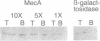Abstract
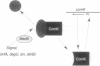
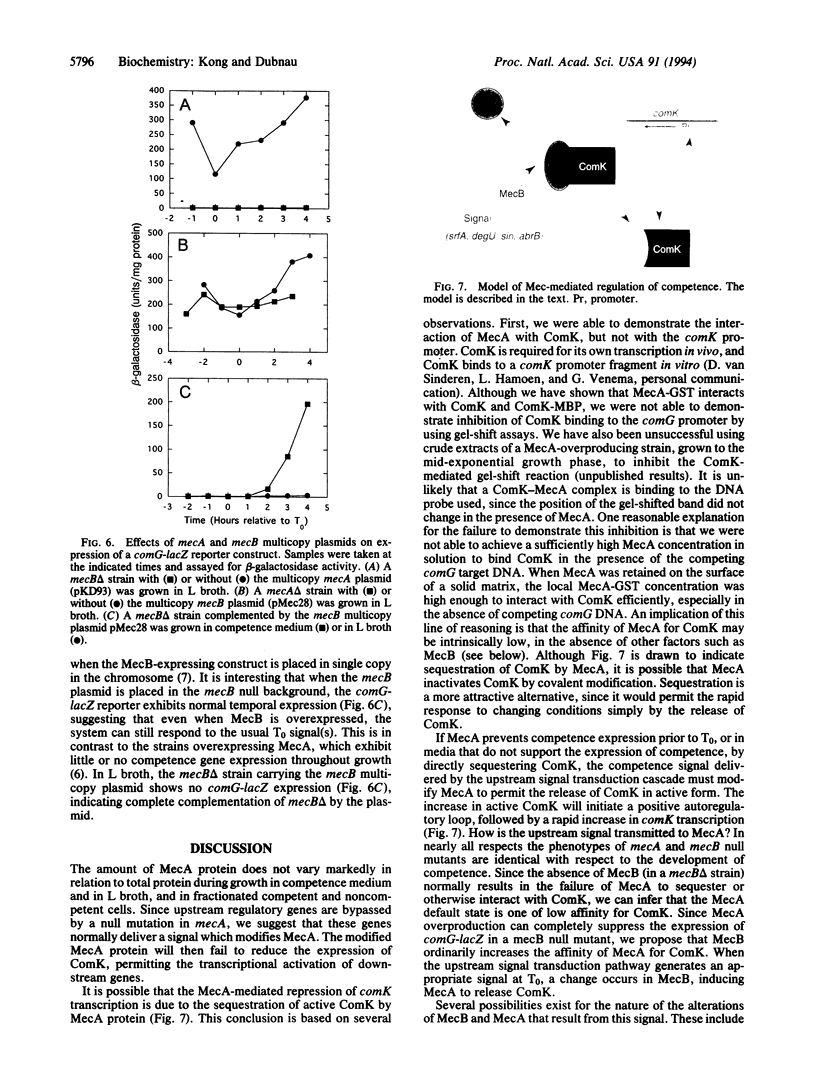
The expression of competence genes in Bacillus subtilis is controlled by a signal transduction cascade which increases the expression of a competence transcription factor (CTF, encoded by comK) during the transition from exponential growth to stationary phase. The transcription of CTF (ComK) is decreased by the product of the mecA gene, and this inhibition is relieved in response to an unknown signal received from upstream in the regulatory pathway. Inactivation of either mecA or another gene, mecB, results in overproduction of ComK. We show here that the concentration of MecA protein does not vary markedly with culture medium, as a function of growth stage, or in competent and noncompetent cells. We also show that MecA can interact directly with ComK. Finally, evidence is presented suggesting that MecB functions prior to MecA in the signaling pathway. A model is discussed which involves the sequestration of ComK by MecA binding and the release of the transcription factor when an appropriate signal is relayed to MecA by MecB.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Breitling R., Dubnau D. A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989 Oct;171(10):5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R., Dubnau D. A membrane protein with similarity to N-methylphenylalanine pilins is essential for DNA binding by competent Bacillus subtilis. J Bacteriol. 1990 Mar;172(3):1499–1508. doi: 10.1128/jb.172.3.1499-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H., Kohchi T., Sano T., Shirai H., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. III. Gene organization of the large single copy region from rbcL to trnI(CAU). J Mol Biol. 1988 Sep 20;203(2):333–351. doi: 10.1016/0022-2836(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Maurizi M. R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992 Dec;56(4):592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Hird S. M., Dyer T. A. Nucleotide sequence of a wheat chloroplast gene encoding the proteolytic subunit of an ATP-dependent protease. Plant Mol Biol. 1990 Dec;15(6):947–950. doi: 10.1007/BF00039435. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Kong L., Siranosian K. J., Grossman A. D., Dubnau D. Sequence and properties of mecA, a negative regulator of genetic competence in Bacillus subtilis. Mol Microbiol. 1993 Jul;9(2):365–373. doi: 10.1111/j.1365-2958.1993.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R., Clark W. P., Katayama Y., Rudikoff S., Pumphrey J., Bowers B., Gottesman S. Sequence and structure of Clp P, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990 Jul 25;265(21):12536–12545. [PubMed] [Google Scholar]
- Maurizi M. R., Clark W. P., Kim S. H., Gottesman S. Clp P represents a unique family of serine proteases. J Biol Chem. 1990 Jul 25;265(21):12546–12552. [PubMed] [Google Scholar]
- Mohan S., Dubnau D. Transcriptional regulation of comC: evidence for a competence-specific transcription factor in Bacillus subtilis. J Bacteriol. 1990 Jul;172(7):4064–4071. doi: 10.1128/jb.172.7.4064-4071.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
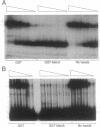
- Msadek T., Kunst F., Rapoport G. MecB of Bacillus subtilis, a member of the ClpC ATPase family, is a pleiotropic regulator controlling competence gene expression and growth at high temperature. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5788–5792. doi: 10.1073/pnas.91.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggiani M., Hahn J., Dubnau D. Suppression of early competence mutations in Bacillus subtilis by mec mutations. J Bacteriol. 1990 Jul;172(7):4056–4063. doi: 10.1128/jb.172.7.4056-4063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Squires C., Squires C. L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992 Feb;174(4):1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosman B., Kooistra J., Olijve J., Venema G. Integration of vector-containing Bacillus subtilis chromosomal DNA by a Campbell-like mechanism. Mol Gen Genet. 1986 Sep;204(3):524–531. doi: 10.1007/BF00331035. [DOI] [PubMed] [Google Scholar]
- Wolfe K. H., Morden C. W., Palmer J. D. Function and evolution of a minimal plastid genome from a nonphotosynthetic parasitic plant. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10648–10652. doi: 10.1073/pnas.89.22.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- van Sinderen D., ten Berge A., Hayema B. J., Hamoen L., Venema G. Molecular cloning and sequence of comK, a gene required for genetic competence in Bacillus subtilis. Mol Microbiol. 1994 Feb;11(4):695–703. doi: 10.1111/j.1365-2958.1994.tb00347.x. [DOI] [PubMed] [Google Scholar]