPC) activity was described decades ago in colony-forming units-fibroblasts (CFU-Fs),52 but the lack of unique cell surface markers and the disparity between lineage tracing models and isolation methods have hampered their characterization. Perivascular human CD146+CD45− MSPCs that reside in the bone marrow contain virtually all of the CFU-F activity and are capable of reconstituting a heterotopic bone marrow niche, suggesting that MSPCs and their progeny contribute to the development of the HSC niche and regulate hematopoiesis.53 A mouse fetal bone CD51+CD105+CD90−CD45−Tie2− progenitor cell population is able to reconstitute the HSC niche by forming donor-derived ectopic bone through endochondral ossification, thus creating a marrow cavity with host-derived vasculature and HSCs.54 Other studies have found that PDGFRα+Sca-1+CD45−Ter119− (PαS) markers identify MSPCs capable of differentiating into osteoblasts, reticular cells, and adipocytes in vivo.55 Transgenic mice that express GFP under the control of the promoter and second intronic enhancer of nestin (Nes-GFP),56 an intermediate filament highly expressed in the brain, allow the prospective identification of perivascular mesenchymal stem cells that are significantly associated with HSCs in the bone marrow. Stem cell activity of these Nes-GFP+ cells is suggested by the fact that this fraction contains all the CFU-F activity in the bone marrow and is able to form clonal spheres that can self-renew, multi-differentiate at the clonal level into the major mesenchymal lineages, and generate hematopoietic activity in vivo upon serial transplantation.57 Nes-GFP+ cells express endogenous nestin57,58 and are also closely associated with sympathetic nerves that regulate the expression of CXCL12 in a circadian fashion.39,57 In further support of the role of sympathetic nerve fibers in regulating the HSC niche, nonmyelinating Schwann cells that express glial fibrillary acidic protein (GFAP) have been reported to activate transforming growth factor-β1, which promotes HSC quiescence.59 Although GFAP+ glial cells are described in close proximity to HSCs, only about a quarter of CD150+CD48−CD41−Lin− HSCs appear in direct contact with them whereas 97% of HSCs were directly associated with CAR cells,36 and 60% were directly associated with Nes-GFP+ MSPCs.57 However, association of HSCs with these various stromal cells requires additional statistical analyses to determine the exact significance of these relationships. Further refinement of cell surface markers that identify MSPC populations demonstrated that PDGFRα+CD51+CD45−Ter119−CD31− stromal cells were enriched for HSC maintenance genes, formed clonal multipotent self-renewing mesenpheres in culture, and were able to reconstitute a bone marrow niche heterotopically.58 These PDGFRα+CD51+ stromal cells largely overlap (∼75%) with Nes-GFP+ MSPCs and contain the majority of the CFU-F activity in both adult mice and human fetal bone marrow.58 They also represent a small subset of CD146+ human skeletal stem cells enriched in MSPC and HSC niche activities.53 Sca-1 is not highly expressed in bone marrow PDGFRα+CD51+ cells, which may suggest that they are stromal cell populations distinct from the previously identified PαS MSPCs.55 Indeed, the latter were isolated from crushed bone with discarded bone marrow,55,60 whereas studies with Nes-GFP+ cells have been carried out with flushed bone marrow. Bones appear to contain a higher concentration of CFU-F (∼10-fold greater than bone marrow) and bone-derived mesenchymal progenitors expressing Sca-1 whereas the population of bone marrow mesenchymal progenitors (based on Nes-GFP) appears mostly negative for Sca-1.58
Endothelial cells that line the sinusoidal blood vessels in the bone marrow may also contribute to HSC regulation. Disruption of VEGFR2 and VE-cadherin–dependent angiogenic signaling pathways that use monoclonal antibodies shows that sinusoidal endothelial cells expand the HSC pool, support self-renewal, and prevent exhaustion of HSCs in both serum-free coculture assays and in vivo through Notch signaling.61 Endothelial-specific deletion of Jagged-1 by VE-cadherin-cre reveals that sinusoidal endothelial cells are directly involved in HSC self-renewal for homeostatic and regenerative hematopoiesis whereas the stromal and perivascular cell compartments, including PDGFRα+CD51+ MSPCs, are not altered in numbers or in their ability to generate CFU-F cells.62 In contrast, CD31hiendomucinhi endothelial cells have been proposed to mediate neoangiogenesis in bone through Notch signaling pathways and to influence perivascular osteoprogenitor levels.63,64 Therefore, the activation state of endothelial cell may play a crucial role in secreting factors that determine the fate of HSPCs.65 Conditional knockdown of stem cell factor (SCF) in endothelial and perivascular cells by using Tie2-cre and leptin receptor (LepR)-cre mice, respectively, led to reductions in HSC frequency in the bone marrow and spleen.66 These functional analyses are consistent with the expression pattern of GFP knocked into the Scf locus, which shows that SCF is largely expressed by perivascular cells and mostly around sinusoids but also near venules and arterioles in the bone marrow.66 The authors have also suggested that hematopoetic cells (Vav1-cre), osteoblasts (Col2.3-cre), and nestin+ cells (constitutive nestin-cre, tamoxifen-inducible nestin-creER) do not represent important contributors of SCF in the bone marrow niche, although the recombination efficiency of Scf deletion in nestin+ cells and osteoblasts was not provided to confirm efficient gene deletion in these cells.66
Recent studies have also determined the contribution of different niche populations in CXCL12 production.67,68 Targeted Cxcl12 deletion in osteoblasts reveals no HSC or myeloprogenitor phenotypes, although the authors have noted lower reconstitution of B and T cells in irradiated mice as well as lower numbers of lymphoid progenitors in the bone marrow using Col2.3-cre mice.68 Conditional deletion in osteoprogenitors using osterix (Osx)-cre results in hematopoietic progenitor mobilization to the blood and spleen and reduced B-lymphoid progenitors.67 These results are in accordance with studies showing that CXCL12 is involved in B-cell maintenance and that depletion of osteoprogenitors or CAR cells leads to reduction in B-lymphoid progenitor cells.69-71 Endothelial deletion (Tie2-cre) of CXCL12 reduces HSC frequency, whereas hematopoietic progenitors and lineage reconstitution levels after transplantation are unaffected.67,68 Deletion of CXCL12 in perivascular stromal cells by using LepR-cre yields no change in HSC number, progenitor number, or reconstitution level but does show an increase in mobilization to blood and spleen. Hematopoietic cells (Vav-cre) and nestin+ MSPCs (Nes-cre) induce no phenotype upon CXCL12 deletion,68 although whether Cre-mediated deletion occurred in nestin+ cells was not demonstrated, thus limiting any conclusions on the contribution of Nes-GFP+ cells in CXCL12 secretion. Conversely, by using transcription factor paired-related homeobox-1 (Prx1-cre), which recombines in osteoblasts and bone marrow stromal cells, a clear reduction in HSC and lymphoid progenitors with an increase in mobilization can be observed.67 Prx1-cre induces recombination in mesenchymal progenitors, which supports the critical role of MSPCs in forming an HSC niche.
One proposed view of the endosteal niche was that it might provide a hypoxic environment for maintaining HSCs in a quiescent state whereas the vascular niche allows HSCs to proliferate and differentiate in an environment in which oxygen is more available.72,73 Expression of E-selectin (found exclusively on endothelial cells) promotes HSC proliferation, whereas E-selectin antagonists promote HSC quiescence and self-renewal.74 The bone marrow space is uniformly occupied by sinusoids, but the endosteal region is also highly vascularized and perfused by arterioles that further subdivide into arterial capillaries that ultimately connect with sinusoids.75 By using 3-dimensional bone marrow imaging, it has been determined that Nes-GFP+ cells with different GFP expression levels discriminate between arterioles (Nes-GFPbright) and sinusoids (Nes-GFPdim); thus, computational simulations have determined that quiescent HSCs preferentially associate with arterioles and that proliferative HSCs move away from arterioles.42 Nes-GFPbright arterioles are themselves quiescent, and their integrity is preserved after treatment with 5-fluorouracil, suggesting that they may provide a shelter for quiescent HSCs after genotoxic insults.42 Furthermore, the pericyte marker NG2 appears to largely label arteriole-associated Nes-GFP+ cells, whereas LepR preferentially labels Nes-GFPdim–associated sinusoids.42 Depletion of NG2+ cells also induces HSC cycling and distribution away from arterioles.42 HSC distribution between proliferative (sinusoids) and quiescent (arteriole) niches may represent specific milieus that regulate distinct HSC pools.
Consistent with the idea that HSC quiescence is associated with a hypoxic niche,76,77 quiescent HSCs are enriched in stabilized transcription factor hypoxia-inducible factor-1α (HIF-1α) allowing them to be maintained and resist stress in hypoxic conditions.78,79 Live in vivo imaging of oxygen concentration by using phosphorescence lifetime sensing nanoprobes provides evidence that the most hypoxic region is located within 40 μm of the bone in the perisinusoidal region whereas there is a modest increase in oxygen concentration within 20 μm of the bone in the endosteal region.80 Interestingly, higher oxygen tension is found around Nes-GFP+ vessels,80 which harbor quiescent HSCs.42 It is possible that the oxygen tension may in fact be much lower just outside the relatively thick-walled arterioles. Other studies have revealed that hypoxia, as sensed by pimonidazole staining, appears HSC autonomous, which raises the possibility that the hypoxia factors may in fact be regulated through different cues.41 Indeed, the microenvironment could also influence the hypoxic and metabolic profile of HSCs because SCF81 and thrombopoietin82 are known to increase HIF-1α levels, which could alter the distribution of hypoxic HSCs. In addition, PαS MSPCs exhibit a hypoxic profile and are enriched in HIF factors that inhibit HSPC expansion and differentiation.83 Therefore, studies in the near future should be able to confirm the existence and the role of a hypoxic niche and its overlap with quiescent and proliferative niches.
Hierarchy and crosstalk in the HSC niche
In the adult bone marrow, MSPCs are responsible for the generation of mesenchymal lineage tissues such as bone, fat, and cartilage as well as bone marrow stromal cells, which constitute an environment that supports HSCs and hematopoiesis (Figure 2). Although the stromal cell composition of the bone marrow is thought to be as complex as its hematopoietic counterpart, the hierarchical relationship among stromal cells and their influence on the niche remains unclear. Myxovirus resistance-1 (Mx-1) induces Cre-mediated recombination in osteogenic progenitors that maintain most of the osteoblast pool at steady state and after tissue stress, whereas Osx-creERT2 induces only a transient source of osteoblasts in adult mice.84 Interestingly, when labeling is induced perinatally, long-lived bone marrow Nes-GFP+ LepR+ stromal cells capable of contributing to tissue regeneration after injury are marked by Osx.85 Nes-GFP+ LepR+ stromal cells indeed appear to contain MSPC activity in the adult bone marrow.85,86 The observation that Osx, which is a transcription factor thought to be specific for the osteolineage, marks the stroma during development was independently reported by other groups.87,88 Interestingly, labeling of Osx+ cells during the fetal stage postnatally marked bone marrow stromal cells, but these cells were short-lived and were replaced by definitive MSPCs during the late fetal and neonatal period.85 These studies raise the interesting possibility of the presence of a primitive and definitive bone marrow stroma, both marked by Osx during ontogeny, whose differential characteristics and functions remain to be defined.
Figure 2.
Hierarchy of the stromal niche cells. MSPC populations are found in both bone and bone marrow compartments and contribute to the generation of mesenchymal lineage cells that generate bone, fat, and cartilage tissues. The relationship between bone-derived MSPCs and marrow MSPCs remains unclear. Stromal cells are also thought to derive from MSPCs, but the nature or origin of most stromal cells is still obscure. Self-renewing mesenchymal cells and their progeny can be prospectively isolated by using specific surface markers and lineage tracing approaches with transgenic labeling to decipher the origin of niche cells. Arrows with solid lines indicate that one cell population that can give rise to another cell population; arrows with dashed lines indicate a relationship between cell populations that remains to be clarified. Mx-1, Myxovirus resistance-1; Prx-1, paired-related homeobox-1.
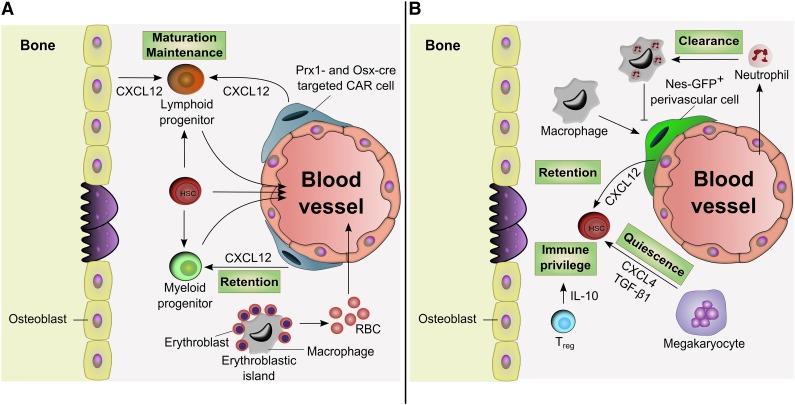
Although much of the focus on bone marrow stroma has been on the HSC niche, emerging data suggest that subsets of stromal cells also contribute to the maintenance of hematopoietic progenitor populations (Figure 3A). For example, conditional deletion of CXCL12 in both osteoblasts and perivascular stromal cells results in a decrease of common lymphoid progenitors, whereas its deletion in osteoprogenitors is associated with a decrease in committed B-lymphoid progenitors.67,68 This is consistent with studies showing that depletion of CAR cells, known to be closely associated with pre-pro B cells,69 reduces both common lymphoid progenitors and pro-B cell levels.70 It is thus possible that the endosteal region might represent a suitable microenvironment for the maintenance of lymphoid progenitors. In addition, macrophages constitute the central unit in erythroblastic islands thereby providing a niche for the maturation of erythropoietic cells in the steady state, after hemolytic or myeloablative stress, and during malignant hematopoiesis.89,90
Figure 3.
Progenitor niches and influence of differentiated progeny. (A) Microenvironment-promoting progenitors. Stromal cells labeled by Prx1-cre or Osx-cre and expressing CXCL12 (CAR cells) have been shown to regulate both lymphoid progenitor maturation and myeloid progenitor retention. Depletion of CXCL12 in Osx-cre–targeted stromal cells depletes B-lymphoid progenitor cells and induces the mobilization of myeloid progenitors from the bone marrow. Macrophages also constitute the central unit of the erythroblastic island allowing erythroblasts to mature and generate red blood cells (RBCs). (B) Regulation by HSC progeny. Macrophages promote HSC retention in the bone marrow by regulating CXCL12 production from Nes-GFP+ perivascular cells. Macrophage-mediated clearance of neutrophils inhibits the retention signals, which allows HSC mobilization. Regulatory T cells (Treg) enable an immune privilege site, protecting HSCs against rejection following allogeneic transplantation. Megakaryocytes localize with HSCs and promote their quiescence. IL-10, interleukin-10.
The progeny of HSPCs supplies our body with mature blood cells which may also constitute important feedback regulators of the HSC niche (Figure 3B). CD169+ macrophages promote HSC retention by regulating CXCL12 production in the bone marrow and by inducing downregulation of HSC maintenance genes in Nes-GFP+ MSPCs.91 Treatment with granulocyte colony-stimulating factor leads to a loss of monocyte and macrophage populations and functions, which is associated with HSC egress to the blood.92,93 Regulatory T cells may also provide an immune privilege site that protects allogeneic HSCs in the niche after transplantation.94 Neutrophil depletion reportedly increased CAR cell population and CXCL12 levels in the bone marrow whereas their clearance by macrophages promoted HSPC mobilization.95 In addition, recent studies showed that megakaryocytes localize specifically with a subset of HSCs, and promotes their quiescence through the production of CXCL4 (also known as platelet factor-4)96 and transforming growth factor-β1.97 FGF1 production by megakaryocytes can also promote HSC expansion under stress.97 Heterogeneity among HSCs suggests that specific molecular cues inside the niche will instruct HSC fate at different levels depending on the targeted subpopulation.98
Niche of hematopoietic malignancies
The importance of identifying and characterizing specific cell populations in the hematopoietic and stromal hierarchy is underscored by their involvement in myeloid neoplasms (Table 1). Interestingly, alteration of the microenvironment by the deficiency in retinoic acid receptor, for example, appears sufficient to induce a myeloproliferative syndrome.103 Simultaneous deletion of retinoblastoma protein in myeloid cells and the stromal compartment can also enhance myeloproliferative diseases.104 In addition, the deletion of Dicer1 in osteoprogenitors (using Osx-cre) but not mature osteoblasts (osteocalcin-cre) promotes myelodysplastic syndrome and acute myeloid leukemia (AML), indicating that alterations in specific stromal cell populations can induce a hematopoietic malignancy.105 Overexpression of β-catenin in osteoblasts (driven by Col1-cre) can also induce myelodysplastic syndrome /AML via increased Jagged-1 expression and Notch signaling.106 Treatment with an inhibitor of gut serotonin restores osteoblast number in leukemic mice and suppresses acute lymphoblastic leukemia and AML progression.107 Because osteoblasts have a high turnover, it is likely that alterations at the MSPC level rather than the osteoblast are driving niche-induced hematopoietic malignancies. The expression of Osx-cre in MSPCs85 and the continuous overexpression of β-catenin in osteoblasts106—a situation unlikely to occur naturally—are also consistent with this idea, although this has not been formally demonstrated. Distinct signals in the microenvironment may also differentially alter malignant transformation, as suggested by the expression of a constitutively active PTH receptor in osteoblasts that inhibits chronic myelogenous leukemia (CML)-like myeloproliferation while enhancing MLL-AF9–driven AML.108 MSPC differentiation toward the osteoblastic lineage in BCR-ABL CML results in increased mature osteoblast numbers99 in contrast to BCR-ABL CML blast crisis.100 Similar expansion of MSPCs has recently been reported for AML, which destroys sympathetic nerves in bone marrow and spleen that are infiltrated with AML. The loss of adrenergic activity in the bone marrow environment leads to the proliferation of nestin+ cells primed to differentiate into the osteoblastic lineage. Blockade of the β2-adrenergic receptor enhanced AML infiltration whereas a β2-adrenergic agonist reduces disease activity.101 Interestingly, a similar effect of JAK2V617F-driven myeloproliferative syndrome is observed on bone marrow innervation but the disease, by contrast, leads to the depletion of nestin+ cells, and this could be rescued by β3-adrenergic agonists.102 Further analyses are needed to dissect the influence of cancer on the bone marrow microenvironment to find the common abnormalities and the cancer type-specific abnormalities.
Table 1.
The HSC niche in acute and chronic myeloid neoplasms
| Model | MSPC | Osteoblasts | Osteoclasts (TRAP+) | Adipocytes | Endothelial cells | SNS fibers | Hematopoietic cells | Reference |
|---|---|---|---|---|---|---|---|---|
| MLL-AF9 AML | ↑ Nes-GFP+ cells ↑ PDGFRα+CD51+ cells ↑ Osteoprecursors (CD51+Sca1–) ↓ NG2+ pericytes ↓ Niche factors |
↓ | ↓ | ↓ Perilipin+ cells | ↑ (CD31+Ter119–CD45–) | ↓ Th+ fibers | NA | 101 |
| BCR-ABL blast crisis CML | ↑CCL3 ↓ CFU-OB |
↓ | ↓ | NA | NA | NA | ↑ CCL3 | 100 |
| BCR-ABL CML | ↑ CFU-F size ↑ Osteoprecursors (CD51+Sca1+) ↓ Niche factors |
↑ | — | NA | ↓ (Lin–CD45–CD31+Sca-1+) | NA | ↑ TPO ↑ CCL3 | 99 |
| JAK2V617F MPN | ↓ Nes-GFP+ cells ↓ Niche factors ↑ IL-1R |
NA | NA | NA | NA | ↓ Th+ Fibers ↓ GFAP+ Schwann cells |
↑ IL-1β | 102 |
CFU-OB; colony-forming unit-osteoblasts; GFAP, glial fibrillary acidic protein; IL-1R, interleukin 1 receptor; MPN, myeloproliferative neoplasm; NA, not available; SNS, sympathetic nervous system; TRAP, tartrate-resistant acid phosphatase; Th, tyrosine hydroxylase; TPO, thrombopoietin.
Concluding remarks
Many, but not all, discrepancies in the literature regarding the osteoblastic vs the vascular niche can be explained by unfaithful mapping of the fate of osteoblast promoters that are in fact expressed throughout the mesenchymal lineage. Uneven recombination efficiencies of Cre recombinase among cell types and transgenic mouse strains also represent meaningful sources of variability and a substrate for controversy. Further understanding the complexity of the HSC niche will be achieved by targeting specific niche cells to shed light on contributions of overlapping cell populations. Recent studies have established a major role for the vasculature in HSC maintenance, and subsets of microenvironments have emerged from these studies. Feedback mechanisms and crosstalk by mature hematopoietic cells have also been established. Although the interplay between the hematopoietic and nonhematopoietic compartments is likely more complex than we imagine, common principles will guide the design of new approaches to tackle hematologic diseases. Hematologic malignancies represent an area that will likely benefit from niche-targeted therapies against cancer-mediated attacks on the healthy microenvironment. For example, therapies that preserve innervation, differentiation, or loss of healthy niche cells in myeloid neoplasms may maintain normal HSC function while preventing the establishment of a cancer-promoting niche. New insights from HSC niches in the extremes of life (development and aging) will allow the identification of new cellular and molecular players involved in the regulation of hematopoiesis. This knowledge will one day be exploited to engineer ex vivo niches for HSC expansion and to allow the discovery of novel pharmacologic approaches for blood diseases.
Acknowledgments
The authors apologize to those whose work was not cited because of space limitations.
This work was supported by the New York State Department of Health (NYSTEM Program) and by the National Institutes of Health R01 grants DK056638 from the National Institute of Diabetes and Digestive and Kidney Diseases and HL069438 from the National, Heart, Lung and Blood Institute (P.S.F.).
Authorship
Contribution: P.E.B. and P.S.F. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul S. Frenette, Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine, Albert Einstein College of Medicine, Price Center, Room 101B, 1301 Morris Park Ave, Bronx, NY 10461; e-mail: paul.frenette@einstein.yu.edu.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 2.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 3.Siminovitch L, McCulloch EA, Till JE. The Distribution of Colony-Forming Cells among Spleen Colonies. J Cell Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 4.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 6.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46(1):65–72. [PubMed] [Google Scholar]
- 8.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 9.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2):7–25. [PubMed] [Google Scholar]
- 10.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133(19):3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 11.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 12.Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208(12):2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissman I, Papaioannou V, Gardner R. Fetal hematopoietic origins of the adult hematolymphoid system. In: Clarkson B, Marks PA, Till JE, editors. Differentiation of Normal and Neoplastic Hematopoietic Cells. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1978. p. 33. [Google Scholar]
- 14.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci USA. 1997;94(13):6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138(6):1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 16.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA. 1995;92(22):10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19(2):257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 19.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95(24):14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102(6):2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 21.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87(2):518–524. [PubMed] [Google Scholar]
- 23.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 26.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4(2):170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012;120(2):295–302. doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120(2):303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosokawa K, Arai F, Yoshihara H, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell. 2010;6(3):194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 34.Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 35.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435(7044):969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 40.Lucas D, Scheiermann C, Chow A, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19(6):695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y, Yin T, Wiegraebe W, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 46.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19(11):2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takakura N, Watanabe T, Suenobu S, et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102(2):199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 49.Rhodes KE, Gekas C, Wang Y, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robin C, Bollerot K, Mendes S, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5(4):385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charbord P, Pouget C, Binder H, et al. A systems biology approach for defining the molecular framework of the hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):376–391. doi: 10.1016/j.stem.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. [PubMed] [Google Scholar]
- 53.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 54.Chan CK, Chen CC, Luppen CA, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 57.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinho S, Lacombe J, Hanoun M, et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 60.Houlihan DD, Mabuchi Y, Morikawa S, et al. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat Protoc. 2012;7(12):2103–2111. doi: 10.1038/nprot.2012.125. [DOI] [PubMed] [Google Scholar]
- 61.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulos MG, Guo P, Kofler NM, et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Reports. 2013;4(5):1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi H, Butler JM, O’Donnell R, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12(11):1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 72.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 73.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 75.Draenert K, Draenert Y. The vascular system of bone marrow. Scan Electron Microsc. 1980;(4):113–122. [PubMed] [Google Scholar]
- 76.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Lévesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–385. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 78.Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 79.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pedersen M, Löfstedt T, Sun J, Holmquist-Mengelbier L, Påhlman S, Rönnstrand L. Stem cell factor induces HIF-1alpha at normoxia in hematopoietic cells. Biochem Biophys Res Commun. 2008;377(1):98–103. doi: 10.1016/j.bbrc.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 82.Kirito K, Fox N, Komatsu N, Kaushansky K. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105(11):4258–4263. doi: 10.1182/blood-2004-07-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guarnerio J, Coltella N, Ala U, Tonon G, Pandolfi PP, Bernardi R. Bone marrow endosteal mesenchymal progenitors depend on HIF factors for maintenance and regulation of hematopoiesis. Stem Cell Reports. 2014;2(6):794–809. doi: 10.1016/j.stemcr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Strecker S, Wang L, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS ONE. 2013;8(8):e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19(2):329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow A, Huggins M, Ahmed J, et al. CD169⁺ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med. 2013;19(4):437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 93.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruns I, Lucas D, Pinho S, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 98.Ema H, Morita Y, Suda T. Heterogeneity and hierarchy of hematopoietic stem cells. Exp Hematol. 2014;42(2):74-82.e2. [DOI] [PubMed]
- 99.Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119(2):540–550. doi: 10.1182/blood-2011-04-348151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 103.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krevvata M, Silva BC, Manavalan JS, et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood. 2014;124(18):2834–2846. doi: 10.1182/blood-2013-07-517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19(11):1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]